Abstract
Purpose.
In this work, we tested the hypothesis that highly targeted delivery of antiglaucoma drugs to the supraciliary space by using a hollow microneedle allows dramatic dose sparing of the drug compared to topical eye drops. The supraciliary space is the most anterior portion of the suprachoroidal space, located below the sclera and above the choroid and ciliary body.
Methods.
A single, hollow 33-gauge microneedle, 700 to 800 μm in length, was inserted into the sclera and used to infuse antiglaucoma drugs into the supraciliary space of New Zealand white rabbits (N = 3–6 per group). Sulprostone, a prostaglandin analog, and brimonidine, an α2-adrenergic agonist, were delivered via supraciliary and topical administration at various doses. The drugs were delivered unilaterally, and intraocular pressure (IOP) of both eyes was measured by rebound tonometry for 9 hours after injection to assess the pharmacodynamic responses. To assess safety of the supraciliary injection, IOP change immediately after intravitreal and supraciliary injection were compared.
Results.
Supraciliary delivery of both sulprostone and brimonidine reduced IOP by as much as 3 mm Hg bilaterally in a dose-related response; comparison with topical administration at the conventional human dose showed approximately 100-fold dose sparing by supraciliary injection for both drugs. A safety study showed that the kinetics of IOP elevation immediately after supraciliary and intravitreal injection of placebo formulations were similar.
Conclusions.
This study introduced the use of targeted drug delivery to the supraciliary space by using a microneedle and demonstrated dramatic dose sparing of antiglaucoma therapeutic agents compared to topical eye drops. Targeted delivery in this way can increase safety by reducing side effects and could allow a single injection to contain enough drug for long-term sustained delivery.
Keywords: drug delivery, glaucoma, microneedle, suprachoroidal
This study shows that microneedles enable targeted injection into the supraciliary space and that antiglaucoma drugs administered in this way show dramatic reduction in dose for similar reductions in IOP compared to topical delivery in rabbits.
Introduction
Glaucoma is the second leading cause of blindness worldwide,1,2 even though well-established pharmacological treatments are available. Poor patient compliance plays a significant role in the loss of vision in patients with glaucoma. Estimated compliance with the use of topical eye drops is as low as 56%,3 which shows that patients cannot manage daily eye drops well. Patients are required to administer eye drops one or more times per day because systemic therapy (e.g., by oral delivery, which typically has higher compliance3–5) with most antiglaucoma drugs causes unacceptable side effects.6,7
Although topical drops administer drug to the eye, they nonetheless are poorly targeted to their specific sites of action within the ocular globe.8 Topical delivery is highly inefficient and requires frequent dosing to overcome poor drug absorption and rapid clearance. Bioavailability of eye drops is typically well below 5%, and what little drug enters the eye is delivered nonspecifically throughout the anterior segment with only a small fraction of the drug reaching its site of therapeutic action.8–10 As with many antiglaucoma drugs, the site of therapeutic action is the ciliary body.11–13 Also, the poorly targeted nature of eye drops leads to extensive systemic exposure, which causes 8% to 53% of glaucoma patients taking topical antiglaucoma drugs to experience side effects.14,15 Poor patient compliance and low bioavailability of glaucoma drugs create the need for a better delivery method.16
In this study, we introduced supraciliary delivery to target drug administration adjacent to the ciliary body to maintain high therapeutic drug levels at the site of pharmacologic action in the targeted tissue. This targeting of the ciliary body can be accomplished by administering drug into the supraciliary space, which is a potential space located below the sclera and above the choroid and ciliary body that can expand to accommodate a fluid or drug formulation. We and others have previously reported using the suprachoroidal space for drug targeting to the choroid and adjacent retina to reach posterior segment targets (Morales-Canton V, et al. IOVS 2013;54:ARVO E-Abstract 3299).17–23 Here, we accessed the ciliary body in the anterior segment by injecting drug into the most anterior portion of the suprachoroidal space, which we are calling the supraciliary space. By localizing the drug in the supraciliary space, we expected to achieve high drug levels locally in the adjacent ciliary body.
Previous studies have accessed the suprachoroidal space to demonstrate highly targeted and reliable delivery to this space and effective drug treatment, with no major safety concerns in animal models and human subjects (Morales-Canton V, et al. IOVS 2013;54:ARVO E-Abstract 3299).17–23 Researchers have accessed the suprachoroidal space using surgically introduced catheters and conventional hypodermic needles, but more recently, our group introduced the use of microneedles as a simple and reliable method for suprachoroidal injection that is expected to be suitable for use as a rapid office procedure.19 A first-in-humans study recently demonstrated injection of bevacizumab into the suprachoroidal space of four human eyes by using a microneedle (Morales-Canton V, et al. IOVS 2013;54:ARVO E-Abstract 3299). In the present study, we adapted this method to target injection into the supraciliary space, as the first study exploring the use of this potential space to deliver therapeutic agents immediately adjacent to the ciliary body and the first study to examine pharmacodynamics of this delivery method.
This study sought to assess the efficacy of supraciliary delivery using a hollow microneedle in the rabbit and compare that delivery to conventional topical delivery. We carried out this assessment by delivering antiglaucoma drugs to the supraciliary space and measuring reduction in intraocular pressure (IOP) over time and comparing IOP to that with topical delivery of the same drugs. Both of the drugs used in this study, sulprostone and brimonidine, have sites of action in the ciliary body,11–13 which suggested that supraciliary targeting should be beneficial.
Sulprostone is a prostaglandin E2 analog that has been shown to lower IOP in the rabbit24–27 but is not used in humans to treat glaucoma. Latanoprost, travoprost, and bimatoprost are prostaglandin F2α analogs commonly used in human clinical therapy,28,29 but rabbits respond poorly to these drugs.30–35 Receptors for the prostaglandin analog F2α are located in both trabecular meshwork and ciliary body in humans.12 Receptors for the prostaglandin E2 analogs (e.g., sulprostone) are found in the ciliary body and iris of the rabbit.11 Although the mechanisms of action of prostaglandin E2 and F2α are different,12,36 the targeting or binding sites for both drugs are in the ciliary body.11,12 We therefore used sulprostone as a model analog with a targeting site similar to other prostaglandin F2α analogs.11,12
Our other drug, brimonidine, is a commonly used clinical agent for antiglaucoma therapy and is active in the rabbit eye too.13 In this study, we measured IOP in the New Zealand White rabbit after bolus injection of sulprostone and brimonidine over a range of different doses and compared the responses to topical eye drops to test the hypothesis that highly targeted delivery of antiglaucoma drugs to the supraciliary space using a hollow microneedle allowed dramatic dose sparing of drug compared to that with topical eye drops.
Materials and Methods
Microneedle Fabrication and Formulation
Microneedles were fabricated from 33-gauge stainless steel needle cannulas (TSK Laboratories, Tochigi, Japan). The cannulas were shortened to approximately 700 to 800 μm in length, and the bevel at the orifice was shaped using a laser (Resonetics Maestro, Nashua, NH, USA), as described previously.37,38 The microneedles were electropolished using an E399 electropolisher (ESMA, South Holland, IL, USA) and cleaned with deionized water. Sulprostone (Cayman Chemical, Ann Arbor, MI, USA) and 0.15% brimonidine tartrate ophthalmic solution (Alphagan P; Allergan, Irvine, CA, USA) were diluted in Hanks' balanced salt solution (HBSS; Cellgro, Manassas, VA, USA). For topical delivery, the final concentration was 0.05 mg/mL sulprostone or 1.5 mg/mL brimonidine tartrate. For supraciliary injection, the solution was diluted to a range of drug concentrations and included 2% carboxymethylcellulose (CMC; Sigma-Aldrich, St. Louis, MO, USA) to increase viscosity and thereby improve localization of the drug at the site of injection.
Anesthesia and Euthanasia
All studies used New Zealand white rabbits of mixed sex weighing between 3 and 4 kg (Charles River Breeding Laboratories, Wilmington, MA, USA). All of the animals were treated according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. For supraciliary injections and for application of topical eye drops, rabbits were anesthetized using 0.5% to 3.0% isoflurane, unless otherwise noted. The isoflurane percentage was slowly increased from 0.5% to 2.5% or 3.0% for 15 minutes. To achieve longer-lasting anesthesia for the supraciliary and intravitreal safety studies measuring IOP immediately after injection, anesthesia was achieved using subcutaneous injection of a mixture of ketamine (25 mg/kg) and xylazine (2.5 mg/kg). This ketamine/xylazine dose was also used during initial studies of screening anesthetics suitable for this study. For brimonidine-treated eyes, proparacaine (a drop of 0.5% solution) was given 1 to 3 minutes before each injection to locally numb the ocular surface. Animals were euthanized using an injection of 150 mg/kg pentobarbital into the ear vein.
