Abstract
Background: Biofortification is a strategy to relieve vitamin A (VA) deficiency. Biofortified maize contains enhanced provitamin A concentrations and has been bioefficacious in animal and small human studies.
Objective: The study sought to determine changes in total body reserves (TBRs) of vitamin A with consumption of biofortified maize.
Design: A randomized, placebo-controlled biofortified maize efficacy trial was conducted in 140 rural Zambian children. The paired 13C-retinol isotope dilution test, a sensitive biomarker for VA status, was used to measure TBRs before and after a 90-d intervention. Treatments were white maize with placebo oil (VA−), orange maize with placebo (orange), and white maize with VA in oil [400 μg retinol activity equivalents (RAEs) in 214 μL daily] (VA+).
Results: In total, 133 children completed the trial and were analyzed for TBRs (n = 44 or 45/group). Change in TBR residuals were not normally distributed (P < 0.0001); median changes (95% CI) were as follows: VA−, 13 (−19, 44) μmol; orange, 84 (21, 146) μmol; and VA+, 98 (24, 171) μmol. Nonparametric analysis showed no statistical difference between VA+ and orange (P = 0.34); both were higher than VA− (P = 0.0034). Median (95% CI) calculated liver reserves at baseline were 1.04 (0.97, 1.12) μmol/g liver, with 59% >1 μmol/g, the subtoxicity cutoff; none were <0.1 μmol/g, the deficiency cutoff. The calculated bioconversion factor was 10.4 μg β-carotene equivalents/1 μg retinol by using the middle 3 quintiles of change in TBRs from each group. Serum retinol did not change in response to intervention (P = 0.16) but was reduced with elevated C-reactive protein (P = 0.0029) and α-1-acid glycoprotein (P = 0.0023) at baseline.
Conclusions: β-Carotene from maize was efficacious when consumed as a staple food in this population and could avoid the potential for hypervitaminosis A that was observed with the use of preformed VA from supplementation and fortification. Use of more sensitive methods other than serum retinol alone, such as isotope dilution, is required to accurately assess VA status, evaluate interventions, and investigate the interaction of VA status and infection. This trial was registered at clinicaltrials.gov as NCT01814891.
Keywords: 13C-retinol dilution, Zambia, biofortified maize, plant carotenoids, retinol
INTRODUCTION
Ensuring optimal vitamin A (VA)7 status is an issue of global health concern. Latest WHO reports estimate that 190 million preschool-aged children and 19.1 million pregnant women are VA deficient based on serum retinol (SR) concentrations <0.7 μmol/L (1). Vitamin A deficiency (VAD) can lead to blindness, anemia, weakened resistance to infection, and increased risk of death (1). Current strategies to alleviate VAD are high-dose supplementation and low-dose fortification with preformed VA (1). Zambia has adopted these strategies with high levels of coverage for supplements (89%, 2007–2011) (2) and fortified sugar (59–99%) (3–5). Sugar in Zambia had a median VA concentration of 8.8 μg/g (range: 0.5–54.9) (6), and daily intake in children was estimated as 9–15 g/d (6, 7), yielding intakes of 77–132 μg preformed retinol/d. Absorption of preformed VA is very efficient; therefore, the potential for hypervitaminosis exists at consistent intakes above requirements (8).
Biofortification of staple crops with provitamin A carotenoids is an alternate method to promote optimal VA status. This approach is sustainable, cost-effective, and potentially wide-reaching (9). Furthermore, negative feedback mechanisms of β-carotene absorption and bioconversion to VA mitigate hypervitaminosis issues associated with preformed VA (10, 11). Provitamin A carotenoid sources are efficacious in humans (12–14). Biofortified orange maize has shown bioefficacy in maintaining VA liver reserves in Mongolian gerbils (15) and in providing VA in single meals to humans (16, 17).
Determining the VA status of an individual or the response to an intervention is difficult (18). Liver VA concentrations are the gold standard because the liver is the primary storage site for VA (19), but these measures are difficult in the field or unethical to directly obtain from humans. SR concentrations are homeostatically controlled over a wide range of physiologic conditions, responding only to severe deficiency or toxicity (20). Isotope dilution is the most sensitive, indirect measure of VA status across a broad range of VA status (18). The deuterated retinol dilution (DRD) technique requires a high dose of isotope (∼17.5 μmol), gives an accurate measure of liver stores (21, 22), and has been used before and after provitamin A, food-based interventions to determine efficacy (13, 14, 23).
13C-Retinol isotope dilution (13C-RID), which has been validated against liver reserves in rats (24) and monkeys (25), as well as correlated with long-term dietary intakes of U.S. women (26), has demonstrated a response to a preformed VA intervention in Thai schoolchildren (27). Because the carbon (not hydrogen) is isotopically labeled, retinol may be analyzed by using gas chromatography–combustion isotope ratio mass spectrometry (GC-CIRMS), which is more sensitive than the gas chromatography–mass spectrometry used for the DRD test (28). This allows much smaller isotope doses to be used, allowing the isotope to act like a true tracer (28).
The focus of this community-based, randomized controlled trial in Zambian preschool children was to evaluate the VA efficacy of orange maize. The 13C-RID test was used to quantitatively determine VA total body reserves (TBRs) and liver concentrations at baseline and after orange maize consumption in comparison with negative and positive control groups.
SUBJECTS AND METHODS
Subjects
All procedures involving human subjects were approved by the Tropical Diseases Research Centre's Ethics Review Committee in Zambia and University of Wisconsin (UW)–Madison's Health Sciences Human Subjects Institutional Review Board. This trial was registered with clinicaltrials.gov as NCT01814891. Written informed consent was obtained from parents or caregivers. The trial was conducted in 2012 in Nyimba District of the Eastern Province of Zambia in preschool children (n = 143 initial enrollment, aged 71.5 ± 6.9 mo) because of the high prevalence of low SR concentrations in a prior survey (6). Nyimba District was also selected because local communities expressed interest in participating following community sensitization programs related to orange maize performed by the National Food and Nutrition Commission (Lusaka, Zambia) and a prior efficacy study orchestrated by UW-Madison (29) in previous years. Four feeding sites were chosen: 2 sites adjacent to the main roadway and 2 sites about 8 km off the paved road.
