Abstract
Objective/background
Aortic pulse wave velocity (PWV), the gold-standard assessment of central arterial stiffness, has prognostic value for cardiovascular disease risk in able-bodied individuals. The aim of this study was to compare aortic PWV in athletes and non-athletes with spinal cord injury (SCI).
Design
Cross-sectional comparison.
Methods
Aortic PWV was assessed in 20 individuals with motor-complete, chronic SCI (C2–T5; 18 ± 8 years post-injury) using applanation tonometry at the carotid and femoral arterial sites. Ten elite hand-cyclists were matched for sex to 10 non-athletes; age and time since injury were comparable between the groups. Heart rate and discrete brachial blood pressure measurements were collected throughout testing.
Outcome measures
Aortic PWV, blood pressure, heart rate.
Results
Aortic PWV was significantly lower in athletes vs. non-athletes (6.9 ± 1.0 vs. 8.7 ± 2.5 m/second, P = 0.044). There were no significant between-group differences in resting supine mean arterial blood pressure (91 ± 19 vs. 81 ± 10 mmHg) and heart rate (60 ± 10 vs. 58 ± 6 b.p.m.).
Conclusion
Athletes with SCI exhibited improved central arterial stiffness compared to non-athletes, which is in agreement with the previous able-bodied literature. This finding implies that chronic exercise training may improve arterial health and potentially lower cardiovascular disease risk in the SCI population.
Keywords: Cardiovascular diseases, Exercise, Pulse wave analysis, Spinal cord injuries, Vascular stiffness
Introduction
Individuals with spinal cord injury (SCI) are forced to extensive immobility due to paralysis and can experience a myriad of autonomic dysfunctions.1 These two adverse alterations may predispose individuals with SCI to amplified risk factors for cardiovascular disease (CVD) relative to able-bodied individuals. In fact, CVD is a leading cause of mortality in the SCI population, with the incidence of CVD occurring at earlier ages compared to able-bodied individuals.2
Increased central arterial stiffness, as a non-traditional risk factor, has been shown to be a major contributor to CVD risk in able-bodied individuals.3 The gold-standard assessment of central arterial stiffness is considered to be aortic pulse wave velocity (PWV),4 which has prognostic value of cardiovascular morbidity and all-cause mortality in the able-bodied population.3
There is a limited number of investigations on aortic PWV in individuals with SCI.5,6 To date, two investigations have demonstrated increased aortic PWV in SCI compared to able-bodied individuals, even when matched for age, sex, weight, height,5 and physical activity levels.6 The importance of physical exercise in the amelioration of arterial stiffness has been clearly demonstrated in able-bodied individuals.7 However, in the SCI population, the effect of high level of physical exercise training on aortic PWV has not been studied. This prompted us to test the hypothesis that high levels of physical exercise training improve central arterial stiffness in the SCI population.
Methods
Participants
Twenty individuals with motor-complete traumatic SCI (C2–T5, 2–29 years post-injury) participated in this study. Ten of these individuals with SCI were elite hand-cyclists and sex matched to ten non-athletes, sedentary individuals with SCI (Table 1). Neurological level of lesion and American Spinal Injury Association Impairment Scale (AIS) classification was determined using the International Standards for Neurological Classification of SCI.8 Athletes were recruited and assessed at the Para-cycling World Championship in Baie-Comeau, Quebec, while non-athletes were recruited from the Spinal Cord Injury Outpatient Program at Vancouver General Hospital.
Table 1 .
