Abstract
Background
One of the major unresolved issues in treating pain is the paradoxical hyperalgesia produced by opiates, and accumulating evidence implicate that EphBs receptors and ephrinBs ligands are involved in mediation of spinal nociceptive information and central sensitization, but the manner in which ephrinB/EphB signalling acts on spinal nociceptive information networks to produce hyperalgesia remains enigmatic. The objective of this research was to investigate the role of ephrinB/EphB signalling in remifentanil-induced hyperalgesia (RIH) and its downstream effector.
Methods
We characterized the remifentanil-induced pain behaviours by evaluating thermal hyperalgesia and mechanical allodynia in a rat hind paw incisional model. Protein expression of EphB1 receptor and ephrinB1 ligand in spinal dorsal horn cord was determined by Western blotting, and Fos was determined by immunohistochemistry assay, respectively. To figure out the manner in which ephrinB/EphB signalling acts with N-methyl-d-aspartic acid (NMDA) receptor, we used MK-801, an antagonist of NMDA receptor, trying to suppressed the hyperalgesia induced by ephrinB1-Fc, an agonist of ephrinB/EphB.
Results
Continuing infusion of remifentanil produced a thermal hyperalgesia and mechanical allodynia, which was accompanied with increased protein expression of spinal-level EphB1 receptor, ephrinB1 ligand and Fos; what appeared above was suppressed by pretreatment with EphB1-Fc, an antagonist of ephrinB/EphB or MK-801, and increased pain behaviours induced by intrathecal injection of ephrinB1-Fc, an agonist of ephrinB/EphB, were suppressed by MK-801.
Conclusions
Our findings indicated that ephrinB/EphB signalling is involved in RIH. EphrinB/EphB signalling might be the upstream of NMDA receptor.
What's already known about this topic?
EphBs receptors and ephrinBs ligands are involved in mediation of spinal nociceptive information and central sensitization.
The combination of EphB receptor and N-methyl-d-aspartic acid receptor induces long-term potentiation that is critical for causing excitation of spinal neuron and pain hyperalgesia.
What does this study add?
EphrinB/EphB signalling is involved in remifentanil-induced hyperalgesia (RIH).
EphrinB/EphB signalling might be the upstream of N-methyl-d-aspartic acid receptor in RIH.
1. Introduction
Opiates are inevitable in the treatment of moderate-to-severe post-operative and chronic pain, while synchronously, these drugs also activate the nocuous mechanism of a sensitization process, causing pain hypersensitivity (Chang et al., 2007). A commonly held view is that pharmacokinetic characteristics of opiates affect the severity of opiate-induced hyperalgesia (OIH) (Celerier et al., 2000; Derrode et al., 2003). For instance, the faster working and short-acting characteristics of remifentanil (Egan et al., 1993) induce a faster and more obvious hyperalgesia compared with other long-acting opiates, which make patients feel more pain and add more difficulties to patients administered analgesia during anaesthesia recovery and post-operative periods. The underlying cellular and molecular mechanism of OIH is very complicated (Bekhit, 2010; Carcoba et al., 2011), which involves multiple intracellular signal transduction pathways. The following mechanisms are proposed: (1) the activation of protein kinase C (involved in opioid receptor desensitization) (Mao et al., 1995); (2) N-methyl-d-aspartic acid (NMDA) receptor pathway activation (Simonnet and Rivat, 2003); (3) increasing release of peptides with opioid antagonistic properties such as cholecystokinin (Maier et al., 1992), neuropeptide FF (Elhabazi et al., 2013) and nociceptin (Harrison and Grandy, 2000); and (4) incremental expression of inflammatory cytokine embracing prostaglandin and interleukin-1. Except those mentioned above, the basic spinal mechanism that likely contributes to OIH is the synaptic long-term potentiation (LTP) after opioid withdrawal (Sandkuhler, 2009). Opiate causes considerable internalization and activation of μ-opioid receptor (Trafton et al., 2000), then leads to postsynaptic G-protein coupling, activation of postsynaptic NMDA receptors and postsynaptic Ca2+ rise, resulting in that synaptic transmission between nociceptive C fibres and neurons in spinal dorsal horn is persistently potentiated (Drdla et al., 2009).
