Abstract
Purpose.
This study tested the hypothesis that highly targeted intrastromal delivery of bevacizumab using coated microneedles allows dramatic dose sparing compared with subconjunctival and topical delivery for treatment of corneal neovascularization.
Methods.
Stainless steel microneedles 400 μm in length were coated with bevacizumab. A silk suture was placed in the cornea approximately 1 mm from the limbus to induce corneal neovascularization in the eyes of New Zealand white rabbits that were divided into different groups: untreated, microneedle delivery, topical eye drop, and subconjunctival injection of bevacizumab. All drug treatments were initiated 4 days after suture placement and area of neovascularization was measured daily by digital photography for 18 days.
Results.
Eyes treated once with 4.4 μg bevacizumab using microneedles reduced neovascularization compared with untreated eyes by 44% (day 18). Eyes treated once with 2500 μg bevacizumab using subconjunctival injection gave similar results to microneedle-treated eyes. Eyes treated once with 4.4 μg subconjunctival bevacizumab showed no significant effect compared with untreated eyes. Eyes treated with 52,500 μg bevacizumab by eye drops three times per day for 14 days reduced the neovascularization area compared with untreated eyes by 6% (day 18), which was significantly less effective than the single microneedle treatment. Visual exam and histological analysis showed no observable effect of microneedle treatment on corneal transparency or microanatomical structure.
Conclusions.
This study shows that microneedles can target drug delivery to corneal stroma in a minimally invasive way and demonstrates effective suppression of corneal neovascularization after suture-induced injury using a much lower dose compared with conventional methods.
Keywords: microneedle, intrastromal, neovascularization, bevacizumab
Intrastromal delivery of bevacizumab using microneedles shows significant dose sparing compared with topical and subconjunctival delivery.
Introduction
Corneal neovascularization is the invasion of blood vessels into the clear cornea, which can cause visual impairment. It is estimated that corneal neovascularization develops in 1.4 million people per year in the United States, and 12% of these patients experience vision loss.1 Corneal neovascularization can be caused by infectious pathogens, inflammatory responses, physical trauma, or degenerative diseases.2 Conventional therapy for corneal neovascularization relies on steroids, such as hydrocortisone and dexamethasone.2 However, steroids carry the risk of serious side effects such as cataract and glaucoma.3
Recently, anti-VEGF treatments have shown promising results for treating corneal neovascularization.4 Currently, topical and subconjunctival injection of bevacizumab is used off-label in the clinic to treat corneal neovascularization.5–7 However, topical administration is extremely inefficient due to the barrier properties of corneal epithelium, and systemic delivery is often accompanied by side effects.2,8–11 Subconjunctival administration is a more efficient and targeted delivery method. However, subconjunctival injection of bevacizumab can cause side effects due to the high dose requirements and may not be suitable for long-term use.2,8–11 Possible side effects with prolonged use of bevacizumab includes thinning or erosive changes to the conjunctiva, sclera, and epithelium.2,12,13 Intrastromal injection of bevacizumab using a hypodermic needle has shown promising results.14–16 Building off these findings, the use of a microneedle could make intrastromal delivery a simple, reliable, and highly targeted procedure.
Microneedles are individual needles or arrays of micrometer-sized needles that are manufactured by adapting the tools of the microelectronics industry.17–19 Solid microneedles were originally used for drug delivery to the skin, either by creating micron-scale holes in the skin to increase permeability20 or by coating drug onto microneedles for rapid or controlled release by dissolution within the skin.21,22 A number of different drugs and vaccines have been delivered to animals and human subjects in this way.23–26
More recently, microneedles have been studied for applications in the eye.27 In a first study, solid microneedles coated with pilocarpine were applied to the cornea and shown to dramatically increase bioavailability of the drug in the anterior segment compared with topical eye drops as evidenced by pupil dilation in rabbits.28 Additional studies have addressed posterior segment delivery using solid and hollow microneedles for drug delivery into a scleral depot,29,30 as well as drug delivery into the suprachoroidal space.31–33
In this study, we tested the hypothesis that highly targeted intrastromal delivery of bevacizumab using coated microneedles allows dramatic dose sparing compared with subconjunctival and topical delivery for treatment of corneal neovascularization. We designed the microneedles to be small enough to penetrate to a depth of a few hundred micrometers into the corneal stroma without crossing the corneal endothelium. This study assesses the efficacy of intrastromal delivery using microneedles in an injury-induced neovascularization model and compares microneedle-based therapy with conventional topical and subconjunctival delivery of bevacizumab. We expect microneedle-based delivery to enable significant dose sparing by targeting bevacizumab precisely at the site of neovascularization in the corneal stroma to suppress blood vessel growth. This study presents the design, fabrication, and characterization of microneedles coated with bevacizumab in both the ex vivo and in vivo rabbit eye to examine the efficacy and safety of this drug delivery method compared with topical and subconjunctival delivery.
Materials and Methods
Fluorescent Labeling of Bevacizumab
Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) was labeled using an antibody/protein labeling kit protocol (SAIVI AlexaFluor 750; Life Technologies, Carlsbad, CA, USA). Briefly, AlexaFluor N-hydroxysuccinimide esters were incubated with the protein in a basic medium (pH 9.3). Labeled protein (bevacizumab) was isolated and purified by gel filtration. The final dye-to-protein ratio (number of AlexaFluor molecules coupled to each protein molecule) was determined to be between 2.5 and 3.5 according to a protocol from Invitrogen. Finally, this solution of labeled protein (8 mg/mL) was mixed with untagged bevacizumab (i.e., Avastin, 25 mg/mL) at a volumetric ratio of 1:1 and was stored in the dark at 4°C.
ELISA of Bevacizumab
A serial dilution of bevacizumab (6.25–50 ng/mL) was used to generate a standard curve. Bevacizumab-coated microneedles were dissolved in PBS and diluted as needed to bring the concentration into the ELISA assay range. Diluted solutions were put in triplicate into wells in a ELISA plate (MaxiSorp; Nunc, Roskilde, Denmark). Plates with VEGF-165 (R&D Systems, Minneapolis, MN, USA) were coated overnight at 4°C in sodium carbonate buffer at pH 9.6. Plates were washed three times with PBS-T (PBS with 0.05% Tween-20) and blocked with 300 μL per well of 1% bovine serum albumin (BSA) in PBS for 2 hours at room temperature. After three washes with 300 μL PBS-T each, 100 μL bevacizumab-containing samples were added in triplicate for 2 hours at room temperature. They were then washed three times with PBS-T as above and 100 μL horseradish peroxidase-labeled goat-anti-human IgG (R&D Systems) in 0.1% BSA per well and then incubated for 2 hours at room temperature. Washing was performed as described and 100 μL TMB (3,3′, 5,5"-tetramethylbenzidine) substrate reagent solution (R&D Systems) was transferred into each well. Reaction was terminated after 20 minutes by adding 50 μL of 0.5 M HCl to each well. Absorbance was measured spectrophotometrically at a wavelength of 450 nm (iMark Microplate Reader; Bio-Rad, Hercules, CA, USA).