Pharmacodynamics Studies
For supraciliary injection, a microneedle was attached to a 50- to 100-μL gas-tight glass syringe containing either a placebo formulation of HBSS or a drug formulation containing a specified concentration of either sulprostone or brimonidine tartrate. The eyelid of the rabbit was pushed back, and the microneedle was inserted into the sclera 3 mm posterior to the limbus in the superior temporal quadrant of the eye. A volume of 10 μL was injected within 5 seconds, and the microneedle was removed from the eye 15 seconds later to reduce reflux of the injected formulation. Topical delivery of sulprostone and brimonidine was achieved by administering an eye drop into the upper conjunctival sack. IOP was measured hourly for 9 hours after drug administration, as described below. Each treatment involved application of just 1 dose of one drug either topically or by supraciliary injection in 1 eye. After a recovery period of at least 14 days, rabbits were used for additional experiments, alternating between the left and right eyes.
Safety Studies
Supraciliary injections of either 10 μL or 50 μL HBSS were performed as described above. Intravitreal injection was performed by inserting a 30-gauge hypodermic needle across the sclera 1.5 mm posterior to the limbus in the superior temporal quadrant of the eye. A volume of 50 μL HBSS was injected within 5 seconds, and the needle was removed from the eye 15 seconds later to reduce reflux. IOP was measured periodically for 1 hour after injection, as described below.
Tonometer Calibration.
The tonometer (TonoVet; icare, Vantaa, Finland) we used for this study is calibrated for use in dogs and cats39 and was therefore recalibrated both in vivo (N = 4) and ex vivo (N = 3) for the rabbit eye.40 Ex vivo rabbit eyes were cannulated using a 25-gauge hypodermic needle (Becton Dickinson, Franklin Lakes, NJ, USA). The needle was inserted 2 to 3 mm posteriorly from the limbus and was connected to a reservoir containing balanced salt solution (BSS, Baxter, Deerfield, IL, USA), elevated to a known height in order to create a controlled pressure inside the eye. The surface of the eye was wetted periodically using saline solution (every 2–3 minutes) to mimic the wetting of the cornea by tear fluid. Final measurements were made after confirming stable IOP for 5 min. Data over a range of IOPs (7.3–22 mm Hg) were collected and used to generate a calibration curve to correct values reported by the TonoVet device to the actual values of IOP in the eyes.
For the in vivo study, rabbits were anesthetized using a subcutaneous injection of a mixture of ketamine (25 mg/kg) and xylazine (2.5 mg/kg). Proparacaine (a drop of 0.5% solution) was given 1 to 3 min before cannulation to locally numb the ocular surface. IOP was controlled in a manner similar to that used in the ex vivo experiments, using an elevated BSS reservoir, and a similar calibration curve was generated. In vivo and in vitro experiments yielded calibration curves of y = 1.18x + 1.82 (R2 = 0.98) and y = 1.01x + 3.08 (R2 = 1.00), respectively, where x = IOP reported by the TonoVet tonometer and y = water column pressure applied to the eye. The resulting calibration curves showed approximately linear relationships with similar slopes. The in vivo calibration curve was used for all data reported in this study.
Intraocular Pressure Measurement
IOP was measured with a hand-held tonometer (TonoVet) in an awake, restrained rabbit. Topical anesthesia was not necessary for the measurement, and no general anesthetic or immobilizing agent was used because the procedure is not painful. We made every effort to avoid artifactual elevation of IOP by avoiding topical anesthesia and by careful and consistent animal handling during each measurement. Each rabbit was acclimatized to the IOP measurement procedure for at least 7 days to obtain a stable background IOP reading. To account for the specific IOP behavior of each rabbit, the initial IOP value (time = 0) reported for each individual eye was an average of measurement over 3 to 4 days, and IOPs over time were reported as changes in IOP relative to the initial average value.
Calculation of Area Under the Curve and Equivalent Dosage
The pharmacodynamic effect of each treatment was characterized by determining the area under the curve (AUC) of the temporal profile of intraocular pressure by numerically integrated use of the trapezoidal rule.41 This pharmacodynamic AUC (AUCPD) is a measure of the strength and duration of the treatment in IOP. To calculate the AUCPD, IOP readings were normalized to the IOP reading prior to treatment. The AUCPD values obtained had units of mm Hg-h and a negative value (because the drugs under study all lowered IOP). However, the negative values were changed to positive values for better representation of the data.
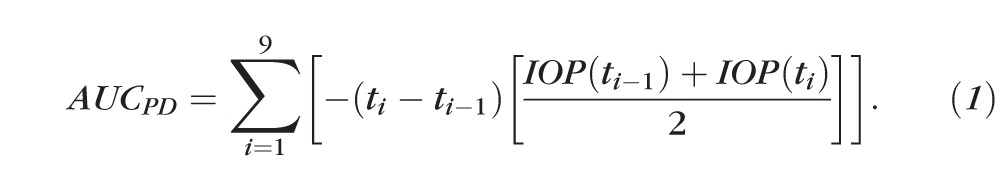 . .
|
where IOP(ti) in mm Hg represents the IOP value measured at time ti in seconds.
An equivalent dosage comparison was made between topical and supraciliary delivery using the following equation, where D is the dose administered and subscripts SC and topical mean suprachoroidal injection and topical administration, respectively.
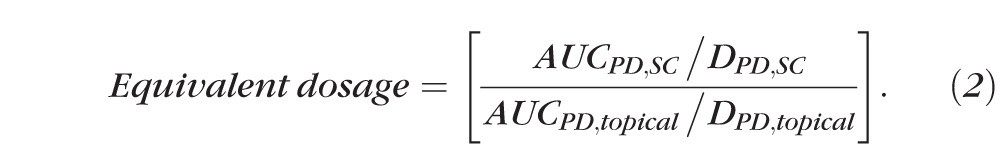 . .
|
Statistical Analysis
Three replicated pharmacodynamics and safety experiments were performed for each treatment group, from which the means and standard errors of mean were calculated. Experimental data were analyzed using two-way analysis of variance (ANOVA) to examine differences between treatments. In all cases, a P value of <0.05 was considered statistically significant. Parametric statistics were used to evaluate the data, as justified by an Anderson-Darling normality test, which showed a normal distribution of IOP measurements in untreated eyes (N = 3, P value = 0.367).
Results
Effect of Anesthesia on Transient IOP Change
Before studying the effect of supraciliary targeting of antiglaucoma drugs, we needed to identify a general anesthetic that did not create artifactual changes in rabbit IOP over the time scale of the experiment. We first tested subcutaneous injection of ketamine/xylazine, which produced deep anesthesia for approximately 2 hours. This anesthetic also produced significant ocular hypotension that lasted for 4 to 5 hours, with a peak IOP decrease of approximately 5 mm Hg at 1 hour after injection of the anesthetic, which was followed by a slow recovery of IOP over time (Fig. 1a).
Figure 1.
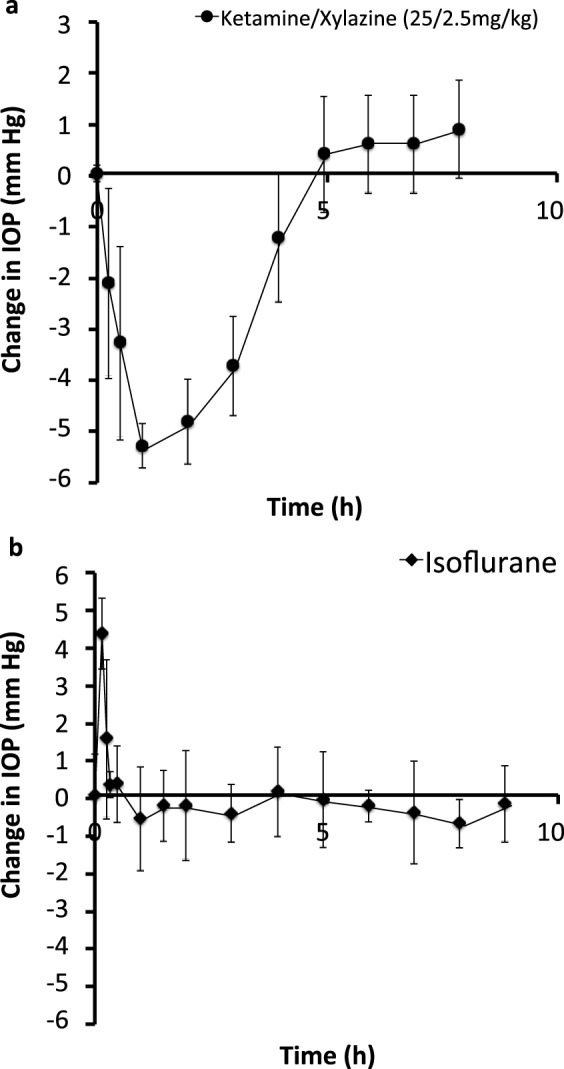
Effect of general anesthetics on IOP in the rabbit eye. (a) Ketamine/xylazine (25/2.5 mg/kg) was administered by subcutaneous injection. (b) Isoflurane (up to 3%) was administered by inhalation for 15 minutes. IOP was measured in both eyes periodically for up to 9 hours. Data points are averages ± SEM (n = 4–6).