Inclusion criteria were relatively healthy children aged 5–7 y living in the study area with weight-for-age and weight-for-height z scores >−3, hemoglobin >70 g/L, no clinical infection at recruitment causing fever, antihelminthic treatment the week before recruitment, and not having received a 200,000 IU VA supplement in the past 6 mo.
Assuming a 16-kg child with a 480-g liver (3% body weight), sample size was calculated by using a theoretical midpoint change of 1125 μg in the orange group's liver stores based on an estimated 15 μg β-carotene/g maize, 75% retention of β-carotene through preparation, 200-g daily maize intake, and a midpoint between the 6 and 12 μg β-carotene/1 μg retinol bioconversion factors (16, 17). In this calculation, daily μg retinol activity equivalent (RAE) intake from the maize was corrected for the estimated average requirement of 275 μg RAEs, which was assumed to be used. Using this difference from a similar child consuming white maize (assuming no change in liver stores), a sample size of 45 would allow an SD of 1.5 times the difference; α was set at 0.05, and power was 0.90. This was increased to a target of 50/group to allow for loss to follow-up.
Biofortified orange maize
The biofortified orange maize for the test group was developed at the International Maize and Wheat Improvement Center as part of its HarvestPlus biofortified maize research project (30). The orange maize variety was created specifically to enable efficacy studies and was formed by making all possible crosses and mixing the resultant seed by using 5 maize lines bred for high provitamin A concentration (17–24 μg/g, predominately β-carotene). Grain of this orange maize variety was produced on a commercial farm in Zambia during November to April immediately preceding the trial. After harvest, the grain was analyzed and had ∼18 μg β-carotene equivalents (βCEs)/g at the time it was placed into a large freezer container in Lusaka. Every 2 weeks, maize (250 kg) was delivered to the study site.
Study design
The paired 13C-RID test was used, meaning each child gave 4 blood samples (i.e., 2 blood draws for each assessment) and served as his or her own control for response to the intervention (28). As illustrated in Figure 1, the study began with a baseline assessment for VA TBRs and other variables of interest, followed by the intervention, a washout period, and a similar endline assessment. The 13C-RID test was administered as follows: after an overnight fast and venous blood draw, 1 μmol 13C2-retinyl acetate in soybean oil (180 μL) was delivered directly into the child's mouth with a positive displacement pipette. The dose was followed with 1.0 mL oil and a high-fat snack (bread with ∼16 g peanut butter) to maximize absorption. After an isotope mixing period of 14 d, another overnight fasting venous blood sample was obtained. During mixing and washout (7-d) periods, all subjects ate white maize and were not supplemented. Treatment groups consisted of a negative control group (VA−, n = 47), who ate white maize and received daily placebo oil; the test group (orange, n = 46), who ate orange maize and received daily placebo oil; and a positive control group (VA+, n = 47), who ate white maize and received daily VA [retinyl palmitate, 400 μg RAEs (the current U.S. Recommended Dietary Allowance for children this age)] in oil. Maize dishes consisted of porridge for breakfast (prepared with maize, water, sugar, salt, and milk or groundnuts; ∼17% maize by weight) and nshima for lunch and dinner (stiff porridge, prepared with maize and water; ∼30% maize by weight). White or orange maize was used for all dishes according to treatment group and was the only difference in the menu among the groups. Unfortified industrial-grade sugar was purchased from a processing company to use in porridge preparation.
FIGURE 1.

Study design. Baseline assessment comprised blood draw 1, isotope dose 1, the 14-d mixing period, and blood draw 2. This was followed by 90 d of intervention and a 7-d washout period. The endline assessment procedure was repeated identically to baseline and comprised blood draw 3, isotope dose 2, the 14-d mixing period, and blood draw 4. Sample size: n = 44, 44, and 45 for VA−, orange, and VA+ groups, respectively. White maize VA– = received placebo oil (214 μL) daily during treatment period and was fed white maize throughout (white shading). Orange maize VA– = received placebo oil (214 μL) daily and orange maize during the treatment period and white maize during mixing and washout periods (light gray shading). White maize VA+ = received VA in oil (400 μg retinol activity equivalents/d in 214 μL) during the treatment period and was fed white maize throughout (dark gray shading). VA, vitamin A.
Labeled retinyl acetate was synthesized by using published procedures (31). Purity, assessed by ultraviolet-Vis spectrophotometry and thin-layer chromatography, was confirmed with HPLC to be ∼99%. The dose was dissolved in soybean oil, purged with nitrogen, and stored frozen in amber vials until use in the field.
Randomization and masking
Once an adequate baseline blood sample was obtained (∼3 mL), children were randomly assigned within a site by picking a card with a colored sticker corresponding to their treatment group from opaque envelopes, and an identification number was assigned. Oil doses (214 μL), identical in appearance, were given during the treatment period only, measured with a positive displacement pipette onto a serving spoon, and administered immediately preceding the lunch meal. Children ate breakfast, lunch, and dinner on site 6 d/wk, and all food intake was measured. The diet was designed to be low in VA (29 μg RAEs/d); menu, nutrient content, and off-site intakes are reported elsewhere (32). Children eating orange maize ate in a separate room to prevent sharing.
In the laboratory, technicians were blinded to treatment groups. All gas chromatograms were integrated by a technician who was not involved in the conduct of the fieldwork.
Food carotenoid analysis
Food samples were analyzed with a modified published procedure (33). Modifications included using 500 μL aqueous potassium hydroxide (50:50), resuspending samples in 150 μL (50:50 methanol/dichloroethane), and injecting 50 μL on the HPLC system for nshima and porridge analysis. For nshima analysis, saponification was carried out at room temperature for 45 min.
13C atom percentage and concentration of serum retinol
Blood collection was performed by the Tropical Diseases Research Centre. Blood (target 7 mL) was collected and centrifuged, and serum was poured into 2 tubes, transported in nitrogen vapor, shipped on dry ice, and stored at −80°C until analysis at UW-Madison.