Microneedle Fabrication and Coating
To make coating formulations, the solution described above containing a mixture of labeled and unlabeled bevacizumab was further diluted with stock solution of bevacizumab (i.e., Avastin, 25 mg/mL) at a volumetric ratio of 1:1. The mixed solution was repeatedly centrifuged using centrifuge filters (Nanosep; Pall Corp., Port Washington, NY) with a 3 kDa molecular weight cutoff until the retentate reached a concentration of 100 mg/mL of bevacizumab. This solution was then immediately mixed with 5% carboxymethylcellulose at a volumetric ratio of 1:3 to make the final coating solution.34
Solid microneedles were fabricated by cutting needle structures from stainless steel sheets (SS304, 75-μm thick; McMaster Carr, Atlanta, GA, USA) using an infrared laser (Maestro Series; Resonetics, Nashua, NH, USA) and then electropolished to yield microneedles of defined geometry that were 400 μm in length, 150 μm in width, 75 μm in thickness, and 55° in tip angle, as described previously.21 Prior to coating, microneedles were treated in a plasma cleaner (PDC-32CG; Harrick Plasma, Inc., Ithaca, NY, USA) to facilitate coating of the formulation on the microneedles. Microneedles were coated by dipping 10 to 40 times into the coating solution at room temperature.
Hollow microneedles were fabricated from borosilicate micropipette tubes (Sutter Instrument, Novato, CA, USA), as described previously.29 A custom, pen-like device with a threaded cap was fabricated to position the microneedle and allow precise adjustment of its length, as described previously.35 This device was attached to a gas-tight, 10-μL glass syringe (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Induction of Corneal Neovascularization
All animal studies adhered to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee. Male and female New Zealand rabbits (2.2–2.5 kg) were anesthetized with ketamine (17 mg/kg); xylazine (8.5 mg/kg); and acepromazine (0.5 mg/kg) subcutaneously. Following topical administration of 0.5% proparacaine hydrochloride to minimize discomfort, a single 7.0-gauge silk suture (TG140; Ethicon, Inc., Blue Ash, OH, USA) was placed at midstromal depth 1 mm away from the limbus of the rabbit cornea to generate corneal neovascularization associated with minor traumatic injury. This silk suture was left in the rabbit cornea for the duration of the experiment to induce neovascularization.36,37 For each animal, a suture was placed in one eye and the companion eye was left untreated.
Measurement of Neovascularization
During the experiment, the rabbit eye was imaged using a digital camera (Canon Rebel T1i; Canon USA, Inc., Melville, NY, USA) with macroscopic lens (Canon MP-E, 65 mm; Canon USA, Inc.) at ×3 magnification every 2 days after placement of the suture. The area of neovascularization was quantified using a graphics editor (Adobe Photoshop; Adobe Systems, Jan Jose, CA, USA).
Experimental Treatment Groups
Prior to all treatment procedures except for topical delivery, rabbits were anesthetized with ketamine (6 mg/kg); xylazine (4 mg/kg); and acepromazine (0.25 mg/kg) subcutaneously. A reduced dose of anesthetic compared with the suture insertion procedure was used to reduce possible stress to the animals. A single drop of topical proparacaine ophthalmic solution was given as anesthesia. The duration of each study was 18 days and, after the suture insertion at the beginning of the experiment, we waited 4 days for neovascularization to develop. All the treatments were done on day 4 except as indicated below. The treatment groups are listed in the Table.
Table.
Treatment Groups
| Ut | Untreated group |
| Top | Topical delivery group |
| Sc-high | High-dose subconjunctival group |
| Sc-low | Low-dose subconjunctival group |
| Mn-1 bolus | 1-microneedle bolus delivery group |
| Mn-4 bolus | 4-microneedle bolus delivery group |
| Mn-1 ×3 | 1-microneedle, 3 doses, delivery group |
| Mn-placebo | 1-microneedle placebo group |
| Mn-hollow | Hollow microneedle bolus delivery group |
Untreated Group (Ut).
Other than applying a suture to the eye, these animals received no further treatments.
Topical Delivery Group (Top).
Topical delivery of bevacizumab was given into the upper conjunctival sack without anesthesia three times per day (at approximately noon, 3:00 PM and 6:00 PM) on days 4 through day 17. Each drop contained 1250 μg of bevacizumab in 50 μL, for a daily dose of 3750 μg of bevacizumab and a total dose of 52,500 μg of bevacizumab over the course of 14 days of treatment.
Subconjunctival Delivery Groups (Sc).
Bevacizumab was injected subconjunctivally with a 30-gauge hypodermic needle at the upper bulbar conjunctiva 4 days after suture placement. The high-dose group (Sc-high) received 2500 μg of bevacizumab (in 100 μL—i.e., Avastin). The low-dose group (Sc-low) received 4.4 μg bevacizumab (Avastin was diluted with HBSS to 100 μL).
Microneedle Delivery Groups (Mn).
Microneedles designed to deliver 1.1 μg bevacizumab were inserted at the site of silk suture placement in the cornea and left in place for 1 min to allow dissolution of the coating. For the one-microneedle bolus delivery group (Mn-1 bolus), a single microneedle (i.e., 1.1 μg bevacizumab) was given as a bolus dose 4 days after suture placement. For the four-microneedle bolus delivery group (Mn-4 bolus), four microneedles (i.e., 4.4 μg of bevacizumab) were given as a bolus dose 4 days after suture placement. For the one microneedle—three doses delivery group (Mn-1 × 3), a single microneedle (i.e., 1.1 μg of bevacizumab) was given as at 4, 6, and 8 days after suture placement (i.e., for a total dose of 3.3 μg bevacizumab). For the microneedle placebo group (Mn-placebo), four microneedles coated with formulation containing no bevacizumab was given as a bolus dose 4 days after suture placement. Finally, for the hollow microneedle bolus delivery group (Mn-hollow), a hollow microneedle was used to inject 2 μL of 25 mg/mL bevacizumab (i.e., Avastin, dose of 50 μg bevacizumab) intrastromally at the site of silk suture placement as a bolus dose 4 days after suture placement. After all of the insertion procedures, the eyelid was left closed for 5 minutes, after which all the tear fluid was wiped off the eye to collect any residual bevacizumab that was not able to penetrate into the cornea using a small piece of a Kimwipe towel. The used towels and microneedles were collected and incubated in HBSS to collect residual bevacizumab.
Fluorescently Labeled Bevacizumab Imaging Study
Prior to imaging, rabbits were anesthetized by subcutaneous injection using ketamine/xylazine/acepromazine at concentrations of 6/4/0.25 mg/kg. Eyes were kept open using a lid speculum for the duration of the imaging procedures. The fluorescence signal intensity in the rabbits was imaged on an in vivo imaging system (IVIS; Xenogen IVIS Spectrum; Caliper Life Sciences, Waltham, MA, USA) at 0, 2, and 4 days postinsertion. Animals were imaged at 745-nm excitation wavelength, 780-nm emission wavelength and 1 second exposure time. Fluorescence intensity was measured as background-subtracted average efficiency within a fixed region of interest centered on the insertion site.
Safety Study
To identify possible microanatomical changes after intrastromal delivery using microneedles, we conducted a histological safety study using four study groups: the untreated group received no suture and no other treatments; the suture-only group received a suture at day 0, but no other treatments; the suture with noncoated microneedles group received a suture on day 0 and four noncoated microneedles inserted at the site of the suture on day 4; and the suture with coated microneedles group received a suture on day 0 and four microneedles each coated with 1.1 μg of bevacizumab inserted at the site of the suture on day 4. Animals were killed on days 1, 6, 10, and/or 18 for histological analysis. Suture placement and microneedle application were carried out as described above. High magnification images were taken every day in all study groups to assess possible gross corneal damage. Corneal tissues were fixed in 10% formalin and embedded in paraffin. Hematoxylin and eosin or periodic acid-Schiff staining was performed.
Statistical Analysis
Replicate pharmacodynamics experiments were done for each treatment group above. Multiple images (3–6) from which the mean and standard error of mean were calculated. Experimental data were analyzed using two-way ANOVA to examine the difference between treatments. In all cases, a value of P < 0.05 was considered statistically significant.