We then tested isoflurane, which was administered by inhalation of an escalating dose over 15 minutes. Anesthesia quickly set in upon initiation of the isoflurane dose and was quickly reversed upon discontinuation of the isoflurane dose. During the 15 minutes of isoflurane administration, IOP was elevated by almost 5 mm Hg but quickly returned to normal after isoflurane administration was stopped and remained unchanged for 9 hours after that (Fig. 1b). We believe the initial, transient ocular hypertension might have been due to both the pharmacological effect of the anesthetic as well as the psychological effect (i.e., startling the rabbit) of administering the inhaled anesthetic.
We concluded from these studies that isoflurane is a suitable anesthetic for the pharmacodynamic experiments in this study, because isoflurane's effects on IOP reversed within 15 to 30 minutes, which is fast enough to permit hourly measurements of IOP without significant artifacts from the anesthetic. However, for the safety experiments in this study, in which IOP was measured multiple times within 1 hour, the rapidly changing effects of isoflurane on IOP would significantly affect IOP measurements. For that reason, we used ketamine/xylazine for the safety study, because the effect of the anesthetic on IOP was relatively small during the first 10 minutes, when the most critical IOP measurements were made in the safety study.
Antiglaucoma Drugs in the Normotensive Rabbit Model
To test our hypothesis regarding drug targeting via the supraciliary space, we next needed to identify antiglaucoma drugs that had pharmacological action at the ciliary body and reduced IOP in the normotensive rabbit model. Our candidates were prostaglandin analogs, adrenergic agonists, and β-blockers that have their pharmacological site of action at the ciliary body.6,11–13 Our first choice was prostaglandin analogs because they are widely used in human clinical medicine,28,29 including for glaucoma treatment.6 Latanoprost, travoprost, and bimatoprost are commonly used prostaglandin analogs,28,29 but rabbits respond poorly to these drugs.30–35 We tested latanoprost in our rabbit model but observed no change in IOP at the standard human dose of 2.5 μg (data not shown).
We therefore used sulprostone as a model prostaglandin analog with its site of pharmacological action to the ciliary body11,12 and an ocular hypotensive effect well documented in reports.25,27 A single topical eye drop of 2.5 μg sulprostone gave a maximum IOP decrease of almost 3.4 mm Hg at approximately 2 hours after drug administration (Fig. 2). Ocular hypotension in the treated eye lasted approximately 8 hours. We also observed changes in IOP in the contralateral (i.e., untreated) eye but to a lesser extent.
Figure 2.
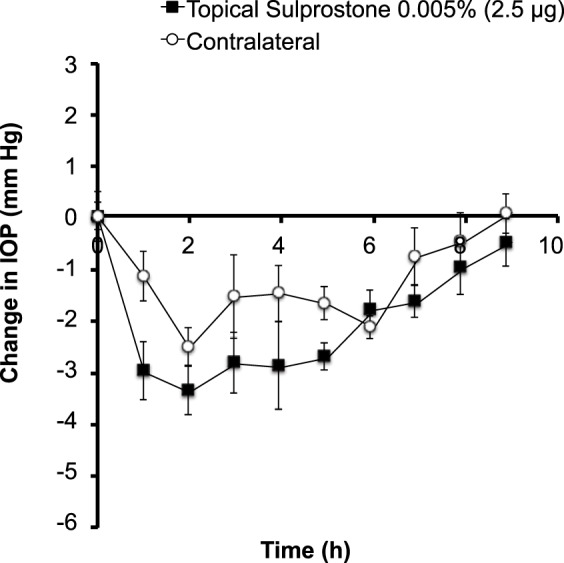
Effect of topical sulprostone administration on IOP in the rabbit eye. A single drop containing 2.5 μg sulprostone was administered to one eye. IOP was then followed for 9 hours in both the treated eye and the untreated/contralateral eye. Data points are averages ± SEM (n = 4).
To more fully test our hypothesis, we decided to study a second drug that lowers IOP by a different mechanism in the ciliary body, as well, and selected brimonidine, an adrenergic agonist widely used in clinical glaucoma therapy. Although the pharmacology and site of action causing an IOP response to brimonidine are species dependent,13 adrenergic agonists have a site of action in the ciliary body in both rabbit13 and human.42–44 A previous study demonstrated α2-adrenoceptor agonism by showing that pretreating the eye with α2-selective antagonists inhibited the IOP response to brimonidine in rabbits.45
We found that topical administration of a single drop (75 μg) of brimonidine produced a peak IOP reduction of approximately 4 mm Hg at 2 hours after drug administration, which slowly returned to normal within 6 hours (Fig. 3). It is notable that the contralateral (untreated) eye also experienced a decrease in IOP with faster kinetics and similar magnitude (Fig. 3), presumably due to systemic distribution of brimonidine. The slower kinetics in the treated eye could be explained by a local brimonidine concentration that was initially too high and only after some clearance of the drug reached the optimal concentration for IOP reduction, whereas the contralateral eye had lower brimonidine concentration from the start due to the nontargeted systemic delivery route. Previous research also showed decreased IOP in the contralateral eye in rabbits, which was produced due to systemic administration after administering brimonidine at high concentrations in the treated eye and was reflected by plasma concentrations high enough to activate central α2-adrenoceptors46 and cardiovascular changes.13
Figure 3.
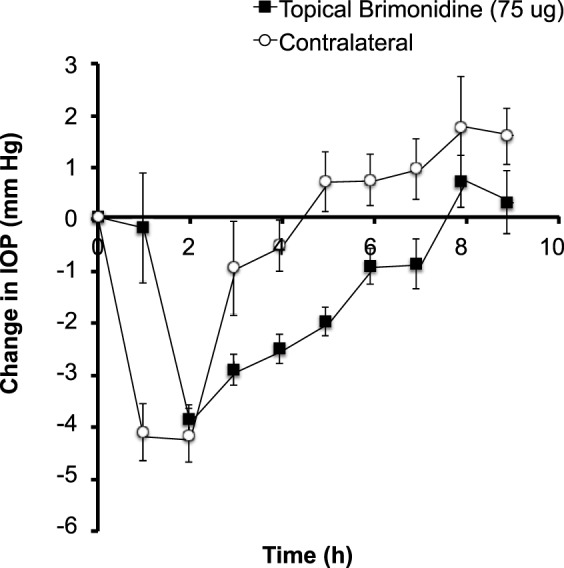
Effect of topical brimonidine administration on IOP in the rabbit eye. A single drop containing (a) 75 μg brimonidine was administered to one eye. IOP was then followed for 9 hours in both the treated eye and the untreated, contralateral eye. Data points are averages ± SEM (n = 5).
Microneedles for Targeted Delivery to the Supraciliary Space
Our next step was to demonstrate targeted injection into the supraciliary space using a microneedle. Because the thickness of sclera above the suprachoroidal space in the rabbit is ~500 μm,47 we prepared microneedles measuring 700 to 800 μm, to be inserted into the base of the sclera and in that way target injection into the suprachoroidal space. The needles were longer than the thickness of the sclera to account for the overlying conjunctiva and for the expected deformation of the sclera during insertion of the microneedle. Figure 4 shows a representative microneedle prepared for supraciliary injection next to a typical eye dropper used for topical administration.
Figure 4.
