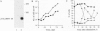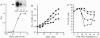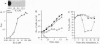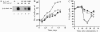Abstract
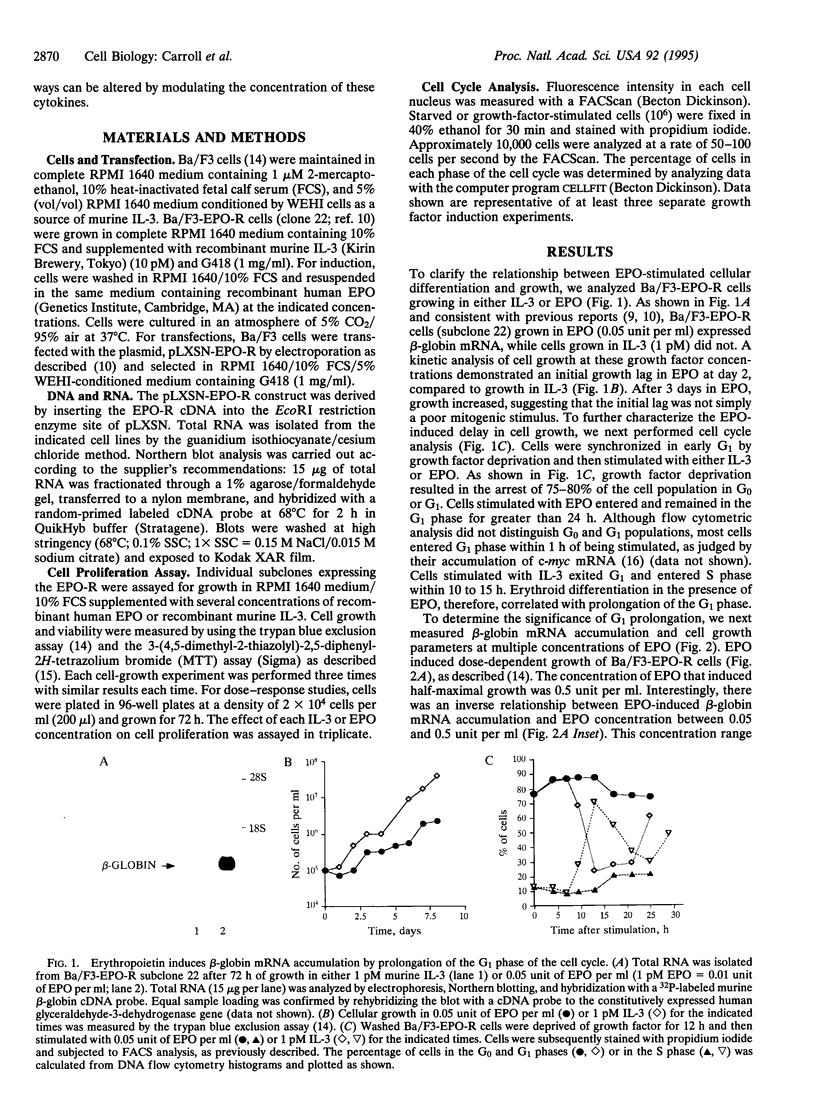
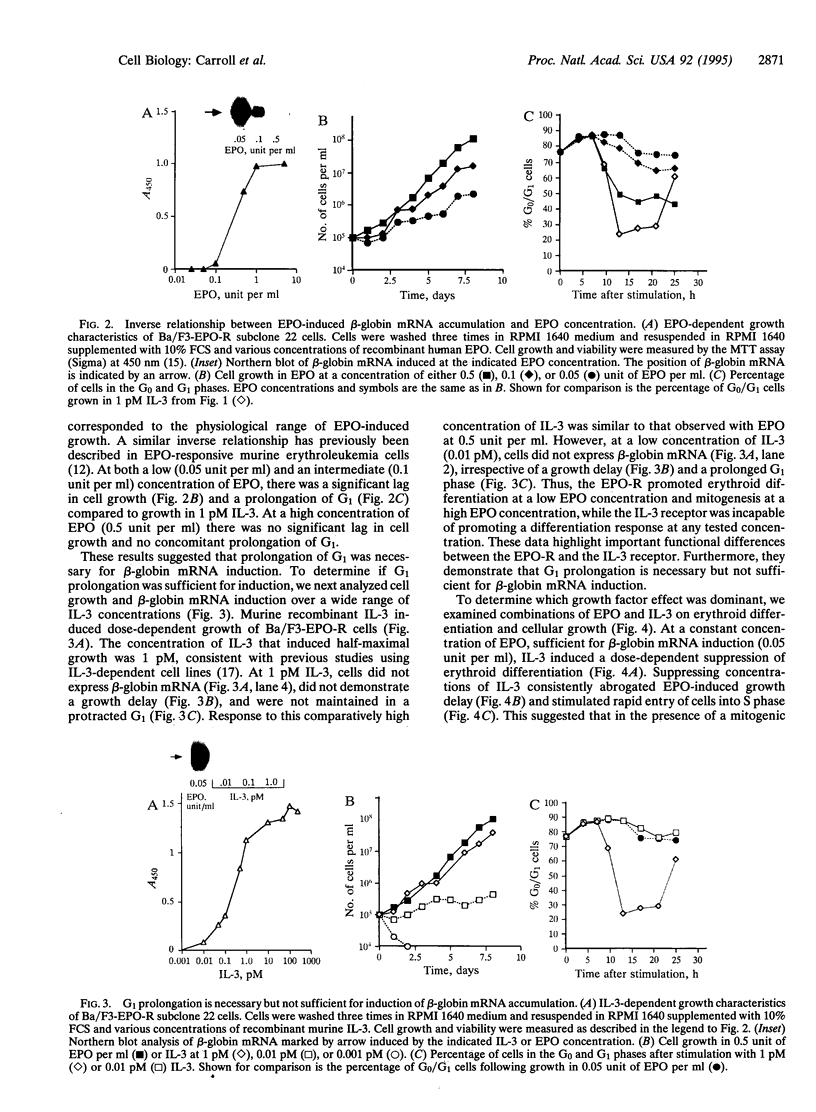
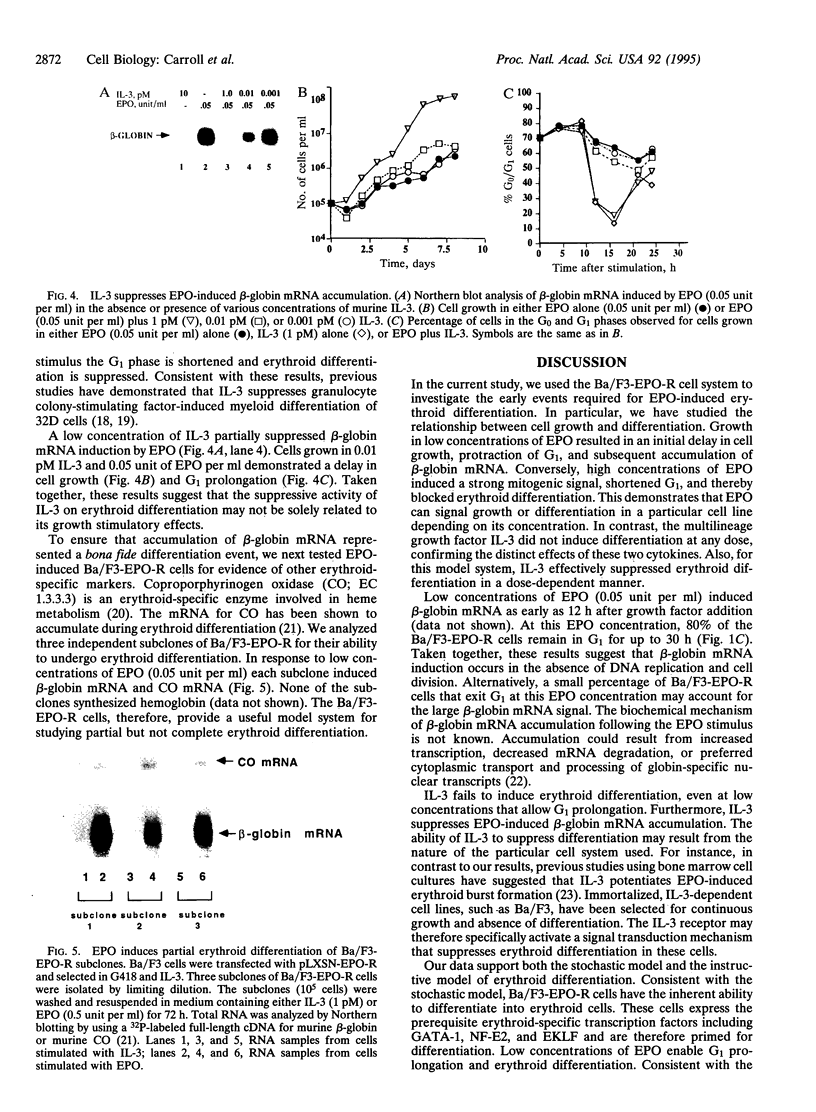
Erythropoietin (EPO), like many other hematopoietic growth factors, can induce either growth or differentiation of hematopoietic cells. Little is known about the molecular basis of this cellular decision, in part because of a paucity of cell lines in which these two phenomena can be dissociated. Ectopic expression of the EPO receptor (EPO-R) in Ba/F3, a murine interleukin 3 (IL-3)-dependent progenitor cell line, confers EPO-dependent cell growth. In these cells (Ba/F3-EPO-R), EPO also induces beta-globin mRNA, a specific marker of erythroid differentiation. Here we show that the induction of erythroid differentiation by EPO requires a delay in cell growth and a prolongation of the (G1) phase of the cell cycle. Interestingly, this effect on G1 prolongation was concentration dependent. At low EPO concentrations (0.05-0.1 unit of EPO per ml; 1 pM EPO = 0.01 unit of EPO per ml), EPO prolonged G1 and induced differentiation; at high concentrations (0.5-10.0 units per ml), EPO shortened G1 and preferentially stimulated growth. IL-3 stimulated Ba/F3 growth but not differentiation at all growth factor concentrations ranging from 0.1 to 500 pM. Moreover, IL-3 suppressed EPO-induced beta-globin induction in a dose-dependent manner. This suppression correlated with the shortening of G1 by IL-3. Taken together, these data demonstrate distinct effects of EPO and IL-3 and a balance between erythroid growth and differentiation that is cell cycle dependent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondurant M. C., Lind R. N., Koury M. J., Ferguson M. E. Control of globin gene transcription by erythropoietin in erythroblasts from friend virus-infected mice. Mol Cell Biol. 1985 Apr;5(4):675–683. doi: 10.1128/mcb.5.