Serum analysis was a modified published procedure (34). Briefly, serum was thawed at room temperature. To 1.5 mL (or all available) serum, ethanol (1 × vol) was added to precipitate proteins, C23 β-apo-carotenol was added as an internal standard, and 3 × 1–mL hexane extractions were performed. Hexane layers were pooled, dried under nitrogen, resuspended in 100 μL methanol, frozen at −80°C for ∼5 min, centrifuged briefly, and injected onto HPLC system 1 for quantification and purification. System 1 contained a Gracesmart C18 (5-μm, 4.6 × 250–mm) column with 92:8 acetonitrile/water at 1.0 mL/min. The retinol fraction was collected, dried under nitrogen, and resuspended in 100 μL methanol for injection onto system 2. System 2 contained an identical column with 100% methanol at 0.7 mL/min. The retinol fraction was collected, dried under vacuum with a SPD1010 SpeedVac (Thermo Scientific), and resuspended in 4–6 μL hexane, and 1–3 μL was injected (to inject approximately 75 ng) on the gas chromatograph. The GC-CIRMS system was published (34). Atom percentage (At%) was directly calculated (Isodat version 2.0; Thermo Scientific) in reference to carbon dioxide, which was calibrated against a sucrose standard (National Institute of Standards and Technology, 8542).
Other biochemical analyses
Serum C-reactive protein (CRP), α-1-acid glycoprotein (AGP), ferritin, and zinc were analyzed to adjust or confirm assumptions. CRP, AGP, and ferritin were analyzed by using ELISA kits (CRP: Cayman Chemical Company; AGP: Abcam; ferritin: Phoenix Pharmaceuticals Inc.). Zinc analyses were performed by UW-Madison Soil Testing Laboratories by using inductively coupled plasma optical emission spectrometry.
Calculation of total body reserves
Vitamin A TBRs were calculated by using the mass-balance equation (35), which balances 13C before and after the dose:
 |
where a is μmol absorbed from the dose (dose × absorption rate), b is TBR in μmol at baseline, and c is TBR in μmol after dosing (c = a + b). Fa, Fb, and Fc are the isotope abundance [13C/total C; At%/100; R/(R + 1), with R being 13C/12C] of the dose, baseline serum, and postdose serum, respectively.
Absorption of a 5-mg dose was 81.2% in 3 children without illness (36), but a 1-mg dose had a higher absorption of 99.2% in 5 children (37). We assumed absorption to be 90% for most children to account for underlying repeated infections, potential micronutrient codeficiencies, and the smaller dose size (0.288 mg), which appears to be absorbed more completely. For children with elevated CRP (>10 mg/L) (38–40) at the time of dosing, absorption was reduced further to 80% (37).
Fa was 0.11 [(13C2 labeled in synthesis + natural abundance 13C)/20 C total], with the equation solved for b, TBR at baseline. Total body reserves were corrected for catabolism and replacement during the mixing period by the following equation:
 |
where k = ln(2)/(half-life of retinol in days), and t is time in days since dosing.
Half-life of VA was calculated from the decay of the μmol tracer of the VA− group from blood draws 2–3 for those subjects having <50 μmol change in TBR (41). These constraints help satisfy assumptions regarding isotopic kinetics (41).
Liver concentrations were calculated by assuming a liver weight of 3% body weight (8, 39, 42). The factor for dose retained in the liver was originally assumed to be 0.5 for a population with a high prevalence of deficiency (24, 42), but after determining the initial TBR results, this assumption was changed to 0.8 to account for the actual VA status of this population, because it is well accepted that the percentage of VA in the liver increases as total body VA stores increase (24, 25, 42).
Statistical analysis and calculation of bioconversion factor
Values are reported as means ± SDs, medians (95% CIs), or percentages. Data were analyzed by using the General Linear Model procedure in the Statistical Analysis System (SAS Institute, version 9.2). Normality of residuals was assessed by the Shapiro-Wilk test. For data with normally distributed residuals, outcomes of interest were evaluated by using 1- or 2-way ANOVA, and differences among treatment groups were determined by using least significant difference tests. For data with nonnormally distributed residuals, nonparametric analysis was carried out on ranked data. Proportions were compared by using χ2 analysis. For calculation of the bioconversion factor, the middle 3 quintiles of change in TBRs from each treatment group were used. The mean change of the VA− group was subtracted from orange and VA+ groups, and μg β-carotene/1 μg retinol from the orange maize was calculated in reference to the VA+ group by the following formula (23):
 |
Significance was P < 0.05. Growth standard z scores were calculated by using WHO macros for child growth standards and run by using R (version 3.0.2; R Core Team) (43).
RESULTS
Subject characteristics
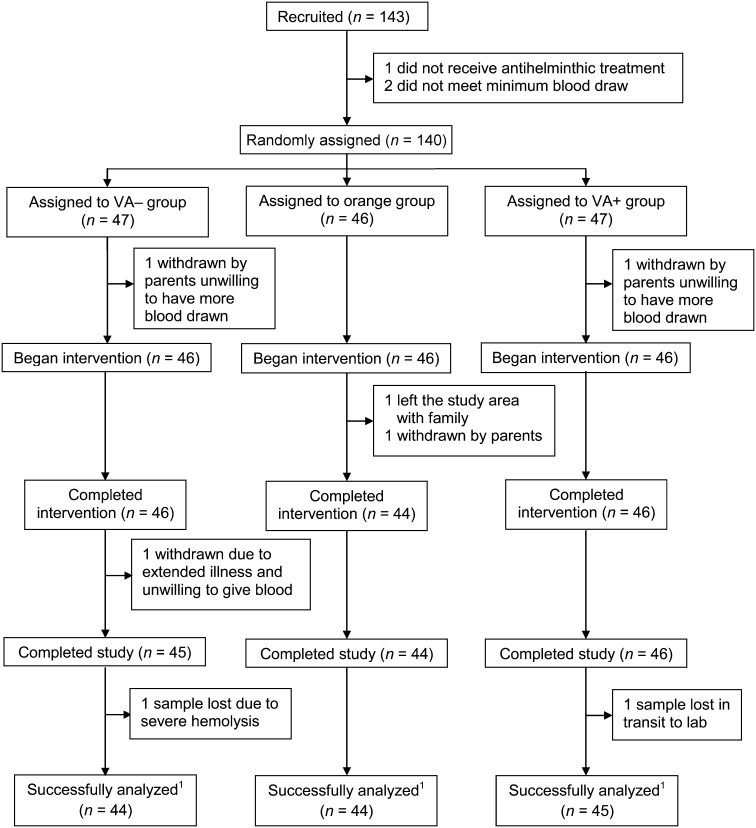
The intervention began in May and went through October 2012. Children (n = 143) were recruited and consented, and 140 met baseline inclusion criteria. Baseline anthropometric data did not differ among groups (Table 1). One hundred thirty-five children completed the trial, and 133 sample sets were successfully analyzed; reasons for dropouts and losses are outlined in Figure 2. At baseline, 8 children (6%) were severely stunted (height-for-age z score <−3), whereas 39 (28%) were moderately stunted (height-for-age z score <−2); 25 (18%) children were undernourished (weight-for-age z score <−2), while none were severely undernourished (weight-for-age z score <−3).