Subject characteristics and outcome parameters of athletes and non-athletes
| Lesion level | AIS | Lesion duration (years) | Height (m) | Weight (kg) | Sex | Age (years) | SBP/DBP (mmHg) | MAP (mmHg) | HR (b.p.m.) | aPWV (m/second) | Physical exercise (hours/week) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Athletes | ||||||||||||
| SCI_01 | C2 | A | 29 | 1.73 | 62 | M | 48 | 101/57 | 72 | 50 | 6.3 | 12 |
| SCI_02 | C4 | B | 23 | 1.76 | 70 | M | 42 | 104/62 | 76 | 53 | 7.8 | 18 |
| SCI_03 | C4 | B | 24 | 1.69 | 55 | F | 42 | 95/52 | 66 | 53 | 5.9 | 12 |
| SCI_04 | C5 | A | 17 | 1.81 | 62 | M | 37 | 112/66 | 82 | 49 | 6.0 | 14 |
| SCI_05 | C5 | B | 18 | 1.78 | 80 | M | 38 | 116/63 | 81 | 61 | 6.5 | 15 |
| SCI_06 | C7 | A | 14 | 1.92 | 71 | M | 54 | 132/80 | 97 | 63 | 8.0 | 21 |
| SCI_07 | C7 | A | 14 | 1.80 | 74 | M | 38 | 116/60 | 78 | 66 | 7.3 | 15 |
| SCI_08 | T2 | A | 12 | 1.80 | 66 | M | 32 | 111/54 | 73 | 63 | 5.8 | 21 |
| SCI_09 | T4 | A | 25 | 1.78 | 59 | F | 41 | 125/75 | 92 | 62 | 8.5 | 21 |
| SCI_10 | T5 | A | 17 | 1.72 | 60 | M | 37 | 125/72 | 90 | 62 | 6.6 | 24 |
| mean ± SD | 19 ± 6 | 1.78 ± 0.06 | 66 ± 8 | 41 ± 6 | 114/64 | 81 ± 10 | 58 ± 6 | 6.9 ± 1.0 | 17 ± 4 | |||
| ±12/9 | ||||||||||||
| Non-athletes | ||||||||||||
| SCI_11 | C4 | A | 16 | 1.93 | 95 | M | 43 | 104/59 | 74 | 71 | 6.0 | 0 |
| SCI_12 | C4 | A | 3 | 1.86 | 88 | M | 58 | 174/102 | 131 | 58 | 14.5 | 0 |
| SCI_13 | C4 | A | 26 | 1.88 | 88 | M | 55 | 148/92 | 111 | 77 | 9.5 | 0 |
| SCI_14 | C4 | A | 2 | 1.65 | 47 | F | 23 | 92/61 | 71 | 51 | 7.1 | 5 |
| SCI_15 | C4 | B | 26 | 1.58 | 68 | M | 42 | 93/59 | 70 | 60 | 6.4 | 0 |
| SCI_16 | C4 | B | 25 | 1.83 | 67 | M | 43 | 135/85 | 102 | 59 | 10.6 | 0 |
| SCI_17 | C5 | A | 25 | 1.93 | 98 | M | 46 | 126/78 | 93 | 61 | 7.5 | 0 |
| SCI_18 | T2 | A | 5 | 1.73 | 91 | M | 33 | 114/71 | 85 | 65 | 8.1 | 3 |
| SCI_19 | T3 | A | 10 | 1.78 | 60 | M | 30 | 106/62 | 90 | 50 | 8.1 | 2 |
| SCI_20 | T3 | A | 28 | 1.60 | 72 | F | 46 | 109/66 | 84 | 43 | 9.1 | 2 |
| mean ± SD | 17 ± 11 | 1.78 ± 0.13 | 77 ± 17 | 42 ± 11 | 120/73 | 91 ± 19 | 60 ± 10 | 8.7 ± 2.5 | 1 ± 2 | |||
| ±26/15 | ||||||||||||
AIS, American Spinal Injury Association Impairment Scale; aPWV, aortic pulse wave velocity; C, cervical; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure T, thoracic.
All individuals with SCI were instructed to abstain from caffeine for 12 hours before aortic PWV testing. Furthermore, prior to testing individuals were asked to empty their bladder to minimize the influence of reflexive sympathetic activation on peripheral vascular tone. None of the individuals reported a history of smoking or CVD, such as coronary artery disease, pulmonary disease, diabetes mellitus, or metabolic syndrome. Each individual's current medication (Table 2) and amount of weekly physical activity (in hours, Table 1) were recorded. This study was approved by the local ethics board and is in accordance to the Declaration of Helsinki. In addition, all individuals provided written informed consent prior to data collection.
Table 2 .
