Abstract
Objectives
Antiretroviral therapy during pregnancy is recommended to reduce the risk of mother-to-child transmission of HIV and for maternal care management. Physiological changes during pregnancy can affect pharmacokinetics, potentially altering pharmacological activity. We therefore evaluated the pharmacokinetics of twice-daily (bid) darunavir in HIV-1-infected pregnant women.
Methods
HIV-1-infected pregnant women receiving an antiretroviral regimen containing darunavir/ritonavir 600/100 mg bid were enrolled in this study. Total and unbound darunavir and total ritonavir plasma concentrations were obtained over 12 h during the second and third trimesters and postpartum. Total darunavir and ritonavir plasma concentrations were determined using a validated high-performance liquid chromatography tandem mass spectrometry assay and unbound darunavir was determined using 14C-darunavir-fortified plasma. Pharmacokinetic parameters were derived using noncompartmental analysis.
Results
Data were available for 14 women. The area under the plasma concentration–time curve from 0 to 12 h (AUC12h) for total darunavir was 17–24% lower during pregnancy than postpartum. The AUC12h for unbound darunavir was minimally reduced during pregnancy vs. postpartum. The minimum plasma concentration (Cmin) of total and unbound darunavir was on average 43–86% and 10–14% higher, respectively, during pregnancy vs. postpartum. The antiviral response (< 50 HIV-1 RNA copies/mL) was 33% at baseline and increased to 73–90% during treatment; the percentage CD4 count increased over time. One serious adverse event was reported (increased transaminase). All 12 infants born to women remaining in the study at delivery were HIV-1-negative; four of these infants were premature.
Conclusions
Total darunavir exposure decreased during pregnancy. No clinically relevant change in unbound (active) darunavir occurred during pregnancy, suggesting that no dose adjustment is required for darunavir/ritonavir 600/100 mg bid in pregnant women.
Keywords: darunavir, HIV, pharmacokinetics, pregnancy, ritonavir
Introduction
Data on the pharmacology of darunavir/ritonavir during pregnancy are limited to case reports 1–8, few of which examined pharmacokinetics 1–3,5–7,9. The effects of pregnancy on the pharmacokinetics of darunavir remain unclear; therefore, further pharmacokinetic studies are needed.
Maternal physiological changes during pregnancy (e.g. blood volume expansion, increased glomerular filtration rate and alterations in hepatic metabolism) may result in altered pharmacokinetics 10. Data support the conclusion that the pharmacokinetic parameters of HIV protease inhibitors (PIs) are changed during pregnancy, leading to lower exposure in pregnant women 3,5,6,11,12. Studying darunavir/ritonavir in HIV-infected pregnant women may provide insights into population-specific pharmacokinetics that could inform treatment strategies and dosing recommendations.
This nonrandomized study was designed to investigate the pharmacokinetic profile, antiviral activity and safety of darunavir/ritonavir [600/100 mg twice daily (bid) or 800/100 mg once daily (qd)], etravirine (200 mg bid) and rilpivirine (25 mg qd) in HIV-infected pregnant women. Here we report only on the group that received darunavir/ritonavir 600/100 mg bid.
Methods
Study design and treatment
HIV-1-infected pregnant women, aged 18 years or older, with pregnancies between 18 and 26 weeks of gestation were included in this multicentre, single-arm, open-label trial. Subjects had to be on the study drug before screening and enrolment, and had to undergo an obstetric examination and have a normal level II ultrasound. The study was approved by a centralized ethics committee [Western institutional review board (IRB)] or a site-specific IRB.
Subjects with any obstetric complications, any neurological conditions requiring medication or any active disease that may have compromised patient safety or study outcomes were excluded from the study. All subjects agreed to comply with protocol requirements and provided written informed consent.
Objectives
The primary objective was to assess the effect of pregnancy on the pharmacokinetics of darunavir/ritonavir administered bid during the second and third trimesters of gestation and postpartum. The secondary objectives were to document antiviral activity, safety and tolerability of darunavir/ritonavir-based regimens during pregnancy and postpartum, to compare darunavir/ritonavir concentrations between maternal serum and cord blood at delivery and to assess the outcomes for infants of women treated with darunavir/ritonavir bid during pregnancy.