Results
Characterization of Microneedles Coated With Bevacizumab
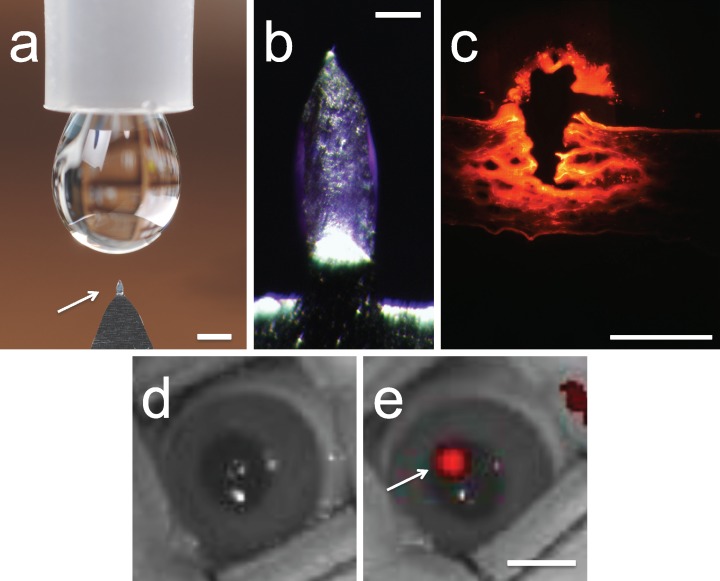
We first designed solid microneedles to penetrate into, but not across, the cornea and in that way deposit drug coated onto the microneedles within the corneal stroma at the site of microneedle penetration. Guided by the average rabbit corneal thickness of 400 μm38 and possible tissue deformation during microneedle insertion, the microneedles used for rabbit corneal insertion were 400 μm in length, 150 μm in width, 75 μm in thickness, and 55° in tip angle (Fig. 1a). These microneedles were coated with a dry film of bevacizumab that was localized to the microneedle shaft and not on the supporting base structure (Fig. 1b). Coatings were applied by dipping repeatedly into a solution of bevacizumab using an automated coating machine. This design enabled efficient delivery of bevacizumab into the corneal stroma at the site of microneedle insertion (Figs. 1c–e).
Figure 1.
Microneedles coated with bevacizumab for targeted intrastromal delivery. (a) A single microneedle (arrow) mounted on a handle for manual application is shown next to a liquid drop from a conventional eye dropper. Scale bar: 1 mm. (b) Magnified view of a microneedle coated with bevacizumab. Scale bar: 200 μm. (c) Histological section of rabbit cornea after delivery of AlexaFluor 750–labeled bevacizumab using a microneedle showing the site of microneedle penetration into the eye (arrow) and distribution of red-labeled bevacizumab in the corneal tissue. Scale bar: 1 mm. Image of a live rabbit eye (d) before treatment and (e) after inserting and removing a microneedle coated with AlexaFluor 750–labeled bevacizumab. The red fluorescence indicates the site of bevacizumab deposition within the corneal stroma (arrow). Scale bar: 5 mm.
Intracorneal-Intrastromal Delivery of Bevacizumab In Vivo
We wanted to quantify in vivo bioavailability of bevacizumab delivered from coated microneedles by tagging the bevacizumab with florescent dye. AlexaFluor 750 dye was tagged to bevacizumab to quantify using ELISA. Microneedles prepared by coating with 10, 20, 30, or 40 dips were inserted into the cornea of an anesthetized rabbit. The amount of bevacizumab coated per microneedle was quantified using ELISA. Coated microneedles were inserted into but not across the cornea for 60 seconds and then removed. We used an insertion time of 60 seconds because we expected it to be sufficient to dissolve most of the coating off the microneedles while minimizing possible patient discomfort and clinical throughput time in future applications. Images shown in Figure 1 indicate that the bevacizumab coating was largely deposited in the corneal stroma.
The amount of bevacizumab coated onto microneedles increased linearly from 1.1 to 7.6 μg per microneedles with increasing number of dip coats (Fig. 2). However, the amount of bevacizumab delivered into the cornea increased linearly with coating amount (Fig. 2). For example, coatings produced using 10 dip coats delivered 52% of the coated bevacizumab into the cornea, with most of the remaining drug still coated on the microneedle, whereas coatings produces using 40 dip coated delivered just 44% of the coated drug. These delivery efficiencies are similar to results from our previous study using fluorescein-coated microneedles in rabbit eyes.28 This effect may be explained by thick coatings on microneedles making insertion into tissue and rapid dissolution in the tissue more difficult. Given these data, we decided to use microneedles coated with 20 dip-coated as a compromise formulation that can deliver 1.14 ± 0.11 μg bevacizumab with reasonable efficiency for the pharmacodynamic tests in this study.
Figure 2.
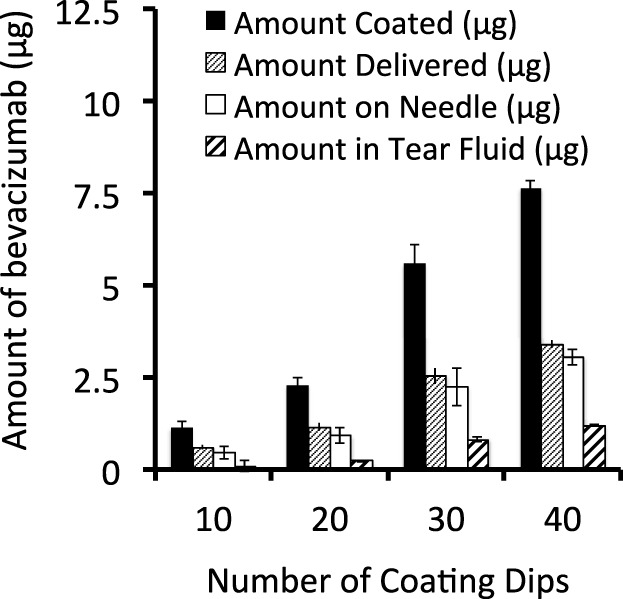
Quantification of bevacizumab coated onto microneedles and delivered into the cornea. Measured coating amount (μg), calculated amount delivered (μg), measured amount left on the needle (μg), and measured amount in tear fluid after the injection (μg). Data show average amount (μg) ± SEM (n = 4 replicates).
Efficacy of Intrastromal Delivery of Bevacizumab Using Microneedles Compared With Topical Delivery
To further assess the capability of microneedles as an intrastromal drug delivery platform, we created injury-induced neovascularization in a rabbit model and delivered bevacizumab using either microneedles and topical eye drops. Topical eye drops of bevacizumab have been used before to treat corneal neovascularization, but with limited efficacy and adverse events associated with prolonged use.12,13,39 We hypothesized that the highly targeted delivery of bevacizumab within the corneal stroma would provide significant dose sparing and better efficacy to suppress injury-induced neovascularization in a rabbit compared with topical delivery.
