Abstract
Background: Growing evidence suggests that dairy consumption is associated with lower type 2 diabetes risk. However, observational studies have reported inconsistent results, and few have examined dairy's association with the underlying disorders of insulin resistance and β-cell dysfunction.
Objective: We investigated the association of the dairy fatty acid biomarkers pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7) with type 2 diabetes traits by evaluating 1) prospective associations with incident diabetes after 5 y of follow-up and 2) cross-sectional associations with directly measured insulin resistance and β-cell dysfunction.
Design: The study analyzed 659 adults without diabetes at baseline from the triethnic multicenter Insulin Resistance Atherosclerosis Study (IRAS). Diabetes status was assessed by using oral-glucose-tolerance tests. Frequently sampled intravenous-glucose-tolerance tests measured insulin sensitivity (SI) and β-cell function [disposition index (DI)]. Serum fatty acids were quantified by using gas chromatography. Logistic and linear regression models were adjusted for demographic, lifestyle, and dietary variables.
Results: Serum 15:0 was a significant biomarker for total dairy intake in the IRAS cohort. It was associated with a decreased incident diabetes risk (OR: 0.73, P = 0.02) and was positively associated with log SI (β: 0.84, P = 0.03) and log DI (β: 2.21, P = 0.02) in fully adjusted models. trans 16:1n−7 was a marker of total partially hydrogenated dietary fat intake and was not associated with outcomes in fully adjusted models.
Conclusions: Serum 15:0, a marker of short-term intake of this fatty acid, was inversely associated with diabetes risk in this multiethnic cohort. This study may contribute to future recommendations regarding the benefits of dairy products on type 2 diabetes risk.
Keywords: dairy, epidemiology, fatty acids, nutrition, type 2 diabetes
See corresponding editorial on page 1407.
INTRODUCTION
Type 2 diabetes, a growing global epidemic, arises from both nonmodifiable and modifiable factors (1). Among the many nutritional exposures that have been investigated in type 2 diabetes, dairy consumption is emerging as a potential protective factor. Three meta-analyses of prospective observational studies have reported that high dairy intake was associated with a lower risk of type 2 diabetes (2–4). However, findings have been inconsistent across individual studies (5–15), and only a limited number of investigations have assessed the relation of dairy intake with the main underlying pathophysiologic traits of type 2 diabetes (1)—namely, insulin resistance (14, 16–18) and β-cell dysfunction (14).
Most previous observational studies measured usual dairy consumption by using self-reported intake from food-frequency questionnaires (FFQs),4 which are susceptible to misclassification error from under- or overreporting (19). A number of dairy bioactives may potentially underlie the associations described in previous studies, including the fatty acid profile, because dairy is a particularly rich source of SFAs and naturally occurring trans fatty acids (TFAs) (4, 20). Despite limited data, current literature suggests that intake of different types of SFAs and TFAs may affect metabolic and cardiovascular disease risk differently (21), and there is growing evidence that certain fatty acids, including those from dairy, may play a role in type 2 diabetes prevention (12). Certain fatty acid biomarkers have been validated as markers for dairy intake, including pentadecanoic acid (15:0) (22–27) and trans-palmitoleic acid (trans 16:1n−7), with fatty acids measured in serum reflecting short-term dietary intake (28–31). Using dairy-derived fatty acid biomarkers has the potential to provide more objective measures of dairy intake, and thus they may help to elucidate the role of dairy on the risk of type 2 diabetes and its underlying disorders.
The current study aimed to investigate the association between dairy biomarkers and type 2 diabetes traits in a large multiethnic cohort by evaluating 1) prospective associations of dairy fatty acid biomarkers with incident diabetes after 5 y of follow-up and 2) cross-sectional associations of dairy fatty acid biomarkers with directly measured insulin resistance and β-cell dysfunction by using frequently sampled intravenous-glucose-tolerance tests (FSIGTs). We hypothesized that pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7), independent of covariates, would be inversely associated with insulin resistance, β-cell dysfunction, and incident type 2 diabetes at 5 y.
SUBJECTS AND METHODS
The Insulin Resistance Atherosclerosis Study (IRAS) is a multicenter epidemiologic study assessing the relation between insulin resistance and subclinical cardiovascular disease. The study consists of a triethnic cohort of 1625 Hispanic, African American, and non-Hispanic white adults aged 40–60 y across a range of glucose tolerance status. Participants were recruited from 4 clinical centers in San Antonio, Texas; San Luis Valley, Colorado; Los Angeles, California; and Oakland, California. Baseline study visits were conducted between October 1992 and April 1994, and 5-y follow-up examinations were conducted from February 1998 to July 1999, with an 81% response rate (32). Participants who did not attend the follow-up examinations were not systematically different in terms of age, sex, ethnicity, or clinic, except for a slightly lower educational attainment compared with those who returned for follow-up (33). Each study center received ethics approval from its institutional review board, and all participants gave informed consent. A comprehensive presentation of the study objectives, design, and recruitment results has been published previously (34).
The present analysis excluded participants with type 2 diabetes at baseline (n = 553) and participants who did not return for follow-up (n = 177). With further exclusions for missing insulin sensitivity and β-cell function measures, as well as 15:0, trans 16:1n−7, and total dairy intake values, the final study sample for the current analysis was 659.
