Abstract
BACKGROUND
Few studies to date have used the cancer diagnosis as a teachable moment to promote healthy behavior changes in survivors of cancer and their family members. Given the role of obesity in the primary and tertiary prevention of breast cancer, the authors explored the feasibility of a mother-daughter weight loss intervention.
METHODS
A randomized controlled trial of a mailed weight loss intervention was undertaken among 68 mother-daughter dyads (n = 136), each comprised of a survivor of breast cancer (AJCC stage 0-III) and her adult biological daughter. All women had body mass indices ≥ 25 kg/m2 and underwent in-person assessments at baseline, 6 months, and 12 months, with accelerometry and exercise capacity performed on a subset of individuals. All women received a personalized workbook and 6 newsletters over a 1-year period that promoted weight loss; exercise; and a nutrient-rich, low-energy density diet. A total of 25 dyads received individually tailored instruction (INDIVIDUAL), 25 dyads received team-tailored instruction (TEAM), and 18 dyads received standardized brochures (CONTROL).
RESULTS
The trial met its accrual target, experienced 90% retention, and caused no serious adverse events. Significant differences in baseline to 12-month changes were observed between INDIVIDUAL versus CONTROL mothers for body mass index, weight, and waist circumference (WC); significant differences also were observed in the WC of corresponding daughters (P < .05). Significant differences were found between INDIVIDUAL versus CONTROL and TEAM versus CONTROL dyads for WC (P = .0002 and .018, respectively), minutes per week of physical activity (P = .031 and .036, respectively), and exercise capacity (P = .047 for both).
CONCLUSIONS
Significant improvements in lifestyle behaviors and health outcomes are possible with tailored print interventions directed toward survivors of cancer and their family members. For greater impact, more research is needed to expand this work beyond the mother-daughter dyad. Cancer 2014;120:2522–2534. © 2014 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Obesity is a major risk factor for the incidence and mortality of several cancers. The results of this randomized controlled trial of weight loss among 136 women diagnosed with breast cancer and their biological daughters suggests that the diagnosis of cancer can be used to motivate healthy diet and exercise behaviors among survivors of cancer and their family members using mailed print interventions.
Keywords: breast neoplasms, survivors, obesity, weight loss, diet, exercise, intervention studies
INTRODUCTION
The link between obesity and the risk of postmenopausal breast cancer is well known, and similar associations are acknowledged for cancers of the endometrium, kidney, esophagus, colorectum, and pancreas.1 Moreover, as improvements in early detection and treatment are made against the backdrop of increasing rates of obesity, more survivors of cancer are now obese and at an increased risk of prevalent comorbidities such as cardiovascular disease, diabetes, and second malignancies (and possibly progressive cancer).2 Therefore, overweight and its attendant risks are fast replacing cachexia as the most prevalent nutritional problem among patients with cancer.2 Organizations such as the American Society of Clinical Oncology are currently developing toolkits that capitalize on the teachable moment of cancer to promote weight control.3
However, the impact of a cancer diagnosis is not confined to the patient alone. In defining the term “cancer survivor,” the National Coalition for Cancer Survivorship includes family, friends, and caregivers,4 because the impact of cancer is far-reaching. Observational studies have suggested that the mother-daughter relationship may be particularly affected by a cancer diagnosis, especially breast cancer.5,6 Sinicrope et al7 have suggested that the mother-daughter relationship can be leveraged specifically to deliver messages regarding cancer prevention and control.
Only a few health promotion interventions to date have capitalized on the mother-daughter bond to promote healthier behaviors, such as contraception,8 exercise,9,10 osteoporosis prevention,11 and weight control.12 Overall, these interventions have been successful, although all have occurred among mother-daughter dyads in which the daughter was a child or adolescent. To our knowledge, to date there are no published reports of mother-daughter interventions among adult dyads, studies that navigate the complex relationship that has been characterized as “the closest and most profound psychological and emotional intergenerational bond,” yet one that is acknowledged as difficult.6,7
The DAMES (Daughters And MothErS Against Breast Cancer) trial endeavored to capitalize on the mother-daughter bond and the teachable moment created by a cancer diagnosis3 to promote weight loss in overweight or obese women recently diagnosed with breast cancer and their overweight or obese daughters. If feasible and promising, the DAMES trial could offer an expedient way to promote both primary and tertiary prevention: tertiary prevention given that obesity is a poor prognostic indicator for cancer-related and overall survival for the patient diagnosed with breast cancer and primary prevention for her daughter who is at increased risk by virtue of family history and weight status.1 Specific aims of this National Cancer Institute-sponsored, 3-armed, randomized controlled trial (RCT) were to explore the feasibility of a mother-daughter weight loss intervention and evaluate whether an individual approach in which mothers and daughters work in parallel to achieve diet and exercise goals or a team-based approach in which mother-daughter dyads work as a team to achieve these goals yielded greater reductions in body mass index (BMI) from baseline to 12-month follow-up. We hypothesized that a mother-daughter intervention would be feasible and that compared with an individual approach, the team-based approach would yield superior results. Support for the team-based approach is provided by literature regarding weight control and exercise in healthy populations in which having a “buddy” results in increased communication, reinforcement, and support,13 and in cancer populations in which dyadic-based interventions have been shown to promote joint problem-solving and reciprocal coping, leading to superior self-efficacy and quality of life, as well as higher rates of adherence and retention.14–16
MATERIALS AND METHODS
Overview
The DAMES trial was a 2-center, single-blind, parallel group RCT in which a total of 68 dyads (each comprised of an overweight or obese postmenopausal survivor of breast cancer and her overweight or obese adult daughter) underwent baseline assessment and were then randomly assigned to 1 of 3 study conditions: 1) a tailored diet and exercise intervention that was delivered in parallel and individually to mothers and daughters (25 dyads) (INDIVIDUAL); 2) a tailored diet and exercise intervention that emphasized the mother-daughter bond in a team-based approach (25 dyads) (TEAM); or 3) an attention control arm that received standardized diet and exercise materials (18 dyads) (CONTROL). Each of the interventions delivered 7 installments (1 workbook followed by 6 newsletters) of mailed materials over a 1-year period. Dyads were reassessed at 6 months and 12 months of follow-up (Fig. 1). The study was conducted from October 2007 through October 2009, approved by the Institutional Review Boards of the Duke University Health System and the University of Texas MD Anderson Cancer Center (UT-MDACC), and registered and reported according to Consolidated Standards of Reporting Trials guidelines (NCT00630591).
Figure 1.