Medication of athletes and non-athletes with SCI
| Athletes | Non-athletes | ||
|---|---|---|---|
| SCI_01 | – | SCI_11 | – |
| SCI_02 | – | SCI_12 | Amitriptyline, Nortriptyline, Pregabalin |
| SCI_03 | Softlax, Dulcolax | SCI_13 | Gabapentin |
| SCI_04 | Baclofen | SCI_14 | Pregabalin, Amitriptyline |
| SCI_05 | Baclofen, Pregabalin | SCI_15 | Baclofen, Gabapentin |
| SCI_06 | Acetan | SCI_16 | Venlafaxine, Zopiclone |
| SCI_07 | – | SCI_17 | – |
| SCI_08 | Detrusitol | SCI_18 | Ditropan |
| SCI_09 | Baclofen, Ditropan, Nitrofurantoin, Citalopram, Cefadroxil | SCI_19 | Ditropan |
| SCI_10 | Ditropan, Nitrofurantoin | SCI_20 | Methadone |
PWV assessment
Following 10 minutes of rest in the supine position, measurements of arterial pressure waveforms were captured using applanation tonometry according to the most recent guidelines.4 A trained rater collected pulse pressure waveforms at the left carotid, and femoral arteries for a minimum of 30 seconds using a handheld tonometer (Model SPT-301; Millar Instruments Inc., Houston, TX, USA), as previously described.9 Aortic PWV was calculated using the equation, PWV = (0.8 × D)/Δt where D is the distance between the carotid and femoral arterial sites measured along the body surface using anthropometric measuring tape held parallel to the body, and Δt is the pulse transit time between those sites.4 Arterial pressure waveforms were bandpass filtered (2–30 Hz), and the arrival of the waveform at each site was identified as the minimum value of the filtered signal. PWV was calculated as the average of two, 10-second samples. If the difference between the values for these two samples was >0.5 m/second, a third 10-second sample was calculated and the median of the three values was reported as the aortic PWV.4 Throughout the PWV assessment, discrete brachial blood pressure (BP) measurements and heart rates (HR) were collected every minute on the left arm using an automated device (Carescape™ V100; GE Healthcare, Milwaukee, WI, USA). Supine brachial BP and HR values are reported as the average values recorded during the PWV measurements.
Statistics
Statistical analyses were performed using SPSS (Version 20.0; IBM Corporation, Armonk, NY, USA). Differences in aortic PWV, resting hemodynamic indices, and demographic parameters between athletes and non-athletes were determined using independent-samples t-tests. Data are presented as mean ± SD, with the significance level set at P < 0.05.
Results
There was no significant difference in age (P = 0.793), time since injury (P = 0.499), weight (P = 0.072), or height (P = 0.966) between athletes and non-athletes (Table 1). The amount of physical exercise, measured as self-reported hours of exercise per week, was as expected significantly (P < 0.001) higher in athletes compared to non-athletes (17 ± 4 vs. 1 ± 2 hours/week).
Resting supine hemodynamic parameters are presented in Table 1. There were no significant differences in systolic BP (P = 0.484), diastolic BP (P = 0.108), mean arterial pressure (P = 0.139), and HR (P = 0.827) between athletes and non-athletes.
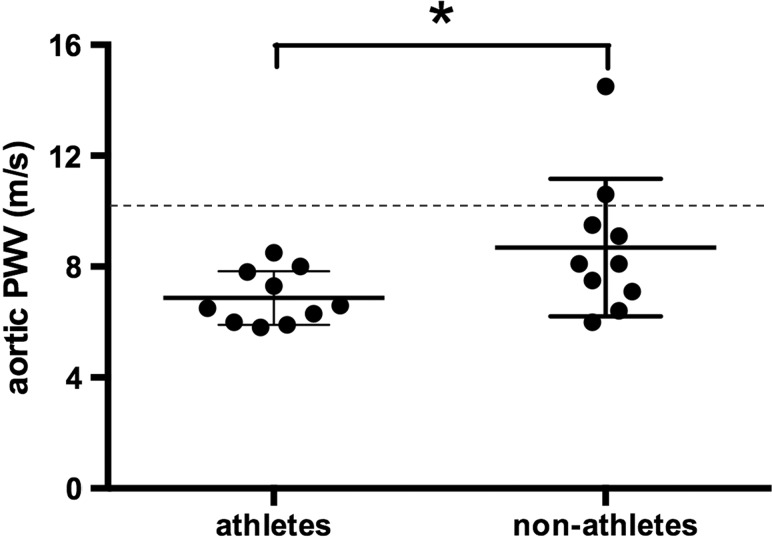
Fig. 1 depicts the difference in aortic PWV assessments between athletes and non-athletes. Aortic PWV was significantly (P = 0.044) lower in athletes as compared with non-athletes (6.9 ± 1.0 vs. 8.7 ± 2.5 m/second).
Figure 1 .