Usual dietary intake over the previous year before baseline was assessed by using a 114-item FFQ modified from the National Cancer Institute's Health Habits and History Questionnaire to include ethnic and regional foods relevant to the study population (35). The validity and reproducibility of this FFQ were established in a subsample of 186 women from the IRAS population by using eight 24-h dietary recalls, followed by a second FFQ (35). Food and beverage intake in the FFQ was quantified through interviews in which participants were asked to recall the frequency of consumption of each food, or groups of foods, over the past year. The FFQ contained 9 frequency options, ranging from “never or less than once a month” to “6 or more times per day,” and 3 portion sizes: “small, medium, or large compared with other men or women about your age.” Servings per day were standardized to the medium serving size for the food intake analyses by multiplying the intake frequency with the portion size after applying a weighting factor (small = 0.5, medium = 1.0, and large = 1.5). One serving, therefore, corresponds to 1 medium-sized portion of the food or food group. Total dairy product intake was calculated by adding 11 dairy food line items from the FFQ: whole milk; 2% milk; skim milk, 1%, or buttermilk; cottage and ricotta cheese; cheese; flavored yogurt (2%, nonfat, or whole); low-fat flavored yogurt (2% or nonfat); ice cream; frozen yogurt or ice milk; milk in coffee or tea; and cream or half-and-half in coffee or tea. Total milk, total cheese, and total yogurt intakes were also calculated. Because trans 16:1n−7 may also be found in foods containing partially hydrogenated fats (29), total partially hydrogenated food intake was calculated by summing the following items from the FFQ: french fries and fried potatoes; salty snacks such as crackers, potato chips, corn chips, tortilla chips, and pretzels; margarine on bread or roll; doughnuts; cookies; cakes; pastry; brownies; sopapillas; and pan dulce. A similar approach for summing sources of hydrogenated fats was recently used by Mozaffarian et al. (29). Nutrient and energy intakes were estimated from the FFQ by using a nutrient database (HHHQ-DIETSYS analysis software, version 3.0; National Cancer Institute, 1993), expanded for additional nutrients.
Clinical examinations were conducted at baseline and follow-up during two 4-h visits, which were administered 1 wk to 30 d apart. Before each clinic visit, participants were asked to fast for 12 h and refrain from heavy exercise and alcohol consumption for 24 h, as well as smoking the morning of the visit. All participants received an oral glucose tolerance test to determine glucose tolerance status (normal, impaired glucose tolerance, or diabetes), based on the 2010 American Diabetes Association criteria for fasting or 2-h postload glucose concentrations, and oral hypoglycemic agent or insulin use (36). FSIGTs were administered following a validated modified protocol (34, 37). Insulin resistance was calculated by using minimal-model analysis (MINMOD, version 3.0, 1994) (38) and expressed as the insulin sensitivity index (SI). Insulin secretion was assessed via acute insulin response, a sensitivity index of β-cell function measured as the mean plasma insulin concentration from 2- to 4-min time points after the initial glucose administration (34). The product of SI and acute insulin response yields the disposition index (DI), an integrated measure of β-cell function reflecting the ability of β cells to compensate for insulin resistance by upregulating insulin secretion (39). DI was used as the measure of β-cell function for this investigation.
Waist circumference and height were measured to the nearest 0.5 cm, and body weight was measured to the nearest 0.1 kg, with the average of the duplicate measurements used in all analyses. A validated physical activity recall was used to determine total estimated energy expenditure over the past year (34). Total estimated energy expenditure (kcal/kg per wk) was calculated by summing energy expenditure activities and energy expenditure from sleep. Smoking status was categorized into 3 groups: never, past, or current. Total usual alcohol intake (g ethanol consumed/d) was evaluated through a separate questionnaire with additional questions about recent use and average lifetime use. Race/ethnicity and age were self-reported. Medical history was assessed by using structured interviews.
Fatty acid analysis
A complete quantitative profile of fatty acids was extracted from participants’ serum samples (which were stored at −70°C) by using methods previously described (Lipomics Technologies Inc.) (40). Briefly, total lipids were extracted in the presence of internal standards according to the method of Folch et al. (41). Fatty acid methyl esters were formed by transesterification of total lipid extracts in sulfuric acid/methanol and were then extracted into hexane and prepared for gas chromatography. Capillary gas chromatography (model 6890; Agilent Technologies) equipped with a 30-m HP-88 capillary column (Agilent Technologies) and a flame ionization detector were used to separate and quantify individual fatty acids. The absolute concentration of each fatty acid in the serum sample was measured by comparing its peak area with the internal standard. A total of 35 serum fatty acids were analyzed and quantified by using these methods. For this study, dairy-derived fatty acids pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7) are the exposures of interest and are expressed as the mole percentage (mol%) of total fatty acids.
Statistical analysis
Baseline characteristics across quintiles of increasing total dairy intake (servings/wk) are presented. Normally distributed variables are presented as means ± SDs, nonnormally distributed variables are presented as medians (IQRs), and categorical variables are presented as number and percentage of participants in each quintile, with differences across quintiles tested by using ANOVA, Kruskal-Wallis tests, and χ2 tests, respectively.
Dietary determinants of dairy fatty acids in serum were assessed by analyzing the correlations of 15:0 and trans 16:1n−7 with total dairy, total milk, total cheese, total yogurt, and total partially hydrogenated food intakes from the FFQ. Furthermore, multivariable-adjusted linear regressions with 15:0 and trans 16:1n−7 as the outcome variable were conducted to assess the contribution of total dairy and total partially hydrogenated food intake to serum concentrations of these dairy fatty acids. The regressions were iteratively adjusted for model 1 (age, sex, and ethnicity), model 2 (physical activity and total energy intake), model 3 (total dairy or total hydrogenated food intake), and model 4 (BMI).
