Abstract
The aim of this work was to describe national surveillance of invasive beta-haemolytic streptococci (BHS) in Denmark and to report overall trends and major findings by groups and types of BHS causing laboratory-confirmed disease from 2005 to 2011. A total of 3063 BHS isolates were received from 2872 patients. Based on confirmed cases the overall annual incidence increased from 6.2 to 8.9 per 100 000 persons between 2005 and 2011. In 2011 the incidences of group A, B, C and G streptococci were 3.1, 2.3, 0.9 and 2.6 per 100 000 persons, respectively. An increase was observed for all groups of BHS, but in particular for group G in men above 65 years of age. Among group A streptococci (GAS), five T-types (1, 28,12, 3,13,B3264 and B3264) represented 71% and five emm-types (1, 28, 3, 89 and 12) 76% of all isolates. Among group B streptococci (GBS) four types (III, Ia, V, Ib) represented 79% of the isolates. Potential coverage for future vaccines against GAS and GBS disease was 76% compared with the 26-valent GAS vaccine and 89% based on GBS serotypes Ia, Ib, II, III and V. The number of reported cases of invasive BHS disease increased in Denmark from 2005 to 2011. Nationwide laboratory-based surveillance of BHS is required to monitor epidemiological changes, explore potential outbreaks and determine potential vaccine coverage.
Keywords: Emm-type, group A B C and G streptococci, invasive disease, T-type, type, typing
Introduction
The beta-haemolytic streptococci (BHS) belonging to the Lancefield groups A, B, C and G (GAS, GBS, GCS and GGS, respectively) cause a broad spectrum of infectious diseases in humans, ranging from meningitis, sepsis and necrotising fasciitis to less severe manifestations such as sore throat and wound infections. The typical clinical presentations vary by serological group; however, all BHS groups can cause both major and minor infections 1. Most infections are community acquired and outbreaks of GAS, GBS and GGS have been reported in healthcare-related settings such as maternity wards or care institutions 2–4.
Laboratory-based surveillance of BHS is relevant because, due to the severity of BHS manifestations in both adults and children, there is a need to explore potential outbreaks and to monitor changes in virulence and potential vaccine coverage. The aim of this work was to describe national surveillance of invasive BHS disease in Denmark, overall trends and major findings according to groups and types of BHS causing invasive disease from 2005 to 2011.
Materials and Methods
Laboratory-based surveillance of BHS in Denmark
The current surveillance system is based on a collaboration between all departments of clinical microbiology (DCMs) in Denmark (15 in 2005 and 13 in 2011 as some DCMs have merged) and the National Neisseria and Streptococcus Reference (NSR) Laboratory at Statens Serum Institut (SSI). Nationwide, the DCMs submit isolates from blood, cerebrospinal fluid and other normally sterile sites to the NSR on a voluntary basis. The isolates are referred with information on the origin of the isolate, including specimen type and date, the unique personal identifier (CPR number), patient age, sex, hospital and ward.
Definitions
A case of invasive BHS disease was defined as isolation of BHS from a normally sterile site. A new case was defined as an invasive isolate with a different Lancefield group within 30 days from the first one or an invasive isolate of any Lancefield group more than 30 days after the first episode, or the isolation of a new type (T-type, emm-type or GBS type) if the group was identical on both occasions.
Bacterial isolates, typing and antibiotic susceptibility testing
The submitted isolates were cultured on 5% horse blood agar (SSI Diagnostica, Hillerød, Denmark) at 36°C in ambient air with 5% CO2. The cultures were inspected for purity of growth, beta-haemolysis and colony morphology, and stored at −80°C. Invasive BHS isolates were from hospitalized patients with a few exceptions, as acutely ill patients are admitted directly to the nearest public hospital in Denmark.
Lancefield groups A, B, C or G were confirmed by agglutination tests using Latex grouping reagents A, B, C and G (Oxoid, Basingstoke, UK) as recommended by the manufacturer. For group C isolates fermentation of trehalose or sorbitol was determined at 36°C overnight (Difco phenol red medium; SSI Diagnostica) and classified as S. dysgalactiae subsp. equisimilis if trehalose was fermented, and as S. equi subsp. zooepidemicus if sorbitol was fermented 5.