Ephs is the largest subfamily of RTKs in the human genome (Kullander and Klein, 2002), and regulates many developmental processes, including tissue border formation, re-angiogenesis, axon guidance and synaptic plasticity (Klein, 2009). It has been proven that Ephs, especially the ephrinB/EphB signalling, participate in nociceptive process. It is known that the induction and the maintenance of neuropathic pain are regulated by neural excitability and synaptic plasticity in both the dorsal root ganglion and the spinal dorsal horn. Our previous study (Yu et al., 2012) and other research (Battaglia et al., 2003; Song et al., 2008) have demonstrated that activated ephrinB/EphB signalling in spinal level participates in the nociceptive process and central sensitization via up-regulating the excitability of nociceptive dorsal root ganglia and wide dynamic range neurons in spinal dorsal horn (Song et al., 2008), decreasing pain threshold. Recent studies also showed that EphB receptor and NMDA receptor induce LTP of spinal dorsal horn neurons (Ruan et al., 2010).
Thus, we conducted a preliminary experiment that showed the expression of EphB1 receptor and ephrinB1 ligand in spinal dorsal horn cord increased in rats, which underwent remifentanil infusion. Whether ephrinB/EphB signalling is involved in remifentanil-induced hyperalgesia (RIH) needs to be proven, and the manner in which ephrinB/EphB signalling involves (or not) NMDA receptor in the development of RIH remains enigmatic. Using antagonists of ephrinB/EphB and NMDA receptors, we evaluated the role of ephrinB/EphB and its relationship with NMDA receptor in animals that received remifentanil infusion in an incisional rat model.
2. Materials and methods
2.1 Animals
Adult, male Sprague-Dawley rats (220–250 g) were employed in our study. Animals were housed under 12/12-h light–dark cycle for at least 3 days before surgery, with free access to food and water. All efforts were made to minimize animal suffering and to reduce the number of animals used. All experimental protocols were in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 80-23, revised 1996), and the protocols were approved by the Zhejiang University Institutional Animal Care and Use Committee.
2.2 Drug administration
EphB1-Fc, an antagonist of ephrinB/EphB, and ephrinB1-Fc, an agonist of ephrinB/EphB, were purchased from R&D Systems, Inc. (Minneapolis, MN, USA); MK-801, an antagonist of NMDA receptor, was purchased from Sigma (St. Louis, MO, USA). These drugs were dissolved in saline (NaCl 0.9%). Rats were anaesthetized with sevoflurane (induction, 3.0% v/v; HengRui, Co., Shanghang, China), then intrathecal injection was applied according to the method described by Mestre et al. (1994). Hamilton microsyringe (GaoGe Co., Shanghai, China) was inserted between the L5 and L6 vertebrae, and a sudden slight flick of the tail implicated entry into the subarachnoid space. A volume of 20 μL drug solution or physiologic saline was injected within a 20-s period into the subarachnoid space, and the injection cannula was kept staying there for a further 10 s.
2.3 RIH model
After intrathecal injection of EphB1-Fc or MK-801 or saline, continuing infusion of remifentanil (Criado and Gomez e Segura, 2003) was applied subcutaneously, and incisional operation (Brennan et al., 1996) was performed at the meantime under sevoflurane anaesthesia (surgery, 1.0% v/v). Remifentanil hydrochloride (RenFu, Co., Yichang, China) was dissolved in saline (NaCl 0.9%, 20 μg/mL) and infused with a dose of 40 μg/kg, at a rate of 0.067 mL/kg/min for 30 min. Control group received the same rate of continuing infusion of physiologic saline subcutaneously. Right hind paw of the rat was sterilized with iodophor. One centimetre of longitudinal incision was made through the skin and fascia of the plantar surface, starting at 0.5 cm from the proximal edge of the heel and extending towards the toes. The underlying muscle was then elevated and incised longitudinally, keeping the muscle origin and insertion intact. After haemostasis with gentle pressure, the skin was closed with two 4-0 nylon sutures and the wound was sterilized with iodophor and covered with erythromycin ointment. The animals were allowed to recover in their cages under a heat source.