Logistic regression analysis was used to evaluate the prospective associations between dairy fatty acids and incident diabetes at 5 y, with sequential adjustment in 3 models. Model 1 was adjusted for demographic variables: age, sex, ethnicity, and center. Model 2 was also adjusted for lifestyle variables: physical activity, smoking status, alcohol intake, and education. Model 3 was adjusted for dietary variables: total energy, fruit and vegetable, red meat, soft drink, and fiber intakes. On the basis of significant Spearman correlations of BMI and waist circumference with 15:0 and the outcome variables, we included these measures of adiposity in additional mechanistic models because these variables are likely on the etiologic pathway between the fatty acid exposures and outcomes. In the cross-sectional study, univariate analyses between outcomes and exposures were conducted by using Spearman's correlations (r). Multiple linear regression analysis was used to assess the cross-sectional association between the dairy fatty acids with insulin resistance (SI) and β-cell dysfunction (DI), adjusted for the same covariates in the logistic regressions. Both SI and DI were skewed, and thus these outcome variables were log transformed to achieve normality. Because some participants had an SI of 0, a constant of 1 was added to the values before being log transformed. Subgroup analyses on a priori variables of interest, including sex, glucose tolerance status, and ethnicity, were conducted for both logistic and linear regressions. Formal tests of interaction for these variables were carried out by using cross-product terms and considered statistically significant when P < 0.05. The assumption of linearity of the associations in the logistic and linear regressions was tested by adding quadratic terms to the models. None of the quadratic terms was statistically significant (all P > 0.05), and thus we concluded that the linearity assumption had not been violated.
We conducted additional analyses by using increasing tertiles of 15:0 and trans 16:1n−7 as the independent variable. In the prospective analyses, we used logistic regression adjusted for the models previously described. For the cross-sectional analyses, we conducted ANOVA, followed by Tukey's post hoc test, to compare the least squares means of log SI and log DI in each fatty acid tertile, adjusted for the models mentioned previously.
All analyses were performed by using SAS 9.2 (SAS Institute Inc.). P < 0.05 was considered significant for all analyses.
RESULTS
Baseline characteristics of participants across quintiles of increasing total dairy intake (servings/wk) are presented in Table 1. There were no differences in age, sex, and educational levels across these categories. Ethnicity differed significantly across quintiles of total dairy intake, with non-Hispanic whites and African Americans having the highest and lowest dairy intakes, respectively. Glucose tolerance status also differed across quintiles, such that the highest percentage of participants with impaired glucose tolerance was in quintile 1, although SI and DI did not significantly differ across quintiles. Total energy intake, percent energy from SFAs, dietary intake of TFAs, and serum 15:0 and trans 16:1n−7 significantly increased across quintiles.
TABLE 1.
Baseline characteristics of the Insulin Resistance Atherosclerosis Study participants (n = 659) overall and across quintiles of total dairy intake from the food-frequency questionnaire1
| Total dairy intake, servings/wk2 |
|||||||
| Characteristic | All | Q1 (0–2.07) | Q2 (2.10–4.10) | Q3 (4.13–6.65) | Q4 (6.69–9.73) | Q5 (9.80–31.08) | P value3 |
| Participants, n (%) | 659 | 132 (20.03) | 131 (19.88) | 133 (20.18) | 132 (20.03) | 131 (19.88) | |
| Age, y | 54.68 ± 8.56 | 54.74 ± 8.84 | 55.52 ± 8.27 | 53.69 ± 7.96 | 55.99 ± 9.24 | 53.50 ± 8.27 | 0.07 |
| Sex, n (%) | 0.16 | ||||||
| Male | 297 (45.07) | 61 (46.21) | 59 (45.04) | 60 (45.11) | 69 (52.27) | 48 (36.64) | |
| Female | 362 (54.93) | 71 (53.79) | 72 (54.96) | 73 (54.89) | 63 (47.73) | 83 (93.36) | |
| Ethnicity, n (%) | <0.0001 | ||||||
| Non-Hispanic white | 277 (42.03) | 36 (27.27) | 50 (38.17) | 56 (42.11) | 66 (50.00) | 69 (52.67) | |
| African American | 152 (23.07) | 55 (41.67) | 32 (24.43) | 28 (21.05) | 16 (12.12) | 21 (16.03) | |
| Hispanic | 230 (34.90) | 41 (31.06) | 49 (37.40) | 49 (36.84) | 50 (37.88) | 41 (31.30) | |
| Glucose tolerance status, n (%) | 0.04 | ||||||
| Normal glucose tolerance | 447 (67.83) | 81 (61.36) | 93 (70.99) | 103 (77.44) | 85 (64.39) | 85 (64.89) | |
| Impaired glucose tolerance | 212 (32.17) | 51 (38.64) | 38 (29.01) | 30 (22.56) | 47 (35.61) | 46 (35.11) | |
| Smoking status, n (%) | 0.0009 | ||||||
| Never | 306 (46.43) | 48 (36.36) | 59 (45.04) | 65 (48.87) | 53 (40.15) | 81 (61.83) | |