The T-type was determined using type-specific antisera (Sevapharma, Prague, Czech Republic) and performed as recommended by the manufacturer on all GAS isolates, GCS isolates 2005–2006 and GGS isolates 2005–2007.
Serotyping was performed on all GBS isolates by the latex agglutination test and the capillary precipitation test using GBS latex and type-specific antisera (SSI Diagnostica) as recommended by the manufacturer. GBS isolates from 2008 to 2011 that were non-typable (NT) by these methods or provided an ambiguous result were also typed with multiplex-PCR using primers for detection of serotypes Ia, Ib, II, III, IV, V, VI, VIII 6, VII and IX 7.
The emm sequence-type was determined as described by Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm).
All isolates were tested by disc diffusion (Oxoid) for susceptibility to erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg) and oxacillin/penicillin (1 μg), followed by MIC determination using Etest (bioMérieux SA, Marcy l'Etoile, France) on isolates that were non-sensitive on the screening tests as described by the manufacturers. EUCAST breakpoints were used (http://www.eucast.org).
Statistical analyses
Information on the size, age and sex distribution of the Danish population was obtained from Statistics Denmark (www.dst.dk) (numbers of inhabitants were 5 411 405 in 2005 and 5 560 628 in 2011). We calculated annual incidence rates per year and by age (0–4, 5–19, 20–64 and ≥65 years) and sex group. All denominators were the number of inhabitants within the stratum in question at the beginning of the year. We divided the study period into an early (2005–2007) and a late (2009–2011) 3-year period and calculated mean incidence rates (IRs) for these two periods. Changes during the study period were ascertained by computing the incidence rate ratios (IRRs) between the mean incidence rates with a 95% confidence interval (CI) and a p-value. Two-sided p-values <0.05 were considered statistically significant. For descriptive purposes we reported medians with interquantile range (IQR). A test for equality of age between the four serogroups was carried out using the Kruskal–Wallis test. Analyses were performed with EpiBasic (available at http://www.folkesundhed.au.dk/uddannelse/software) and calculations confirmed with Stata 11 (StataCorp, College Station, TX, USA).
Results
From 2005 to 2011 the NSR received 3063 invasive BHS isolates from 2872 patients, which constituted 2978 cases of invasive disease.
Overall annual incidences of invasive beta-haemolytic streptococcal disease
The annual incidence of BHS causing invasive disease in Denmark increased from 6.2 per 100 000 persons in 2005 to 8.9 in 2011, peaking at 9.2 per 100 000 in 2009. The increase was statistically significant (IRR 1.3, 95% CI 1.2–1.4, p <0.0001) and it was observed for all groups of BHS (GAS, IRR 1.3, 95% CI 1.1–1.5, p 0.0002; GBS, IRR 1.4, 95% CI 1.2–1.6, p 0.0002; GCS, IRR 2.0, 95% CI 1.5–2.8, p <0.0001; GGS, IRR 1.2, 95% CI 1.1–1.4, p 0.0015). During the most recent year, 2011, the annual incidences were 3.1 for GAS, 2.6 for GGS, 2.3 for GBS and 0.9 for GCS per 100 000 persons (Fig.1). Most isolates originated from blood and the proportion of isolates from CSF was highest for GBS and GAS (Table1).
Fig 1.
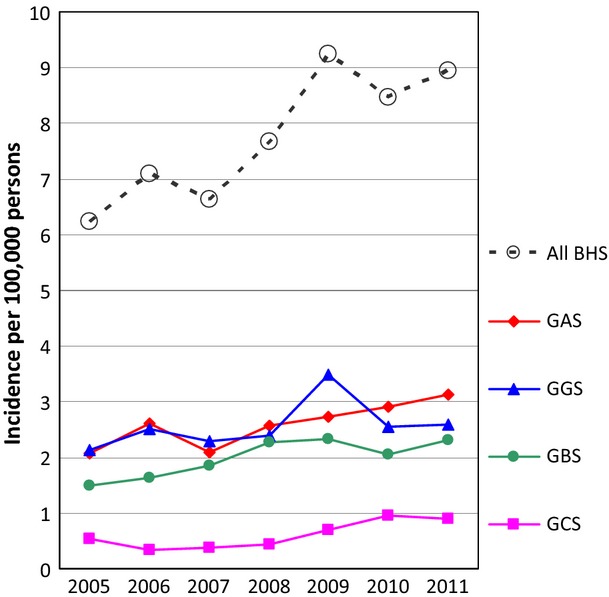
Annual incidences of invasive beta-haemolytic streptococci (BHS) and group A, B, C and G streptococci (GAS, GBS, GCS and GGS, respectively) in Denmark from 2005 to 2011.