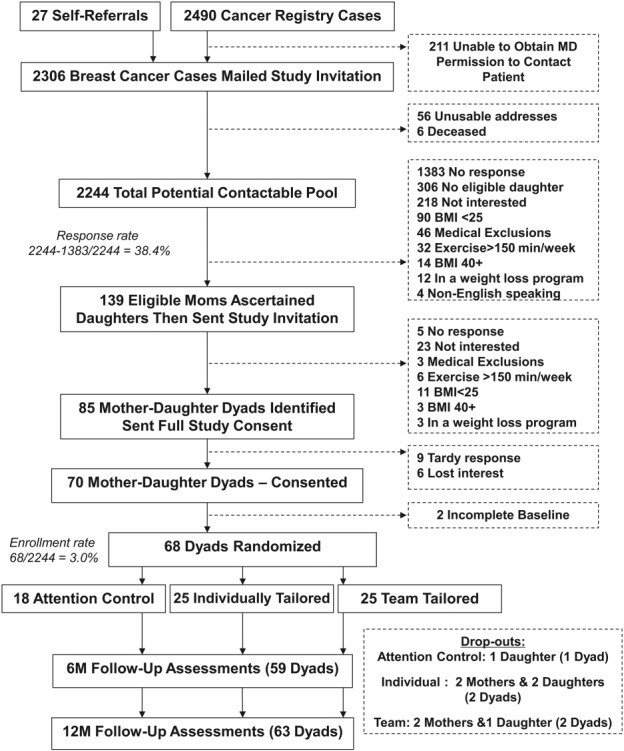
CONSORT (Consolidated Standards Of Reporting Trials) diagram for the current study is shown. BMI indicates body mass index.
Eligibility
Women diagnosed with AJCC stage 0 to III breast cancer who had completed primary treatment but were within 5 years of diagnosis with no evidence of progressive disease or second primary tumors and who had a biological daughter who was aged ≥ 21 years were eligible. Dyad daughters had to have no previous diagnoses of cancer, with the exception of nonmelanoma skin cancer. Both mothers and daughters had to meet the following criteria: 1) a BMI of 25 kg/m2 to 39.9 kg/m2; 2) no preexisting medical condition(s) that would preclude adherence to an unsupervised exercise program (eg, untreated stage 3 hypertension, severe orthopedic conditions or being scheduled for a hip or knee replacement, paralysis, end-stage renal disease, dementia, unstable angina, history of a recent myocardial infarction, or congestive heart failure or pulmonary conditions requiring hospitalization or oxygen use within 6 months) or to a diet high in fruits and vegetables (ie, taking pharmacologic doses of warfarin); 3) ability to speak and write English and the completion of at least the sixth grade and thereby the ability to comprehend the intervention materials; 4) community dwelling in the United States, Puerto Rico, or Guam (regions in which there was visiting nurse coverage by Examination Management Services Inc [Scottsdale, Ariz]); 5) not currently exercising at least 150 minutes per week as assessed by the Leisure-Time Exercise Questionnaire of Godin et al17; and 6) not currently enrolled in a weight loss program.
Accrual/Enrollment
Dyad members were recruited through Web sites, flyers, community presentations, and listserves; however, the primary route for recruitment was through the cancer registries of Duke University and UT-MDACC. Letters of invitation with accompanying screening surveys (see criteria above) were mailed to stage-eligible and age-eligible cases, and a preaddressed, postage-paid envelope was provided for return. Women returning the completed screener received an incentive of 10 postage stamps. Permission and information needed to contact daughters was obtained from survivors of breast cancer who were deemed eligible, and a similar invitation and survey was then posted to daughters. Once an eligible dyad was identified, an in-person baseline assessment was scheduled at Duke University or UT-MDACC (if participants resided within a 60-mile radius and agreed to report) or through a home visit with a visiting nurse for those who were unable or unwilling to travel. Signed informed consent was obtained before baseline data were collected.
Measures
The following objective and self-reported measures were taken at baseline and repeated at 6 months and 12 months of follow-up unless otherwise indicated.
Clinical assessments
Participants' heights and weights were measured while they were wearing light clothing and no shoes.18 Weights were assessed on calibrated and zeroed scales. Heights (taken only at baseline) were performed at maximal upright stance on the inhale with the use of a Frankfort plane. BMI was calculated in kg/m2 and served as an outcome of primary interest. Waist circumference was assessed at the level of the iliac crest at exhale using a nonstretch tape measure.18
Systolic and diastolic blood pressure were measured after participants had been seated for at least 5 minutes and on the arm ipsilateral to the affected breast. Participants with blood pressures above 179 mm Hg systolic or 109 mm Hg diastolic were placed “on hold” until written clearance was obtained by their physician to participate in the unsupervised exercise intervention.
A symptom-limited cardiopulmonary exercise test (CPET) to assess exercise capacity (VO2peak) was performed on a convenience sample of 47 participants who lived within a 60-mile radius of Duke University or UT-MDACC and reported to these institutions for their assessments. The CPET was performed on an electronically braked cycle ergometer with breath-by-breath gas analysis (MedGraphics; St. Paul, Minn) (TrueOne; Parvo Medics, Sandy, Utah) with continuous 12-lead electrocardiographic monitoring according to CPET guidelines for clinical populations.19,20 Oxyhemoglobin saturation was monitored continuously using pulse oximetry, and blood pressure was measured noninvasively by manual auscultatory sphygmomanometry every 2 minutes. Data were recorded as the highest 30-second average within the last 2 minutes of the CPET.
Self-reported measures
Two-part telephone interviews were administered at all 3 timepoints and included the following components: 1) 2 random 24-hour dietary recalls were conducted by trained interviewers using the interactive Nutrition Data System-Revised software (NCC Food and Nutrient Database System Version 2006, Minneapolis, Minn) and data regarding total energy intake; energy from solid fats, added sugars, and alcohol; servings of fruits and vegetables, legumes, total and whole grains, dairy, and meat; and intakes of sodium and saturated fat were used to derive a Healthy Eating Index score as defined by Guenther et al21 and using methods described by Miller et al22; 2) physical activity using the Leisure-Time Exercise Questionnaire of Godin et al17; 3) health-related quality of life using the Short Form-36 instrument23; 4) 2 items on self-efficacy: How confident or sure are you that you could walk or do another type of exercise for at least 30 minutes on 5 or more days of the week? How confident or sure are you that you could regularly limit the number of calories you eat or drink? (examples of portion control, substitution of low-energy for high-energy density foods, and behavioral strategies for consuming fewer calories were provided [anchors: very sure (1), sure (2), somewhat sure (3), unsure (4), and very sure (5)])24; 5) social support related to healthful behavior change with respect to diet and exercise, using validated scales developed by Sallis et al25; and 6) self-reported weight. Sociodemographic factors, such as age, race, marital and smoking status, educational attainment, income adequacy, and distance (in miles) between the residences of the mother and daughter were collected only at baseline. Response cards and food portion visuals were mailed to participants before the scheduled telephone call to enhance the quality of the data collected and to expedite the survey. At 6-month and 12-month follow-up, all participants were asked whether they experienced any hospitalizations or changes in health; details for each event were gathered along with information to discern whether the event was or was not attributable to the intervention.