| Past | 259 (39.30) | 55 (41.67) | 53 (40.46) | 56 (42.11) | 58 (43.94) | 37 (28.24) | |
| Current | 94 (14.26) | 29 (21.97) | 19 (14.50) | 12 (9.02) | 21 (15.91) | 13 (9.92) | |
| Alcohol intake category, g ethanol/wk | 3.52 (0–39.54) | 2.24 (0–34.35) | 7.26 (0–57.82) | 0 (0–15.47) | 8.86 (0–63.92) | 3.52 (0–25.92) | 0.007 |
| Highest educational level completed, n (%) | 0.48 | ||||||
| <12 y | 87 (13.20) | 21 (15.91) | 16 (12.21) | 12 (9.02) | 20 (15.15) | 18 (13.74) | |
| ≥12 y | 572 (86.80) | 111 (84.09) | 115 (87.79) | 121 (90.98) | 112 (84.85) | 113 (86.26) | |
| BMI, kg/m2 | 27.24 (24.83–27.24) | 27.24 (24.81–29.99) | 26.58 (24.10–29.33) | 28.09 (25.56–30.80) | 27.20 (24.77–29.55) | 27.71 (24.75–33.07) | 0.04 |
| Waist circumference, cm | 90.28 ± 12.52 | 90.53 ± 12.74 | 87.87 ± 11.79 | 89.92 ± 11.06 | 91.53 ± 11.57 | 91.59 ± 14.92 | 0.10 |
| Total estimated energy expenditure, kcal/kg per wk | 272.28 (250.88–305.33) | 272.03 (247.84–304.72) | 270.05 (251.20–303.87) | 267.22 (249.16–295.37) | 284.22 (255.35–320.87) | 272.96 (251.64–309.56) | 0.05 |
| Insulin sensitivity, ×10−4 min−1 (μU/mL)−1 | 1.71 (0.95–3.01) | 1.73 (0.98–2.80) | 1.92 (1.00–3.25) | 1.73 (1.00–3.11) | 1.53 (0.92–2.50) | 1.65 (0.85–3.16) | 0.47 |
| Acute insulin response, μU/mL | 51.5 (29.5–87.0) | 50.50 (30.00–92.25) | 51.0 (24.5–93.0) | 60.0 (36.0–84.0) | 49.5 (30.5–82.0) | 49.5 (25.0–81.5) | 0.49 |
| Disposition index | 85.68 (43.44–154.59) | 103.86 (43.10–151.16) | 89.70 (48.02–149.49) | 98.05 (57.38–179.41) | 66.28 (41.22–140.27) | 76.44 (36.25–153.00) | 0.07 |
| Dietary variables | |||||||
| Total energy intake, kcal/wk | 12,416.84 (8999.48–16,181.69) | 9559.99 (7181.22–13,685.13) | 11,457.43 (7858.01–13,914.89) | 12,257.03 (9288.20–15,780.76) | 13,505.43 (10,679.84–17,341.15) | 15,005.27 (11,425.64–19,989.34) | <0.0001 |
| % Energy from fat | 35.44 ± 8.44 | 34.68 ± 8.66 | 35.62 ± 9.51 | 35.64 ± 8.84 | 34.94 ± 7.91 | 36.29 ± 7.14 | 0.57 |
| % Energy from SFA | 12.05 ± 3.39 | 11.18 ± 3.42 | 11.74 ± 3.66 | 12.17 ± 3.31 | 12.25 ± 3.25 | 12.88 ± 3.08 | 0.001 |
| Dietary intake TFA, g/wk | 15.05 (7.56–25.90) | 11.69 (6.09–21.00) | 12.67(6.23–22.19) | 14.81 (9.57–22.54) | 16.59 (8.62–29.89) | 19.75 (11.83–33.32) | <0.0001 |
| Total dairy consumption, servings/wk | 5.53 (2.59–8.75) | 0.96 (0.35–1.54) | 3.05 (2.59–3.54) | 5.53 (4.80–6.13) | 8.05 (7.28–8.75) | 12.46 (11.06–15.23) | <0.0001 |
| Total cheese consumption, servings/wk | 1.51 (0.49–3.26) | 0.28 (0–0.58) | 0.98 (0.32–1.96) | 1.58 (0.77–3.01) | 2.24 (0.98–3.89) | 3.5 (1.96–7.00) | <0.0001 |
| Total milk consumption, servings/wk | 0.98 (0–3.50) | 0 (0-0.21) | 0.56 (0–0.98) | 0.98 (0.21–3.50) | 2.54 (0.56–4.66) | 3.71 (1.75–7.00) | <0.0001 |
| Total yogurt consumption, servings/wk | 0.210 (0–1.12) | 0 (0–0.21) | 0.11 (0–1.05) | 0.21 (0–1.12) | 0.56 (0–2.52) | 0.88 (0–3.82) | <0.0001 |
| Serum dairy fatty acid, mol% of total fatty acids | |||||||
| Pentadecanoic acid (15:0) | 0.25 ± 0.06 | 0.23 ± 0.05 | 0.24 ± 0.05 | 0.26 ± 0.05 | 0.25 ± 0.06 | 0.26 ± 0.07 | <0.0001 |
| Trans-palmitoleic acid (trans 16:1n−7) | 0.30 ± 0.10 | 0.30 ± 0.11 | 0.32 ± 0.10 | 0.32 ± 0.09 | 0.30 ± 0.10 | 0.29 ± 0.09 | 0.02 |
Total dairy intake was defined as sum of whole milk; 2% milk; skim milk, 1%, or buttermilk; cottage and ricotta cheese; cheese; flavored yogurt (2%, nonfat, or whole); low-fat flavored yogurt (2% or nonfat); ice cream; frozen yogurt, ice milk; milk in coffee or tea; and cream or half-and-half in coffee or tea. Continuous variables are presented as means ± SDs if normal or medians (IQRs) if distribution is nonnormal. Categorical variables are presented as n (%). Q, quintile; TFA, trans fatty acid.
Range of total dairy intake per quintile.
For continuous variables, P values are from ANOVA or Kruskal-Wallis comparison across quintiles for normal and skewed variables, respectively. For categorical variables, P values are from χ2 value for comparison across quintiles.
Total intakes of dairy (r = 0.20, P < 0.0001), milk (r = 0.13, P = 0.0006), and cheese (r = 0.16, P < 0.0001) were positively correlated with 15:0. trans 16:1n−7 was not significantly correlated with either 15:0 or reported intakes of dairy foods (data not shown). In contrast, intake of total partially hydrogenated foods, another dietary source of trans 16:1n−7, was positively and significantly correlated with serum trans 16:1n−7 (r = 0.18, P < 0.0001). Linear regression analysis showed that 15:0 in serum was independently and positively associated with total dairy intake, whereas trans 16:1n−7 was negatively associated with total dairy intake (Table 2). Conversely, 15:0 was negatively and trans 16:1n−7 was positively associated with total partially hydrogenated food intake (Table 3).