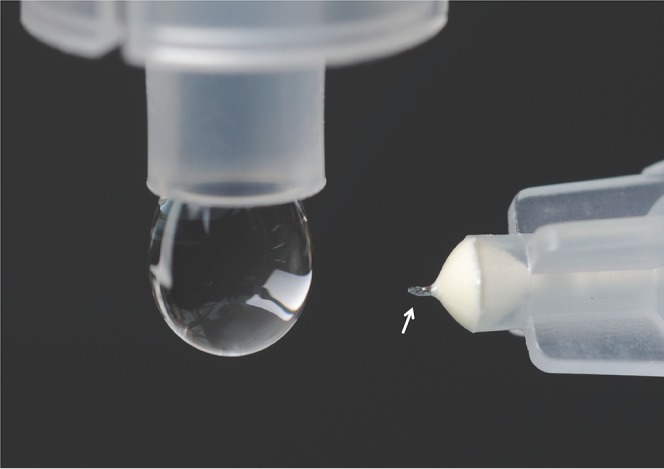
Hollow microneedle (arrow), 720 μm in length, is shown next to a liquid drop of approximately 50-μL volume from a conventional eye dropper.
Previous studies of injections made in this way have targeted the suprachoroidal space with the objective of having the injected formulation flow away from the site of injection and travel circumferentially around the eye for broad coverage of the choroidal surface, especially toward the posterior pole. In this study, we had the opposite objective. Our goal was to localize the injected formulation at the site of injection immediately above the ciliary body and minimize flow to other parts of the eye.
To accomplish this goal, we increased the viscosity of the injected formulation by adding 2% w/v (weight/volume percent) CMC. The viscosity of this solution at rabbit body temperature of 39°C was 80.5 ± 3.7 Pa/s at a shear rate of 0.1 s−1, which is approximately 80,000 times more viscous than water at room temperature. Injection of this high-viscosity formulation into the rabbit eye by using a microneedle was able to localize the injection near the site of injection. As shown in Figure 5a, the dye injected in this way spread over an area within just a few millimeters from the site of injection. The degree of spread depended on the amount of fluid injected, such that there was more spread when larger volumes were used (data not show).
Figure 5.
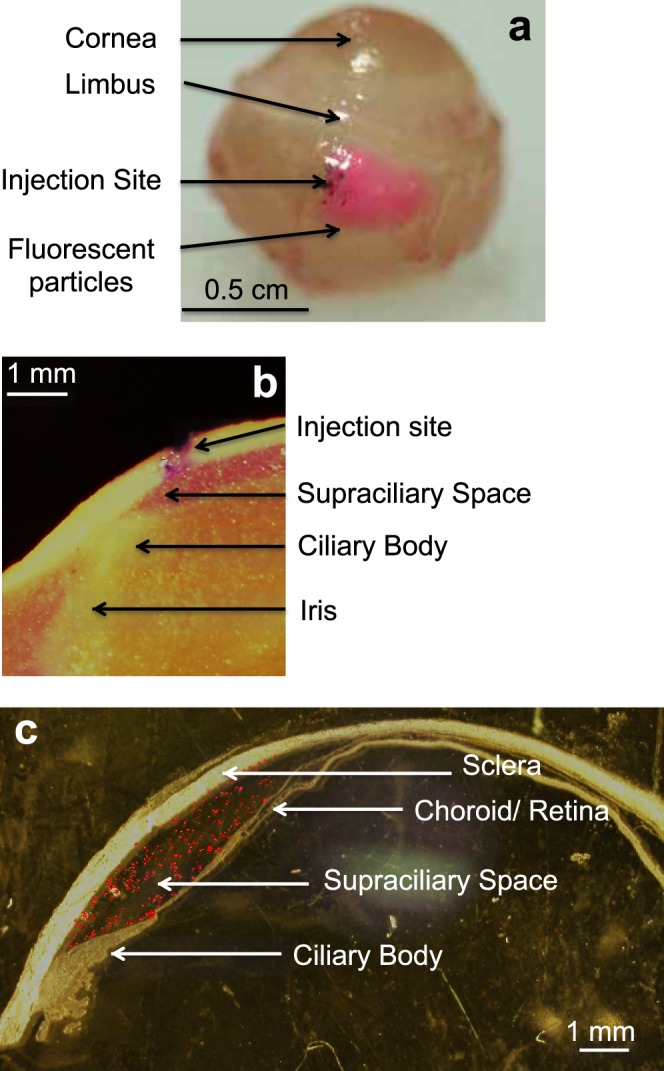
Supraciliary targeting of injections using a high-viscosity formulation injected using a microneedle. (a) Injection of 10 μL red fluorescing particles (1-μm diameter, 0.5% w/v) into the rabbit eye ex vivo spread only a few millimeters from the site of injection. The whole eye was imaged by digital camera 60 min after injection. (b) Injection of 50 μL red fluorescing particles (1-μm diameter, 0.5% w/v) into the rabbit eye ex vivo was localized to the supraciliary space, directly above and adjacent to the ciliary body. The eye was frozen immediately after injection, prepared as frozen sections, and imaged by brightfield microscopy. (c) Injection of 30 μL red fluorescing microparticles (10-μm diameter, 0.5% w/v) into a human cadaver eye was localized to the supraciliary space. The eye was fixed in formalin immediately after injection, prepared as frozen sections, and a brightfield image was overlaid on a fluorescent image to show ocular anatomy with fluorescence in the same histological section.
Histological examination demonstrated that the injection was localized to the supraciliary space. As shown in Figure 5b, the injected dye can be seen in the expanded supraciliary space bounded by the ciliary body on the lower anterior boundary, the choroid on the lower central and posterior boundary and the sclera on the upper boundary of the rabbit eye. In Figure 5c, a similar experiment was conducted in a human eye, which similarly shows supraciliary localization of the injected fluorescent particles. While the supraciliary space is significantly expanded immediately after injection, when these tissues were frozen for analysis, we believe that this space closes down again as fluid flows away and is absorbed, based on our unpublished data for suprachoroidal injections and other data discussed further below.
We also determined the possible effect of supraciliary injection of 2% CMC in 10 μL on IOP over the course of our experiments. As shown in Figure 6, there was no apparent effect of this injection on IOP at the hourly time points over the course of a 9-h study. Two-way ANOVA comparison of the isoflurane-only group (Fig. 2) to the placebo group (Fig. 6) showed no statistically significant differences, with P values of 0.05 and 0.07 for treated and contralateral eyes, respectively.
Figure 6.
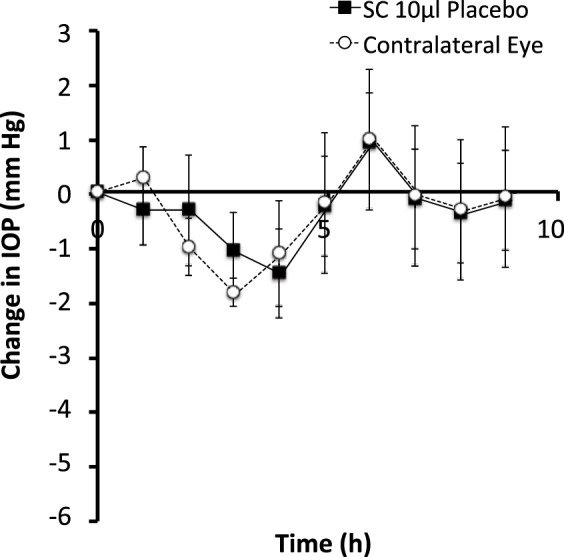
Effect of supraciliary injection on IOP in the rabbit eye. A single injection of 10 μL of a 2% w/v solution of CMC was administered to one eye. IOP was then followed for 9 hours in both the treated eye and untreated, contralateral eye. Data points are averages ± SEM (n = 3).
Pharmacodynamics of Sulprostone After Supraciliary Delivery
Having completed the initial experiments for anesthesia, topical delivery, and supraciliary targeting, we were now able to test the central hypothesis of this study regarding the effects of antiglaucoma drugs targeted to the supraciliary space. We therefore injected sulprostone into the supraciliary space over a range of doses (0.025 μg–0.005 μg in 10 μL) in rabbits.
Supraciliary delivery of sulprostone at a dose of 0.025 μg in 10 μL (i.e., a dose 100 times lower than the topical dose shown in Fig. 2) produced an IOP decrease of ~3.1 mm Hg within 1 hour that persisted at that level for at least 9 hours (Fig. 7a). IOP was similarly decreased in the contralateral eyes but to a lesser extent.
Figure 7.