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Nagata Y., Kishi A., Sakamaki K., Miyajima A., Yamamoto M., Engel J. D., Todokoro K. Induction of erythroid-specific gene expression in lymphoid cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11593–11597. doi: 10.1073/pnas.90.24.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Nagata Y., Machide M., Kishi A., Amanuma H., Sugiyama M., Todokoro K. Tyrosine kinase activation through the extracellular domains of cytokine receptors. Nature. 1993 Apr 15;362(6421):646–648. doi: 10.1038/362646a0. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D. Hematopoietic growth factors and the regulation of differentiative decisions. Curr Opin Cell Biol. 1994 Dec;6(6):804–808. doi: 10.1016/0955-0674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993 Sep 24;74(6):1079–1087. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Beaumont C., de Verneuil H., Nordmann Y. Accumulation of porphobilinogen deaminase, uroporphyrinogen decarboxylase, and alpha- and beta-globin mRNAs during differentiation of mouse erythroleukemic cells. Effects of succinylacetone. J Biol Chem. 1985 Aug 15;260(17):9630–9635. [PubMed] [Google Scholar]
- Holmes K. L., Palaszynski E., Fredrickson T. N., Morse H. C., 3rd, Ihle J. N. Correlation of cell-surface phenotype with the establishment of interleukin 3-dependent cell lines from wild-mouse murine leukemia virus-induced neoplasms. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6687–6691. doi: 10.1073/pnas.82.19.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Imada K., Tsudo M., Kodaka T., Itoh K., Arima N., Hattori T., Okuma M., Uchiyama T. Interleukin-2 (IL-2) induces erythroid differentiation and tyrosine phosphorylation in ELM-I-1 cells transfected with a human IL-2 receptor beta chain cDNA. Biochem Biophys Res Commun. 1992 Oct 15;188(1):352–357. doi: 10.1016/0006-291x(92)92392-b. [DOI] [PubMed] [Google Scholar]