2.4 Measurement of thermal hyperalgesia
Thermal hyperalgesia was measured using the paw withdrawal latency (PWL) according to the method described by Hargreaves et al. (1988). Rat was placed in clear plastic chambers (7 × 9 × 11 cm) and allowed to acclimatize to the environment for 30 min before testing to avoid stress response. The radiant heat was given directly on the plantar surface of the right hind paw and was ceased at once the rat lifted up or licked the hind paw. The time to the endpoint was considered as the PWL. The radiant heat intensity was kept unchanging during the test, and an automatic 25-s cut-off was used to prevent tissue damage. Thermal stimuli were delivered three times to each hind paw at 5-min intervals, and the average was taken as the PWL.
2.5 Measurement of mechanical allodynia
Mechanical allodynia was measured using the paw withdrawal threshold (PWT) with von Frey filaments according to the ‘up-down’ method described by Chaplan et al. (1994). Rat was placed in plastic boxes (20 × 25 × 15 cm) on a metal mesh floor and allowed to acclimate for 30 min. We used the von Frey filament to touch the plantar surface of the right hind paw for 6–8 s, and marked the withdrawal of the paw followed by clear flinching movements as positive response. The measurement began with medium-strength von Frey filament (2 g), then changed to an adjacent higher degree filament if led no positive response; on the contrary, changed to an adjacent lower degree filament if led a positive response. Different stimuli were taken at 30-s intervals to eliminate the effect of last stimulus. Repeat the procedures above until the first positive and negative response (or negative and positive response) straddled; 50% of the two filament forces was the response threshold. Measurements were taken five times and the average was taken as the PWL.
2.6 Western blotting
The spinal lumbosacral enlargement of rat was quickly extracted and stored in liquid nitrogen. Tissue samples were homogenized in lysis buffer (containing phenylmethylsulfonyl fluoride, 100:1) and split on ice for 30 min. The homogenates were centrifuged at 13,000 g for 15 min at 4 °C. The supernatant was collected, and protein concentration was measured according to the Bradford method (Bradford, 1976). Samples were heated at 100 °C for 5 min to denature, then electrophoresed on a precast 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto 0.45-mm polyvinylidene fluoride membrane. The membranes were incubated overnight at 4 °C with the rabbit anti-EphB1 antibody (1:50) or rabbit anti-ephrinB1 antibody (1:200), or primary rabbit anti-glyceraldehyde phosphate dehydrogenase antibody (1:10,000). The membranes were extensively washed with Tris-buffered saline Tween 20 (BiYunTian Co.,JiangSu, China) and incubated for 1 h with the secondary antibody (1:500) at room temperature. The immune complexes were detected by enhanced chemiluminescence Western detection reagents. And Western blot densitometry analysis was performed using Quantity One 4.6.2 (Bio-Rad, Hercules, CA, USA).
2.7 Immunohistochemistry
Rats were anaesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal injection) and were subjected to sternotomy followed by intracardial perfusion with 200 mL of saline and 400 mL 4% paraformaldehyde. The spinal cord of L4–L5 was removed and postfixed in 4% paraformaldehyde overnight and was subsequently dehydrated in 30% sucrose solution. Fifteen micrometer transverse series sections were cut on a cryostat and stored in phosphate buffer. After washing with phosphate-buffered saline, the tissue sections were incubated in the same buffer saline containing 0.3% Triton X-100 (JiangLai Co., Shanghai, China) at room temperature for 30 min. For the Fos protein assay, the sections were incubated in primary polyclonal rabbit anti-Fos antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight and were then incubated in biotinylated goat anti-rabbit (1:200) at 37 °C for 1 h. Finally, the sections were treated with 0.05% diaminobenzidine for 5–10 min. Sections were rinsed in phosphate-buffered saline, mounted on gelatin-coated slides, air-dried, dehydrated with alcohol, cleared with xylene and coverslipped for microscopic examination. We examined five L4–L5 spinal cord sections per animal; these five scarves selected contain the greatest number of positive neurons. For each animal, we counted the total number of positive neurons in the bilateral spinal cord I–V and X lamina of the selected five sections, regardless of the intensity of the staining.