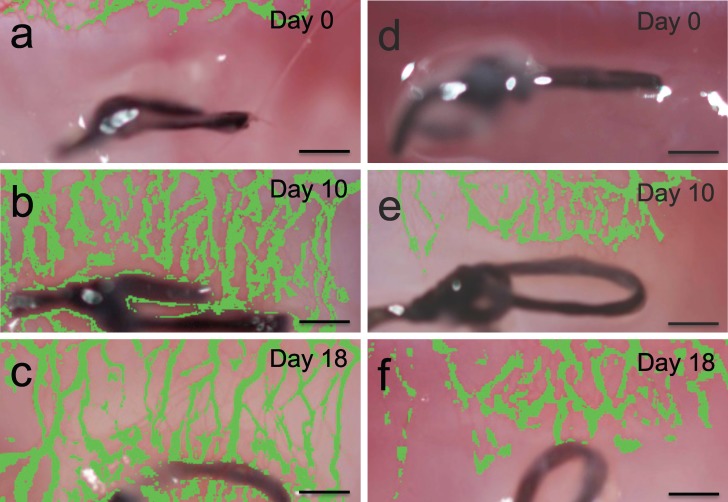
A suture was inserted into the mid-space of the cornea. All treatments were then started after 4 days, once significant neovascularization had developed. Changes in vascularization area in the eyes was measured using image analysis to compare the pharmacodynamics of topical and microneedle delivery. As negative controls, a group of rabbits were left untreated and another group of rabbits were treated with four placebo microneedles (Mn-placebo; coated with drug-free formulation). The untreated and placebo microneedle groups showed similar changes in corneal neovascularization with no statistical difference (P = 0.11), where the neovascularization area increased until day 10 and then decreased slightly until day 18 (Figs. 4a–c). The peak neovascularization area for the untreated group was 0.60 ± 0.06 mm2 on day 10 and by day 18 area was 0.49 ± 0.05 mm2 (Fig. 5).
Figure 4.
Corneal neovascularization after suture-induced injury. Representative photographs of rabbit cornea in vivo 0 days (a), 10 days (b), and 18 days (c) after applying a suture with no treatment. Photographs of rabbit cornea in vivo 0 days (d), 10 days (e), and 18 days (f) after applying a suture, followed by bolus administration of bevacizumab on day 4 using Mn-4 bolus. Area of the blood vessel growth has been false-colored to show the neovascularization area more clearly. Scale bars: 500 μm.
Figure 5.
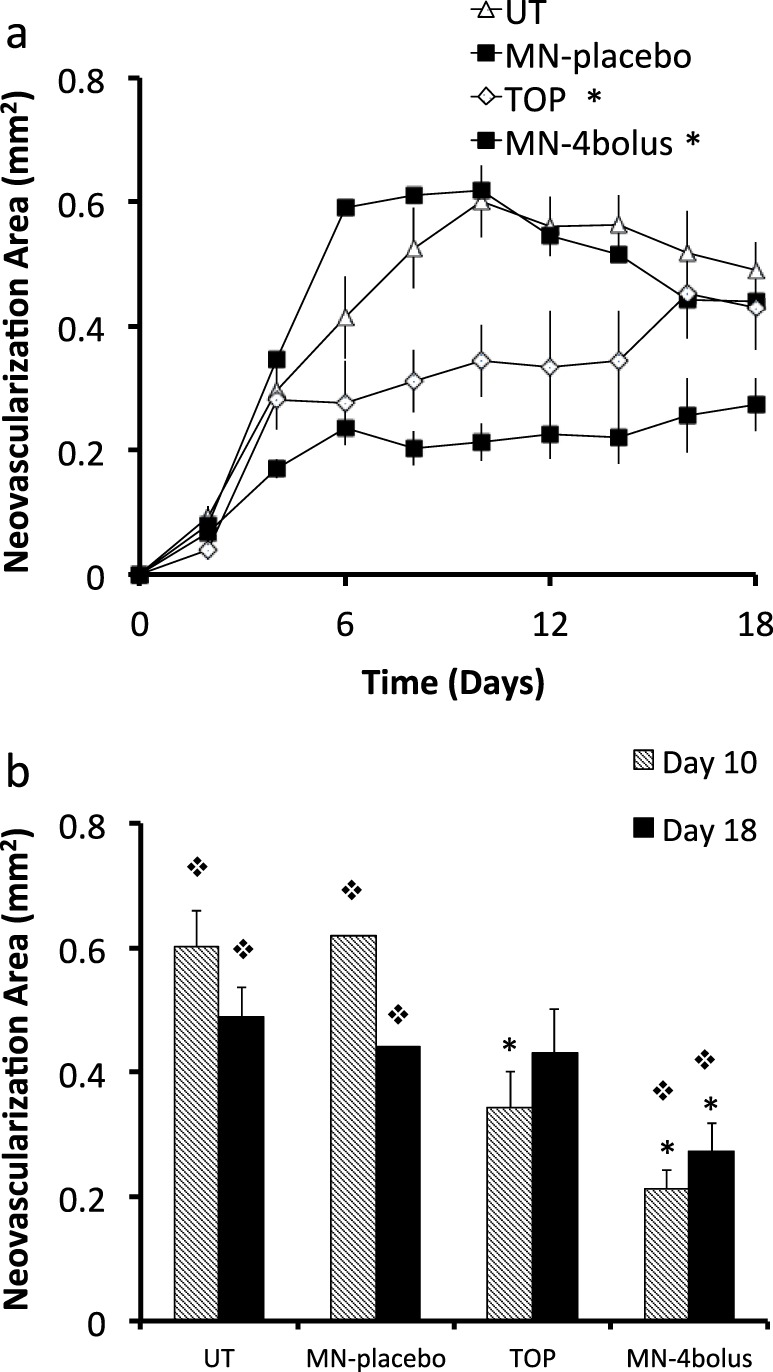
Quantification of corneal neovascularization after suture-induced injury and treatment with bevacizumab by topical and intrastromal routes. (a) Neovascularization area versus time and (b) comparison between neovascularization area at days 10 and 18 for four treatment groups: Ut, Mn-placebo, Top, and Mn-4 bolus. * Significant difference compared with the untreated group (P < 0.05). φ Significant difference compared with the Top group (P < 0.05). Data show average neovascularization area (mm2) ± SEM (n = 5 to 6 replicates).
For the topical delivery group, three topical eye drops were given every day from day 4 through the end of the experiment (day 18), which is a total of 52,500 μg of bevacizumab over a period of 14 days (i.e., 3750 μg/d). Topical eye drops reduced neovascularization compared with the untreated eyes by 44% (day 10) and 6% (day 18; Fig. 5). The topical eye drops group showed an immediate inhibition of the blood vessel growth after starting the treatment at day 4. However, neovascularization area increased steadily after that until the end of the experiment. At day 18, the topical eye drops group showed no significant difference versus the untreated eyes (one-way ANOVA, P = 0.36). Two-way ANOVA analysis showed that the change in neovascularization area for the topical group over time was significantly different from the untreated group (P < 0.0001). This is consistent with literature data that topical administration of bevacizumab can reduce corneal neovascularization.7,39,40
For the microneedles group (Mn-4 bolus), eyes were treated one time with 4.4 μg of bevacizumab using four microneedles. This small dose administered using microneedles reduced neovascularization area compared to the untreated eyes by 65% (day 10) and 44% (day 18; Figs. 4d–f, 5.) Two-way ANOVA analysis showed that the microneedles group was significantly more effective at reducing corneal neovascularization compared to the untreated group (P < 0.0001) and the topical group (P < 0.0001), even though the microneedles group used 9722 times less bevacizumab compared to topical delivery.