TABLE 1.
Baseline anthropometric data for Zambian children (n = 140) enrolled in a bioefficacy trial investigating biofortified orange maize by randomized treatment groups1
| VA− (n = 47) | Orange (n = 46) | VA+ (n = 47) | P value2 | |
| Age,3 mo | 69.0 (65.2, 72.8)4 | 69.0 (66.5, 71.5) | 71.0 (67.7, 74.3) | 0.99 |
| Weight,3 kg | 17.1 (15.9, 18.3) | 17.4 (16.4, 18.3) | 16.8 (16.3, 17.3) | 0.89 |
| Height, cm | 107.3 ± 6.35 | 108.1 ± 5.3 | 107.4 ± 4.7 | 0.75 |
| Hemoglobin, g/L | 114 ± 13 | 117 ± 12 | 118 ± 9 | 0.23 |
| Sex, % male | 51.1 | 47.8 | 61.7 | 0.67 |
| Malaria, % | 17.0 | 8.7 | 8.5 | 0.35 |
| BMI,3 kg/m2 | 14.9 (14.6, 15.2) | 14.7 (14.5, 14.9) | 14.6 (14.4, 14.8) | 0.34 |
| Height-for-age z score | −1.53 ± 1.18 | −1.40 ± 0.91 | −1.53 ± 0.88 | 0.76 |
| Weight-for-age z score | −1.18 ± 0.93 | −1.17 ± 0.79 | −1.31 ± 0.81 | 0.64 |
| BMI-for-age z score3 | −0.33 (−0.57, −0.09) | −0.48 (−0.67, −0.29) | −0.52 (−0.69, −0.35) | 0.33 |
Baseline data were taken during recruitment day, which included the baseline blood draw 1 for 13C-natural abundance. The VA– group received white maize with placebo oil, the orange group received orange maize with placebo oil, and the VA+ group received white maize with VA in oil (400 μg retinol activity equivalents/d in 214 μL). VA, vitamin A.
P values were determined by testing the null hypothesis that each variable is equal among treatment groups by using ANOVA or χ2 test.
Indicates nonnormally distributed residuals; P value reflects nonparametric analysis.
Median; 95% CI in parentheses (all such values).
Mean ± SD (all such values).
FIGURE 2.
Trial profile for a randomized, controlled efficacy study that fed orange maize to Zambian children for 90 d compared with positive and negative controls. VA− group received white maize throughout and placebo oil (214 μL) daily during the treatment period. Orange group received white maize during mixing/washout periods and orange maize and placebo oil (214 μL) daily during the treatment period. VA+ group received white maize throughout and VA in oil (400 μg retinol activity equivalents/d in 214 μL) during the treatment period. 1All 4 blood draws for the subject were analyzed for 13C-retinol content with adequate signal. VA, vitamin A.
Food carotenoid analysis
The nshima, porridge, and dry maize in the orange maize group contained 4.09 ± 0.69, 2.60 ± 0.36, and 17.9 ± 1.14 μg βCEs/g, respectively. Nshima content did not differ by site (P = 0.45) or time (P = 0.80), and porridge content did not differ by recipe (P = 0.54), site (P = 0.096), or time (P = 0.056). Retention of provitamin A carotenoids from ground maize to cooked nshima was ∼78%. Dry white maize contained 0.089 ± 0.050 μg βCEs/g but was not detectable in nshima or porridge in the white maize group (limit of detection: 0.005 μg βCEs/g).
Dietary intakes
Dietary intakes for the trial are published (32). Briefly, total food intake among groups did not differ, but the orange maize group ate less nshima (276 ± 36 compared with 288 ± 26 g/serving, P = 0.008) because this maize takes up less water and is more nutrient dense [0.28 ± 0.02 (orange) compared with 0.26 ± 0.02 (white) g dry weight/g nshima, P = 0.0085] than the white maize after cooking. Meal compliance was 98.4% and similar among groups. The orange maize group consumed 49,000 g nshima per child (4.09 μg βCEs/g) and 22,000 g porridge per child (2.60 μg βCEs/g) during the trial. This equates to 257.6 mg βCEs or 2.86 mg βCEs/d per child. VA dosing compliance was 98.7% in the VA+ group, for a mean VA dose intake of 35.53 mg or 394.8 μg RAEs/d per child.
Total body reserves, liver concentration, and bioconversion factor
The calculated half-life of retinol for a subset of the children in the VA− group (n = 19) was 136 ± 33 d. The TBR and liver VA concentrations at baseline and endline, as well as their respective changes, were calculated (Table 2). Change in TBR residuals was not normally distributed (P < 0.0001); median (95% CI) changes were as follows: VA−: 13 (−19, 44); orange: 84 (21, 146); and VA+: 98 (24, 171) μmol; nonparametric analysis showed that there were no statistical differences between VA+ and orange groups (P = 0.34), but both were higher than the VA− group (Figure 3; P = 0.0034). Median (95% CI) calculated liver reserves for all subjects at baseline were 1.04 (0.97, 1.12) μmol/g liver, with 59% >1 μmol/g, the current subtoxicity cutoff. None were <0.1 μmol/g, a more recently suggested, higher than traditional, deficiency cutoff (18).
TABLE 2.