Evaluations
Intensive pharmacokinetic samplings over 12 h were performed at three study visits: during the second trimester (24–28 weeks), during the third trimester (34–38 weeks) and 6–12 weeks postpartum. Eight blood draws were performed during each visit: predose and 1, 2, 3, 4, 6, 9 and 12 h postdose. Darunavir (total and unbound) and ritonavir (total) plasma concentrations were measured. Cord blood and maternal plasma concentrations of total darunavir and ritonavir were also measured on the day of delivery. Total darunavir and ritonavir plasma concentrations were determined using a previously validated high-performance liquid chromatography tandem mass spectrometry assay with a lower limit of quantification of 5.00 ng/mL for both compounds 13. The unbound darunavir concentration was determined by fortifying plasma samples with 14C-darunavir. The unbound 14C-darunavir was separated using ultrafiltration. Total and unbound 14C-darunavir concentrations were measured using liquid scintillation counting. The unbound fraction of darunavir was calculated as the ratio of the unbound concentration in the filtrate to the total concentration in the plasma before centrifugation.
Human serum albumin and α1-acid glycoprotein (AAG) were measured because darunavir is highly protein bound and the concentration of these proteins usually decreases during pregnancy as a result of haemodilution 14. Efficacy and safety assessments were completed at each visit. Adherence was self-reported and based on the 4 days preceding the visit. Infants' HIV-1 statuses were determined by polymerase chain reaction testing, reported within 16 weeks postpartum.
Statistical analysis
Total and unbound pharmacokinetic parameters were derived from the plasma concentration–actual time data using noncompartmental analysis (WinNonlin Professional™ 4.1; Pharsight, Mountain View, CA). The minimum and maximum plasma concentrations (Cmin and Cmax, respectively) along with time to reach Cmax (tmax) were obtained by inspection of the plasma concentration–time profiles. The area under the plasma concentration–time curve from 0 to 12 h (AUC12h) was determined using the linear trapezoidal rule. The primary pharmacokinetic parameters used in the statistical analysis were AUC12h, Cmin and Cmax on the logarithmic scale. The least squares means (LSMs) of the primary parameters for each treatment were estimated with a linear mixed-effects model, controlling for period as a fixed effect and subject as a random effect. A 90% confidence interval (CI) was constructed around the difference between the LSMs of test and reference. Both the difference between the LSMs and the 90% CI were retransformed to the original scale. Period effects were considered significant at the 5% level. Safety and antiviral activity were summarized using descriptive statistics (sas/stat® version 9.2; SAS Institute Inc., Cary, NC).
Results
Population and baseline characteristics
Sixteen women were enrolled in this part of the trial and received darunavir/ritonavir 600/100 mg bid (Supplementary Table S1). The median age was 24 years (range 18–35 years), and 63% were Black or African American. Most (80%) subjects had a CD4 count ≥ 350 cells/μL, 33% had a viral load < 50 HIV-1 RNA copies/mL, 53% had a viral load between 50 and 400 copies/mL and 14% had a viral load ≥ 400 copies/mL. Sixty-three per cent were previously treated with one protease inhibitor (PI)-based regimen.
Disposition
Of 16 subjects, 14 had at least one intensive pharmacokinetic visit and were included in the pharmacokinetic analysis, and 12 completed the treatment phase of the study. A total of five subjects discontinued (four during treatment before delivery; one during follow-up) (Table S1). Two subjects discontinued because of adverse events (AEs): one for increased transaminases related to study drugs and one for unrelated premature delivery (premature delivery led to an inability to fulfill protocol requirements as the patient missed the postpartum visit). Two subjects discontinued because of virological failure related to suboptimal adherence as documented by the principal investigator [one patient reported 87.5% adherence (AUC12h 69 970 ng/h/mL; Cmin 4140 ng/mL) in the 4 days prior to the second trimester visit and 75% adherence in the 4 days prior to the third trimester visit (no pharmacokinetic data were available), and the other patient reported 62.5% adherence in the 4 days prior to the second trimester visit; third trimester adherence rate and pharmacokinetic data were not available]. One subject was ineligible to continue the trial because of a failed drug screening.
Pharmacokinetics of total and unbound darunavir
Mean total and unbound darunavir plasma concentrations were higher during the postpartum period compared with the second and third trimesters of pregnancy (Fig. 1a,b).
Figure 1.