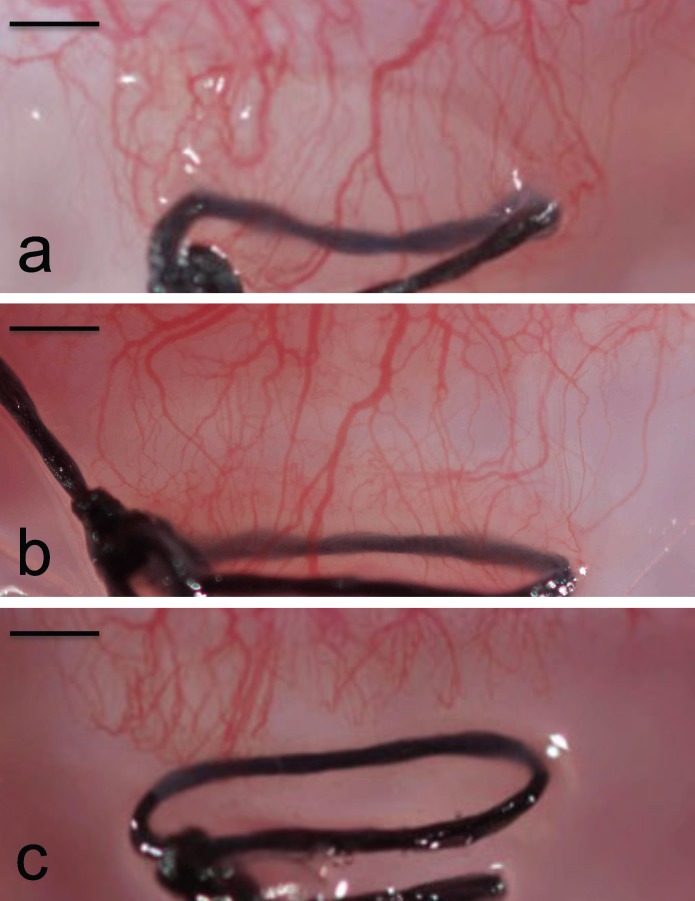
The fact that intrastromal delivery of just 4.4 μg of drug using microneedles outperformed the administration of 52,500 μg of topical bevacizumab shows the inefficiency of the topical delivery and the highly targeted nature of intrastromal delivery (Fig. 3). This low bioavailability of bevacizumab by topical delivery can be explained by the strong barrier properties of the cornea to macromolecules and the rapid clearance of topical formulations from the precorneal space.8,41 In possible future clinical use, the dose sparing enabled by intrastromal delivery may reduce the risk of adverse events associated with prolonged topical administration of bevacizumab.2,6,12,13
Figure 3.
Corneal neovascularization after suture-induced injury. Photographs of rabbit cornea in vivo 18 days after applying a suture (a) with no treatment (Ut), (b) with a single treatment of bevacizumab using topical eye drops (Top), and (c) with a single treatment of bevacizumab using solid microneedles (Mn-4 bolus). Scale bars: 500 μm.
Efficacy of Intrastromal Delivery of Bevacizumab Using Microneedles Compared With Subconjunctival Delivery
Next we sought to compare the pharmacodynamics of subconjunctival versus microneedle delivery methods. There are reports in the literature showing inhibition of blood vessel growth via subconjunctival delivery of bevacizumab.42–45 We hypothesized that highly targeted delivery of bevacizumab within the corneal stroma would provide significant dose sparing to suppress injury-induced neovascularization compared to subconjunctival delivery.
We measured changes in neovascularization area in eyes treated with Sc-high and Sc-low of bevacizumab. Based on the reported effective dose in literature,42–44 2500 μg (i.e., 100 μL of a 25 μg/μL bevacizumab solution) was given as a bolus on day 4 for the high-dose subconjunctival injection. For the low-dose subconjunctival injection, we decided to match the microneedle dose that was able to inhibit neovascularization (see Fig. 5). For this group, 4.4 μg of bevacizumab was given as a bolus on day 4.
Eyes treated with a low dose of 4.4 μg of bevacizumab by subconjunctival injection (SC-low) had no significant effect on neovascularization compared to the untreated eyes (UT) (Fig. 6, two-way ANOVA, P = 0.05). For the high-dose subconjunctival injection (SC-high), eyes treated with 2500 μg of bevacizumab significantly reduced neovascularization compared to the untreated eye by 62% (day 10) and 29% (day 18; Fig. 6, two-way ANOVA, P < 0.0001) and was not significantly different compared with the Mn-4 bolus group (Fig. 6, two-way ANOVA, P = 0.45). Although the pharmacodynamic responses for the microneedle group and high-dose subconjunctival group were similar, the microneedle group received 568 times less bevacizumab. This effect can be explained by the highly targeted nature of intrastromal delivery using microneedles.
Figure 6.
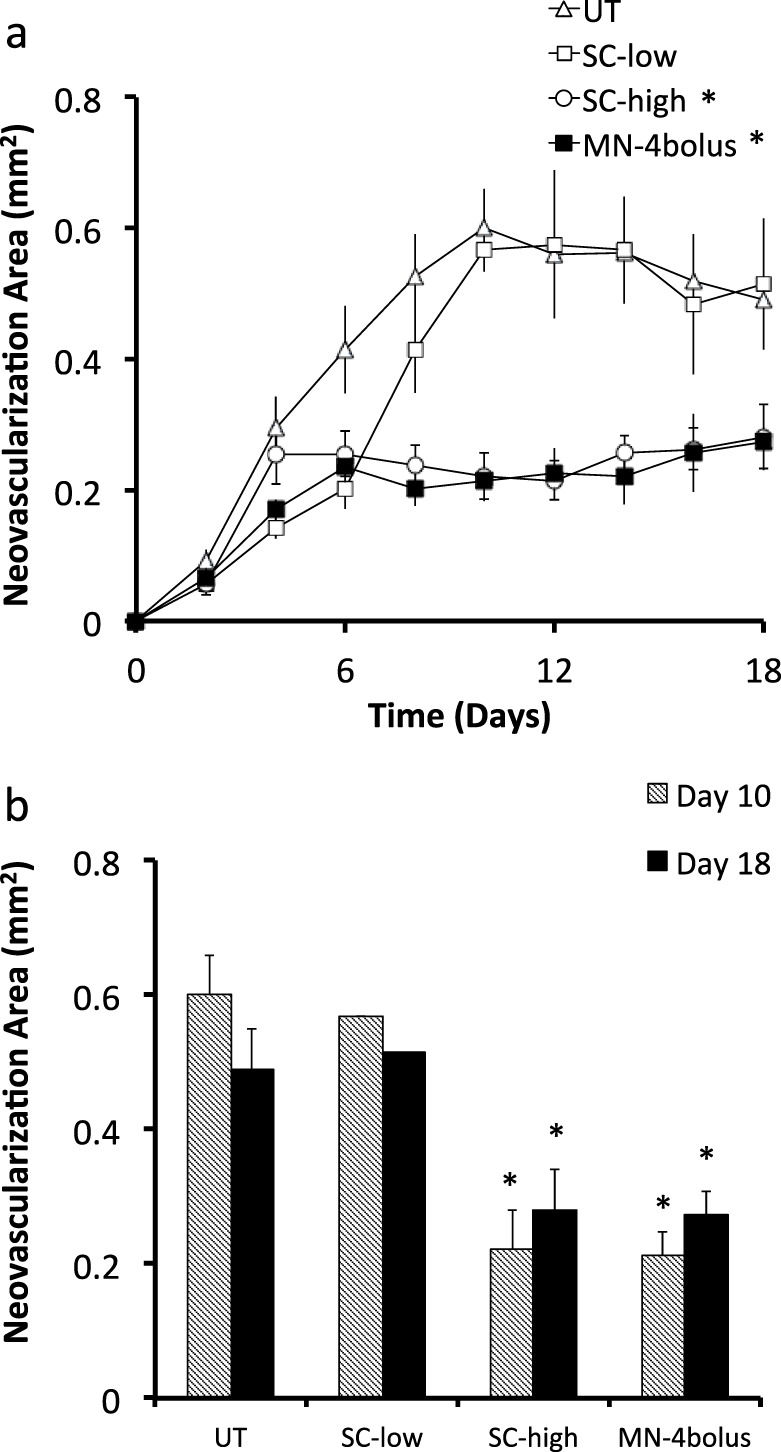
Quantification of corneal neovascularization after suture-induced injury and treatment with bevacizumab by subconjunctival and intrastromal routes. (a) Neovascularization area versus time and (b) comparison between neovascularization area at days 10 and 18 for four treatment groups: Ut, Sc-low, Sc-high, and Mn-4 bolus. * Indicates a significant difference compared with the untreated group (P < 0.05). Data show average neovascularization area (mm2) ± SEM (n = 5 to 6 replicates).