Table 1.
Characteristics of invasive beta-haemolytic streptococci (BHS) and group A, B, C and G streptococci (GAS, GBS, GCS and GGS, respectively) in Denmark from 2005 to 2011
| GAS (n = 994) | GBS (n = 767) | GCS (n = 233) | GGS (n = 984) | All BHS (n = 2978) | |
|---|---|---|---|---|---|
| Isolates from clinical material (%) | |||||
| Blood | 93.1 | 93.2 | 97.4 | 96.9 | 94.7 |
| Cerebrospinal fluid | 1.4 | 4.0 | 0.4 | 0.3 | 1.6 |
| Other sterile material | 5.5 | 2.7 | 2.2 | 2.9 | 3.7 |
| Patient information, age (years) | |||||
| Median age | 63.2 | 62.2 | 68.7 | 71.2 | 66.2 |
| Age interquartile rangea | 42.3–75.2 | 35.1–75.9 | 58.0–79.0 | 60.5–81.7 | 50.3–78.6 |
| Age range | 0–99 | 0–98 | 0–100 | 0–100 | 0–100 |
| Age group (years) related incidence | |||||
| 0–4 | 2.54 | 6.31 | 0.09 | 0.35 | 9.29 |
| 5–19 | 0.45 | 0.08 | 0.06 | 0.06 | 0.65 |
| 20–64 | 1.95 | 1.18 | 0.38 | 1.51 | 5.02 |
| ≥65 | 7.56 | 5.75 | 2.32 | 10.36 | 25.98 |
| All ages | 2.59 | 2.00 | 0.61 | 2.56 | 7.76 |
| Sex and age group (years) related incidence | |||||
| Incidence in men | |||||
| 0–4 | 2.31 | 6.33 | 0.09 | 0.43 | 9.15 |
| 5–19 | 0.49 | 0.05 | 0.03 | 0.05 | 0.63 |
| 20–64 | 1.90 | 1.23 | 0.48 | 1.75 | 5.36 |
| ≥65 | 8.23 | 7.36 | 2.85 | 14.34 | 32.78 |
| All ages | 2.54 | 2.17 | 0.69 | 3.09 | 8.49 |
| Incidence in women | |||||
| 0–4 | 2.78 | 6.29 | 0.09 | 0.27 | 9.43 |
| 5–19 | 0.40 | 0.12 | 0.09 | 0.06 | 0.66 |
| 20–64 | 2.00 | 1.12 | 0.28 | 1.27 | 4.68 |
| ≥65 | 7.03 | 4.50 | 1.91 | 7.27 | 20.71 |
| All ages | 2.64 | 1.83 | 0.52 | 2.05 | 7.05 |
| Sex incidence ratio (M:F) | 0.96 | 1.19 | 1.33 | 1.51 | 1.20 |
| Antibiotic resistant (%) | |||||
| Penicillin | 0 | 0 | 0.9b | 0 | |
| Erythromycin | 1.9 | 9.2 | 1.1 | 7.7 | |
| Clindamycin | 0.5 | 5.4 | 0.3 | 1.1 | |
| Tetracycline | 4.3 | 48.1 | 0.3 | 22.9 | |
Kruskal–Wallis equality-of-populations range test, χ2 = 165.011 with 3 d.f., p 0.0001.
MIC values of two isolates were 0.5 and 1 mg/L and above the breakpoint of 0.25 mg/L; the MIC determinations were confirmed by a different laboratory.
Sex, age distributions and age group-related incidences
In the surveillance period a slightly higher proportion of the BHS isolates originated from men compared with isolates from women (IRR 1.2, 95% CI 1.1–1.3, p <0.0001). This tendency was most conspicuous for GGS isolates and statistically significant for all groups (GBS, IRR 1.2, 95% CI 1.0–1.4, p 0.021; GCS, IRR 1.3, 95% CI 1.0–1.7, p 0.030; GGS, IRR 1.5, 95% CI 1.3–1.7, p <0.0001) except for GAS isolates (IRR 1.0, 95% CI 0.9–1.1, p 0.51) (Table1).