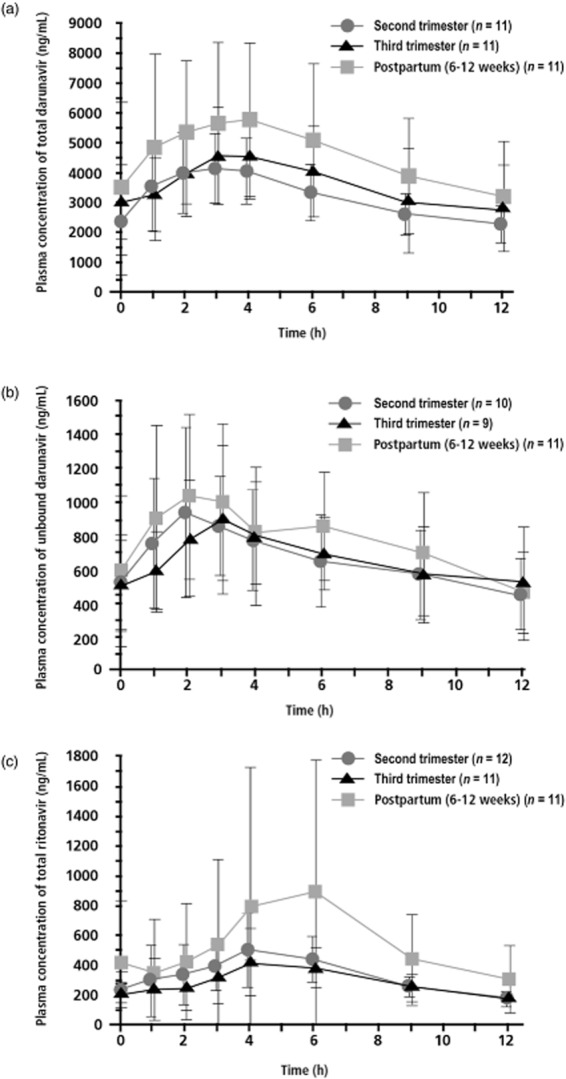
Mean (standard deviation) plasma concentration–time curves for total darunavir (a), unbound darunavir (b) and total ritonavir (c) after administration of darunavir/ritonavir 600/100 mg twice daily (bid), during the second and third trimesters and postpartum.
Pharmacokinetic parameters for total and unbound darunavir are provided in Table 1. Data for unbound darunavir were not available for all patients as not all plasma samples were available for plasma protein binding analysis. The maximum plasma concentration of total darunavir during the second and third trimesters was 28 and 19% lower, respectively, vs. postpartum based on the LSM ratios. The median tmax for total darunavir was 3 h in all periods examined. Total darunavir Cmin was on average 43 and 86% higher during the second and third trimesters, respectively, vs. postpartum. Individual Cmin values for total darunavir are provided in Figure S1a. Total darunavir AUC12h during pregnancy was 24 and 17% lower in the second and third trimesters, respectively, vs. postpartum. Unbound darunavir Cmax was 22 and 18% lower during the second and third trimesters, respectively, vs. postpartum. The median tmax of unbound darunavir was approximately 3 h during pregnancy and 2 h postpartum. Unbound darunavir Cmin was 10 and 14% higher during the second and third trimesters, respectively, vs. postpartum. Individual Cmin values for unbound darunavir are provided in Figure S1b. Unbound darunavir AUC12h was 8 and 7% lower during the second and third trimesters, respectively, vs. postpartum. The free fraction of darunavir (ratio of unbound plasma concentration vs. total plasma concentration) was slightly higher during pregnancy vs. postpartum (data not shown).
Table 1.