Accelerometry
Objective data on physical activity were captured for all participants accrued at the Duke University site via accelerometry (Actigraph, Fort Walton Beach, Fla). Programmed actigraphs were mailed to subjects along with instructions for the collection of 1 weeks' worth of data. Procedures analogous to those described by Sloane et al26 were used to excerpt data that were then correlated with measures of self-report.
Randomization
After complete baseline data were collected for both members of each dyad, dyads were randomly assigned by an off-site statistician to 1 of 3 arms (INDIVIDUAL, TEAM, or CONTROL) within 2 strata defined based on the race of the mother (white/nonwhite).
Interventions
Common elements
All groups received a welcome letter informing them that they would receive 6 additional informational mailings on a bimonthly basis over the next year and 6 brief surveys. All participants were informed that they would receive an incentive of $5 for each survey completed and returned within a 3-week window. This letter also was accompanied by a workbook that was personalized with the participant's name. Participants were encouraged to call a toll-free study telephone number if they had questions or problems or needed to report an adverse event.
In addition to equal contact, interventions also shared equal content with all materials written at a sixth-grade reading level. Intervention materials reinforced goals proposed by the American Cancer Society27 and the US dietary guidelines.28 Materials promoted portion control and diets high in nutrients and low in energy as well as 150 minutes per week of aerobic exercise and twice-weekly strength training. However, interventions differed with respect to tailoring.
INDIVIDUAL arm
Mothers and daughters assigned to this arm each received individually tailored print materials. For example, the initial workbook was not only personalized with the participant's name, but the initial pages also delineated individual weight goals and the kilocalorie levels required to achieve desired rates of weight loss using the Mifflin-St. Jeor equation (kcal/day = −161 + 10(wt) + 6.25 (ht) −5(age)).29 In addition, the 3 major foods contributing the highest percentage of kilocalories to each participant's diet were identified from the dietary recalls performed at baseline, and individuals were either directed to lower-calorie substitutes or provided with guidance on portion control. Introductory pages also included tailored feedback on how current intakes of saturated fat and fruits and vegetables as well as physical activity compared with the national guidelines. In keeping with social cognitive theory, which provided the primary behavioral framework for the interventions,24 participants were encouraged to keep records of their food intake and physical activity (self-monitoring), as well as to problem-solve on overcoming perceived barriers to healthy behaviors and to set incremental and achievable goals.24 As stated, participants were surveyed bimonthly on their progress and plans, as well as their perceived barriers and readiness to pursue lifestyle changes and responses were used to inform tailored messages.30 The 6 subsequent newsletters provided tailored messages regarding progress toward goals, along with appropriate reinforcement (if progress was charted) or encouragement (if progress was stalled) and feedback on portion control and overcoming barriers. Newsletter messages also were framed on readiness to pursue lifestyle changes, and thus elements of the transtheoretical model of behavior change were drawn on to engage participants with the level of information best able to motivate them.30 Mothers and daughters assigned to this arm also received supplies and equipment to assist them with self-monitoring, such as logbooks and reference manuals (T-Factor 2000; WW Norton and Company, New York, NY) or Web sites (eg, mypyramid.org) to tally their intakes of kilocalories and saturated fat. They also received portion control tableware (Portion Doctor; Portion Health Products, St. Augustine Beach, Fla); iPods with prerecorded selections to set walking pace (Apple, Cupertino, Calif); and shoe chips (Nike Inc, Beaverton, Ore) to monitor steps taken, minutes of physical activity, and kilocalories burned. Figure 2 shows an illustration of the study materials.
Figure 2.

Illustration of intervention materials used in the DAMES (Daughters and MothErS Against Breast Cancer) trial is shown.
TEAM arm
Mothers and daughters assigned to the team-based intervention received information and supplies identical to those in the individual arm, but also received information on their other team member. Here, concepts of interdependence theory (ie, structuring goals to guide mother-daughter interactions to ultimately achieve outcomes) and the theory of communal coping (ie, cooperative problem-solving to deal with individual and common stressors) were drawn on to leverage the mother-daughter bond by encouraging effective communication between partners that would enhance their sense of confidence in planning, coordinating, and carrying forth strategies to increase mutual benefit.31,32 As an example, if a dyad member was charting success at meeting exercise goals, their next newsletter would provide positive reinforcement and also encourage them to share (in a helpful way) what had worked for them with their partner. Likewise, if a dyad member was experiencing a setback, they were provided with suggestions to get back on track and their partner was encouraged to provide them with helpful support.
CONTROL arm
Mothers and daughters assigned to this arm received a copy of the National Cancer Institute brochure Facing Forward (NIH Publication No. 10-2424) and the American Institute for Cancer Research publication Facts on Weight Management and Cancer, which were included in their binder personalized with their name. Subsequent brochures were mailed on a bimonthly basis and included American Institute for Cancer Research brochures (New American Plate, A Healthy Weight for Life, Getting Active-Staying Active, and Moving Toward a Plant-based Diet) and pamphlets from the American Heart Association (Managing Your Weight and Cholesterol, Blood Pressure and Weight Tracker) and the American College of Sports Medicine (Fit Over 40). These brochures were accompanied by a cover letter that encouraged the participant to read the brochure and then place it in their binder for easy reference. Bimonthly surveys assessed the perceived helpfulness of each brochure.
Statistical Analysis
DAMES was a feasibility trial and therefore an emphasis was placed on the accrual, delivery, and acceptance of the intervention, attrition, and adverse events. Preestablished benchmarks were the achievement of: 1) targeted accrual within a 9-month period; 2) “good” adherence as noted by the completion of at least 4 of 6 of the written surveys; 3) a retention rate of at least 80%; and 4) an absence of serious adverse events that were directly attributable to the intervention. General linear models were used to test for arm differences in adherence as measured by the number of completed surveys. Because other feasibility endpoints were dichotomous, their arm differences were tested using chi-square tests.
Because weight loss was the goal of each intervention, changes in BMI from baseline to 12 months were also of interest. The sample size for this feasibility study was derived using assumed differences between the control and each experimental arm of 0.48 (effect sizes in the “medium-to-large range” as defined by Cohen33) using a 2-sided alpha of .05 and a power of 0.80. The effect of the interventions by arm on other measures of adiposity (eg, body weight and waist circumference), health-related outcomes (eg, blood pressure and cardiorespiratory fitness), and quality of life were explored in a similar fashion. To estimate the arm effects on change in outcomes from baseline to 12 months, repeated-measures models were used to account for the covariance between the mothers and daughters. In addition, general linear models were used to estimate these arm effects within mothers and daughters separately. Although testing largely compared both experimental arms with the control group, for BMI (a key outcome) the 2 experimental groups also were compared with one another. Because this was a feasibility study, a 2-sided alpha level of .05 was used for all tests without attempting to control for multiplicities. All analyses were performed using SAS statistical software (version 9.3; SAS Institute Inc, Cary, NC).