TABLE 2.
Regression analysis assessing the contribution of total dairy intake from the food-frequency questionnaire to serum concentrations of pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7) in 659 adults in the Insulin Resistance Atherosclerosis Study1
| Dependent variable | n | β ± SEM | P value |
| 15:0 | |||
| Model 12 | 659 | 0.002 ± 0.0005 | 0.001 |
| Model 23 | 646 | 0.002 ± 0.0005 | 0.002 |
| Model 34 | 646 | 0.002 ± 0.0005 | 0.0002 |
| Model 45 | 645 | 0.002 ± 0.0005 | 0.0001 |
| trans 16:1n−7 | |||
| Model 12 | 659 | −0.002 ± 0.0008 | 0.021 |
| Model 23 | 646 | −0.002 ± 0.0009 | 0.07 |
| Model 34 | 646 | −0.003 ± 0.0009 | 0.004 |
| Model 45 | 645 | −0.003 ± 0.0009 | 0.004 |
Total dairy intake was defined as sum of whole milk; 2% milk; skim milk, 1%, or buttermilk; cottage and ricotta cheese; cheese; flavored yogurt (2%, nonfat, or whole); low-fat flavored yogurt (2% or nonfat); ice cream; frozen yogurt, ice milk; milk in coffee or tea; and cream or half-and-half in coffee or tea.
Model 1: total dairy intake adjusted for age, sex, and ethnicity.
Model 2: additionally adjusted for physical activity and total energy intake.
Model 3: additionally adjusted for total hydrogenated food intake.
Model 4: additionally adjusted for BMI.
TABLE 3.
Regression analysis assessing the contribution of total partially hydrogenated food intake from the food-frequency questionnaire to serum concentrations of pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7) in 659 adults in the Insulin Resistance Atherosclerosis Study1
| Dependent variable | n | β ± SEM | P value |
| 15:0 | |||
| Model 12 | 659 | −0.0006 ± 0.0004 | 0.11 |
| Model 23 | 646 | −0.0007 ± 0.0004 | 0.06 |
| Model 34 | 646 | −0.001 ± 0.0004 | 0.008 |
| Model 45 | 645 | −0.001 ± 0.0004 | 0.010 |
| trans 16:1n−7 | |||
| Model 12 | 659 | 0.003 ± 0.0007 | <0.0001 |
| Model 23 | 646 | 0.003 ± 0.0007 | <0.0001 |
| Model 34 | 646 | 0.003 ± 0.0007 | <0.0001 |
| Model 45 | 645 | 0.003 ± 0.0007 | <0.0001 |
Total partially hydrogenated food was defined as the sum of french fries and fried potatoes; salty snacks such as crackers, potato chips, corn chips, tortilla chips, or pretzels; margarine on bread or roll; doughnuts; cookies; cakes; pastry; brownies; sopapillas; and pan dulce.
Model 1: total partially hydrogenated food intake adjusted for age, sex, and ethnicity.
Model 2: additionally adjusted for physical activity and total energy intake.
Model 3: additionally adjusted for total hydrogenated food intake.
Model 4: additionally adjusted for BMI.
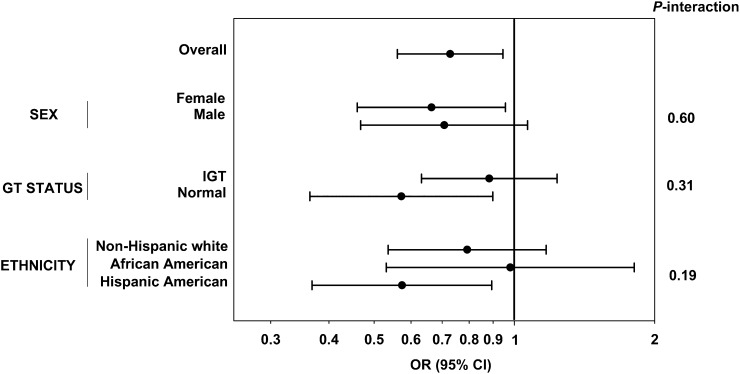
In the prospective analysis, 103 of 659 participants developed diabetes after 5 y of follow-up. In multivariate logistic regression, 15:0 was associated with a 27% decreased risk for incident type 2 diabetes in the fully adjusted model, which included demographic, lifestyle, and dietary variables (model 3: OR per SD: 0.73; 95% CI: 0.56, 0.95; P = 0.02) (Figure 1). After further adjustment for adiposity variables in a mechanistic model, the associations persisted, with additional adjustment for BMI (OR: 0.76; 95% CI: 0.58, 0.99; P = 0.04) or waist circumference (OR: 0.77; 95% CI: 0.59, 1.00; P = 0.05). None of the interaction terms tested in the effect modification analyses were statistically significant (P > 0.05) (Figure 1).
FIGURE 1.
Logistic regression analyses, overall and stratified by subgroups, for incident diabetes risk with pentadecanoic acid (15:0) in 659 adults in the Insulin Resistance Atherosclerosis Study. ORs per SD and 95% CIs of each regression are shown, as well as P-interaction values from the effect modification analyses. ORs were adjusted for age, sex, ethnicity, center, physical activity, smoking status, alcohol intake, education, and total energy, fruit and vegetable, red meat, soft drink, and fiber intakes. Overall, model 3: OR per SD: 0.73; 95% CI: 0.56, 0.95; P = 0.02. GT, glucose tolerance; IGT, impaired glucose tolerance.