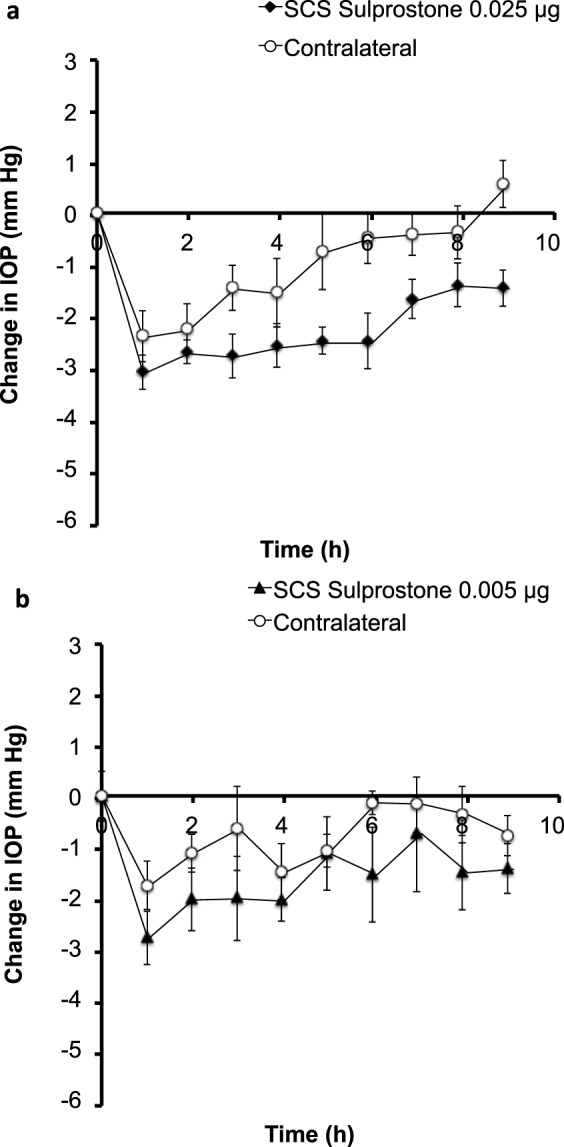
Effect of a supraciliary injection of sulprostone on IOP in the rabbit eye. A single injection of (a) 0.025 μg and (b) 0.005 μg sulprostone in 10 μL was administered to one eye. IOP was then followed for 9 hours in both the treated eye and untreated, contralateral eye. Data points are averages ± SEM (n = 4–6).
Supraciliary delivery of 0.005 μg sulprostone in 10 μL (i.e., a dose 500 times lower than the topical dose) produced a peak IOP drop of ~2.8 mm Hg at 1 hour after drug administration (Fig. 7b). IOP levels increased over time, but ocular hypotension persisted for approximately 6 hours in the treated eye and was statistically significant compared to that of placebo treated eyes (P < 0.0001). However, responses of the contralateral eyes were not significantly different from placebo-treated eyes (P = 0.159).
Overall, sulprostone was found to lower IOP in a dose-dependent manner (Fig. 8a). Based on a rough comparison, topical delivery of 2.5 μg sulprostone and supraciliary delivery of 0.025 μg sulprostone in 10 μL showed similar levels of initial IOP reduction, although the effect lasted longer after supraciliary delivery. To provide a more quantitative measure of the supraciliary dose equivalent to topical delivery, we determined and compared the AUCPD for pharmacodynamic data in the topically and supraciliary-treated eyes (Fig. 8b). Comparison of these values gave a ratio of 101, which indicates that the supraciliary dose needed to achieve a similar pharmacodynamic response was ~100-fold less than that for topical delivery. We believe that this dramatic dose sparing was achieved by highly targeted delivery of sulprostone to its site of action in the ciliary body.
Figure 8.
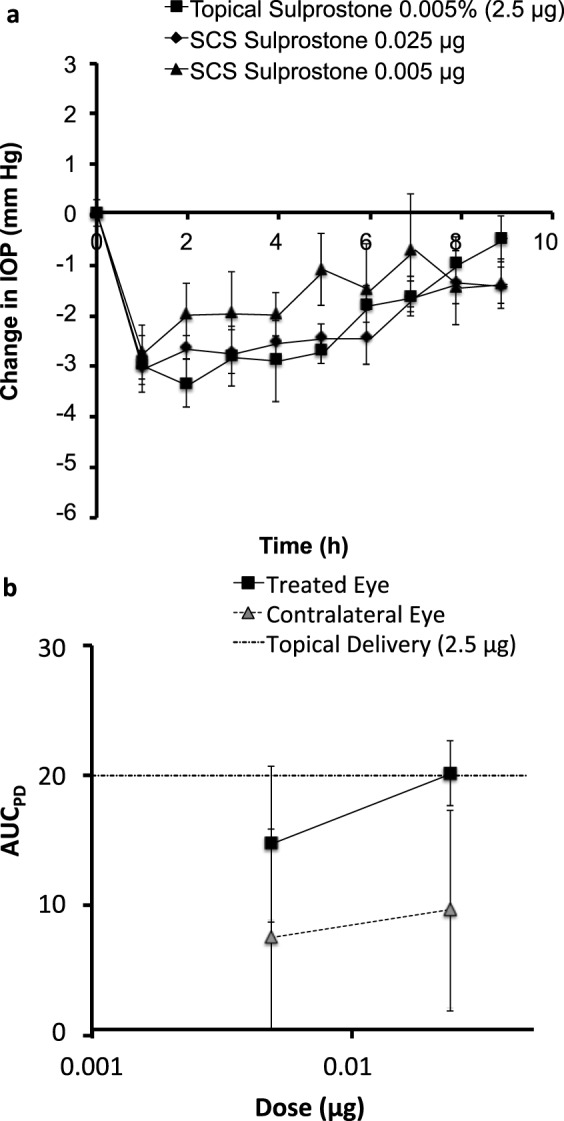
Comparison of decrease in IOP by supraciliary delivery versus topical delivery of sulprostone. (a) Data from Figures 2 and 7 are graphed together to show the dose-response relationship after supraciliary delivery and to facilitate comparison with topical delivery in the treated eyes. (b) Pharmacodynamic area under the curve (AUCPD) after supraciliary delivery in treated and contralateral eyes and in comparison with topical delivery. AUCPD was calculated using Equation 1.
Pharmacodynamics of Brimonidine After Supraciliary Delivery
To assess the generality of dose sparing by targeting antiglaucoma drugs to the supraciliary space, we carried out similar experiments to study supraciliary delivery of brimonidine over a range of concentrations (0.015–0.15 μg in 10 μL) in rabbits. Similar to sulprostone, brimonidine produced a concentration-dependent drop in IOP at doses much lower than those used for topical delivery.
Supraciliary delivery of brimonidine at a dose of 1.5 μg in 10 μL (i.e., a dose 50 times lower than the topical dose shown in Fig. 3a) produced an IOP decrease of ~3.3 mm Hg within 1 hour that persisted at that level for approximately 9 hours (Fig. 9a). IOP was similarly decreased in the contralateral eye but to a lesser extent.
Figure 9.
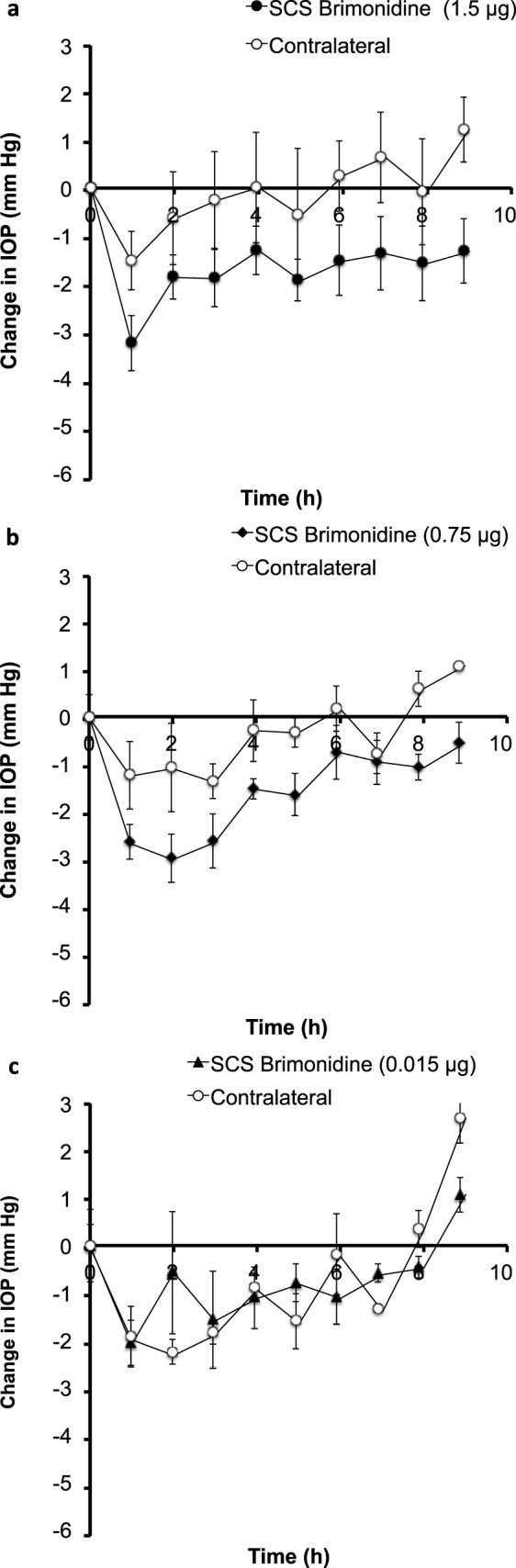
Effect of supraciliary injection of brimonidine on IOP in the rabbit eye. A single injection of (a) 1.5 μg, (b) 0.75 μg, and (c) 0.015 μg of brimonidine in 10 μL was administered to one eye. IOP was then followed for 9 hours in both the treated eye and untreated, contralateral eye. Data points are averages ± SEM (n = 3–5).