Effect of Bevacizumab Dose on Efficacy of Intrastromal Delivery Using Microneedles
In the experiments so far, a single bevacizumab dose of 4.4 μg was given by microneedles. We therefore investigated other intrastromal doses to better optimize the dosing regimen.
We first investigated a lower dose of 1.1 μg given as a bolus on day 4 using a single microneedle (Mn-1 bolus). The average neovascularization area was 34% (day 10) and 10% (day 18) lower after this Mn-1 bolus compared with the Ut group (Fig. 7, two-way ANOVA, P = 0.001). However, the Mn-1 bolus was not as effective at reducing neovascularization compared to the higher Mn-4 bolus group (Fig. 7, two-way ANOVA, P < 0.0009). This shows that an intrastromal bolus of 1.1 μg bevacizumab is effective, but a bolus of 4.4 μg bevacizumab is more effective.
Figure 7.
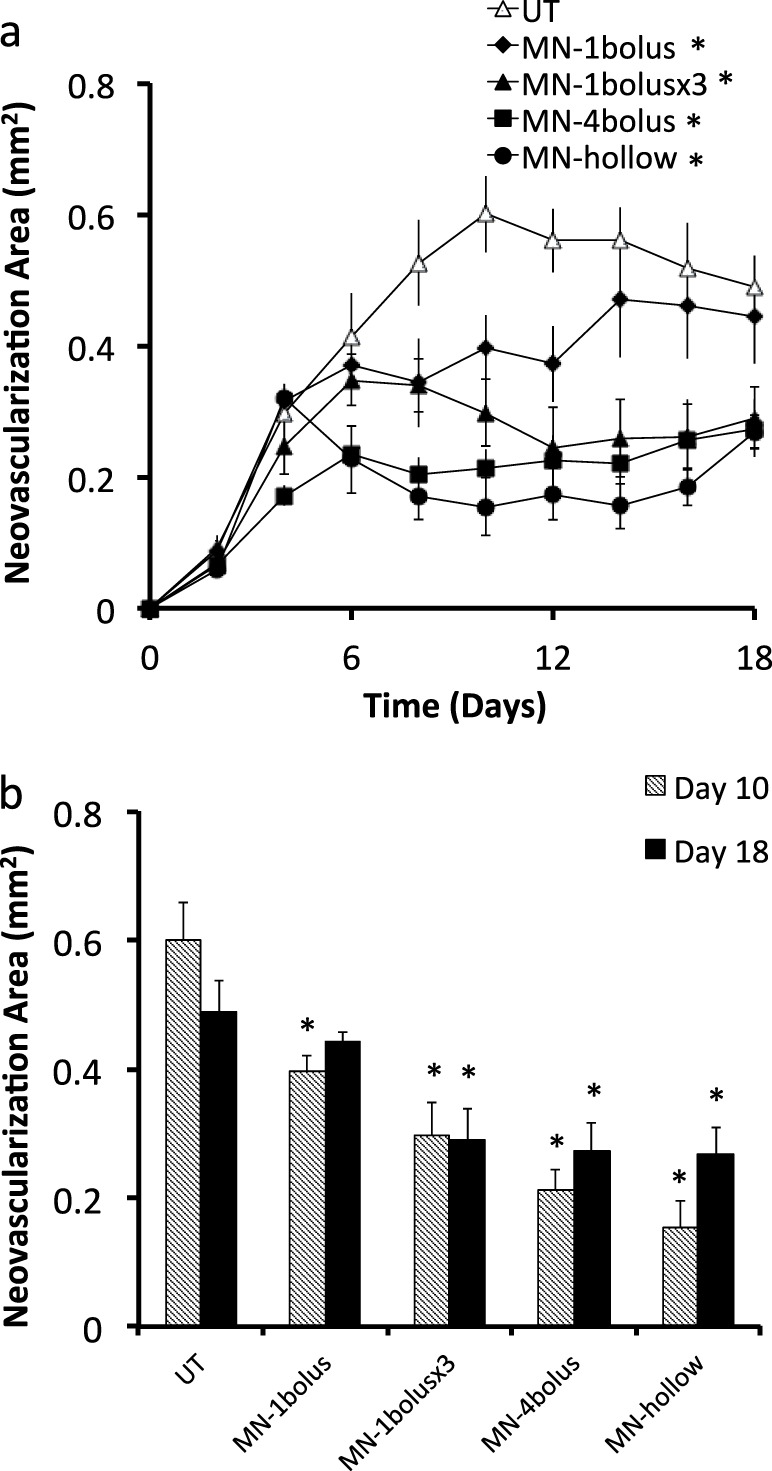
Quantification of corneal neovascularization after suture-induced injury and treatment with bevacizumab as a function of dose by intrastromal routes. (a) Neovascularization area versus time and (b) comparison between neovascularization area at days 10 and 18 for five treatment groups: Ut and Mn-1 bolus on day 4; Mn-1 bolus ×3 on days 4, 6, and 8; Mn-4 bolus on day 4; and 50 μg on day 4 (Mn-hollow). * Indicates a significant difference compared with the untreated group (P < 0.05). Data show average neovascularization area (mm2) ± SEM (n = 4–6 replicates).
We next investigated administration of bevacizumab as multiple sequential doses, in which eyes were treated with one microneedle administering 1.1 μg bevacizumab on days 4, 6 and 8 (i.e., for a total of 3.3 μg bevacizumab). This protocol (Mn-1 bolus ×3) reduced neovascularization area by 50% (day 10) and 41% (day 18), which was significantly better compared with the Ut group (Fig. 7, two-way ANOVA, P < 0.0009), but was not as effective as the Mn-4 bolus group (Fig. 7, two-way ANOVA, P = 0.019). The three-dose protocol appeared to have a delayed effect on inhibiting neovascularization, where the first dose had only a partial effect, but after the third dose inhibition of neovascularization was equivalent to that achieved with the Mn-4 bolus. This shows that multiple small doses can effective, but administration of a single bolus dose should be simpler in possible future clinical practice.
We finally investigated bolus intrastromal administration of an even higher dose of 50 μg of bevacizumab. This high dose would have required the use of 46 coated microneedles, which is impractical. We therefore injected this larger dose with a hollow microneedle (Mn-hollow; 2 μL of a 25 μg/μL bevacizumab solution) and found that it reduced neovascularization compared with Ut eyes by 74% (day 10) and 45% (day 18), (Fig. 7, two-way ANOVA, P < 0.0009) and was not significantly different compared with the Mn-4 bolus group (Fig. 7, two-way ANOVA, P = 0.154). This shows that giving a bolus dose more than 4.4 μg of bevacizumab did not provide additional improvement. However, this comparison is complicated by the fact that the high dose (Mn-hollow) was given as a liquid solution that spread over a larger area in the corneal stroma, in contrast to the solid formulation (Mn-4 bolus) that dissolved off the solid microneedles at the sites of microneedle insertion.