Aortic PWV in athletes and non-athletes with SCI. Dashed line indicates the clinical cut-off value for increased CVD risk.4 *P < 0.05
Discussion
Our study is the first to investigate the influence of a high physical exercise training level in individuals with SCI on aortic PWV, a prognostic index of CVD risk in the able-bodied population. We report significantly lower aortic PWV in athletes with high level of physical exercise training (mean 17 hours/week) compared to sex-matched non-athletes with SCI of comparable age and time since injury. This finding is in line with previous reports in the able-bodied literature comparing trained vs. untrained individuals.10 The difference in aortic PWV between athletes and non-athletes with SCI was 1.8 m/second. The lower central arterial stiffness in athletes might be interpreted as clinically relevant, since it has been shown that a 1.0 m/second change in aortic PWV translates into a 15% increased risk of CVD mortality in the able-bodied population.3 On the other hand, both of our assessed groups are still below the clinical cut-off value for aortic PWV of 10 m/second, with values above this indicating an increased risk for CVD in able-bodied individuals.4
To date, aortic PWV in the SCI population has only been investigated by two studies.5,6 Applying the PWV equation used in our investigation, the first study demonstrated an average aortic PWV for their middle-aged (46 ± 8 years) individuals with SCI of 10.2 m/second,5 whereas the second study reported an average aortic PWV of 5.8 m/second for its younger (30 ± 7 years) and physically active SCI sample.6 The average aortic PWV for our middle-aged non-athletic sample was 8.7 m/second lying in between the former investigations, whereas aortic PWV in our middle-aged athletic group was 6.9 m/second. While it is difficult to draw conclusions on the values obtained in our SCI groups based on the limited and varied evidence on aortic PWV in SCI, it is important to note that aortic PWV of elite hand-cycling athletes is even lower than the reference value of 7.2 ± 2.6 m/second of able-bodied normotensive individuals (40–49 years old).11
It has been shown that transient changes in BP can alter PWV.12 However, our reported hemodynamic indices were not significantly different between athletes and non-athletes and were therefore unlikely to account for group differences in aortic PWV.
In conclusion, this study suggests for the first time that a high level of physical exercise training in individuals with SCI attenuates arterial stiffness measured as aortic PWV and might reduce CVD risk in this population. However, the value of aortic PWV as a predictor for CVD risk in the SCI population still needs to be established.
Disclaimer statements
Contributors MH is the primary author and was responsible for study conception, data collection, data analyses, data interpretation, and drafting of the manuscript. KDC was responsible for study design, data collection, data analyses, data interpretation, and drafting of the manuscript. CRW was responsible for data collection and drafting of the manuscript. CMG was responsible for participant recruitment and drafting of the manuscript. AVK is the senior author and was responsible for study conception, data interpretation, drafting of the manuscript, and final approval of the manuscript.
Funding This study has been funded through the following fellowships: Swiss National Science Foundation (MH), Heart and Stroke Foundation (KDC), Craig H. Neilsen and Michael Smith Foundation for Health Research (CRW).
Conflicts of interest None.
Ethics approval Research Ethics Board of University of British Columbia.
References
- 1.Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol 2009;169(2):157–64. [DOI] [PubMed] [Google Scholar]
- 2.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007;86(2):142–52. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55(13):1318–27. [DOI] [PubMed] [Google Scholar]
- 4.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30(3):445–8. [DOI] [PubMed] [Google Scholar]
- 5.Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med 2009;32(1):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips AA, Cote AT, Bredin SS, Krassioukov AV, Warburton DE. Aortic stiffness increased in spinal cord injury when matched for physical activity. Med Sci Sports Exerc 2012;44(11):2065–70. [DOI] [PubMed] [Google Scholar]
- 7.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 2008;105(4):1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie KD, Hubli M, Krassioukov AV. Applanation tonometry: a reliable technique to assess aortic pulse wave velocity in spinal cord injury. Spinal Cord 2014;52(4):272–5. [DOI] [PubMed] [Google Scholar]
- 10.Laurent P, Marenco P, Castagna O, Smulyan H, Blacher J, Safar ME. Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J Am Soc Hypertens 2011;5(2):85–93. [DOI] [PubMed] [Google Scholar]
- 11.Collaboration TRVfAS. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010;31(19):2338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nye ER. The effect of blood pressure alteration on the pulse wave velocity. Br Heart J 1964;26:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]