In univariate analyses, 15:0 was positively correlated with SI (r = 0.14, P = 0.0003) and DI (r = 0.11, P = 0.006), whereas trans 16:1n−7 was negatively correlated with SI (r = −0.12, P = 0.003) and not significantly correlated with DI. Multiple regression analyses showed that 15:0 was positively associated to both log SI (β: 0.84; SEM: 0.38; P = 0.03) and log DI (β: 2.21; SEM: 0.93; P = 0.02) after adjustment for demographic, lifestyle, and dietary variables (Table 4). Further adjustment for BMI (Table 4) and waist circumference (data not shown) in mechanistic models attenuated results to nonsignificance, although the direction and magnitude of these associations were similar. trans 16:1n−7 was negatively associated with log SI after adjustment for age, sex, ethnicity, and study center (β: −0.56; SEM = 0.21; P = 0.0096). Further adjustment with lifestyle and dietary variables attenuated results to nonsignificance. trans 16:1n−7 was not associated with log DI. None of the interaction terms tested in the effect modification analyses were statistically significant (P > 0.05) (data not shown).
TABLE 4.
Cross-sectional multiple regression analyses for log SI and log DI with pentadecanoic acid (15:0) and trans-palmitoleic acid (trans 16:1n−7) in 659 adults in the Insulin Resistance Atherosclerosis Study1
| Log SI |
Log DI |
||||
| Independent variable | n | β ± SEM | P value | β ± SEM | P value |
| 15:0 | |||||
| Model 12 | 659 | 0.689 ± 0.370 | 0.06 | 1.763 ± 0.895 | 0.049 |
| Model 23 | 645 | 0.909 ± 0.382 | 0.018 | 2.205 ± 0.927 | 0.018 |
| Model 34 | 645 | 0.844 ± 0.381 | 0.027 | 2.212 ± 0.931 | 0.018 |
| Model 45 | 644 | 0.504 ± 0.334 | 0.13 | 1.628 ± 0.881 | 0.07 |
| trans 16:1n−7 | |||||
| Model 12 | 659 | −0.556 ± 0.214 | 0.010 | −0.264 ± 0.520 | 0.61 |
| Model 23 | 645 | −0.439 ± 0.225 | 0.05 | 0.078 ± 0.546 | 0.89 |
| Model 34 | 645 | −0.415 ± 0.223 | 0.06 | 0.096 ± 0.548 | 0.86 |
| Model 45 | 644 | −0.375 ± 0.195 | 0.05 | 0.137 ± 0.516 | 0.79 |
DI, disposition index; SI, insulin sensitivity index.
Model 1 was adjusted for age, sex, ethnicity, and center.
Model 2: additionally adjusted for physical activity, smoking status, alcohol intake, and education.
Model 3: additionally adjusted for total energy, fruit and vegetable, red meat, soft drink, and fiber intakes.
Model 4: additionally adjusted for BMI.
Additional analyses across tertiles of 15:0 and trans 16:1n−7 yielded similar results. Briefly, participants in the highest tertile of 15:0 had significantly lower type 2 diabetes risk after 5 y compared with those in the lowest tertile in the fully adjusted model (tertile 3 compared with tertile 1, model 3: OR: 0.47; 95% CI: 0.26, 0.86; P-trend: 0.05). Further adjustment for BMI did not change the association appreciably (tertile 3 compared with tertile 1, model 4: OR: 0.53; 95% CI: 0.29, 0.9; P-trend = 0.12). In cross-sectional analyses, those in the highest tertile of 15:0 had higher least squares mean values of log SI and log DI than those in the lowest tertile in fully adjusted models (log SI model 3: P = 0.003; log DI model 3: P = 0.0002). The significant positive associations were maintained after additional adjustment for BMI (data not shown). There was no association with log SI or log DI across tertiles of trans 16:1n−7 in fully adjusted models (data not shown).
DISCUSSION
In the present study, we found that serum 15:0 was a significant biomarker for total dairy intake in this multiethnic cohort. In addition, 15:0 was positively associated with SI and DI, as well as a decreased incident diabetes risk after 5 y of follow-up, independent of demographic, lifestyle, and dietary variables. Further adjustment for BMI attenuated the cross-sectional results, suggesting that the associations may be partially mediated by adiposity. In contrast, trans 16:1n−7 was negatively associated with SI, although further adjustment with lifestyle and dietary variables attenuated this result to nonsignificance. Although there was some evidence of stronger associations of 15:0 with diabetes and its underlying traits in specific subgroups, these results should be interpreted cautiously because the interaction terms were nonsignificant.
Few previous studies have examined the associations between 15:0 (42) and trans 16:1n−7 (28, 29) biomarkers with type 2 diabetes risk, as well as underlying disorders of insulin resistance and β-cell function in type 2 diabetes. These studies largely demonstrated that higher concentrations of these fatty acids were associated with a lower risk in diabetes. In agreement with the present study, a sex- and age-matched nested case-referent prospective study of 159 Swedish participants without diabetes at baseline showed that a higher proportion of 15:0 in erythrocyte membranes was associated with a 29% decrease in incident diabetes (OR: 0.71; 95% CI: 0.52, 0.97; P = 0.033) after 5 y of follow-up with limited adjustment for confounding variables (alcohol intake, BMI, glycated hemoglobin, smoking, and physical activity) (42). On the other hand, the European Prospective Investigation into Cancer and Nutrition cohort did not find significant decreases in diabetes risk with concentrations of 15:0 (43, 44). Furthermore, one previous cross-sectional study found no significant association of 15:0 with insulin resistance or β-cell function (45).