Supraciliary delivery of 0.75 μg brimonidine in 10 μL (i.e., a dose 100 times lower than the topical dose) produced a peak IOP drop of ~3 mm Hg at 2 hours after drug administration that persisted for approximately 5 hours (Fig. 7b). The contralateral eye showed a similar but smaller drop in IOP. Statistical analysis showed significant differences for treated (P < 0.001) eyes but not for contralateral eyes (P = 0.915).
Supraciliary delivery of 0.015 μg of brimonidine in 10 μL (i.e., doses 500 times lower than the topical dose) showed no significant IOP changes in treated (P = 0.20) and contralateral eyes (P = 0.26).
Supraciliary delivery of brimonidine reduced IOP in a dose-dependent matter (Fig. 10a). Compared to topical delivery of 75 μg of brimonidine, a 100-fold lower dose of 0.75 μg of brimonidine by supraciliary delivery showed a similar duration and magnitude of ocular hypotension (Fig. 10a). By calculating AUCPD values (Fig. 10b), we estimated the supraciliary dose needed to obtain a similar pharmacodynamic response was 115-fold less than topical delivery.
Figure 10.
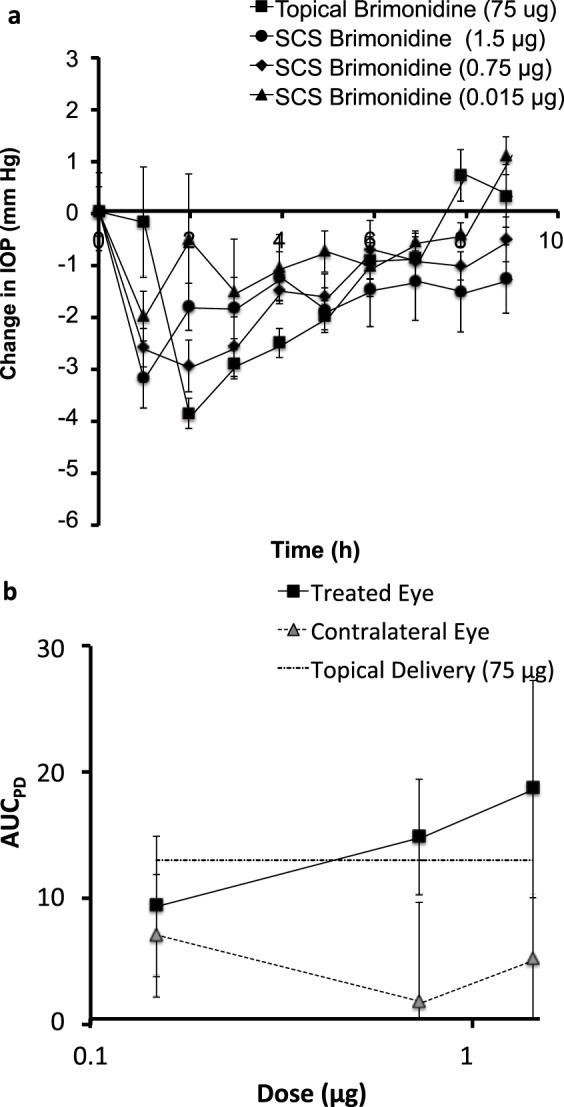
Comparison of decrease in IOP caused by supraciliary delivery versus topical delivery of brimonidine. (a) Data from Figures 3a and 9 are graphed together to show the dose-response relationship after supraciliary delivery and to facilitate comparison with topical delivery in the treated eyes. (b) Pharmacodynamic area under the curve (AUCPD) after supraciliary delivery in treated and contralateral eyes and in comparison with topical delivery. AUCPD was calculated using Equation 1.
It is notable that in the rabbit model studied here, decreased IOP was seen in both the treated eyes and to a lesser extent in the contralateral eyes (Fig. 9). Ocular hypotension in contralateral eyes is believed to be due to systemic absorption.48 Both we and other investigators13 have observed similar contralateral responses after topical delivery of brimonidine (Fig. 3).
Safety of Microneedle Injection Into the Supraciliary Space
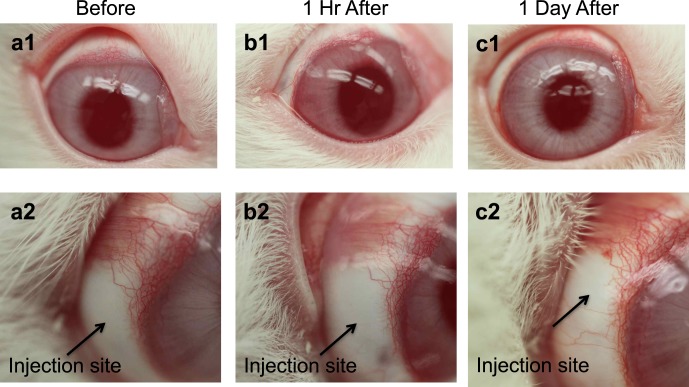
Injections into the supraciliary space using microneedles were well tolerated, and no injection-related complications were observed, such as bleeding or squinting. After injection, the needle insertion site was not visually apparent on the conjunctival surface, indicating only very minor trauma (Fig. 11). We did not observe any inflammation, redness, or pain-related response after the injection. No apparent vision loss was observed in any of the rabbits.
Figure 11.
Representative images of a rabbit eye (a) before (b) 1 hour after and (c) one day after supraciliary injection of the placebo formulation (2% CMC in 10 μL). All images are of the same rabbit eye. The lower row of images shows magnified views of the injection site of the eyes shown in the upper row of images.
To further assess safety, we measured IOP elevation associated with supraciliary and intravitreal injection. Note that this is the short-lived elevation in IOP caused by the injection itself (as opposed to the longer-term IOP reduction caused by the antiglaucoma drugs presented above). For this study, ketamine/xylazine was used for general anesthesia because it provides a relatively steady IOP between 1 hour and 2 hours after injection (Fig. 1). Rabbits given an intravitreal injection of 50 μL of HBSS 1 hour after induction of anesthesia were found to have a peak IOP increase of 36 ± 1 mm Hg due to the injection (Fig. 12). IOP then decreased exponentially until it stabilized at 30 to 40 minutes after the injection. This reaction is similar to that seen in human patients, where intravitreal injection can increase IOP by ~30 mm Hg.49 Considering intravitreal injection is well tolerated in human patients when only topical anesthesia is used and is safely performed millions of times per year,50 we expect that this temporary increase in IOP is safe and well tolerated.
Figure 12.
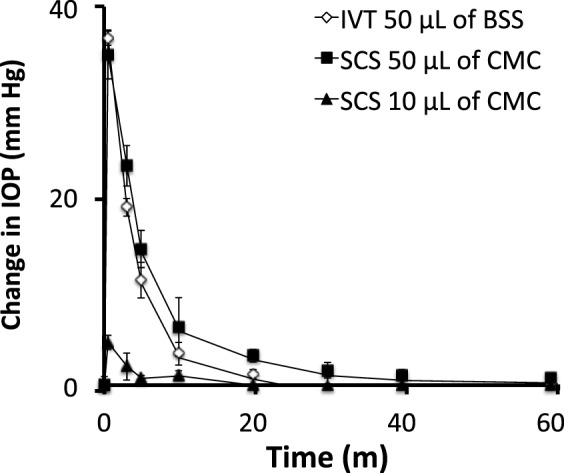
IOP increase due to injection of 50 μL of HBSS into the intravitreal space (IVT) and 10 μL and 50 μL of 2% carboxymethylcellulose placebo formulation (CMC) into the supraciliary space (SCS).