Primary and secondary measures of status by treatment group in Zambian children1
| VA− | Orange | VA+ | P value2 | |
| Total body reserves of retinol,3 μmol | ||||
| Baseline4 | 686 (567, 805)5 [44] | 685 (590, 779) [44] | 723 (630, 816) [45] | 0.35 |
| Endline4 | 665 (560, 769)b [44] | 806 (664, 947)a [44] | 811 (679, 944)a [45] | 0.0040 |
| Change4 | 13 (−19, 44)b [44] | 84 (21, 146)a [44] | 98 (24, 171)a [45] | 0.0034 |
| Liver retinol concentration,3 μmol/g | ||||
| Baseline4 | 1.02 (0.83, 1.22) [44] | 1.04 (0.94, 1.15) [44] | 1.11 (0.97, 1.25) [45] | 0.29 |
| Endline4 | 0.96 (0.83, 1.10)b [44] | 1.09 (0.90, 1.28)a [44] | 1.17 (0.98, 1.35)a [45] | 0.0042 |
| Change4 | −0.04 (−0.10, 0.02)b [44] | 0.06 (−0.07, 0.19)a [44] | 0.11 (0.00, 0.22)a [45] | 0.0055 |
| Serum retinol concentration,6 μmol/L | ||||
| Baseline | 0.99 ± 0.29 [42]7 | 0.96 ± 0.27 [43] | 0.97 ± 0.25 [43] | 0.83 |
| Endline4 | 0.97 (0.86, 1.09) [43] | 0.94 (0.83, 1.06) [43] | 0.97 (0.90, 1.05) [43] | 0.39 |
| Change | 0.042 ± 0.26 [41] | 0.065 ± 0.25 [43] | −0.030 ± 0.21 [43] | 0.16 |
| Prevalence of low serum retinol (% <0.7 μmol/L)6 | ||||
| Baseline | 16.7 [42] | 16.3 [43] | 18.6 [43] | 0.97 |
| Endline | 7.0 [43] | 4.7 [43] | 14.0 [43] | 0.33 |
| Prevalence of elevated C-reactive protein (% >10 mg/L)6 | ||||
| Baseline | 19.4 [36] | 17.1 [41] | 17.5 [40] | 1.00 |
| Endline | 9.8 [41] | 4.7 [43] | 8.1 [37] | 0.71 |
| Prevalence of elevated α-1-acid glycoprotein (% >1.2 g/L)6 | ||||
| Baseline | 95.0 [40] | 88.4 [43] | 97.7 [43] | 0.94 |
| Endline | 82.9 [41] | 68.3 [41] | 76.2 [42] | 0.75 |
| Prevalence of low serum ferritin (% <12 μg/L)6 | ||||
| Baseline | 17.5 [40] | 14.0 [43] | 9.3 [43] | 0.63 |
| Serum zinc,8 μg/L | ||||
| Endline4 | 915 (811, 1018) [40] | 930 (843, 1017) [38] | 916 (849, 983) [41] | 0.82 |
| Hemoglobin,6 g/L | ||||
| Endline4 | 117 (113, 121) [44] | 117 (113, 120) [44] | 120 (117, 123) [45] | 0.88 |
| Anthropometric changes6 | ||||
| Weight change, kg | 1.1 ± 0.7 [44] | 1.2 ± 0.6 [44] | 0.9 ± 0.6 [45] | 0.075 |
| Height change,4 cm | 3.1 (2.9, 3.2) [44] | 3.1 (2.7, 3.4) [44] | 3.0 (2.8, 3.2) [45] | 0.62 |
The number of participants analyzed is in brackets. Treatment groups with uncommon superscript lowercase letters are statistically different; a > b. The VA− group received white maize with placebo oil, the orange group received orange maize with placebo oil, and the VA+ group received white maize with VA in oil (400 μg retinol activity equivalents/d in 214 μL). VA, vitamin A.
P values were determined by testing the null hypothesis that each variable is equal among treatment groups by using ANOVA or χ2 test.
Baseline is calculated from blood draws 1 and 2, endline is calculated from blood draws 3 and 4, and change is the difference between endline and baseline.
Indicates nonnormally distributed residuals; P value reflects nonparametric analysis.
Median; 95% CI in parentheses (all such values).
Baseline measurements were taken during blood draw 1, endline measurements were taken during blood draw 3, and change is the difference between endline and baseline.
Mean ± SD (all such values).
Measurement taken at blood draw 4.
FIGURE 3.
A: Schematic boxplot (SAS Institute, version 9.2) of change in TBRs of VA (in μmol) by treatment group. Line is the median; box is first through third quartiles; whiskers are to the most extreme point within fences; lower and upper fences are first or third quartile plus or minus 1.5 times the interquartile range, respectively; and points outside the fences are represented individually. Treatment groups with uncommon lowercase letters are statistically different (nonparametric analysis). Overall ANOVA P = 0.0034. Sample size: n = 44, 44, and 45 for VA−, orange, and VA+ groups, respectively. VA− group received white maize with placebo oil (white shading). Orange group received orange maize with placebo oil (light gray shading). VA+ group received white maize with VA in oil (400 μg retinol activity equivalents/d in 214 μL) (dark gray shading). B: Individual raw data ranked by treatment group. TBR, total body reserve; VA, vitamin A.
The bioconversion factor for the provitamin A carotenoids from the orange maize was estimated to be 10.4 μg βCEs/1 μg retinol. Retrospective power analysis revealed that for change in TBRs, we had a power of 0.76 to detect a difference between the VA− and orange groups and a power of 0.95 to detect a change between the VA− and VA+ groups.
Serum retinol and morbidity biomarkers
Change in SR showed no treatment effect (Table 2, P = 0.16) and was not correlated to change in TBRs (r2 = 0.0016, P = 0.80). At baseline, 22 children (17%) had concentrations <0.7 μmol/L. Comparing all 4 time points, SR did not show a treatment (P = 0.12) or a treatment-by-time interaction (P = 0.82) but had a time effect (P < 0.0001) and increased from the first to second blood draw (0.95 ± 0.27–1.08 ± 0.29 μmol/L, P = 0.0038) while all subjects were on a low VA diet during the isotope mixing period.
Subjects with an elevated CRP at baseline (n = 21) had a significantly lower SR than did those with a normal CRP (n = 94) (0.83 ± 0.28 compared with 1.02 ± 0.25, P = 0.0029) and a higher prevalence of SR <0.7 μmol/L (33% compared with 10%, P = 0.0053). Subjects with a positive malaria smear at baseline (n = 16) had a significantly higher prevalence of elevated CRP than did subjects with a negative smear (56% compared with 12%, P = 0.0002). Subjects with an elevated AGP at baseline (n = 110) had a significantly lower SR than did those with a normal AGP (n = 8) (0.96 ± 0.26 compared with 1.26 ± 0.26, P = 0.0023) and a higher prevalence of SR <0.7 μmol/L (17.3% compared with 0%, P < 0.0001). CRP and AGP were positively and significantly correlated (r2 = 0.32, P < 0.0001).