Mean (± standard deviation)* pharmacokinetic parameters and statistical results for total and unbound darunavir and total ritonavir, after administration of darunavir/ritonavir 600/100 mg twice daily (bid), during the second and third trimesters and postpartum
| Postpartum (6–12 weeks) (reference) | Second trimester of pregnancy | Third trimester of pregnancy | LSM ratio (90% CI) |
||
|---|---|---|---|---|---|
| Second trimester vs. reference | Third trimester vs. reference | ||||
| Pharmacokinetics of total darunavir | |||||
| n | 11 | 11† | 11 | 11†:11 | 11:11 |
| C0h (ng/mL) | 3 497 ± 2 922 | 2 403 ± 1 161 | 3 028 ± 1 236 | ND | ND |
| Cmin (ng/mL) | 2 711 ± 2 268 | 1 980 ± 840 | 2 498 ± 1 193 | 1.43 (0.39–5.22) | 1.86 (0.49–7.04) |
| Cmax (ng/mL) | 6 499 ± 2 411 | 4 601 ± 1 125 | 5 111 ± 1 517 | 0.72 (0.61–0.86) | 0.81 (0.69–0.96) |
| C12h (ng/mL) | 3 194 ± 1 847 | 2 268 ± 624 | 2 807 ± 1 446 | ND | ND |
| tmax (h) | 3.00 (1.00–6.00) | 3.00 (0.93–5.83) | 3.00 (2.00–11.88) | ND | ND |
| AUC12h (ng/h/mL) | 55 300 ± 27 020 | 38 950 ± 10 010 | 43 700 ± 16 400 | 0.76 (0.63–0.90) | 0.83 (0.72–0.97) |
| CL/F (L/h) | 12.79 ± 5.19 | 16.45 ± 4.65 | 15.60 ± 5.73 | ND | ND |
| Pharmacokinetics of unbound darunavir‡ | |||||
| n | 11§ | 6¶ | 7# | 6**:11†† | 7‡‡:11†† |
| C0h (ng/mL) | 592.4 ± 446.9 | 526.3 ± 281.8 | 504.2 ± 270.1 | ND | ND |
| Cmin (ng/mL) | 420.6 ± 293.1 | 413.1 ± 243.9 | 414.8 ± 243.8 | 1.10 (0.59–2.06) | 1.14 (0.59–2.20) |
| Cmax (ng/mL) | 1 173 ± 481.0 | 933.7 ± 371.8 | 923.4 ± 286.3 | 0.78 (0.52–1.18) | 0.82 (0.57–1.16) |
| C12h (ng/mL) | 464 ± 241 | 456 ± 209 | 519 ± 336 | ND | ND |
| tmax (h) | 2.03 (1.00–4.00) | 2.92 (0.93–8.83) | 3.00 (1.00–11.88) | ND | ND |
| AUC12h (ng/h/mL) | 9 178 ± 3 956 | 7 425 ± 2 999 | 7 294 ± 2 408 | 0.92 (0.66–1.30) | 0.93 (0.69–1.24) |
| CL/F (L/h) | 77.66 ± 35.46 | 91.63 ± 33.86 | 91.20 ± 32.72 | ND | ND |
| Pharmacokinetics of total ritonavir | |||||
| n | 11 | 11§§ | 11¶¶ | 11##:11 | 11***:11 |
| C0h (ng/mL) | 418.8 ± 419.4 | 241.0 ± 120.4 | 210.2 ± 63.64 | ND | ND |
| Cmin (ng/mL) | 232.5 ± 246.1 | 149.7 ± 69.92 | 137.1 ± 39.47 | 1.08 (0.31–3.81) | 1.22 (0.41–3.59) |
| Cmax (ng/mL) | 1 016 ± 881.8 | 569.8 ± 245.6 | 515.3 ± 206.4 | 0.66 (0.41–1.08) | 0.63 (0.40–0.98) |
| C12h (ng/mL) | 309 ± 224 | 177 ± 53 | 179 ± 99 | ND | ND |
| tmax (h) | 6.00 (2.02–12.08) | 4.14 (0.93–6.08) | 4.05 (1.00–9.10) | ND | ND |
| AUC12h (ng/h/mL) | 6 746 ± 6 032 | 3 929 ± 1 203 | 3 532 ± 1 130 | 0.72 (0.44–1.17) | 0.67 (0.43–1.04) |
| CL/F (L/h) | 25.98 ± 24.95 | 27.80 ± 8.754 | 31.05 ± 9.715 | ND | ND |
LSM, least squares mean; CI, confidence interval; C0h, predose plasma concentration; ND, not determined; Cmin, minimum plasma concentration; Cmax, maximum plasma concentration; C12h, plasma concentration at 12 hours; tmax, time to reach the maximum plasma concentration; AUC12h, area under the plasma concentration–time curve over 12 h; CL/F, apparent clearance.
Median (range) for tmax.
n = 10 for AUC12h.
Data for unbound darunavir were not available for all patients because of poor sample quality (protein in the filtrate) or low sample volume.
n = 10 for C0h and Cmin.
n = 10 for C0h, n = 9 for Cmin, and n = 7 for Cmax and tmax.
n = 11 for C0h, and n = 8 for Cmin, Cmax and tmax.
n = 9 for Cmin, and n = 7 for Cmax.
n = 10 for Cmin.
n = 8 for Cmin and Cmax.
n = 12 for C0h, Cmin, Cmax and tmax.
n = 10 for Cmin.
n = 12 for Cmin and Cmax.
n = 10 for Cmin.