RESULTS
Feasibility Endpoints
Accrual
The DAMES trial achieved its accrual target and also met racial/ethnic benchmarks within the established time frame. However, the 3% accrual rate was much lower than the 10% rate that was initially forecast and therefore the study required a patient pool that was triple the original estimate. Approximately 38% of respondents completed the screening questionnaire, and among responders the leading reasons for nonparticipation in the trial were the absence of a biological daughter and overall disinterest (a frequent written-in comment suggested that respondents would be interested in participating if they could partner with someone other than their daughter). Women diagnosed with breast cancer (mothers) who either refused, failed to respond, or were ineligible to participate in the trial did not differ from those who were enrolled in terms of race, age, or stage of disease (all P > .05). Characteristics of the overall study sample are reported in Table 1 and did not differ by arm assignment with respect to tumor features, demographics, study site, smoking status, or BMI. The study sample was diverse in terms of race/ethnicity and socioeconomic status. The majority of the mothers had stage I or II breast cancer, and most women had a BMI of ≥ 30 kg/m2 (obese).
Table 1.
Study Sample Characteristicsa
| Characteristic | Mothers (n=68) | Daughters (n=68) |
|---|---|---|
| AJCC Stage of disease, no. (%) | ||
| 0 | 12 (18%) | — |
| I | 29 (43%) | — |
| II | 21 (31%) | — |
| III | 3 (4%) | — |
| Missing | 3 (4%) | — |
| Mean time since diagnosis, mo (SD) | 24 (13) | — |
| Distance between mother-daughter dyad, miles | ||
| Mean (SD) | 75 (86) | |
| Range | 0-646 | |
| Race | ||
| Non-Hispanic white | 100 (74%) | |
| Hispanic white | 10 (7%) | |
| African-American | 24 (18%) | |
| Asian | 2 (1%) | |
| Site, no. (%) | ||
| Duke University | 21 (30.8%) | |
| The University of Texas MD Anderson Cancer Center | 47 (69.2%) | |
| Age, y | ||
| Mean (SD) | 61.3 (7.4) | 32.9 (1.4) |
| Range | 46-80 | 21-54 |
| Educational status, no. (%) | ||
| Less than high school | 1 (1.5%) | 0 (0%) |
| High school graduate | 18 (26.9%) | 7 (10.3%) |
| Some college/junior college/trade school | 25 (37.3%) | 24 (35.3%) |
| College graduate/postgraduate | 23 (34.3%) | 37 (54.4%) |
| Income, no. (% less than $40K/y) | 21 (31%) | 19 (28%) |
| Current smoker, no. (%) | 2 (2.9%) | 9 (13.2%) |
| Mean BMI (SD), kg/m2 | 31.0 (2.6) | 32.9 (1.4) |
Abbreviations: BMI, body mass index; SD, standard deviation.
No differences were observed between the 3 study groups with regard to these characteristics and therefore data are presented for the overall sample.
Adherence and retention
Table 2 shows data regarding adherence as ascertained by completion of the intermittent surveys. The benchmark of completion of at least 4 of the 6 surveys (67%) was only achieved in the control group, which had significantly higher completion rates compared with either of the intervention arms (P = .0019). Response rates were also found to be significantly higher among mothers compared with daughters across all 3 study arms (P = .0342). Retention rates varied from 84% to 100% across arms (90% overall); no significant differences were observed between arms or for any sociodemographic or disease-related characteristics.
Table 2.
Adherence (Completion Rates) to Periodic Surveys Used to Inform Newsletters
| Survey Completionabc Mean (SD) | 67% Survey Completion Rate % of Sample | 100% Survey Completion Rate % of Sample | |
|---|---|---|---|
| INDIVIDUAL (25 dyads) | |||
| Dyad | 3.44 (2.41) | 53% | 30% |
| Mothers | 4.00 (2.23) | 68% | 36% |
| Daughters | 2.88 (2.49) | 48% | 24% |
| TEAM (25 dyads) | |||
| Dyad | 3.53 (2.12) | 66% | 28% |
| Mothers | 3.96 (2.15) | 68% | 36% |
| Daughters | 3.20 (2.08) | 44% | 20% |
| ATTENTION CONTROL (18 dyads) | |||
| Dyad | 4.94 (1.35) | 86% | 43% |
| Mothers | 5.06 (1.11) | 89% | 39% |
| Daughters | 4.83 (1.58) | 83% | 47% |
Abbreviation: SD, standard deviation.
Mean completion from possible 6 surveys.
P value by randomization status (P=.0019).
P value for mothers versus daughters (P=.0342).
Adverse events
A total of 95 health-related events were reported among the 136 women over the 1-year study period, 9 of which were serious. No differences were noted between study arms and only 2 events (1 report in the CONTROL arm and other in the INDIVIDUAL arm) were attributable to the intervention (nonserious knee and/or hip soreness with exercise).
Effects
Table 3 documents precision estimates for various study outcomes by arm across time. Significant reductions in BMI were observed in mothers assigned to the INDIVIDUAL arm compared with those in the CONTROL arm, although such differences were not observed among daughters and only a trend was seen in dyads overall. Similar findings were observed in the INDIVIDUAL arm for weight loss as measured as a continuous variable. It is interesting to note that 39.1% of mothers as well as 39.1% of daughters in the INDIVIDUAL arm lost a clinically significant amount of body weight (≥ 5% of their baseline weight), whereas the respective rates among mothers and daughters in the TEAM arm and CONTROL arm were 21.7% and 33.3% and 27.8% and 35.3%, respectively; no significant between-arm differences were observed. Although analyses were performed on actual weights, a significant correlation (ρ = 0.940; P < .0001) was observed between actual and self-reported weight (data not shown). No significant differences were observed between the INDIVIDUAL and TEAM arms for BMI.
Table 3.