- Johnson P., Chung S., Benchimol S. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 1993 Mar;13(3):1456–1463. doi: 10.1128/mcb.13.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Sherr C. J. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kohno H., Furukawa T., Yoshinaga T., Tokunaga R., Taketani S. Coproporphyrinogen oxidase. Purification, molecular cloning, and induction of mRNA during erythroid differentiation. J Biol Chem. 1993 Oct 5;268(28):21359–21363. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Mueller T. J. The role of erythropoietin in the production of principal erythrocyte proteins other than hemoglobin during terminal erythroid differentiation. J Cell Physiol. 1986 Feb;126(2):259–265. doi: 10.1002/jcp.1041260216. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Sawyer S. T., Bondurant M. C. Splenic erythroblasts in anemia-inducing Friend disease: a source of cells for studies of erythropoietin-mediated differentiation. J Cell Physiol. 1984 Dec;121(3):526–532. doi: 10.1002/jcp.1041210311. [DOI] [PubMed] [Google Scholar]
- Krantz S. B., Goldwasser E. Specific binding of erythropoietin to spleen cells infected with the anemia strain of Friend virus. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7574–7578. doi: 10.1073/pnas.81.23.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboi E., Carroll M., D'Andrea A. D., Mathey-Prevot B. Erythropoietin receptor signals both proliferation and erythroid-specific differentiation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11351–11355. doi: 10.1073/pnas.90.23.