Matched maternal and cord plasma concentrations were available for 10 subjects. Excluding one outlier resulting from a possible switch of the samples, the mean [standard deviation (SD)] total darunavir concentrations were higher in maternal plasma vs. cord plasma [2324 (1056) ng/mL vs. 383 (322) ng/mL, respectively] when assessed in nine women and their infants. The median cord : maternal plasma ratio was 0.1455 (range 0.01407–0.3621).
Pharmacokinetics of ritonavir
Mean total ritonavir plasma concentrations were higher during the postpartum period compared with the second and third trimesters of pregnancy (Fig. 1c). Median total ritonavir plasma concentrations peaked 4 h after drug administration during pregnancy and at 6 h in the postpartum period (Table 1). Total ritonavir Cmax during the second and third trimesters of pregnancy was 34 and 37% lower, respectively, vs. postpartum based on the LSM ratios. Total ritonavir Cmin was on average 8 and 22% higher during the second and third trimesters, respectively, vs. postpartum. Individual Cmin values for total ritonavir are provided in Figure S1c. Total ritonavir AUC12h during pregnancy was 28 and 33% lower in the second and third trimesters, respectively, vs. postpartum.
Matched maternal and cord plasma concentrations were available for eight subjects. Excluding one outlier resulting from a possible switch of the samples, the mean (SD) total ritonavir concentrations were higher in maternal plasma vs. cord plasma [429 [428] ng/mL vs. 17 [24] ng/mL, respectively) when assessed in seven women and their infants. The median cord : maternal plasma ratio was 0.1076 (range 0.04432–0.114).
Albumin and α1-acid glycoprotein concentrations
Mean baseline albumin and AAG concentrations were 31 and 617 g/L and postpartum concentrations were 38 and 790 g/L, respectively, resulting in a 22 to 28% decrease during pregnancy vs. postpartum (Fig. S2).
Antiviral activity
The response rate (< 50 copies/mL) increased significantly from 33% (five of 15) at baseline (Table S1) to 73% (eight of 11) and 90% (nine of 10) during the second and third trimesters, respectively, and was 80% (eight of 10) at 12 weeks postpartum (Fig. S3a). Three patients had detectable viral loads during pregnancy; two patients had detectable viral load during the second but not the third trimester [72 copies/mL in one patient (100% reported adherence in both trimesters) and 123 copies/mL in the other (87.5% reported adherence in the second trimester, and 100% reported adherence in the third)]. The third patient had detectable viral load during both trimesters (813 copies/mL in the second trimester and 59 copies/mL in the third trimester), with 100% reported adherence for both trimesters. Of these three patients with detectable viral load during pregnancy, two had undetectable viral load in the postpartum and follow-up periods. CD4 count is significantly lower in HIV-1-negative women during pregnancy compared with the 12-week postdelivery period 15; therefore, the percentage of CD4 cells was plotted instead of absolute CD4 count. The percentage of CD4 cells increased from 30% at baseline to 35% at 2–5 weeks postpartum (Fig. S3b). All 12 infants born to women who stayed on treatment until delivery were HIV negative. One of these infants was born to a patient who discontinued the study but remained on study treatment during follow-up.
Safety
The most common AEs, occurring with an incidence of ≥ 25%, were infections and infestations (44%), gastrointestinal disorders (25%) and premature labour (25%). Of 12 infants, four were born prematurely (at weeks 30, 36, 36 and 37) and no congenital abnormalities occurred. One serious AE possibly related to study medication was reported (increased transaminase).