Mean Values (SD) for Study Outcomes Across Study Arms at Baseline, 6 Months, and 12 Months
| Condition | Baseline | 6 Months | 12 Months | Baseline to 12-Month Change Scoresa | P for Baseline to 12-Month Changeb Intervention Versus Controlc | ||
|---|---|---|---|---|---|---|---|
| Team | Individual | ||||||
| BMI, kg/m2 | |||||||
| Mothers | Control | 30.7 (2.6) | 30.6 (2.9) | 30.4 (3.1) | −0.33 (1.12) | .40 | .03 |
| Individual | 31.6 (3.4) | 30.2 (4.1) | 30.1 (4.0) | −1.40 (1.72) | |||
| Team | 30.8 (3.3) | 29.6 (3.1) | 29.6 (2.9) | −0.74 (1.63) | |||
| Daughters | Control | 33.3 (5.7) | 32.8 (5.9) | 32.8 (5.5) | −0.97 (2.96) | .73 | .46 |
| Individual | 32.5 (5.0) | 31.1 (5.2) | 30.9 (5.7) | −1.38 (2.79) | |||
| Team | 32.6 (7.3) | 31.5 (6.7) | 31.4 (6.3) | −1.07 (2.81) | |||
| Test for dyad | .46 | .11 | |||||
| Body weight, kg | |||||||
| Mothers | Control | 81.6 (9.3) | 81.3 (9.9) | 80.7 (10.1) | −0.87 (2.97) | .35 | .04 |
| Individual | 83.2 (8.8) | 80.3 (10.8) | 79.7 (10.2) | −3.77 (4.80) | |||
| Team | 82.6 (13.4) | 79.6 (12.6) | 78.8 (9.6) | −2.09 (4.30) | |||
| Daughters | Control | 93.1 (18.7) | 91.5 (19.3) | 91.4 (17.7) | −2.78 (8.39) | .63 | .40 |
| Individual | 87.5 (14.5) | 83.6 (15.1) | 83.1 (16.5) | −3.65 (7.35) | |||
| Team | 89.1 (23.7) | 86.0 (21.4) | 85.8 (20.0) | −3.09 (8.00) | |||
| Test for dyad | .38 | .09 | |||||
| Waist circumference, cm | |||||||
| Mothers | Control | 94.7 (8.8) | 93.8 (9.1) | 93.7 (9.7) | −1.0 (3.7) | .12 | .004 |
| Individual | 97.4 (8.9) | 92.6 (8.7) | 90.7 (7.4) | −6.5 (6.7) | |||
| Team | 96.1 (10.5) | 93.7 (9.9) | 91.4 (8.4) | −3.7 (5.4) | |||
| Daughters | Control | 97.3 (12.9) | 97.3 (14.7) | 97.2 (13.2) | −1.0 (6.9) | .10 | .03 |
| Individual | 95.9 (11.9) | 91.7 (13.1) | 90.1 (13.6) | −5.3 (5.9) | |||
| Team | 94.9 (14.5) | 92.1 (14.1) | 90.8 (13.4) | −4.1 (6.9) | |||
| Test for dyad | .018 | .0002 | |||||
| Systolic blood pressure | |||||||
| Mothers | Control | 123.1 (10.2) | 124.9 (17.9) | 122.1 (20.5) | −1.0 (20.1) | .49 | .29 |
| Individual | 132.3 (14.7) | 128.9 (15.0) | 130.0 (17.6) | −2.0 (18.1) | |||
| Team | 125.1 (12.8) | 124.0 (11.7) | 118.8 (10.4) | −5.1 (17.8) | |||
| Daughters | Control | 114.2 (10.1) | 111.8 (15.3) | 116.7 (14.4) | +1.6 (12.0) | .88 | .57 |
| Individual | 115.7 (8.5) | 112.9 (7.8) | 115.2 (14.5) | −0.7 (12.6) | |||
| Team | 116.0 (11.2) | 113.1 (13.1) | 117.8 (14.9) | +2.1 (11.4) | |||
| Test for dyad | .66 | .87 | |||||
| Physical activity (minutes of moderate plus vigorous/wk) | |||||||
| Mothers | Control | 31.9 (79.9) | 60.8 (112.0) | 32.4 (66.0) | +0.5 (99.1) | .18 | .09 |
| Individual | 39.8 (88.4) | 75.5 (94.9) | 73.0 (73.9) | +32.4 (105.2). | |||
| Team | 32.4 (58.8) | 90.4 (100.0) | 63.8 (77.1) | +30.5 (108.9) | |||
| Daughters | Control | 10.0 (24.7) | 29.7 (54.4) | 34.9 (63.5) | +24.9 (68.9) | .13 | .23 |
| Individual | 35.3 (56.2) | 124.0 (106.9) | 66.2 (82.1) | +27.9 (95.1) | |||
| Team | 25.0 (51.7) | 84.5 (80.7) | 71.8 (62.3) | +45.7 (78.7) | |||
| Test for dyad | .036 | .031 | |||||
| Physical activity (MET hr/wk from questionnaire) | |||||||
| Mothers | Control | 4.5 (7.7) | 6.9 (13.1) | 4.1 (5.6) | +0.1 (8.3) | .12 | .06 |
| Individual | 5.1 (10.7) | 8.6 (8.3) | 9.0 (8.9) | +3.1 (10.8) | |||
| Team | 5.6 (6.5) | 10.8 (9.6) | 8.3 (8.7) | +2.9 (11.6) | |||
| Daughters | Control | 2.7 (3.5) | 6.2 (6.5) | 6.5 (8.1) | +2.8 (8.1) | .29 | .74 |
| Individual | 6.4 (8.5) | 16.9 (11.5) | 7.8 (10.0) | +1.8 (10.8) | |||
| Team | 4.4 (7.6) | 10.8 (10.7) | 9.6 (7.6) | +4.6 (10.7) | |||
| Test for dyad | .06 | .13 | |||||
| Energy intake, kcal/d | |||||||
| Mothers (mean [SD]) | Control | 1671 (550) | 1337 (452) | 1400 (487) | −270 (566) | .76 | .14 |
| Individual | 1570 (605) | 1195 (365) | 1189 (429) | −402 (586) | |||
| Team | 1698 (485) | 1406 (374) | 1369 (399) | −319 (425) | |||
| Daughters (mean [SD]) | Control | 1571 (556) | 1481 (545) | 1561 (786) | +19 (706) | .63 | .72 |
| Individual | 1691 (508) | 1482 (452) | 1545 (553) | −112 (584) | |||
| Team | 1776 (618) | 1542 (522) | 1559 (476) | −183 (638) | |||
| Test for dyad | .62 | .35 | |||||
| Diet quality index (HEI-2005) | |||||||
| Mothers | Control | 58.9 (8.7) | 56.8 (7.9) | 58.9 (10.7) | 0.0 (12.7) | .21 | .30 |
| Individual | 62.6 (9.0) | 64.8 (7.3) | 63.7 (11.9) | +2.0 (14.0) | |||
| Team | 57.5 (10.7) | 63.4 (11.3) | 62.7 (11.7) | +4.8 (8.2) | |||
| Daughters | Control | 54.2 (9.3) | 57.0 (13.3) | 55.9 (13.9) | +1.6 (12.3) | .45 | .74 |
| Individual | 53.7 (9.0) | 59.5 (12.2) | 57.1 (10.8) | +2.8 (10.7) | |||
| Team | 53.7 (10.0) | 55.2 (10.9) | 58.2 (12.3) | +5.0 (14.0) | |||
| Test for dyad | .15 | .35 | |||||
| Self-efficacy for exercise (very unsure [5] to very sure [1]) | |||||||
| Mothers | Control | 1.9 (1.1) | 2.1 (1.1) | 2.2 (1.0) | +0.2 (0.9) | .58 | .12 |
| Individual | 2.2 (1.1) | 2.0 (1.2) | 1.7 (1.0) | −0.4 (1.5) | |||
| Team | 1.8 (0.8) | 2.0 (1.0) | 2.0 (1.1) | +0.1 (1.0) | |||
| Daughters | Control | 1.9 (1.1) | 2.2 (1.4) | 2.2 (1.0) | +0.2 (1.3) | .94 | .48 |
| Individual | 1.7 (0.9) | 1.9 (1.0) | 1.8 (0.9) | +0.1 (0.8) | |||
| Team | 1.6 (0.9) | 2.0 (1.0) | 2.0 (1.5) | +0.4 (1.4) | |||
| Test for dyad | .69 | .09 | |||||
| Self-efficacy for adhering to a healthy weight loss diet (very unsure [5] to very sure [1]) | |||||||