We similarly injected 50 μL of a 2% CMC formulation into the supraciliary space of the rabbit eye and observed a transient increase in IOP that peaked at 35 ± 3 mm Hg and decayed in under 1 hour, which is similar to the effects of conventional intravitreal injection (Fig. 12). We also injected 10 μL of formulation into the supraciliary space and found the peak IOP increase was 5 ± 1 mm Hg, which then disappeared within 20 minutes. Considering the similar magnitude and kinetics of IOP change by these intravitreal and supraciliary injections, we hypothesized that the safety profile of supraciliary delivery may be similar to that of intravitreal injection. In fact, supraciliary injection may be safer than intravitreal injection, considering that intravitreal and supraciliary injections are performed at the same site of the eye (i.e., pars plana),50 but supraciliary injection uses a needle that penetrate an order of magnitude less deeply into the eye.
Discussion
In current therapy, antiglaucoma drugs with a site of action in the ciliary body are almost always given by topical eye drops. This approach has a very low bioavailability, with typically less than 5% of the applied drug being absorbed into the eye and only a tiny fraction of that reaching its therapeutic target in the ciliary body.51,52 As a result, eye drops must be given at least daily and there can be side effects from systemic delivery of the poorly absorbed drug.53,54
This study introduced the idea of targeting the ciliary body by injection into the adjacent supraciliary space. We accessed this space, located just a few hundred micrometers below the conjunctival surface, by using a hollow microneedle designed to be just long enough to penetrate to the base of the sclera. Injection at this site filled the supraciliary space with a formulation designed with high viscosity that inhibited its flow away from the site of injection, thereby creating a depot next to the ciliary body. When the antiglaucoma drugs sulprostone and brimonidine were injected in this way, they were able to reduce IOP at doses 2 orders of magnitude lower than those required for similar pharmacodynamics using topical eye drops. These results show the highly targeted nature of supraciliary delivery and suggest opportunities to improve glaucoma therapies.
Targeted delivery reduces the amount of drug administered. This can improve safety and patient acceptance, due to reduced side effects. For example, the incidence of iris darkening in patients receiving prostaglandin analogs is as high as 35%,55,56 and brimonidine causes dry mouth in 33% of patients.7 By targeting drug delivery to the ciliary body, side effects caused at off-target sites of action could be reduced.
Targeted delivery also facilitates development of sustained-release therapies that eliminate the need for patients to comply with daily eyed drop regimens. For example, brimonidine is used clinically at a daily topical dose of 75 μg given 3 times per day.57 The daily dose of brimonidine administered to the supraciliary space appears to be approximately 100 times less than the topical dose. This means that the supraciliary daily dose is roughly 2.25 μg, and a 3-month supply would be 67.5 μg. While these calculations suggest the feasibility of injecting controlled-release microparticles into the supraciliary space, additional pharmacokinetics study will be needed to develop such controlled-release microparticles.
If this vision for sustained-release drug therapy can be realized, it could have a dramatic effect on patient compliance with glaucoma therapy. Current therapy requires many patients to administer eye drops on at least a daily basis. Compliance with such dosing schedules is very low, in the range of 56%.3 Many glaucoma patients visit their ophthalmologists every 6 months for routine examinations. In this way, glaucoma patients could receive supraciliary injections of sustained-release medication during their regular doctor's visits and thereby eliminate the need for compliance with topical eye drop therapy.
From a practical standpoint, we believe that supraciliary injections could be relatively easily introduced into clinical practice. Currently, retina specialists give millions of intravitreal injections per year at the pars plana, located 2 to 5 mm from the limbus.58,59 Supraciliary targeting requires placement of microneedles at the same site, which should be straightforward for an ophthalmologist to do. Assuring microneedles go to the right depth at the base of the sclera is determined by microneedle length, which is designed to match approximate scleral thickness. We believe variations of scleral thickness could be compensated for by the pliable nature of the choroid. In related work accessing the suprachoroidal space, microneedles have been used for hundreds of suprachoroidal injections in rabbits37 (Verhoeven RS, et al. IOVS 2013;54:ARVO E-Abstract 119) and to a lesser extent in pigs23 and were recently reported for use in four human (Morales-Canton V, et al. IOVS 2013;54:ARVO E-Abstract 3299) subjects, without significant complications. Furthermore, in all the unpublished and published works19,37,60 from our laboratory, we never experienced failed injection due to the variation of scleral thickness. However, further studies are required to more fully assess safety.
Conclusions
This study provides the first demonstration of supraciliary injection that targeted delivery of antiglaucoma drugs to their site of action in the ciliary body. Choice of the correct anesthetic facilitated these studies, as both isoflurane and ketamine/xylazine affected IOP but with different pharmacodynamics. Choice of the right drug was also important; we studied sulprostone, which is a model prostaglandin analog drug that works in rabbits, and brimonidine, which is an α2-adrenergic agonist drug that works in both rabbits and humans and shows significant effects in the contralateral eye after unilateral drug administration in the rabbit. We found that microneedles enable targeted injection into the supraciliary space and that antiglaucoma drugs administered in this way show reductions in dose of 2 orders of magnitude for similar reductions in IOP compared to topical delivery. The rabbits tolerated supraciliary injections well with no adverse events or notable findings regarding safety. We conclude that targeted drug delivery to the supraciliary space using a microneedle enables dramatic dose sparing of antiglaucoma therapeutics compared to topical eye drops.
Acknowledgments
We thank Bryce Chiang at Georgia Institute of Technology for the helpful discussions and assistance in the laboratory. This work was carried out at the Institute for Bioengineering and Bioscience and the Center for Drug Design. Yoo C. Kim, Henry F. Edelhauser, and Mark R. Prausnitz hold microneedle patents and/or patent applications, and Mark Prausnitz and Henry Edelhauser have significant financial interest in Clearside Biomedical, a company developing microneedle-based products for ocular delivery. This potential conflict of interest has been disclosed and is overseen by Georgia Institute of Technology and Emory University.
Supported by NEI EY017045 (YCK, HFE, MRP) and NEI EY022097 (YCK, MRP).
Disclosure: Y.C. Kim, P; H.F. Edelhauser, Clearside Biomedical (I, C), P; M.R. Prausnitz, Clearside Biomedical (I, S), P
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 3. Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adher. 2011; 5: 441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 5. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001; 23: 1296–1310. [DOI] [PubMed] [Google Scholar]
- 6. Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000; 59: 411–434. [DOI] [PubMed] [Google Scholar]
- 7. Camras CB, Toris CB, Tamesis RR. Efficacy and adverse effects of medications used in the treatment of glaucoma. Drugs & Aging. 1999; 15: 377–388. [DOI] [PubMed] [Google Scholar]
- 8. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006; 58: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 9. Burstein NL, Anderson JA. Corneal penetration and ocular bioavailability of drugs. J Ocul Pharmacol. 1985; 1: 309–326. [DOI] [PubMed] [Google Scholar]
- 10. Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004; 269: 1–14. [DOI] [PubMed] [Google Scholar]
- 11. Csukas S, Bhattacherjee P, Rhodes L, Paterson CA. Prostaglandin E2 binding site distribution and subtype classification in the rabbit iris-ciliary body. Prostaglandins. 1992; 44: 199–208. [DOI] [PubMed] [Google Scholar]