DISCUSSION
Orange maize was efficacious in Zambian children, as determined by the 13C-RID test in the presence of high liver stores, with 59% having concentrations >1.0 μmol/g liver at baseline. While the orange maize was expected to be efficacious, the absence of VAD and high liver stores were unexpected findings given 2009 VAD estimates of 56.4% with SR <0.7 μmol/L (unadjusted for infection) or 22% with modified relative dose-response tests >0.06 in younger Zambian children aged 2–5 y (6). However, children aged 6–59 mo are given high-dose supplements semiannually, sugar has been fortified since 1998 with more complete coverage in recent years, and dietary intake of VA was adequate for 99.6% of children aged 2–5 y in the prior survey (6); all 3 sources allow TBR to build over time. VA status is reported as TBR (μmol or mmol), which relies on fewer assumptions, and liver concentration (μmol/g) to compare with published values. Validation against liver reserves is on a concentration basis and accounts for body weight. Baseline TBRs in mmol (0.73) were much higher than those in Filipino children (0.08–0.09) (13), Chinese children (0.09–0.13 and 0.27) (14, 44), Mexican children (0.17) (45), and Nicaraguan children before sugar fortification (0.39) (8).
Although the isotope dilution test is the most sensitive biomarker of VA status, it relies on assumptions that must be carefully considered for the population and the specific test used. It is important to note the distinction between isotope dilution methods. Methods using 13C combined with GC-CIRMS are more sensitive than deuterium or 13C on gas chromatography–mass spectrometry, allowing for smaller isotope doses to give an adequate analytic signal (1 compared with 17.5 μmol) (28). This is important for considering the fraction of dose absorbed and the specific activity ratio of serum/liver.
Similar absorption (∼80%) was noted for 5- and 60-mg VA doses in Zambian children (36), a 1-mg dose was almost completely absorbed (99.2%) in the absence of acute infection in Indian children (37), and a 1-mg dose was completely absorbed in monkeys (25). Although there was relatively high AGP, children in this study were absorbing the daily VA doses well (∼70% retention), as indicated by the increase in TBR in the VA+ group. Together, these data justify 90% absorption of the dose or 80% absorption in the children with elevated CRP and no fever on the day of dosing. Furthermore, we administered antihelminthic treatment before beginning the study to optimize the validity of the 13C-RID test and the intervention. If a population has a high intestinal parasitic load, assumptions used in the isotope dilution equation and efficacy of orange maize may differ.
A factor of 0.65 is used to correct for differences in isotope enrichment between serum and liver with larger deuterated doses (21), based on radioactivity studies in rats (46). This ratio in humans was 0.80 with large deuterated doses (∼30 mg), but the DRD equation has not been updated (22). This ratio was omitted in our equation because it was ∼1 in rats (24) and monkeys (25) when smaller doses of 13C and GC-CIRMS were used and blood samples were taken fasting. The ratio of 1 is likely because of the physiologic nature of small doses along with a low VA diet during the mixing period. The correction for catabolism of VA during the mixing period assumes first-order elimination of VA (21, 41). The dose decay, after mixing with the body pool, was measured from 14 to 130 d. The calculated half-life (136 d) is close to that in adults (154 d) (42, 47) but longer than that in younger children aged 1–2 y (32 d) (39). Our children are older, are free of severe infection, and have large stores of VA, and the dose was followed for a longer time.
This study was powered based on change in TBR, and SR did not respond even though TBR showed a treatment effect. Change in TBR by using isotope dilution coupled with no response in SR has been observed frequently (13, 23, 27). Nonsignificant differences in SR with and without VA supplementation have also been observed (40). This is because SR is homeostatically controlled over a broad range of liver reserves (18, 28). Our baseline assessment of TBR showed no correlation to SR (R2 = 0.018, P = 0.090), and SR predicted a 17% VAD prevalence: a moderate public health concern. SR from blood draw 4 misclassified 22% as VA deficient, indicating a severe public health concern. Conversely, in a recent study using 13C-RID in Thai children with SR >0.7 μmol/L, 64% were deficient based on liver concentrations <0.1 μmol/g (27). A high prevalence of elevated AGP in our study was positively and significantly correlated with CRP (indicating chronic infection) (48), which is correlated with a reduction in SR (29). Misclassification by SR may be a result of this chronic infection. Together, these data demand further use of stable isotope methods to more accurately assess VA status, evaluate VA interventions, and investigate the interaction of VA status, infection, and SR.
The unexpected finding of high liver stores in these Zambian children raises concern for implementing multiple interventions to improve VA status in the same groups. Supplementation and fortification both use preformed VA, which can lead to hypervitaminosis A because it is efficiently absorbed and stored (8, 9). When multiple interventions are in place, as in our study population, VA intakes can easily exceed requirements, and stores can build over time, especially in the background of adequate VA in the diet. This could just be limited to this age group in Zambia (due to just finishing a 4.5-y VA supplementation, having fortified sugar available their entire lives, and their mothers consuming fortified sugar during pregnancy and lactation). However, when supplemented at just the amount of the U.S. Recommended Dietary Allowance (400 μg RAEs/d) for 90 d, the VA+ group stored ∼70% of these doses, reaching concentrations further above the subtoxicity cutoff (increasing from 1.18 to 1.44 μmol/g); we should note, however, that daily VA supplementation may overestimate response in liver store values as seen with the DRD test (49).
Recently, a fortified rice intervention using preformed VA increased liver reserves from 0.10 to 0.17 μmol/g in just 50 d (27). In that study, 75–80% of the additional VA intake from the fortified rice grains (890 μg RAEs/d) was stored in the liver. Similarly, evaluation of Nicaraguan sugar fortification demonstrated that liver reserves rose drastically, with mean liver concentrations increasing from 0.57 to 1.20 μmol/g after only 1 y of implementation (8). TBRs of these Nicaraguan children after fortification (0.93 mmol) were identical to our VA+ dosed group (0.95 mmol). These data, along with the current study, suggest that consumption of VA above required levels can lead to an increase in TBR in a short period.