| Mothers | Control | 2.1 (0.9) | 2.1 (0.9) | 2.3 (0.8) | +0.3 (1.0) | .09 | .56 |
| Individual | 1.9 (0.8) | 1.7 (0.7) | 2.1 (0.9) | +0.3 (1.0) | |||
| Team | 2.0 (0.9) | 1.9 (0.8) | 1.9 (0.8) | −0.1 (0.8) | |||
| Daughters | Control | 2.0 (1.1) | 1.9 (0.8) | 1.9 (0.8) | −0.1 (1.1) | .69 | .78 |
| Individual | 1.7 (0.9) | 1.9 (0.9) | 1.7 (0.8) | +0.1 (1.1) | |||
| Team | 1.8 (0.8) | 2.1 (1.0) | 1.8 (1.0) | −0.1 (1.0) | |||
| Test for dyad | .16 | .56 | |||||
| Frequency of receiving social support for adhering to routine exercise and a healthy weight loss diet (times per wk) | |||||||
| Mothers | Control | 9.1 (16.6) | 12.2 (18.5) | 9.3 (18.5) | +0.1 (4.0) | .71 | .15 |
| Individual | 10.9 (17.3) | 15.1 (20.7) | 8.0 (14.1) | −3.1 (8.0) | |||
| Team | 6.7 (6.4) | 7.8 (12.5) | 7.6 (9.7) | +1.0 (8.2) | |||
| Daughters | Control | 14.2 (23.6) | 14.5 (24.7) | 11.0 (16.6) | −3.9 (14.0) | .37 | .29 |
| Individual | 7.9 (11.5) | 17.1 (20.9) | 8.0 (9.3) | −0.1 (11.8) | |||
| Team | 8.4 (10.6) | 6.6 (6.8) | 6.0 (5.3) | −0.7 (6.5) | |||
| Test for dyad | .72 | .55 | |||||
| Quality of life (SF-36) physical quality of life | |||||||
| Mothers | Control | 45.3 (8.5) | 45.4 (7.7) | 46.2 (9.0) | +0.9 (4.9) | .16 | .73 |
| Individual | 44.3 (8.3) | 43.8 (9.7) | 45.8 (9.2) | +2.2 (10.4) | |||
| Team | 44.3 (11.9) | 44.3 (12.9) | 42.8 (11.4) | −2.3 (6.5) | |||
| Daughters | Control | 48.2 (8.7) | 50.5 (10.4) | 52.0 (7.7) | +4.2 (5.6) | .88 | .77 |
| Individual | 51.8 (7.4) | 53.5 (9.5) | 53.5 (6.9) | +2.0 (7.1) | |||
| Team | 50.7 (6.8) | 53.8 (3.8) | 54.0 (5.5) | +3.0 (5.8) | |||
| Test for dyad | .26 | .93 | |||||
| Quality of life (SF-36) mental quality of life | |||||||
| Mothers | Control | 53.7 (8.5) | 56.3 (8.5) | 56.1 (5.8) | +2.4 (7.4) | .46 | .35 |
| Individual | 56.6 (8.2) | 58.7 (7.2) | 55.5 (10.4) | −1.9 (10.0) | |||
| Team | 52.1 (11.7) | 54.0 (8.9) | 53.6 (12.4) | +0.6 (10.7) | |||
| Daughters | Control | 47.1 (13.4) | 49.1 (12.7) | 47.9 (10.8) | +1.4 (7.9) | .10 | .28 |
| Individual | 51.3 (9.7) | 51.3 (10.7) | 52.2 (10.7) | +2.0 (12.9) | |||
| Team | 51.6 (11.1) | 51.6 (7.4) | 54.3 (6.9) | +2.9 (12.8) | |||
| Test for dyad | .64 | .94 | |||||
Abbreviations: BMI, body mass index; HEI-2005, Healthy Eating Index-2005; MET, metabolic equivalent task; SD, standard deviation; SF-36, Short Form-36.
Change scores are based on participants with complete data at baseline and at 12-month follow-up (ie, 18 of 18 CONTROL mothers, 17 of 18 CONTROL daughters, 23 of 25 INDIVIDUAL mothers, 23 of 25 INDIVIDUAL daughters, 23 of 25 TEAM mothers, and 24 of 25 TEAM daughters).
Note that P values reflect testing on change from baseline to 12-month follow-up and are based on residualized change scores on all available data (which differ slightly from the change scores depicted in the previous column); the P values shown are unadjusted for other factors such as race, age, and education because these variables did not appreciably alter the value.
Bold type indicates statistical significance.
Waist circumference proved to be a more sensitive measure of adiposity and dyads assigned to both the TEAM and INDIVIDUAL arms experienced significant reductions compared with those assigned to the CONTROL arm. Significant differences also were observed among mothers and daughters in the CONTROL versus INDIVIDUAL arms. Likewise, VO2peak change scores were found to be significantly improved in both of the intervention arms compared with the CONTROL arm (TEAM, +1.28 mL.kg.−1min−1 [2.77] and INDIVIDUAL, +1.64 [1.85] vs CONTROL, −0.52 [2.53]; P = .047 for both). No between-arm differences were observed with regard to blood pressure and quality of life.
Compared with controls, dyads in both the TEAM and INDIVIDUAL arms experienced significantly greater increases in self-reported minutes of moderate to vigorous physical activity (supported by trends of total metabolic equivalent task [MET] hours per week of physical activity captured via self-report and accelerometry, both of which were found to be significantly correlated [Spearman ρ, 0.300; P = .009]). No between-arm differences were noted with regard to energy intake or diet quality. Similarly, no between-arm differences were observed with regard to potential mediators of the intervention (eg, social support or self-efficacy). Significant correlations were found between adherence (as measured by survey completion) and change in BMI in both the CONTROL and INDIVIDUAL arms, but not for the TEAM arm; Pearson correlation coefficients were ρ of 0.417 (P = .015), ρ of 0.294 (P = .048), and ρ of −0.038 (P = .802), respectively.
DISCUSSION
To the best of our knowledge, the DAMES trial is the first lifestyle intervention that attempted to capitalize on the teachable moment of a cancer diagnosis together with leveraging the mother-daughter bond to promote intergenerational lifestyle change in mothers with breast cancer and their biological daughters. Similar to previous studies conducted in healthy mother-daughter dyads that were aimed at healthful dietary changes and increased exercise, the overall retention rate for the DAMES trial was 90% and therefore was comparable to the 78% to 100% range established by these trials,10,12 while also reporting no serious adverse events attributable to the intervention.