2.8 Experimental protocol
2.8.1 Experiment 1: the changes of mechanical and thermal hyperalgesia, EphB1 receptor, ephrinB1 ligand and Fos protein expression in RIH rats
Experiments were performed in rats receiving one of the following treatments: animals underwent a surgical incision without remifentanil infusion: control group (CON, n = 6); animals underwent a surgical incision with remifentanil infusion: remifentanil group (RIH, n = 6). Withdrawal threshold and latency to mechanical and thermal stimulation, respectively, were performed at baseline (24 h before incision) and 2 and 6 h, as well as 1, 2, 3, 4 and 5 days after remifentanil incision. The protein expression of EphB1 receptor, ephrinB1 ligand and Fos of both CON and RIH rats was assayed on days 1 and 3, respectively, after remifentanil incision.
2.8.2 Experiment 2: the effects EphB1-Fc and MK-801 have on mechanical and thermal hyperalgesia and Fos expression in RIH rats
Experiments were performed in rats receiving one of the following treatments: control group (CON, n = 6, as described above); remifentanil group (RIH, n = 6, as described above); animals underwent a remifentanil incision with EphB1-Fc (5 μg/20 μL) injection: EphB1-Fc group (EphB1-Fc, n = 6); animals underwent a remifentanil incision with MK-801(10 μg/20 μL) injection: MK-801 group (MK-801, n = 6). Withdrawal threshold and latency to mechanical and thermal stimulation and the protein expression of Fos were assayed in all four groups. The experimental time points were the same as in experiment 1.
2.8.3 Experiment 3: the effects MK-801 have on mechanical and thermal hyperalgesia in ephrinB1-Fc pretreatment rats
Experiments were performed in rats receiving one of the following treatments: animals received intrathecal injection with saline: control group (CON, n = 6); animals received ephrinB1-Fc (2 μg/20 μL): ephrinB1-Fc group (ephrinB1-Fc, n = 6); animals received MK-801 (20 μg/20 μL): MK-801 group (MK-801, n = 6); animals received MK-801 (20 μg/10 μL) and ephrinB1-Fc (2 μg/10 μL) sequentially: MK-801 + ephrinB1-Fc group (MK-801 + ephrinB1-Fc, n = 6). Withdrawal threshold and latency to mechanical and thermal stimulation, respectively, were performed at baseline (24 h before drug administration) and 2, 6, 8, 12, 24 and 48 h after drug administration.
2.9 Statistical analysis
All data were given as the mean ± standard deviation. Statistical analysis between two samples was performed using Student's t-test. Statistical comparison of more than two groups was performed using one-way analysis of variance test. Statistical analyses of data were generated by GraphPad Prism 5 (Graph Pad Software, Inc., San Diego, CA, USA). All value of p less than 0.05 was considered statistically significant.
3. Results
3.1 Remifentanil induced a significant decrease in mechanical and thermal pain threshold
At 2 h after plantar incision, a significant decrease in both mechanical and thermal nociceptive thresholds was observed (4.06 ± 0.95 g and 8.38 ± 1.50 s) compared with control group (7.83 ± 0.98 g and 11.34 ± 2.02 s). On day 1 after manipulation, remifentanil induced a significant decrease in both mechanical and thermal hypersensitivity (about 55% ± 3% and 41% ± 2%, respectively) (p < 0.01, group RIH compared with group CON, n = 6 per group; Fig. 1A and B).
Fig 1.