The median age of patients with BHS was 66.2 years; however, patients with GBS or GAS infections had a lower median age whereas the median age of patients with GGS infections was higher, 71.2 years (Table1).
The age group-related incidence of BHS disease was highest for adults above 64 years of age and this was the case for all groups of BHS except GBS (Table1), where the incidence in children below 5 years of age was the highest (93.8% of these children were below 3 months of age). A statistically significant increase in the incidence of BHS disease was observed for all age groups but one (0–4 years, IRR 1.5, 95% CI 1.1–2.0, p 0.012; 20–64 years, IRR 1.3, 95% CI 1.1–1.5, p 0.0001; ≥65 years, IRR 1.3, 95% CI 1.1–1.4, p <0.0001); the exception was the age group 5–19 years (IRR 1.5, 95% CI 0.8–2.9, p 0.20). The increase was most predominant for GAS and GBS disease in children below 5 years and for GAS and GBS disease among adults above 64 years of age (Fig.2); the increase, however, was only statistically significant for GBS and GCS in age group 20–64 years and for GAS, GCS and GGS disease in age group ≥65 years (Appendix S1). The increase was statistically significant for GAS, GBS, GCS and GGS in both men and women and for some notable subgroups: men aged ≥65 years (GCS and GGS), women aged 20–64 years (GBS, GCS and GGS) and women aged ≥65 years (GGS).
Fig 2.
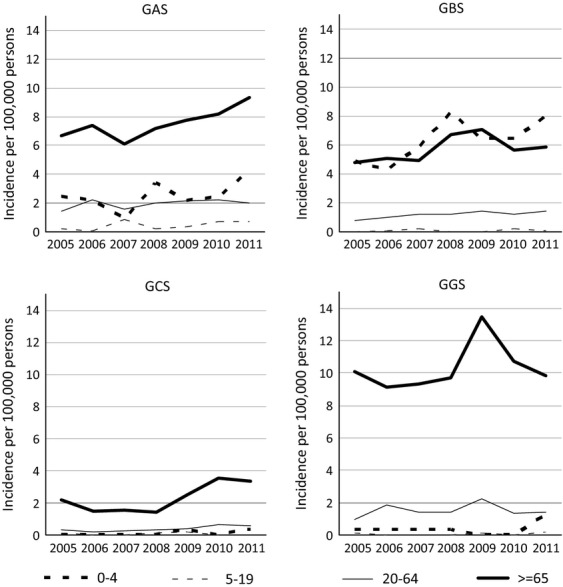
Streptococcal group and age group-related incidences of invasive beta-haemolytic streptococci group A, B, C and G streptococci (GAS, GBS, GCS and GGS, respectively) in Denmark, 2005–2011.
Invasive GGS infections showed some remarkable characteristics. First of all, the highest annual incidence of BHS disease (14.3 per 100 000 persons) was observed for this group in men above 64 years (Table1), and secondly, there was a distinct incidence peak in 2009 (Fig.2) associated with an incidence among men (19.0 per 100 000), twice as high as in women (9.2 per 100 000).
Patients with recurrent infection
Three per cent of the patients (n = 88) experienced more than one episode of invasive disease, 76 patients had two episodes, nine patients had three episodes and three patients suffered from four episodes.
Of the 76 patients with two episodes, GGS was the causative agent of the first episode in most patients (47%), followed by GBS (32%), GAS (13%) and GCS (8%). In 71% of the patients the recurrent infections were with the same group, 48% of which were with GGS and 28% with GBS. Of the 22 patients with recurrent infection with a different group, the first episode was caused by GGS followed by GBS or GCS in ten patients, and caused by GBS followed by GGS or GCS in eight patients. The recurrence occurred in 33% of patients between 2 and 3 months after the first episode and in 29% of patients after 3 months but within a year after the first episode.
Of the nine patients with three episodes of infections six patients had three episodes with GGS, and in the last three patients GGS caused two or one of the three episodes. Two of the three patients had four episodes of infections that were with the same group (GBS and GCS) and one patient had four episodes with three different groups (GAS, GCS and GBS).