Safety of Intrastromal Delivery of Bevacizumab
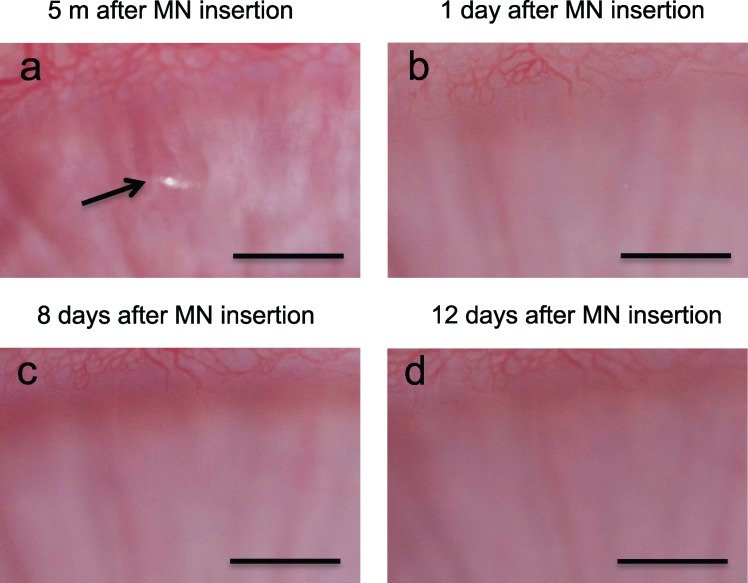
We analyzed rabbit corneas with and without microneedle treatment and with and without suture placement to assess the safety of microneedle insertion by both magnified inspection of the corneal surface in vivo and histological examination of tissue sections obtained at various times after microneedle treatment. Figure 8 shows a representative set of images of the corneal surface of a rabbit in vivo after insertion of a noncoated microneedle. Immediately after insertion and removal of the microneedle, a small puncture in the corneal epithelium is evident with a size on the order of 200 μm (Fig. 8a). By the next day, it was not possible to locate the insertion site due to apparent repair of the epithelium (Fig. 8b). Similarly, at later times the corneal surface continued to look intact and normal (Figs. 8c, 8d). We also examined eyes treated with bevacizumab-coated microneedles and again saw only a microscopic puncture in the corneal epithelium that disappeared within 1 day and was not associated with any complications (data not shown). We examined these injection sites on a daily basis throughout the 18-day experiments, but we never saw evidence of corneal opacity in any of the 22 eyes treated with microneedles in this study.
Figure 8.
Representative high-magnification bright-field microscopy images of the rabbit cornea in vivo at the site of insertion of a noncoated microneedle. Arrow points to site of microneedle insertion. Scale bars: 1 mm.
In addition to examining the corneal surface, we killed animals at different time points to look for changes in corneal microanatomical structure. Histological analysis was carried out by an investigator who is board certified in both ophthalmology and anatomic pathology. In comparison with untreated eyes (Fig. 9a), eyes treated by insertion of noncoated microneedles exhibited no significant changes in microanatomical structure of the cornea; no evidence of the corneal puncture could be found (Figs. 9b–d). There was also no significant presence of macrophages or vascularization observed. We also compared histological sections from eyes that only had a suture applied (Figs. 9e, 9f) to eye that had been sutured and then treated 4 days later with 1.1 μg bevacizumab using a microneedle (Figs. 9g, 9h). In the sutured eyes, there were large numbers of inflammatory cells present, but there were no notable differences seen between sutured eyes with and without microneedle treatment.
Figure 9.
Representative histological images showing histologic findings of cornea after microneedles treatment and/or suture placement: (a) untreated eye: microneedle-treated eye imaged 1 day (b), 6 days (c), and 14 days (d) after insertion and removal of an uncoated microneedle; suture-treated eye imaged 10 days (e) and 18 days (f) after suture placement; and suture- and microneedle-treated eye imaged 10 days (g) and 18 days (h) after suture placement (microneedle treatment was performed on day 4 using a microneedle coated with 1.1 μg bevacizumab). Scale bars: 100 μm.
Altogether, these data indicate that insertion of microneedles into the corneal is minimally invasive and thereby has few or no adverse effects on the eye. Overall, no adverse effects were seen in any experiments in this study. Although this analysis suggests that microneedle insertion into the eye may be well tolerated, additional studies are needed to assess safety more fully.
Discussion
In this study, we sought to assess the capability of coated microneedles to deliver a protein therapeutic to treat corneal neovascularization and compare this approach with subconjunctival and topical delivery. A series of experiments were conducted to measure neovascularization area in the rabbit eye using a number of different drug delivery methods. In vivo studies showed that bevacizumab can be rapidly delivered into the rabbit cornea using microneedles with approximately 50% bioavailability and have a long-lasting effect to suppress corneal neovascularization for at least 2 weeks.
In the injury-induced corneal neovascularization model, administration of 52,500 μg bevacizumab by topical delivery had an initial effect on neovascularization, but no longer significantly decreased neovascularization area by the end of the study on day 18 compared with the untreated group. In contrast, 4.4 μg bevacizumab given by microneedle delivery decreased neovascularization area of 44% on day 18 compared with the untreated group. This shows that topical eye drops provided a very inefficient way to deliver a protein therapeutic to the cornea. This is because a very small amount of drug is able to diffuse across the corneal epithelium, which has tight junctions around epithelial cells that block penetration of molecules.9 We thought that the damage caused by the relatively large 7-gauge silk suture might enhance topical penetration, but suture-induced damage was not enough to allow enough drug absorption to suppress neovascularization for 14 days.
Subconjunctival injection of 2500 μg bevacizumab reduced neovascularization compared with the untreated eye by 29% on day 18. This high-dose subconjunctival injection gave statistically the same response as 4.4 μg bevacizumab administered directly into the cornea by coated microneedles. The microneedle group was able to achieve the same response as the high-dose subconjunctival group using a dose 463 times smaller in the rabbit eye. We expect that the required dose will be even lower in the human eye because bevacizumab-binding is 7- to 8-fold greater in humans than rabbits,46 thereby enabling a longer residence time of bevacizumab in the cornea.
A safety study was done to examine the effect of intrastromal delivery using microneedles on corneal opacity and microanatomical structure. We expected that the small incision made into cornea using microneedles should not change corneal opacity because the small amount of damage it causes. Much more invasive surgery, such as cataract removal and LASIK, is preformed in millions patients each year in United States47 alone without causing corneal opacity. We also expected minimal concern with possible infection due to damage in corneal epithelium since corneal epithelium is one of the fastest healing tissues in the body.9 Throughout the safety and pharmacodynamics study, more than 8000 high-magnification images were taken around the microneedle insertion site and, at all times, there was never any evidence in change in corneal transparency. Moreover, these images revealed no other adverse effects either.
Recently, there has been an increasing number of reports showing the efficacy of off-label use of bevacizumab in treating corneal neovascularization.5–7 However, penetration of high-molecular weight (149 kDa) bevacizumab into intact corneal epithelium is difficult. The resulting high dose requirement for topical administration can result in unwanted side effects with prolonged use, including thinning or erosive changes to the conjunctiva, sclera, and epithelium.2,12,13 Subconjunctival injection provides more efficient delivery. However, there is still the possibility of causing side effects due to high dose requirements and this method may not be suitable for long-term use.2,12,13 Targeted delivery to the site of action using microneedles that is highly dose sparing should reduce side effects and possible complications associated with high doses required by conventional delivery methods. We believe microneedles provide a simple and reliable way to give bevacizumab into the cornea at the site of action.