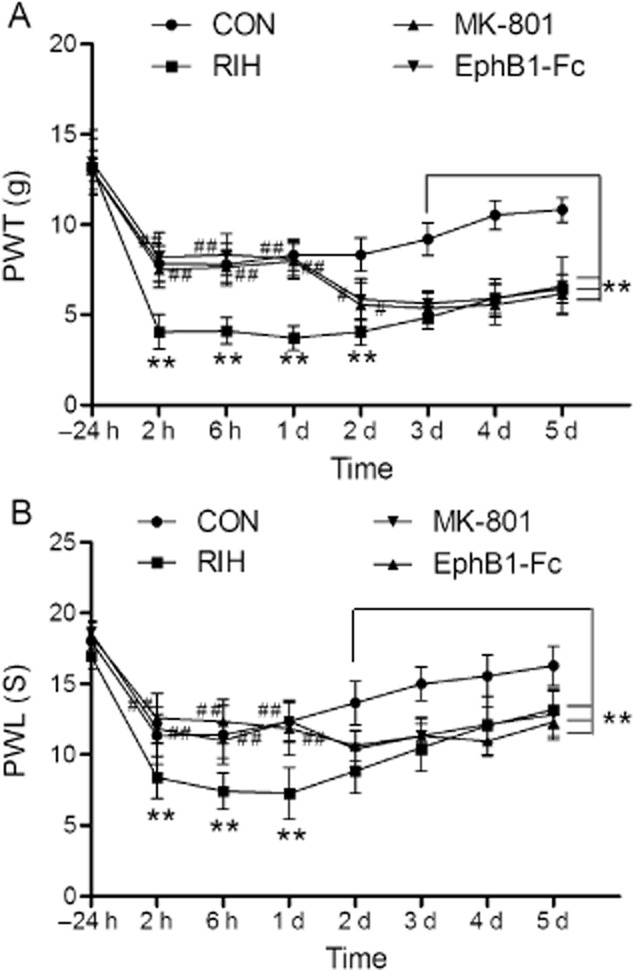
The effects of EphB1-Fc and MK-801 on mechanical allodynia (A) and thermal hyperalgesia (B) induced by remifentanil in rats. Data were shown as the mean ± standard deviation. **p < 0.01, compared with group CON; #p < 0.05, ##p < 0.01, compared with group remifentanil-induced hyperalgesia (RIH); n = 6 rats in each group. PWL, paw withdrawal latency; PWT, paw withdrawal threshold.
3.2 Remifentanil increased protein expression of EphB1 receptor, ephrinB1 ligand and Fos in spinal dorsal horn cord
Like the behaviour tests that the peak hyperalgesia appeared on day 1 and lasted for at least 5 days, there was a significant increase in EphB1 receptor and ephrinB1 ligand protein levels in spinal dorsal horn cord 1 and 3 days after remifentanil incision. The EphB1 receptor level was increased by approximately 174% ± 5% and 170% ± 5% on days 1 and 3, respectively, whereas the ephrinB1 ligand level was increased by approximately 48% ± 2% and 37% ± 2% on days 1 and 3, respectively, compared with group CON (p < 0.01, group RIH compared with group CON, n = 6 per group; Fig. 2A and B).
Fig 2.
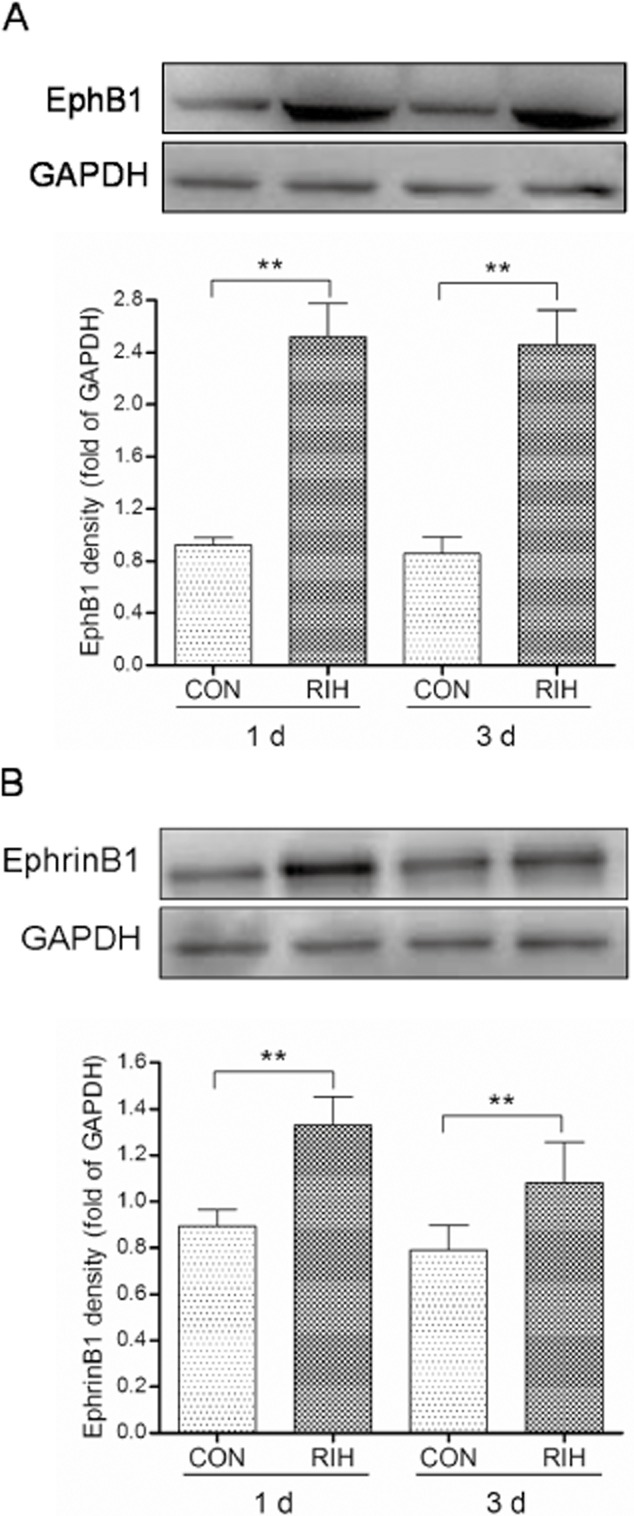
EphB1 receptor (A) and ephrinB1 ligand (B) protein levels on the first and third day after incision. The fold change for the density of EphB1 receptor or ephrinB1 ligand normalized to the glyceraldehyde phosphate dehydrogenase (GAPDH) level. Data were shown as the mean ± standard deviation. **p < 0.01, compared with group CON; n = 6 rats in each group.