Discussion
Lower exposures of total darunavir/ritonavir 600/100 mg bid were observed during pregnancy compared with postpartum. Total darunavir Cmax was 28 and 19% lower, and AUC12h was 24 and 17% lower during the second and third trimesters, respectively, vs. postpartum. These data are consistent with published reports demonstrating a decrease in total drug exposure (AUC) of darunavir/ritonavir 600/100 mg bid during pregnancy ranging from 29 1 to 36% 9. Lower levels of drug exposure during pregnancy vs. postpartum have been observed with other HIV PIs 11,12,16–19. However, unlike previous reports, the current study also assessed free plasma darunavir levels, which were higher during pregnancy vs. postpartum. As a result, the difference in unbound Cmax and AUC12h at postpartum compared with values found during pregnancy was minimal. The small number of patients with pharmacokinetic data for unbound darunavir, six in the second trimester and seven in the third trimester, limited the ability to draw definitive conclusions; however, we hypothesize that the lower total drug exposure of darunavir during pregnancy might be partially compensated for by higher free drug, resulting from lower protein binding to darunavir. The antiviral activity of darunavir/ritonavir in HIV-infected pregnant women was effective in preventing mother-to-child transmission and in suppressing HIV in pregnant women, consistent with findings in pregnant 1,3,5,9 and nonpregnant HIV-infected individuals 20. Despite lower total PI levels in studies in pregnant women, subjects achieved desired immunovirological responses and protection from transmission of HIV to their infants 3,5,6,9,11,12. Taken together, the findings that unbound concentrations of darunavir remained nearly unchanged during pregnancy relative to postpartum and that clinical responses were maintained suggest that no a priori dose adjustment is needed in darunavir/ritonavir when dosed bid in pregnant women.
Total ritonavir Cmax was 34 and 37% lower, and AUC12h was 28 and 33% lower during the second and third trimesters, respectively, vs. postpartum, similar to findings in previously published reports 11. The levels of darunavir/ritonavir transferred from maternal to cord plasma were low, with median cord : maternal ratios of 0.1455 and 0.1076, respectively, consistent with other reports demonstrating low transplacental passage of PIs 3,7,9.
It is interesting to note that Cmin values were on average higher during the second and third trimesters, respectively, vs. postpartum for both darunavir (43 and 86% higher, respectively) and ritonavir (8 and 22% higher, respectively). This is a novel observation and further investigation is warranted. Plasma concentration values at 12 hours have also been presented in Table 1 in order to provide additional context to these data.
In this study, darunavir/ritonavir bid treatment was generally well tolerated in HIV-infected pregnant women, with few discontinuations because of AEs (12%). The observed AE profile partially overlaps with that found previously in nonpregnant adults 20, and it is unclear how many AEs may actually have been associated with pregnancy independent of darunavir/ritonavir bid treatment.
A limitation of this study may be the use of the 6- to 12-week postpartum levels as the reference. Darunavir/ritonavir pharmacokinetic parameters after administration of darunavir/ritonavir 600/100 mg bid – as part of an antiretroviral regimen during pregnancy and postpartum – were not clinically different compared with historical controls.
Darunavir/ritonavir 600/100 mg bid administered with other antiretrovirals during pregnancy resulted in consistent unbound (active) drug exposure during pregnancy and postpartum, was effective in preventing mother-to-child transmission and in suppressing HIV and was generally well tolerated, with few discontinuations because of AEs. Data from this small pharmacokinetic study, while not definitive, suggest that darunavir/ritonavir 600/100 mg bid may be a treatment option for pregnant women in need of highly active antiretroviral therapy.
Acknowledgments
We thank the subjects, staff at the study sites and principal investigators for their participation in the trial. The authors also acknowledge internal study support staff, and Francesca Balordi, PhD, and Jennifer Granit, PhD, Medicus International New York, for editorial assistance. Funding for the study and for editorial support was provided by Janssen Services LLC.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1 Individual minimum plasma concentrations of total darunavir (A), unbound darunavir (B) and total ritonavir (C) after administration of darunavir/ritonavir 600/100 mg twice daily (bid), during the second and third trimesters and postpartum.
Fig. S2 Mean (standard error) plasma concentration–time curves for albumin (A) and α1-acid glycoprotein (AAG) (B) assessed at various time-points throughout the study.
Fig. S3 Antiviral activity expressed as percentage response rate (A) (only patients who completed the study in its entirety are included) and status of the immune system expressed as median percentage CD4 count (B) assessed at various time-points throughout the study.
Table S1 Baseline demographics, disease characteristics and study discontinuations.
References
- Capparelli EV, Best BM, Stek A, et al. Pharmacokinetics of darunavir once or twice daily during and after pregnancy. Rome, Italy, July 15–16, 2011. Poster P72 3rd International Workshop on HIV Pediatrics.