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Miyata K., Yoshimura A. Proliferation and erythroid differentiation through the cytoplasmic domain of the erythropoietin receptor. J Biol Chem. 1994 Feb 25;269(8):5976–5980. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Gross A. J., Ogawa M. A stochastic model of self-renewal and commitment to differentiation of the primitive hemopoietic stem cells in culture. J Cell Physiol. 1982 Dec;113(3):455–458. doi: 10.1002/jcp.1041130314. [DOI] [PubMed] [Google Scholar]
- Nijhof W., Wierenga P. K., Sahr K., Beru N., Goldwasser E. Induction of globin mRNA transcription by erythropoietin in differentiating erythroid precursor cells. Exp Hematol. 1987 Aug;15(7):779–784. [PubMed] [Google Scholar]
- Ohashi H., Maruyama K., Liu Y. C., Yoshimura A. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):158–162. doi: 10.1073/pnas.91.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. T., Hankins W. D. The functional form of the erythropoietin receptor is a 78-kDa protein: correlation with cell surface expression, endocytosis, and phosphorylation. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6849–6853. doi: 10.1073/pnas.90.14.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A., Niemeyer C. M., Nathan D. G., Ekern S. C., Bieber F. R., Yang Y. C., Wong G., Clark S. C. Stimulation of human hematopoietic colony formation by recombinant gibbon multi-colony-stimulating factor or interleukin 3. J Clin Invest. 1987 Sep;80(3):818–823. doi: 10.1172/JCI113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Bird T. A., Morella K. K., Mosley B., Gearing D. P., Baumann H. Distinct regions of the human granulocyte-colony-stimulating factor receptor cytoplasmic domain are required for proliferation and gene induction. Mol Cell Biol. 1993 Apr;13(4):2384–2390. doi: 10.1128/mcb.13.4.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]