Unlike previous studies, the present study did not find serum trans 16:1n−7 to be correlated with total dairy intake or serum 15:0 or to be inversely associated with diabetes risk. In the Cardiovascular Health Study, Mozaffarian et al. (28) found that trans 16:1n−7 was highly correlated with other biomarkers of dairy fat intake, including 15:0 (r = 0.64), and that higher concentrations of circulating trans 16:1n−7 were associated with a lower risk of incident diabetes (quintile 5 compared with quintile 1: HR: 0.38, 95% CI: 0.24, 0.62; P-trend < 0.001). Furthermore, this study found that trans 16:1n−7 was associated with a 16.7% lower insulin resistance as measured by HOMA-IR (P-trend < 0.001). A more recent study by Mozaffarian et al. (29) also found trans 16:1n−7 to be significantly associated with lower incident diabetes in a multiethnic cohort (Multi-Ethnic Study of Atherosclerosis), with similar findings across different ethnicity subgroup analyses. In contrast, high concentrations of trans 16:1n−7 in a European cohort were not significantly associated with a lower diabetes risk (43, 44), and adipose tissue trans 16:1n−7 was not associated with diabetes prevalence in a Costa Rican cohort (46). Inconsistencies in the results of these studies are perhaps due to differences in population characteristics, dairy intake behaviors, biological media used to measure fatty acids, and covariates used in the analyses.
Presently, the mechanism underlying the inverse relation of 15:0 with diabetes risk is not known. It is possible that 15:0, given its significant correlation with total dairy intake, may be a marker for other beneficial components of dairy, such as calcium, vitamin D, magnesium, protein, probiotics, or prebiotics (2, 4, 47). Alternatively, because previous studies have shown effects of other types of SFAs on β cells and insulin-sensitive tissues (48), 15:0 may have an as yet to be described direct effect on one or more of the traits underlying diabetes.
Optimal dietary biomarkers for epidemiologic research are those that cannot be endogenously produced in the body. In terms of fatty acids, these include odd-numbered, branch-chained, and trans fatty acids (19). A number of fatty acids have been validated as biomarkers of dairy intake, including 15:0 and trans 16:1n−7. Studies have shown that 15:0, measured in adipose tissue (22, 24, 26, 27), serum (23–26), plasma (30), and erythrocyte (30), is a valid biomarker for dairy intake with strong correlations between the fatty acid and dairy intake measured through dietary records. Serum fatty acids, in particular, represent short-term dietary intake (31). Our findings are consistent with these aforementioned validation studies on 15:0. In agreement with a previous study (29), partially hydrogenated foods were a source of trans 16:1n−7 in this cohort, as demonstrated by the significant positive association with total partially hydrogenated food intake in the fully adjusted linear regression. Trans 16:1n−7 in plasma (28–30, 49) and erythrocytes (30) has also been shown to be highly correlated with self-reported dairy intake. In contrast, trans 16:1n−7 was not significantly correlated with total dairy intake or 15:0 in our study. The samples in which the fatty acid measures were conducted in the IRAS cohort were collected in the early 1990s, before large-scale reformulation of foods to reduce trans fat content (29, 50). This may explain the high correlation found in the current study between trans 16:1n−7 and total partially hydrogenated food intake and not with total dairy intake or 15:0.
There are several strengths to this study. To our knowledge, this is the first study to simultaneously examine the association of 15:0 and trans 16:1n−7 with incident diabetes, as well as its main underlying pathophysiologic traits of insulin resistance and β-cell dysfunction. The design of the IRAS cohort also allowed for the evaluation of these associations across multiple ethnicities and glucose tolerance status. In addition, insulin resistance and β-cell dysfunction were assessed precisely by using FSIGT. Other observational studies on dairy fatty acids and diabetes outcomes did not have measures with this degree of precision (28, 29, 42, 43). As previously mentioned, using biomarkers in our analyses gave us a more objective measure of dairy intake compared with estimates obtained from FFQ data. Moreover, we adjusted for a broad range of potential demographic, lifestyle, and dietary confounders in the analyses. On the other hand, given the observational design, this study is limited in that it cannot infer causal relationships between the exposure and outcomes. Also, although adjustments for several potential confounders were considered in the analyses, other confounding factors may still be unaccounted for.
In conclusion, serum 15:0, a marker of short-term intake of this fatty acid, was a significant and independent biomarker for total dairy intake in the IRAS cohort. This fatty acid was positively associated with insulin sensitivity and β-cell function, as well as a 27% decreased risk of incident diabetes after 5 y. Unlike previous studies, trans 16:1n−7 was not correlated with total dairy intake in this cohort but rather with intake of partially hydrogenated fats. Further studies are required to evaluate the association between dairy fatty acid biomarkers and diabetes outcomes and its mechanisms to inform future public health recommendations regarding dairy intake.
Acknowledgments
The authors’ responsibilities were as follows—LEW and SMH: contributed to the IRAS design; IDS and AJH: designed the current study; SMW: conducted the fatty acid analyses; IDS: wrote the manuscript and analyzed the data with analytical insights from AJH; SMW, ADL, LEW, MJR, SMH, CL, and AJH: revised and provided critical feedback on the manuscript; and AJH: had primary responsibility for the final content. All authors read and approved the final version of the manuscript. SMW is an employee of Metabolon, Inc., which sells diagnostics for the management of metabolic disorders. AJH holds a Tier II Canada Research Chair in Diabetes Epidemiology. None of the funders had any role in the design, analysis, interpretation, or presentation of the results. None of the other authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DI, disposition index; FFQ, food-frequency questionnaire; FSIGT, frequently sampled intravenous-glucose-tolerance test; IRAS, Insulin Resistance Atherosclerosis Study; SI, insulin sensitivity index; TFA, trans fatty acid.