Although provitamin A carotenoids are an ideal choice for meeting optimal VA status because of their efficacy and regulation of absorption and bioconversion, the orange maize group continued to absorb and convert β-carotene to VA, increasing liver stores, even though their liver reserves were also >1.0 μmol/g. This may be explained by the “last in–first out” hypothesis of VA metabolism—that is, recently ingested VA will be preferentially secreted into circulation for use by tissues, whereas stores are maintained in the liver for a future period of low VA intake (46, 50). Further elucidation of VA stores with long-term consumption of provitamin A carotenoids should be investigated. β-Carotene is regarded as safe, even at doses of 180 mg/d. Carotenodermia is a adverse effect with high consumption (>30 mg/d), but βC is not carcinogenic, mutagenic, embryotoxic, or teratogenic and does not appear to cause hypervitaminosis A (when assessed for clinical symptoms or by SR) (51, 52). It is unclear whether β-carotene could lead to high VA stores over time, as our children were also consuming preformed VA from study foods (e.g., small dried fish) and off-site intake. Regulation presumably occurred because the bioconversion factor obtained (10.4 μg βCEs/1 μg retinol) is higher than other estimates from maize in humans from single test meals [i.e., 3.2 (16) and 6.5 (17)].
Our findings using the 13C-RID test demonstrate that orange maize is an efficacious VA source in humans. Contrary to previous thinking, baseline estimates showed no VAD and high liver stores in rural Zambian children. We hypothesize that multiple years of high-dose supplements and sugar fortification on top of an already adequate diet (6) has led to excessive stores in these children. More sensitive measures of VA status other than SR, notably stable isotope methods, should be used for population and intervention evaluation.
Acknowledgments
We thank Christopher Davis for maintaining laboratory equipment, Michael Grahn for doing ELISA analyses, and Peter Crump for statistical consultation. We also thank Fabiana Moura, HarvestPlus, for her valuable and timely advice and support during the preparatory phase of this multi-institutional intervention.
The authors’ responsibilities were as follows—BG: produced the first draft of the manuscript, involved in orchestration of the fieldwork, and analyzed all samples on the GC-CIRMS; CK: coordinated procurement of supplies and communication among partners; SAA: involved in developing field materials, orchestrating fieldwork, and corresponding with the human subjects’ review committee; SS: involved in aspects of fieldwork, especially those related to dietary records; JC: point of contact for the Zambian ethics committee: JC and NK: supervised the team from the Tropical Diseases Research Centre in the collection of blood samples, analysis of hemoglobin and malaria slides, and paperwork related to releasing the serum and food samples from Zambia; MM: hired field staff workers and coordinated supply procurement from Lusaka; KP: developed the biofortified maize for the trial, made seed available for planting in Zambia, and helped oversee its growth; CM: coordinated with the local government for the conduct of the trial, oversaw all National Food and Nutrition Commission staff, and met with SAT to discuss all aspects of the trial; SAT: designed the study as principal investigator, involved in all aspects of this study, including manuscript revision, and is the guarantor of the study; all authors read and approved the final version of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AGP, α-1-acid glycoprotein; At%, atom %; CRP, C-reactive protein; DRD, deuterated retinol dilution; GC-CIRMS, gas chromatography/combustion/isotope ratio mass spectrometry; orange, orange maize with placebo oil; RAE, retinol activity equivalent; SR, serum retinol; TBR, total body reserve; UW, University of Wisconsin; VA, vitamin A; VAD, vitamin A deficiency; VA−, white maize with placebo oil; VA+, white maize with vitamin A in oil (400 retinol activity equivalents in 214 μL daily) 13C-RID, 13C-retinol isotope dilution; βCE, β-carotene equivalent.
REFERENCES
- 1.WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva (Switzerland): World Health Organization; 2009. [Google Scholar]
- 2.Vitamin A supplementation coverage rate (% of children ages 6–59 months): World Development Indicators [Internet]. Washington, DC: The World Bank [cited 2014 May 10]. Available from: http://data.worldbank.org/indicator/SN.ITK.VITA.ZS.
- 3.Fiedler JL, Lividini K, Kabaghe G, Zulu R, Tehinse J, Bermudez O, Jallier V, Guyondet C. Assessing Zambia's industrial fortification options: getting beyond changes in prevalence and cost-effectiveness. Food Nutr Bull 2013;34:501–19. [DOI] [PubMed] [Google Scholar]
- 4.Kafwembe EM. The vitamin A status of Zambian children in a community of vitamin A supplementation and sugar fortification strategies as measured by the modified relative dose response (MRDR) test. Int J Vitam Nutr Res 2009;79:40–7. [DOI] [PubMed] [Google Scholar]
- 5.Clewes C, Kankasa C. Report of the National Survey to evaluate the impact of vitamin A interventions in Zambia in July and November 2003 [Internet]. Lusaka (Zambia); National Food and Nutrition Commission of Zambia. 2003 [cited 2013 Dec 12]. Available from: http://www.micronutrient.org/nutritiontoolkit/ModuleFolders/12.Data_entry_analysis_and_report_writing/Examples/Report_of_survey_in_Zambia_%28Sept2005%29.pdf.
- 6.Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr 2012;15:1688–96. [DOI] [PubMed] [Google Scholar]
- 7.Serlemitsos J, Fusco H. Vitamin A fortification of sugar in Zambia [Internet]. Washington, DC: USAID. 2001 [cited 2014 May 19]. Available from: http://www.a2zproject.org/~a2zorg/pdf/zambiasugar.PDF.