We examined the effect of remifentanil treatment on spinal-level Fos protein expression. In the RIH group, it was increased by approximately 35% ± 2% and 27% ± 1.5% 1 and 3 days after surgery, respectively, compared with group CON (p < 0.01, group RIH compared with group CON, n = 6 per group; Fig. 3A–D).
Fig 3.
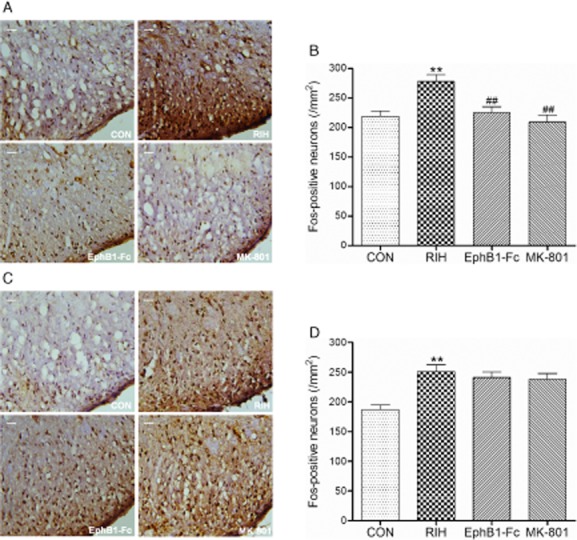
The expression of Fos in the spinal cord dorsal horn of the rats in each group at first (A and B) and third day (C and D) after surgery. Scale bar represented 100 μm. Data were shown as the mean ± standard deviation. **p < 0.01, compared with group CON; ##p < 0.01, compared with group remifentanil-induced hyperalgesia (RIH); n = 6 rats in each group.
3.3 Blocking of EphB and NMDA receptor inhibited remifentanil-induced pain behaviours and down-regulated the Fos expression in RIH rats
We also found that the decrease in pain threshold produced by remifentanil on day 1 was suppressed with EphB1-Fc and MK-801 antagonist (p < 0.01, group EphB1-Fc and group MK-801 compared with group RIH, n = 6 per group; Fig. 1A and B). On day 1 after manipulation, the PWT of group EphB1-Fc (8.11 ± 0.97 g) and group MK-801 (7.97 ± 0.99 g) approximated the PWT of group CON (8.31 ± 0.87 g); the PWL of group EphB1-Fc (11.84 ± 1.87 s) and group MK-801 (12.37 ± 1.49 s) also approximated the PWL of group CON (12.34 ± 1.38 s). The PWT and PWL descended to RIH level again on day 2 (p > 0.05, group EphB1-Fc and group MK-801 compared with group RIH, n = 6 per group; Fig. 1A and B).