REFERENCES
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 3.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids 2010;45:925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr 2011;65:1027–31. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med 2005;165:997–1003. [DOI] [PubMed] [Google Scholar]
- 6.Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health 2007;61:695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fumeron F, Lamri A, Emery N, Bellili N, Jaziri R, Porchay-Baldérelli I, Lantieri O, Balkau B, Marre M, DESIR Study Group. Dairy Products and the Metabolic Syndrome in a Prospective Study, DESIR. J Am Coll Nutr 2011;30:454S–63S. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006;29:1579–84. [DOI] [PubMed] [Google Scholar]
- 9.Louie JC, Flood VM, Rangan AM, Burlutsky G, Gill TP, Gopinath B, Mitchell P. Higher regular fat dairy consumption is associated with lower incidence of metabolic syndrome but not type 2 diabetes. Nutr Metab Cardiovasc Dis 2013;23:816–21. [DOI] [PubMed] [Google Scholar]
- 10.Margolis KL, Wei F, de Boer IH, Howard BV, Liu S, Manson JE, Mossavar-Rahmani Y, Phillips LS, Shikany JM, Tinker LF, et al. A diet high in low-fat dairy products lowers diabetes risk in postmenopausal women. J Nutr 2011;141:1969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morcillo S, Atencia JA, Martin F, Ortega A, Bilbao JR, Rubio-Martín E, Rojo-Martíneza G, Esteva I, Valdésa S, Olveira G, et al. Consumption of cows’ milk is associated with lower risk of type 2 diabetes mellitus: a cross-sectional study. Int Dairy J 2012;26:162–5. [Google Scholar]
- 12.Sluijs I, Forouhi NG, Beulens JW, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB, et al. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr 2012;96:382–90. [DOI] [PubMed] [Google Scholar]
- 13.Soedamah-Muthu SS, Masset G, Verberne L, Geleijnse JM, Brunner EJ. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr 2013;109:718–26. [DOI] [PubMed] [Google Scholar]
- 14.Struijk EA, Heraclides A, Witte DR, Soedamah-Muthu SS, Geleijnse JM, Toft U, Lau CJ. Dairy product intake in relation to glucose regulation indices and risk of type 2 diabetes. Nutr Metab Cardiovasc Dis 2013;23:822–8. [DOI] [PubMed] [Google Scholar]
- 15.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 2006;29:2238–43. [DOI] [PubMed] [Google Scholar]
- 16.Akter S, Kurotani K, Nanri A, Pham NM, Sato M, Hayabuchi H, Mizoue T. Dairy consumption is associated with decreased insulin resistance among the Japanese. Nutr Res 2013;33:286–92. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Livingston KA, Fox CS, Meigs JB, Jacques PF. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr Res 2013;33:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma B, Lawson AB, Liese AD, Bell RA, Mayer-Davis EJ. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose-dependent association. Am J Epidemiol 2006;164:449–58. [DOI] [PubMed] [Google Scholar]
- 19.van Dam RM, Hunter D. Biochemical indicators of dietary intake In:Willett W. editor. Nutritional epidemiology. 3rd ed. Oxford (United Kingdom): Oxford University Press; 2013. p. 150–212. [Google Scholar]
- 20.Jensen RG. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci 2002;85:295–350. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 22.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 2002;76:750–7. [DOI] [PubMed] [Google Scholar]
- 23.Biong A, Berstad P, Pedersen J. Biomarkers for intake of dairy fat and dairy products. Eur J Lipid Sci Technol 2006;108:827–34. [Google Scholar]
- 24.Brevik A, Veierød MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr 2005;59:1417–22. [DOI] [PubMed] [Google Scholar]
- 25.Smedman AE, Gustafsson IB, Berglund LG, Vessby BO. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr 1999;69:22–9. [DOI] [PubMed] [Google Scholar]
- 26.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr 2001;131:828–33. [DOI] [PubMed] [Google Scholar]
- 27.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 1998;68:291–5. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 2007;86:929–37. [DOI] [PubMed] [Google Scholar]
- 31.Arab L. Biomarkers of fat and fatty acid intake. J Nutr 2003;133(Suppl 3):925S–32S. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, Haffner SM. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liese AD, Nichols M, Hodo D, Mellen PB, Schulz M, Goff DC, D'Agostino RB. Food intake patterns associated with carotid artery atherosclerosis in the Insulin Resistance Atherosclerosis Study. Br J Nutr 2010;103:1471–9. [DOI] [PubMed] [Google Scholar]
- 34.Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF, et al. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–72. [DOI] [PubMed] [Google Scholar]
- 35.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiologic study. Ann Epidemiol 1999;9:314–24. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beard JC. The insulin sensitivity index in nondiabetic man: correlation between clamp-derived and IVGTT-derived values. Diabetes 1986;35:362–9. [DOI] [PubMed] [Google Scholar]
- 38.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–22. [DOI] [PubMed] [Google Scholar]
- 39.Bergman RN. Minimal model: perspective from 2005. Horm Res 2005;64(Suppl 3):8–15. [DOI] [PubMed] [Google Scholar]
- 40.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 2002;43:1809–17. [DOI] [PubMed] [Google Scholar]
- 41.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 42.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2008;18:503–10. [DOI] [PubMed] [Google Scholar]
- 43.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am J Clin Nutr 2011;93:127–42. [DOI] [PubMed] [Google Scholar]
- 44.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. Am J Clin Nutr 2010;92:1214–22. [DOI] [PubMed] [Google Scholar]
- 45.Kratz M, Marcovina S, Nelson JE, Yeh MM, Kowdley KV, Callahan HS, Song X, Di C, Utzschneider KM. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not β-cell function in humans. Am J Clin Nutr 2014;99:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro-Webb N, Ruiz-Narváez EA, Campos H. Cross-sectional study of conjugated linoleic acid in adipose tissue and risk of diabetes. Am J Clin Nutr 2012;96:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol 2011;48:257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid–mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr 2009;139:1–4. [DOI] [PubMed] [Google Scholar]
- 49.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr 2010;91:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unnevehr L, Jagmanaite E. Getting rid of trans fats in the US diet: Policies, incentives and progress. Food Policy 2008;33:497–503. [Google Scholar]