All of the patients except for one with recurrent infections were above 19 years of age and most of the patients (66%) were above 65 years of age and men (68%).
T-type and emm-type distribution among invasive GAS isolates
Eighteen different T-types were found among the 994 GAS isolates tested and 13 of these T-types occurred in more than five cases. Five T-types represented 71% of the isolates: 1 (25%), 28 (18%), 12 (12%), 3,13,B3264 (9%) and B3264 (7%). Eleven per cent of the isolates were non-typeable (NT). T-type 1 was the predominant one, varying from 20% in 2007 to 30% in 2008; exceptions were the years 2005 and 2010, when T-type 28 was the predominant type, accounting for 25% and 32%, respectively (Fig.3a).
Fig 3.
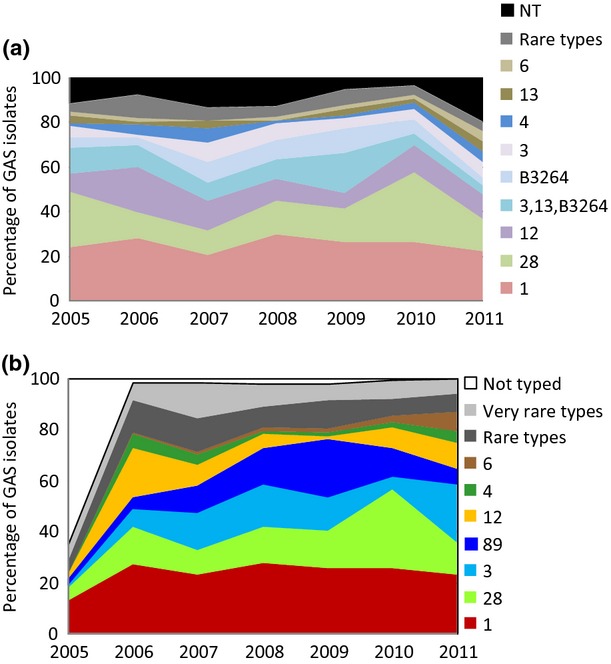
(a) T-type (A) and emm-type (B) distribution of invasive group A streptococcal isolates in Denmark from 2005 to 2011.
Fifty-eight different emm-types were found among 910 typed isolates and they comprised 81 types and subtypes. Of note, only 20 types and subtypes were represented by more than five cases. Together, 5 emm-types, 1 (26%), 28 (17%), 3 (13%), 89 (11%) and 12 (9%), represented 76% of the typed isolates. In most years emm-type 1 was predominant, varying from 23% to 28% of all isolates, except for the years 2010 when type 28 constituted 31% and 2011 when types 1 and 3 both accounted for 23% of all isolates (Fig3b).
The 26-valent M-protein vaccine based on the emm-types 1.0, 1.2, 2, 3, 5, 6, 11, 12, 13, 14, 18, 19, 22, 24, 28, 29, 33, 43, 59, 75, 76, 77, 89, 92, 101 and 114 8 would cover 76% of the isolates. Combining T- and emm-types, 135 different types were found, though only 21 occurred in more than five cases during the surveillance period. Despite the high diversity among the typed isolates, 52% were distributed among only four combinations of T- and emm-types: 1–1 (23%), 28–28 (15%), 12–12.0 (8%) and B3264–89 (6%).
T-type and emm-type distribution among invasive GGS isolates
Four different T-types were found among the 376 GGS isolates tested from 2005 to 2007. Of these, 12% were T-type 25 and 5% were T-types 2, 4 or 28. A majority of the isolates (84%) were non-typable; therefore T-typing of GGS isolates was discontinued in 2008. Sixty-four different emm-types and subtypes were found among the 844 typed GGS isolates from 2006 to 2011 and 19 of these types were represented by more than five cases. Five emm-types, stG6 (20%), stG485 (16%), stG643 (13%), stC74a (11%) and stG480 (7%), constituted 66% of the typed isolates.
Emm-type stG6 was the predominant type, varying from 15% to 21% per year; in 2008 and 2010 type stG480 constituted the same proportion as type stG6 (15% and 17%, respectively, Fig.4).
Fig 4.