- 12. Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008; 53 (suppl 1); S107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burke J, Kharlamb A, Shan T, et al. Adrenergic and imidazoline receptor-mediated responses to UK-14,304-18 (brimonidine) in rabbits and monkeys. A species difference. Ann N Y Acad Sci. 1995; 763: 78–95. [DOI] [PubMed] [Google Scholar]
- 14. Timoptic 0.25% , 0.5% [package insert]. Whitehouse Station, NJ: Merck & Co; 2005. [Google Scholar]
- 15. Bhatt R, Whittaker KW, Appaswamy S, Desai A, Fitt A, Sandramouli S. Prospective survey of adverse reactions to topical antiglaucoma medications in a hospital population. Eye. 2005; 19: 392–395. [DOI] [PubMed] [Google Scholar]
- 16. Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am J Health Syst Pharm. 2005; 62: 691–699. [DOI] [PubMed] [Google Scholar]
- 17. Einmahl S, Savoldelli M, D'Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002; 43: 1533–1539. [PubMed] [Google Scholar]
- 18. Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011; 52: 4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011; 28: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzo S, Ebert FG, Bartolo ED, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012; 32: 776–784. [DOI] [PubMed] [Google Scholar]
- 21. Tetz M, Rizzo S, Augustin AJ. Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results. Ophthalmologica. 2012; 227: 183–189. [DOI] [PubMed] [Google Scholar]
- 22. Wang M, Liu W, Lu Q, et al. Pharmacokinetic comparison of ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits. Neurosurgery. 2012: 32; 2158–2164. [DOI] [PubMed] [Google Scholar]
- 23. Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013; 54: 2483–2492. [DOI] [PubMed] [Google Scholar]
- 24. Waterbury LD, Eglen RM. Ocular hypotensive activity of sulprostone, a potent and selective prostanoid EP1/EP3 receptor agonist. Clin Pharmacol Ther. 1990; 47: 150–150. [Google Scholar]
- 25. Liu JH, Jumblatt JE. Neuromodulatory effect of sulprostone on the circadian elevation of intraocular pressure in rabbits. Curr Eye Res. 1993; 12: 975–980. [DOI] [PubMed] [Google Scholar]
- 26. Liu JHK, Shieh BE, Jumblatt JE. Sulprostone decreases intraocular-pressure in rabbits by prejunctional inhibition of norepinephrine release. Invest Ophthalmol Vis Sci. 1993; 34: 921–921. [Google Scholar]
- 27. Waterbury LD, Eglen RM, Faurot GF, Cooper G. EP3, but not EP2, FP, or TP prostanoid-receptor stimulation may reduce intraocular pressure. Invest Ophthalmol Vis Sci. 1990; 31: 2560–2567. [PubMed] [Google Scholar]
- 28. Bernstein P. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator, multicenter study. Am J Ophthalmol. 2004; 137: 387–388; author reply 388–389. [DOI] [PubMed] [Google Scholar]
- 29. Koz OG, Ozsoy A, Yarangumeli A, Kose SK, Kural G. Comparison of the effects of travoprost, latanoprost and bimatoprost on ocular circulation: a 6-month clinical trial. Acta Ophthalmol Scand. 2007; 85: 838–843. [DOI] [PubMed] [Google Scholar]
- 30. Orihashi M, Shima Y, Tsuneki H, Kimura I. Potent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbits. Biol Pharm Bull. 2005; 28: 65–68. [DOI] [PubMed] [Google Scholar]
- 31. Morales M, Gomez-Cabrero A, Peral A, Gasull X, Pintor J. Hypotensive effect of profilin on rabbit intraocular pressure. Eur J Pharmacol. 2007; 567: 145–148. [DOI] [PubMed] [Google Scholar]
- 32. Impagnatiello F, Borghi V, Gale DC, et al. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp Eye Res. 2011; 93: 243–249. [DOI] [PubMed] [Google Scholar]
- 33. Ohashi M, Mayama C, Ishi K, Araie M. Local effect of topical FP-receptor agonists on retinal vessels of the ipsilateral posterior retina in normal rabbit eyes. Clin Exp Ophthalmol. 2008; 36: 767–774. [DOI] [PubMed] [Google Scholar]
- 34. Akaishi T, Kurashima H, Odani-Kawabata N, Ishida N, Nakamura M. Effects of repeated administrations of tafluprost, latanoprost, and travoprost on optic nerve head blood flow in conscious normal rabbits. J Ocul Pharmacol Ther. 2010; 26: 181–186. [DOI] [PubMed] [Google Scholar]
- 35. Kurashima H, Watabe H, Sato N, Abe S, Ishida N, Yoshitomi T. Effects of prostaglandin F(2alpha) analogues on endothelin-1-induced impairment of rabbit ocular blood flow: comparison among tafluprost, travoprost, and latanoprost. Exp Eye Res. 2010; 91: 853–859. [DOI] [PubMed] [Google Scholar]
- 36. Jumblatt JE, Ohia SE, Hackmiller RC. Prejunctional modulation of norepinephrine release in the human iris-ciliary body. Invest Ophthalmol Vis Sci. 1993; 34: 2790–2793. [PubMed] [Google Scholar]
- 37. Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012; 53: 4433–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel SR, Edelhauser HF, Prausnitz MR. Targeted drug delivery to the eye enabled by microneedles. In: Kompella UB, Edelhauser HF. eds Drug Product Development for the Back of the Eye. New York: Springer; 2011; 331–360. [Google Scholar]
- 39. icare. Tonovet User's and Maintenance Manual. Instruction Manual v.1.2 11/08. 2008. Available at: http://www.icaretonometer.com/wp-content/uploads/2012/12/Icare_VET_manual_low_res.pdf. Accessed September 16, 2014. [Google Scholar]
- 40. Pereira FQ, Bercht BS, Soares MG, da Mota MG, Pigatto JA. Comparison of a rebound and an applanation tonometer for measuring intraocular pressure in normal rabbits. Vet Ophthalmol. 2011; 14: 321–326. [DOI] [PubMed] [Google Scholar]
- 41. Atkinson KE. An Introduction to Numerical Analysis. 2nd ed. New York: Wiley; 1989; xvi, 693. [Google Scholar]
- 42. Huang Y, Gil DW, Vanscheeuwijck P, Stamer WD, Regan JW. Localization of alpha 2-adrenergic receptor subtypes in the anterior segment of the human eye with selective antibodies. Invest Ophthalmol Vis Sci. 1995; 36: 2729–2739. [PubMed] [Google Scholar]
- 43. Toris CB, Gleason ML, Camras CB, Yablonski ME. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995; 113: 1514–1517. [DOI] [PubMed] [Google Scholar]
- 44. Toris CB, Camras CB, Yablonski ME. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999; 128: 8–14. [DOI] [PubMed] [Google Scholar]
- 45. Burke JA, Potter DE. Ocular effects of a relatively selective alpha 2 agonist (UK-14, 304-18) in cats, rabbits and monkeys. Curr Eye Res. 1986; 5: 665–676. [DOI] [PubMed] [Google Scholar]
- 46. Angelov OV, Wiese AG, Tang-Liu DD, Acheampong AA, Ismail IM, Brar BS. Preclinical safety profile of brimonidine. Eur J Ophthalmol. 1996; 6: 21–25. [DOI] [PubMed] [Google Scholar]
- 47. Tsonis PA. Animal Models in Eye Research. London: Academic Press; 2008. [Google Scholar]
- 48. Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996; 41 (suppl 1): S9–S18. [DOI] [PubMed] [Google Scholar]
- 49. El Chehab H, Le Corre A, Agard E, Ract-Madoux G, Coste O, Dot C. Effect of topical pressure-lowering medication on prevention of intraocular pressure spikes after intravitreal injection. Eur J Ophthalmol. 2013; 23: 277–283. [DOI] [PubMed] [Google Scholar]
- 50. Van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JSAG. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011; 31: 1449–1469. [DOI] [PubMed] [Google Scholar]
- 51. Acheampong AA, Shackleton M, John B, Burke J, Wheeler L, Tang-Liu D. Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab Dispos. 2002; 30: 421–429. [DOI] [PubMed] [Google Scholar]
- 52. Acheampong AA, Shackleton M, Tang-Liu DD. Comparative ocular pharmacokinetics of brimonidine after a single dose application to the eyes of albino and pigmented rabbits. Drug Metab Dispos. 1995; 23: 708–712. [PubMed] [Google Scholar]
- 53. Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008; 53 (suppl 3); S93–S105. [DOI] [PubMed] [Google Scholar]
- 54. Beckers HJ, Schouten JS, Webers CA, van der Valk R, Hendrikse F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 1485–1490. [DOI] [PubMed] [Google Scholar]
- 55. Chiba T, Kashiwagi K, Kogure S, et al. Iridial pigmentation induced by latanoprost ophthalmic solution in Japanese glaucoma patients. J Glaucoma. 2001; 10: 406–410. [DOI] [PubMed] [Google Scholar]
- 56. Alm A, Schoenfelder J, McDermott JA. 5-year, multicenter, open-label, safety study of adjunctive latanoprost therapy for glaucoma. Arch Ophthalmol. 2004; 122: 957–965. [DOI] [PubMed] [Google Scholar]
- 57. Schuman JS. Clinical experience with brimonidine 0.2% and timolol 0.5% in glaucoma and ocular hypertension. Surv ophthalmol. 1996; 41 (suppl 1): S27–S37. [DOI] [PubMed] [Google Scholar]
- 58. Peyman GA, Meffert SA, Conway MD. Vitreoretinal Surgical Techniques. 2nd ed. Valley Stream, NY: Martin Dunitz Publications; 2001. [Google Scholar]
- 59. Gross JG, Freeman WR, Goldbaum MH, Mendez TL. Useful adjuncts for vitreoretinal surgery. Br J Ophthalmol. 1989; 73: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim YC, Edelhauser HF, Prausnitz MR. Particle-stabilized emulsion droplets for gravity-mediated targeting in the posterior segment of the eye. Adv Healthc Mater. 2014; 3: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]