Although the DAMES trial was undertaken to assess feasibility and was not fully powered to test for differences in outcomes, several statistically significant improvements in physical activity and fitness and reductions in adiposity (as measured by BMI, body weight, and waist circumference) were observed with both minimal interventions of personally and iteratively tailored print materials. Moreover, the magnitude of effects also appears clinically significant for many of these outcomes. For example, increases in the Healthy Eating Index of 2 points as noted in the mothers and daughters on the INDIVIDUAL arm are considered slightly above a “small effect,” whereas improvements of roughly 5 points, as noted among TEAM mothers and daughters, are considered significant.34 The doubling of the number of minutes of physical activity within the intervention arms and the finding that these increases equate to an annual weight loss of 0.97 kg to 1.59 kg also bodes for clinical significance. However, the large variation in physical activity, particularly among daughters on the TEAM arm, is cause for caution and may be influenced somewhat by 3 participants who became marathon runners over the course of the study year (these women were obvious outliers, but because their data were valid we included them in our analysis). Finally, the weight loss literature indicates that reductions of 5% body weight are found to improve several health parameters, such as serum glucose, lipids, and blood pressure.35 Thus, the finding that these minimal interventions resulted in a weight loss of ≥ 5% in a substantial percentage of participants (ie, 21.7%-39.1%) is also clinically significant. Perhaps the reason that we were unable to detect differences between the INDIVIDUAL and TEAM tailored interventions was due to a lack of power, especially among mothers, in whom the percentage achieving 5% weight loss was most discrepant and did not align with our original hypothesis (ie, that the team-based approach would produce effect sizes of the greatest magnitude).
The benefits of family-based approaches for weight loss may depend partly on the nature of the relationship between family members and the age/developmental stage of the target. Supporting this idea, a review conducted by McLean et al36 found that programs that treated overweight couples together resulted in greater weight loss for both partners, but that programs that treated overweight children and their overweight parents only yielded positive results for the children (to the best of our knowledge, however, to date this has only been explored in dependent children). Given the complex nature of family relationships, more research is needed to understand how best to involve family members in diet and exercise interventions. Indeed, family involvement can either entail enlisting a family member as a supporter of the survivor's behavior change or actively involving and treating the family member along with the survivor.36,37 The latter approach is consistent with family systems38 and interpersonal theories39 and emphasizes relationship factors such as closeness, communication, and the quality of the relationship in the behavior change process.39 Although this main outcomes analysis did not examine relationship characteristics as potential moderators of effect, studies suggest that we may also need to carefully consider family member characteristics when deciding whether a communal or social support approach is more appropriate. For example, Brownell et al40 found that obese adolescents who were treated alone lost significantly more weight than obese adolescents who attended a weight loss program with their mothers (who were not obese). However, in an intervention in which overweight adults were encouraged to invite up to 3 partners of their choosing to join them in a weight loss program, Gorin et al41 found that those with at least 1 successful partner lost significantly more weight at 6 months, 12 months, and 18 months. Together, these studies suggest that more work is needed to determine whether survivors of cancer and their family members can benefit from communal approaches. Moreover, data from the current study also suggest that the impact of communal approaches may vary based on outcome. For example, compared with the INDIVIDUAL-based approach, the TEAM-based approach appeared to generate nearly double the increase in diet quality, but exhibited fewer improvements in weight loss. Although the cause of this variable effect is unknown, one potential explanation could be that the TEAM-based intervention tied into mother-daughter communications that have been traditionally practiced, such as recipe sharing and food preparation.42 Weaker than expected effects within the TEAM-based approach for other outcomes may have been due to the lack of intensive skill training in areas of active listening, requesting assistance, and providing optimal support.43
Perhaps the more important lesson learned from the current study lies not in the comparative effect of the interventions but rather in the study population (ie, mothers and daughters). As indicated in the literature, the mother-daughter relationship is complex, and although it can be positive, it also is subject to strain.44 The added pressure of a breast cancer diagnosis and issues surrounding weight control can further stress the relationship,6 making participation in a mother-daughter weight loss intervention perhaps less productive than if survivors of cancer were provided free choice in selecting a teammate for the partner-based intervention, as in the aforementioned study by Gorin et al.41 Through implementation of the current study, we found that focusing recruitment solely on mother-daughter dyads served as a considerable barrier because many survivors of breast cancer did not have biological daughters or did not want to participate in a weight loss intervention with them. Likewise, similar barriers were observed among daughters. Our team has conducted several mailed print interventions and the response rate to the DAMES trial was considerably lower; our response rate was 3%, compared with the response rate of 42% experienced in FRESH START, which recruited 543 survivors of breast and prostate cancer over a similar time period.45 In addition, the absence of relative improvements in perceived social support or satisfaction with the mother-daughter relationship, especially within the TEAM arm, after working toward a common goal over the 1-year intervention period are of interest, although it could be posited that high baseline levels may have reduced the ability to detect change. Nevertheless, the observation that adherence was significantly greater in the CONTROL arm compared with the INTERVENTION arm, and that adherence was only related to BMI in the CONTROL and INDIVIDUAL arms but not among members of the TEAM arm, suggests that other factors may be responsible (eg, the intervention spurring membership in weight loss and fitness programs).
The lack of changes in social support or self-efficacy as well as the lack of an association between these measures and changes in objective outcomes are disconnects, particularly because the intervention was grounded in social cognitive theory,24 interdependence theory,31 and the theory of communal coping.32 However, similar disconnects have been found in fully powered trials either for global behavior change46 or for behavior change within specific domains.47 Therefore, the finding that we observed changes in BMI but did not observe changes in these intermediate constructs is hardly a rare phenomenon and also could be due to ceiling effects or a lack of statistical power. Indeed, the lack of statistical power was a primary limitation of the current study, but one that was balanced by numerous strengths, including a strong RCT design; objective measures; excellent retention; and, even if enrollment was low, the sample accrued was diverse and not biased in terms of race, age, or stage of disease.
Therefore, the data from the current study, plus the resulting precision estimates that demonstrate the benefits of a minimal intervention comprised of 1 workbook and a series of 6 iteratively tailored newsletters, are compelling and call for future interventions that are directed toward survivors of cancer and selected partners. Given the evidence of a greater impact with partner-based interventions that emanate from other studies, there is a need to test a similar intervention in other patient-partner dyads; to test the added value of intensive skill training in active listening and other supportive techniques; and to experiment with other means of dissemination, such as Web-based platforms to achieve broader reaching impact.
FUNDING SUPPORT
Funding for this research was provided by the following National Institutes of Health grants: R21 CA122143, P30 CA16672, and P30 CA13148 (to Dr. Demark-Wahnefried) and K01 CA134550 (to Dr. Hughes).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Coalition for Cancer Survivorship. 2013. canceradvocacy.org/. Accessed October 3.