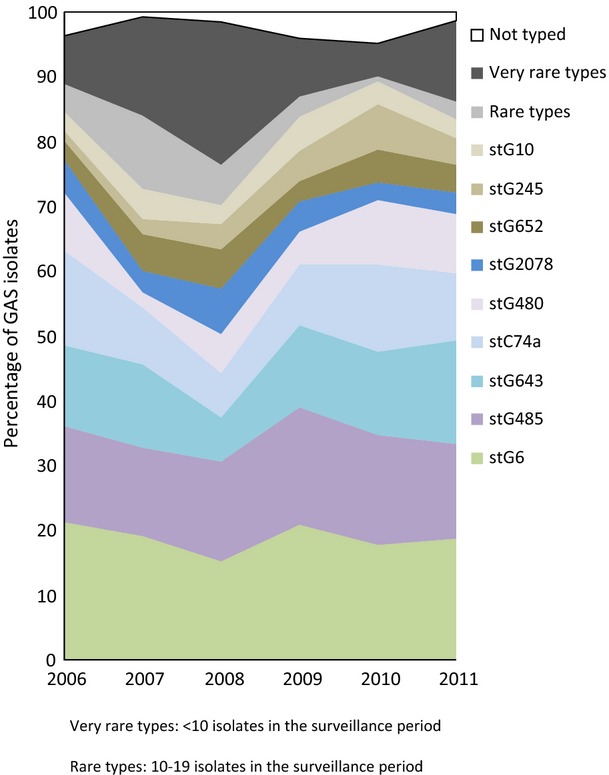
Emm-type distribution of invasive group G streptococcal isolates in Denmark from 2006 to 2011.
The incidence peak of GGS in 2009 was due to an increase of the existing and already predominant types (Fig.4) in more than half of the DCMs and there was no evidence of clustering within the population serviced by any of the DCMs.
T-type and emm-type distribution among invasive GCS isolates
In total, 233 invasive GCS isolates were received in the surveillance period, 94% of these were S. dysgalactiae subsp. equisimilis and 4% were S. equi subsp. zooepidemicus. Four different T-types (2, 4, 25 and 28 (one isolate of each)) were found among the 47 GGS isolates tested from 2005 to 2006. A major part of the isolates (91%) were NT and T-typing of GCS isolates was discontinued in 2007. Fourteen different emm-types were found among the 190 typed GCS isolates from 2006 to 2011 and seven of these types were represented by more than five cases. The three emm-types stG62647 (47%), stG43 (13%) and stG485 (11%) constituted 72% of the isolates.
Type distribution among invasive GBS isolates
All nine known types were found among the 767 GBS isolates. Seventy-nine per cent of the isolates were represented by the four types III (34%), Ia (18%), V (15%) and Ib (12%). Nine per cent of the isolates were NT by the phenotypic testing methods. From 2008 to 2011, 49 NT isolates were tested by PCR to determine the type, and the results were included in the overall type distributions. The predominant type during the surveillance period was type III, varying from 21% to 44% of the isolates per year (Fig.5). A future vaccine based on serotypes Ia, Ib, II, III and V 9 would potentially cover 89% of the isolates.
Fig 5.
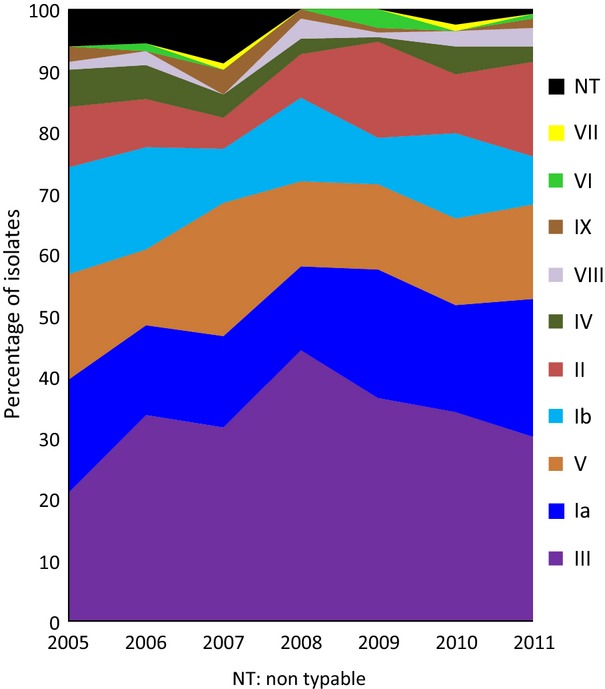
Type distribution of invasive group B streptococcal isolates in Denmark from 2006 to 2011.