In conclusion, this study showed that microneedles can be used to target delivery of bevacizumab to the cornea for suppression of corneal neovascularization. This approach was found to be equally effective as subconjunctival injection and superior to topical eye drops, yet used orders of magnitude less drug. Clinical exam and microanatomical analysis showed no observable adverse effects in eyes treated with microneedles, and the reduced dose enabled by microneedles suggests that bevacizumab delivery by this method may have fewer drug-induced side effects compared to other less-efficient delivery methods. This study sets the stage not only for future treatment of corneal neovascularization using bevacizumab, but also provides the foundation for targeted delivery of other agents to the corneal stroma using microneedles.
Acknowledgments
We thank Bernard McCarey and Damian Berezovsky at Emory University for their helpful discussions. This work was carried out at the Institute for Bioengineering and Bioscience and the Center for Drug Design, Development and Delivery at Georgia Tech. Yoo C. Kim, Henry F. Edelhauser, and Mark R. Prausnitz hold microneedle patents and/or patent applications, and Mark Prausnitz and Henry Edelhauser have significant financial interest in Clearside Biomedical, a company developing microneedle-based products for ocular delivery. This potential conflict of interest has been disclosed and is overseen by Georgia Institute of Technology and Emory University.
Supported by the NEI EY017045 (YCK, HFE, MRP), NEI EY022097 (YCK, MRP), NIH NEI EY06360 (HEG), and an unrestricted departmental grant from Research to Prevent Blindness, Inc. (HEG).
Disclosure: Y.C. Kim, P; H.E. Grossniklaus, None; H.F. Edelhauser, Clearside Biomedical (I, C), P; M.R. Prausnitz, Clearside Biomedical (I, S), P
References
- 1. Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009; 88: 495–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maddula S, Davis DK, Maddula S, Burrow MK, Ambati BK. Horizons in therapy for corneal angiogenesis. Ophthalmology. 2011; 118: 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aydin E, Kivilcim M, Peyman GA, Esfahani MR, Kazi AA, Sanders DR. Inhibition of experimental angiogenesis of cornea by various doses of doxycycline and combination of triamcinolone acetonide with low-molecular-weight heparin and doxycycline. Cornea. 2008; 27: 446–453. [DOI] [PubMed] [Google Scholar]
- 4. Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011; 30: 927–938. [DOI] [PubMed] [Google Scholar]
- 5. Acar BT, Halili E, Acar S. The effect of different doses of subconjunctival bevacizumab injection on corneal neovascularization. Int Ophthalmol. 2013; 33: 507–513. [DOI] [PubMed] [Google Scholar]
- 6. Bock F, Konig Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 281–284. [DOI] [PubMed] [Google Scholar]
- 7. Yoeruek E, Ziemssen F, Henke-Fahle S, et al. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008; 86: 322–328. [DOI] [PubMed] [Google Scholar]
- 8. Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998; 87: 1479–1488. [DOI] [PubMed] [Google Scholar]
- 9. Kaufman PL, Alm A, Adler FH. eds Adler's Physiology of the Eye: Clinical Application. 10th ed. St. Louis; London: Mosby; 2003: pp. xvii, 876. [Google Scholar]
- 10. Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006; 33: S26–S34. [DOI] [PubMed] [Google Scholar]
- 11. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007; 96: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008; 115: e33–e38. [DOI] [PubMed] [Google Scholar]
- 13. Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009; 247: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 14. Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011; 30: 215–218. [DOI] [PubMed] [Google Scholar]
- 15. Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011; 30: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 16. Oh JY, Kim MK, Wee WR. Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009; 28: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 17. Donnelly RF. Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010; 17: 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012; 161: 645–655. [DOI] [PubMed] [Google Scholar]
- 19. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012; 64: 1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: A novel approach to transdermal drug delivery. J Pharm Sci. 1998; 87: 922–925. [DOI] [PubMed] [Google Scholar]
- 21. Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007; 117: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matriano JA, Cormier M, Johnson J, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002; 19: 63–70. [DOI] [PubMed] [Google Scholar]
- 23. Mikolajewska P, Donnelly RF, Garland MJ, et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. 2010; 27: 2213–2220. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010; 16: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010; 5: e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011; 28: 159–165. [DOI] [PubMed] [Google Scholar]
- 27. Patel SR, Edelhauser HF, Prausnitz MR. Targeted drug delivery to the eye enabled by microneedles. In: Kompella UB, Edelhauser HF. eds Drug Product Development for the Back of the Eye. New York: Springer; 2011; 331–360. [Google Scholar]
- 28. Jiang J, Gill HS, Ghate D, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007; 48: 4038–4043. [DOI] [PubMed] [Google Scholar]
- 29. Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009; 26: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palakurthi NK, Correa ZM, Augsburger JJ, Banerjee RK. Toxicity of a biodegradable microneedle implant loaded with methotrexate as a sustained release device in normal rabbit eye: a pilot study. J Ocul Pharmacol Ther. 2011; 27: 151–156. [DOI] [PubMed] [Google Scholar]
- 31. Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012; 53: 4433–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013; 54: 2483–2492. [DOI] [PubMed] [Google Scholar]
- 33. Kim YC, Edelhauser HF, Prausnitz MR. Particle-stabilized emulsion droplets for gravity-mediated targeting in the posterior segment of the eye. Adv Healthc Mater. 2014; 3: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007; 24: 1369–1380. [DOI] [PubMed] [Google Scholar]
- 35. Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011; 28: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seta F, Patil K, Bellner L, et al. Inhibition of VEGF expression and corneal neovascularization by siRNA targeting cytochrome P450 4B1. Prostaglandins Other Lipid Mediat. 2007; 84: 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holzer MP, Solomon KD, Vroman DT, et al. Photodynamic therapy with verteporfin in a rabbit model of corneal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 2954–2958. [DOI] [PubMed] [Google Scholar]
- 38. Chan TPS, Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest Ophthalmol Vis Sci. 1983; 24: 1408–1410. [PubMed] [Google Scholar]
- 39. Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010; 51: 2411–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007; 125: 834–836. [DOI] [PubMed] [Google Scholar]
- 41. Sasaki H, Yamamura K, Nishida K, Nakamura J, Ichikawa M. Delivery of drugs to the eye by topical application. Prog Retin Eye Res. 1996; 15: 583–620. [Google Scholar]
- 42. Chen WL, Lin CT, Lin NT, et al. Subconjunctival injection of bevacizumab (avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2009; 50: 1659–1665. [DOI] [PubMed] [Google Scholar]
- 43. Erdurmus M, Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 1577–1579. [DOI] [PubMed] [Google Scholar]
- 44. Kim T-I, Kim SW, Kim S, Kim T, Kim EK. Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea. 2008; 27: 349–352. [DOI] [PubMed] [Google Scholar]
- 45. Papathanassiou M, Theodossiadis PG, Liarakos VS, Rouvas A, Giamarellos-Bourboulis EJ, Vergados IA. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Ophthalmol. 2008; 145: 424–431. [DOI] [PubMed] [Google Scholar]
- 46. Avastin EPAR– Scientific Discussion. European Medicine Agency; 2006. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000582/WC500029262.pdf. Accessed July 17, 2014. [Google Scholar]
- 47. Solomon KD, Fernandez de Castro LE, Sandoval HP, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009; 116: 691–701. [DOI] [PubMed] [Google Scholar]