- 5.Kim Y, Wellisch DK, Spillers RL. Effects of psychological distress on quality of life of adult daughters and their mothers with cancer. Psychooncology. 2008;17:1129–1136. doi: 10.1002/pon.1328. [DOI] [PubMed] [Google Scholar]
- 6.Wiggs CM. Mothers and daughters: intertwining relationships and the lived experience of breast cancer. Health Care Women Int. 2011;32:990–1008. doi: 10.1080/07399332.2011.603858. [DOI] [PubMed] [Google Scholar]
- 7.Sinicrope PS, Brockman TA, Patten CA, et al. Factors associated with breast cancer prevention communication between mothers and daughters. J Womens Health (Larchmt) 2008;17:1017–1023. doi: 10.1089/jwh.2007.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noone J, Young HM. Rural mothers' experiences and perceptions of their role in pregnancy prevention for their adolescent daughters. J Obstet Gynecol Neonatal Nurs. 2010;39:27–36. doi: 10.1111/j.1552-6909.2009.01082.x. [DOI] [PubMed] [Google Scholar]
- 9.Ransdell LB, Dratt J, Kennedy C, O'Neill S, DeVoe D. Daughters and mothers exercising together (DAMET): a 12-week pilot project designed to improve physical self-perception and increase recreational physical activity. Women Health. 2001;33:101–116. [PubMed] [Google Scholar]
- 10.Ransdell LB, Taylor A, Oakland D, Schmidt J, Moyer-Mileur L, Shultz B. Daughters and mothers exercising together: effects of home- and community-based programs. Med Sci Sports Exerc. 2003;35:286–296. doi: 10.1249/01.MSS.0000048836.67270.1F. [DOI] [PubMed] [Google Scholar]
- 11.Winzenberg TM, Oldenburg B, Frendin S, De WL, Jones G. A mother-based intervention trial for osteoporosis prevention in children. Prev Med. 2006;42:21–26. doi: 10.1016/j.ypmed.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Stolley MR, Fitzgibbon ML. Effects of an obesity prevention program on the eating behavior of African American mothers and daughters. Health Educ Behav. 1997;24:152–164. doi: 10.1177/109019819702400204. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JP, Raglin JS, Jastremski CA. Twelve month adherence of adults who joined a fitness program with a spouse vs without a spouse. J Sports Med Phys Fitness. 1995;35:206–213. [PubMed] [Google Scholar]
- 14.Kim Y, Kashy DA, Wellisch DK, Spillers RL, Kaw CK, Smith TG. Quality of life of couples dealing with cancer: dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med. 2008;35:230–238. doi: 10.1007/s12160-008-9026-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Carver CS, Spillers RL, Crammer C, Zhou ES. Individual and dyadic relations between spiritual well-being and quality of life among cancer survivors and their spousal caregivers. Psychooncology. 2011;20:762–770. doi: 10.1002/pon.1778. [DOI] [PubMed] [Google Scholar]
- 16.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 17.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77:359–362. [PubMed] [Google Scholar]
- 18.Lohman T, Martorell R, Roche A. Anthropometric Standardization Manual. Champaign. IL: Human Kinetics; 1988 [Google Scholar]
- 19.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Arena R, Sietsema K, et al. American Heart Association Exercise, Cardiac Rehabilitation; Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 21.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1854–1864. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Miller PE, Mitchell DC, Harala PL, Pettit JM, Smiciklas-Wright H, Hartman TJ. Development and evaluation of a method for calculating the Healthy Eating Index-2005 using the Nutrition Data System for Research. Public Health Nutr. 2011;14:306–313. doi: 10.1017/S1368980010001655. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 24.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs. NJ: Prentice Hall; 1986. [Google Scholar]
- 25.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 26.Sloane R, Snyder DC, Demark-Wahnefried W, Lobach D, Kraus WE. Comparing the 7-day physical activity recall with a triaxial accelerometer for measuring time in exercise. Med Sci Sports Exerc. 2009;41:1334–1340. doi: 10.1249/MSS.0b013e3181984fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle C, Kushi LH, Byers T, et al. 2006 Nutrition, Physical Activity and Cancer Survivorship Advisory Committee; American Cancer Society. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 28.United States Department of Agriculture Center For Nutrition Policy and Promotion. US Dietary Guidelines. 2010. cnpp.usda.gov/dietaryguidelines.htm. Accessed October 3, 2013.
- 29.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105:775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Kelley HH, Holmes JG, Kerr NL, Reis HT, Rusbult CE, Van Lange PAM. An Atlas of Interpersonal Situations. New York: Cambridge University Press; 2003. [Google Scholar]
- 32.Lyons RF, Michelson KD, Sullivan MJ, Coyne JC. Coping as a communal process. J Soc Pers Relations. 1998;15:579–605. [Google Scholar]
- 33.Cohen J. NJ: Lawrence Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. Hillsdale. [Google Scholar]
- 34.Zhang S, Midthune D, Guenther PM, et al. A new multivariate measurement error model with zero-inflated dietary data, and its application to dietary assessment. Ann Appl Stat. 2011;5:1456–1487. doi: 10.1214/10-AOAS446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. Int J Obes Relat Metab Disord. 2003;27:987–1005. doi: 10.1038/sj.ijo.0802383. [DOI] [PubMed] [Google Scholar]
- 37.Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. Int J Obes Relat Metab Disord. 1997;21:715–737. doi: 10.1038/sj.ijo.0800495. [DOI] [PubMed] [Google Scholar]
- 38.Wiehs KL, Fisher L, Baird M. Families, health, and behavior. Family Systems Health. 1999;20:7–46. [Google Scholar]
- 39.Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc Sci Med. 2006;62:1369–1380. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Brownell KD, Kelman JH, Stunkard AJ. Treatment of obese children with and without their mothers: changes in weight and blood pressure. Pediatrics. 1983;71:515–523. [PubMed] [Google Scholar]
- 41.Gorin A, Phelan S, Tate D, Sherwood N, Jeffery R, Wing R. Involving support partners in obesity treatment. J Consult Clin Psychol. 2005;73:341–343. doi: 10.1037/0022-006X.73.2.341. [DOI] [PubMed] [Google Scholar]
- 42.Ristovski-Slijepcevic S, Chapman GE, Beagan BL. Being a ‘good motherÇ: dietary governmentality in the family food practices of three ethnocultural groups in Canada. Health (London) 2010;14:467–483. doi: 10.1177/1363459309357267. [DOI] [PubMed] [Google Scholar]
- 43.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22:1688–1704. doi: 10.1002/pon.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi AS, Rossi PH. Of Human Bonding: Parent-Child Relationships Across the Lifecourse. New York: Aldine; 1990. [Google Scholar]
- 45.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 46.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosher CE, Fuemmeler BF, Sloane R, et al. Change in self-efficacy partially mediates the effects of the FRESH START intervention on cancer survivors' dietary outcomes. Psychooncology. 2008;17:1014–1023. doi: 10.1002/pon.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]