- 8.Ribaya-Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr 2004;80:1291–8. [DOI] [PubMed] [Google Scholar]
- 9.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf 2008;7:373–81. [Google Scholar]
- 10.Ribaya-Mercado JD, Solon FS, Solon MA, Cabal-Barza MA, Perfecto CS, Tang G, Solon JA, Fjeld CR, Russell RM. Bioconversion of plant carotenoids to vitamin A in Filipino school-aged children varies inversely with vitamin A status. Am J Clin Nutr 2000;72:455–65. [DOI] [PubMed] [Google Scholar]
- 11.Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 2013;288:9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang G, Hu Y, Yin S, Wang Y, Dallal GE, Grusak MA, Russell RM. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr 2012;96:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ribaya-Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr 2007;85:1041–9. [DOI] [PubMed] [Google Scholar]
- 14.Tang G, Gu X, Hu S, Xu Q, Qin J, Dolnikowski GG, Fjeld CR, Gao X, Russell RM, Yin S. Green and yellow vegetables can maintain body stores of vitamin A in Chinese children. Am J Clin Nutr 1999;70:1069–76. [DOI] [PubMed] [Google Scholar]
- 15.Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr 2008;100:786–93. [DOI] [PubMed] [Google Scholar]
- 16.Muzhingi T, Gadaga TH, Siwela AH, Grusak MA, Russell RM, Tang G. Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am J Clin Nutr 2011;94:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Nugroho A, Rocheford T, White WS. Vitamin A equivalence of the β-carotene in β-carotene–biofortified maize porridge consumed by women. Am J Clin Nutr 2010;92:1105–12. [DOI] [PubMed] [Google Scholar]
- 18.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94:658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raica N, Scott J, Lowry L, Sauberlich HE. Vitamin A concentration in human tissues collected from five areas in the United States. Am J Clin Nutr 1972;25:291–6. [DOI] [PubMed] [Google Scholar]
- 20.Bausch J, Rietz P. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol 1977;31:99–112. [PubMed] [Google Scholar]
- 21.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen H, III, Jones A, Anderson D, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 22.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 1997;66:67–74. [DOI] [PubMed] [Google Scholar]
- 23.Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, Brown KH. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr 2004;80:705–14. [DOI] [PubMed] [Google Scholar]
- 24.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 25.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009;139:2000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentine AR, Davis CR, Tanumihardjo SA. Vitamin A isotope dilution predicts liver stores in line with long-term vitamin A intake above the current Recommended Dietary Allowance for young adult women. Am J Clin Nutr 2013;98:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014;144:519–24. [DOI] [PubMed] [Google Scholar]
- 28.Haskell M, Ribaya-Mercado J. Vitamin A Tracer Task Force. Handbook on vitamin A tracer dilution methods to assess status and evaluate intervention programs. Washington, DC: HarvestPlus; 2005. [Google Scholar]
- 29.Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 30.Pixley KV, Palacios-Rojas N, Babu R, Mutale R, Surles R, Simpungwe E. Biofortification of maize with provitamin A carotenoids Tanumihardjo SA. editor. Carotenoids in human health New York: Springer Science and Business Media; 2013. p. 271–92. [Google Scholar]
- 31.Tanumihardjo SA. Synthesis of 10, 11, 14, 15-13C4-and 14, 15-13C2-retinyl acetate. J Labelled Comp Radiopharm 2001;44:365–72. [Google Scholar]
- 32.Schmaelzle S, Kaliwile C, Arscott SA, Gannon BM, Masi C, Tanumihardjo SA. Nutrient and nontraditional food intakes by Zambian children in a controlled feeding trial. Food Nutr Bull 2014;35:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howe JA, Tanumihardjo SA. Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J Agric Food Chem 2006;54:7992–7. [DOI] [PubMed] [Google Scholar]
- 34.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C Natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med (Maywood) 2009;234:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman KJ, Brenna JT. High sensitivity tracer detection using high-precision gas chromatography–combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal Chem 1992;64:1088–95. [DOI] [PubMed] [Google Scholar]
- 36.Aklamati EK, Mulenga M, Dueker S, Buchholz B, Peerson J, Kafwembe E, Brown KH, Haskell MJ. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J Nutr 2010;140:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivakumar B, Reddy V. Absorption of labelled vitamin A in children during infection. Br J Nutr 1972;27:299–304. [DOI] [PubMed] [Google Scholar]
- 38.Clyne B, Olshaker J. The C-reactive protein. J Emerg Med 1999;17:1019–25. [DOI] [PubMed] [Google Scholar]
- 39.Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4]retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr 2003;77:681–6. [DOI] [PubMed] [Google Scholar]
- 40.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken (NY): John Wiley; 2005. [Google Scholar]
- 42.Olson JA. Recommended dietary intakes (RDI) of vitamin A in humans. Am J Clin Nutr 1987;45:704–16. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. WHO Anthro (version 3.2.2, January 2011) and macros [Internet]. 2011 [cited 2013 Oct 22]. Available from: http://www.who.int/childgrowth/software/en/.
- 44.Tang G, Qin J, Hao L, Yin S, Russell RM. Use of a short-term isotope-dilution method for determining the vitamin A status of children. Am J Clin Nutr 2002;76:413–8. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Teros V, Quihui-Cota L, Méndez-Estrada R, Grijalva-Haro M, Esparza-Romero J, Valencia M, Green MH, Tang G, Pacheco-Moreno BI, Tortoledo-Ortiz O, et al. Vitamin A–fortified milk increases total body vitamin A stores in Mexican preschoolers. J Nutr 2013;143:221–6. [DOI] [PubMed] [Google Scholar]
- 46.Hicks VA, Gunning DB, Olson JA. Metabolism, plasma transport and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr 1984;114:1327–33. [DOI] [PubMed] [Google Scholar]
- 47.Sauberlich HE, Hodges R, Wallace D, Kolder H, Canham J, Hood J, Raica N, Jr, Lowry LK. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 1974;32:251–75. [DOI] [PubMed] [Google Scholar]
- 48.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am J Clin Nutr 2000;71:1582–8. [DOI] [PubMed] [Google Scholar]
- 49.Ribaya-Mercado JD, Mazariegos M, Tang G, Romero-Abal ME, Mena I, Solomons NW, Russell RM. Assessment of total body stores of vitamin A in Guatemalan elderly by the deuterated-retinol-dilution method. Am J Clin Nutr 1999;69:278–84. [DOI] [PubMed] [Google Scholar]
- 50.McLaren DS. The luxus vitamins-A and B12. Am J Clin Nutr 1981;34:1611–6. [DOI] [PubMed] [Google Scholar]
- 51.Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL. Evaluation of vitamin A toxicity. Am J Clin Nutr 1990;52:183–202. [DOI] [PubMed] [Google Scholar]
- 52.Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Vitamin A. In: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001. p. 82–161. [PubMed]