- Furco A, Gosrani B, Nicholas S, et al. Successful use of darunavir, etravirine, enfuvirtide and tenofovir/emtricitabine in pregnant woman with multiclass HIV resistance. AIDS. 2009;23:434–435. doi: 10.1097/QAD.0b013e32832027d6. [DOI] [PubMed] [Google Scholar]
- Ivanovic J, Bellagamba R, Nicastri E, et al. Use of darunavir/ritonavir once daily in treatment-naive pregnant woman: pharmacokinetics, compartmental exposure, efficacy and safety. AIDS. 2010;24:1083–1084. doi: 10.1097/QAD.0b013e32833653b2. [DOI] [PubMed] [Google Scholar]
- Jaworsky D, Thompson C, Yudin MH, et al. Use of newer antiretroviral agents, darunavir and etravirine with or without raltegravir, in pregnancy: a report of two cases. Antivir Ther. 2010;15:677–680. doi: 10.3851/IMP1558. [DOI] [PubMed] [Google Scholar]
- Pacanowski J, Bollens D, Poirier JM, et al. Efficacy of darunavir despite low plasma trough levels during late pregnancy in an HIV-hepatitis C virus-infected patient. AIDS. 2009;23:1923–1924. doi: 10.1097/QAD.0b013e32832e534b. [DOI] [PubMed] [Google Scholar]
- Pinnetti C, Tamburrini E, Ragazzoni E, De Luca A, Navarra P. Decreased plasma levels of darunavir/ritonavir in a vertically infected pregnant woman carrying multiclass-resistant HIV type-1. Antivir Ther. 2010;15:127–129. doi: 10.3851/IMP1473. [DOI] [PubMed] [Google Scholar]
- Ripamonti D, Cattaneo D, Cortinovis M, Maggiolo F, Suter F. Transplacental passage of ritonavir-boosted darunavir in two pregnant women. Int J STD AIDS. 2009;20:215–216. doi: 10.1258/ijsa.2008.008515. [DOI] [PubMed] [Google Scholar]
- Sued O, Lattner J, Gun A, et al. Use of darunavir and enfuvirtide in a pregnant woman. Int J STD AIDS. 2008;19:866–867. doi: 10.1258/ijsa.2008.008075. [DOI] [PubMed] [Google Scholar]
- Colbers A, Moltó J, Ivanovic J, et al. A comparison of the pharmacokinetics of darunavir, atazanavir and ritonavir during pregnancy and post-partum. Seattle, WA, USA, March, 2012 [Poster 1013] 19th Conference on Retroviruses and Opportunistic Infections.
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Acosta EP, Bardeguez A, Zorrilla CD, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48:430–436. doi: 10.1128/AAC.48.2.430-436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- Scholler-Gyure M, Kakuda TN, Sekar V, et al. Pharmacokinetics of darunavir/ritonavir and TMC125 alone and coadministered in HIV-negative volunteers. Antivir Ther. 2007;12:789–796. [PubMed] [Google Scholar]
- Notarianni LJ. Plasma protein binding of drugs in pregnancy and in neonates. Clin Pharmacokinet. 1990;18:20–36. doi: 10.2165/00003088-199018010-00002. [DOI] [PubMed] [Google Scholar]
- Towers CV, Rumney PJ, Ghamsary MG. Longitudinal study of CD4+ cell counts in HIV-negative pregnant patients. J Matern Fetal Neonatal Med. 2010;23:1091–1096. doi: 10.3109/14767050903580359. [DOI] [PubMed] [Google Scholar]
- Cespedes MS, Castor D, Ford SL, et al. Steady-state pharmacokinetics, cord blood concentrations, and safety of ritonavir-boosted fosamprenavir in pregnancy. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e318285d918. doi: 10.1097/QAI.0b013e318285 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie F, Zorrilla C, Josipovic D, et al. Safety and exposure of once-daily ritonavir-boosted atazanavir in HIV-infected pregnant women. HIV Med. 2011;12:570–579. doi: 10.1111/j.1468-1293.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56:412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KB, Dumond JB, Prince HA, et al. Protein binding of lopinavir and ritonavir during four phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013;63:51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Individual minimum plasma concentrations of total darunavir (A), unbound darunavir (B) and total ritonavir (C) after administration of darunavir/ritonavir 600/100 mg twice daily (bid), during the second and third trimesters and postpartum.
Fig. S2 Mean (standard error) plasma concentration–time curves for albumin (A) and α1-acid glycoprotein (AAG) (B) assessed at various time-points throughout the study.
Fig. S3 Antiviral activity expressed as percentage response rate (A) (only patients who completed the study in its entirety are included) and status of the immune system expressed as median percentage CD4 count (B) assessed at various time-points throughout the study.
Table S1 Baseline demographics, disease characteristics and study discontinuations.