Antibiotic resistance among invasive BHS isolates
The proportions of GAS and GCS isolates resistant to erythromycin, clindamycin and tetracycline were low (<5%), whereas the proportions of erythromycin and clindamycin-resistant GBS and GGS isolates ranged from 1.1% to 9.2%. In contrast, almost half of the GBS isolates were resistant to tetracycline (Table1). Two group C blood isolates from 2010 were found to have a penicillin MIC value of 0.5 and 1 mg/L (i.e. above the breakpoint of 0.25 mg/L).
Discussion
An increase of invasive BHS disease was observed in Denmark from 2005 to 2011 based on voluntary submission of invasive BHS isolates to the national reference laboratory as an integral part of the Danish surveillance system. In 2011 the incidences reached 3.1 per 100 000 persons for GAS, 2.6 for GGS, 2.3 for GBS and 0.9 for GCS. For invasive GAS disease similar incidence rates have been reported in the UK (3.1 per 100 000), Sweden (3.1) and the USA (3.5) 10,11). For the other groups of streptococci, however, the incidences in Denmark seem to be lower than in comparable populations in industrialised countries. For example, the incidence of GGS was 4.3 in Finland and 3.2 in the USA 12,13 and the incidence of GBS was reported to be higher in the USA (7.3 14 including all ages and 7.5 in adults above 17 years 15); these incidence differences may at least in part reflect differences in the surveillance methods used.
The number of cases of invasive BHS disease in Denmark is probably higher than we were able to detect with our current surveillance system. First of all, patients with invasive BHS disease but with negative (or missing) cultures will be missed in this system, and secondly, our surveillance system is based on voluntary submission of isolates. A previous study from 1999 to 2002 found that on average 79% of GAS, 58% of GBS, 56% of GCS and 66% of GGS isolates found by the DCMs were sent to the reference laboratory 16. The proportion of isolates submitted may have increased and this can be the reason for the observed increase in incidence of BHS during the surveillance period; however, we do not believe that the peak of invasive GGS in 2009 or the higher proportion of men with GGS disease results from a changed submission rate because such a change should affect other groups of BHS as well. This is supported by an earlier study from the northern part of Denmark reporting a nearly six-fold increase in the annual incidence of GGS bacteriaemia cases (from 3.0 to 17.2 per 100 000) when comparing 1981–1987 with 1994–1999 17.
Our study shows that the incidence of invasive BHS disease is highest among adults above 65 years of age, except for invasive GBS disease, which is also high among children below 3 months of age.
Typing of invasive GAS isolates showed predominance of T- and emm-types 1, 3, 89 and 12, which are also common types in other European countries and the USA 11,18. Similarly, the GBS strains causing invasive disease, types Ia, III and V, have been shown to be major types in the USA 14 and Sweden 19, and for GCS and GGS isolates the emm-types stG6, stG485 and stG643 are also predominant types in the USA 20.
Typing of isolates with respect to components that may be applied in vaccine formulations such as GBS capsule components (the type) and M-protein components (emm-type) may contribute to decisions on vaccine formulations and help to estimate vaccine coverage. Our findings on a potential vaccine coverage for future vaccines of 76% for the 26-valent GAS vaccine and 89% for a future GBS vaccine (including serotypes Ia, Ib, II, III and V) are in accordance with reports from other countries 9,21.
In conclusion, based on national surveillance of bacteriologically confirmed invasive BHS disease we document an increase over the 7-year period 2005–2011 for all Lancefield groups and this is most pronounced for GGS in men. Type distribution and potential vaccine coverage in Denmark are compatible with other Western countries. Future studies should explore in more detail the background of the observed incidence changes. A recently introduced national database updated in real-time by the DCMs (The Danish Microbiology Database (MiBa)) 22 may be useful for future epidemiological studies. However, continuation of typing and characterization of BHS isolates is important to explore potential outbreaks, monitor changes in virulence and evaluate potential vaccine coverage.
Acknowledgments
We acknowledge and express our thanks to all DCMs in Denmark for consistent submission of streptococcal isolates to the NSR Laboratory for the nationwide laboratory-based surveillance of invasive BHS in Denmark. This work was part of the national surveillance of severe streptococcal infections funded by the Danish Ministry of the Interior and Health.
Transparency Declaration
The authors declare that they have no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplementary
Appendix S1. Supplementary data on the statistical analysis
References
- 1.Stevens DL, Kaplan EL. Streptococcal infections: clinical aspects, microbiology, and molecular pathogenesis. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 2.MacFarquhar JK, Jones TF, Woron AM, et al. Outbreak of late-onset group B Streptococcus in a neonatal intensive care unit. Am J Infect Control. 2010;38:283–288. doi: 10.1016/j.ajic.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Arnold KE, Schweitzer JL, Wallace B, et al. Tightly clustered outbreak of group A streptococcal disease at a long-term care facility. Infect Control Hosp Epidemiol. 2006;27:1377–1384. doi: 10.1086/508820. [DOI] [PubMed] [Google Scholar]
- 4.Utsumi M, Makimoto K, Quroshi N, Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing. 2010;39:299–305. doi: 10.1093/ageing/afq029. [DOI] [PubMed] [Google Scholar]
- 5.Facklam R. What happened to the Streptococci: overview of taxonomic and nomenclature changes. Clin Micro Rev. 2002;15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poyart C, Tazi A, Reglier-Poupet H, et al. Multiplex PCR assay for rapid and accurate capsular typing of broup B streptococci. J Clin Microbiol. 2007;45:1985–1988. doi: 10.1128/JCM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Meth. 2010;80:212–214. doi: 10.1016/j.mimet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun. 2002;70:2171–2177. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards MS. Group B streptococcal conjugate vaccine. Hum Vaccin. 2008;4:444–448. doi: 10.4161/hv.4.6.6507. [DOI] [PubMed] [Google Scholar]
- 10.Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46:2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Loughlin RE, Roberson A, Cieslak PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 12.Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J. Clinical presentations and epidemiology of beta-haemolytic streptococcal bacteraemia: a population-based study. Clin Micro Infect. 2009;15:286–288. doi: 10.1111/j.1469-0691.2008.02672.x. [DOI] [PubMed] [Google Scholar]
- 13.Broyles LN, Van Beneden C, Beall B, et al. Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009;48:706–712. doi: 10.1086/597035. [DOI] [PubMed] [Google Scholar]
- 14.Skoff TH, Farley MM, Petit S, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis. 2009;49:85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 15.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299:2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 16.Ekelund K, Skinhøj P, Madsen J, Konradsen HB. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: epidemiological and clinical aspects. Clin Micro Infect. 2005;11:569–576. doi: 10.1111/j.1469-0691.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- 17.Hindsholm M, Schønheyder HC. Clinical presentation and outcome of bacteraemia caused by beta-haemolytic streptococci serogroup G. APMIS. 2002;8:554–558. doi: 10.1034/j.1600-0463.2002.11007806.x. [DOI] [PubMed] [Google Scholar]
- 18.Luca-Harari B, Darenberg J, Neal S, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47:1155–1165. doi: 10.1128/JCM.02155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persson E, Berg S, Trollfors B, et al. Serotypes and clinical manifestations of invasive group B streptococcal infections in western Sweden 1998–2001. Clin Micro Infect. 2004;10:791–796. doi: 10.1111/j.1469-0691.2004.00931.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad Y, Gertz RE, Li Z, et al. Genetic relationships deduced from emm and multilocus sequence typing of invasive Streptococcus dysgalactiae subsp. equisimilis and S. canis recovered from isolates collected in the United States. J Clin Microbiol. 2009;47:2046–2054. doi: 10.1128/JCM.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Sakota V, Jackson D, Franklin AR, Beall B. Array of M protein gene subtypes in 1064 recent invasive group A streptococcus isolates recovered from the active bacterial core surveillance. J Infect Dis. 2003;188:1587–1592. doi: 10.1086/379050. [DOI] [PubMed] [Google Scholar]
- 22.Voldstedlund M, Haahr M. 2012. MiBa. Epi-News.; 25. Available at: http://www.ssi.dk/English/News/EPI-NEWS/2012.aspx (last accessed 16 September 2013)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary
Appendix S1. Supplementary data on the statistical analysis