Pretreatment with EphB1-Fc or MK-801 both also significantly inhibited the remifentanil-induced increase of Fos expression in spinal dorsal horn cord on day 1: EphB1-Fc and MK-801 reduce the Fos level by approximately 20% ± 1.5% and 25% ± 1.5%, respectively (p < 0.01, group EphB1-Fc and group MK-801 compared with group RIH, n = 6 per group; Fig. 3A and B), without affecting the Fos protein level on day 3 (p > 0.05, group EphB1-Fc and group MK-801 compared with group RIH; Fig. 3C and D).
3.4 Inhibition of spinal-level NMDA receptor suppressed pain behaviours induced by intrathecal injection of ephrinB1-Fc
The result above demonstrated that activation of ephrinB/EphB signalling was required in RIH; we then figured out the manner in which ephrinB/EphB signalling acts with NMDA receptor. We found that ephrinB1-Fc, an agonist of ephrinB/EphB, markedly reduced both PWT by 67% ± 3% and PWL by 17% ± 1% in normal rats 2 h after drug administration (p < 0.01, group ephrinB1-Fc compared with group CON, n = 6 per group; Fig. 4A and B), and lasted for about 24 h (p < 0.01, group ephrinB1-Fc compared with group CON, n = 6 per group; Fig. 4A and B).
Fig 4.
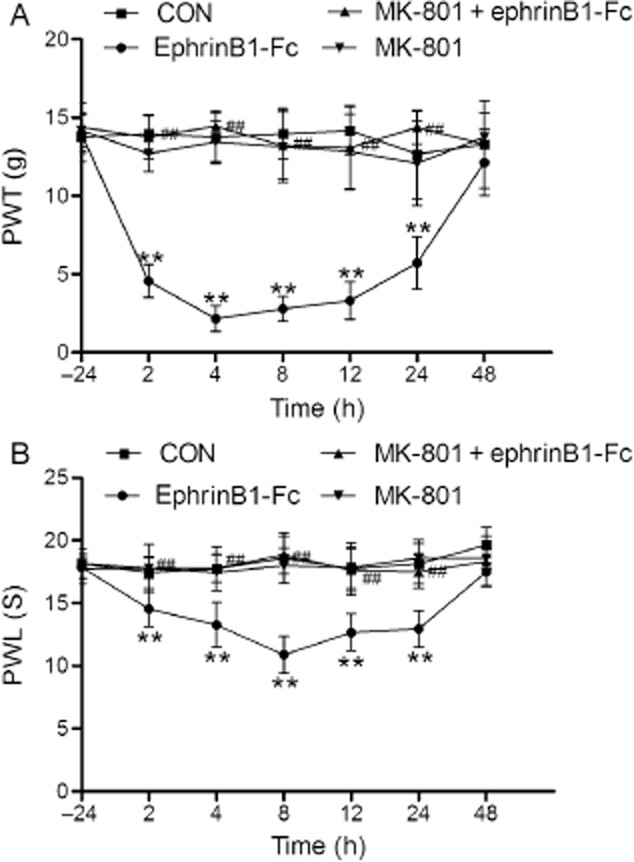
The effect of ephrinB1-Fc and MK-801 on mechanical allodynia (A) and thermal hyperalgesia (B) in normal rats. Data were shown as the mean ± standard deviation. **p < 0.01, compared with group CON; ##p < 0.01, compared with group ephrinB1-Fc; n = 6 rats in each group. PWL, paw withdrawal latency; PWT, paw withdrawal threshold.
The pain behaviours induced by ephrinB1-Fc were suppressed by pretreatment with MK-801 (p < 0.01, group MK-801 + EphB1-Fc compared with group ephrinB1-Fc, n = 6 per group; Fig. 4A and B). At 2 h after drug administration, the PWT of group MK-801 + EphB1-Fc (13.75 ± 1.37 g) approximated the PWT of group CON (13.96 ± 1.23 g); the PWL of group MK-801 + EphB1-Fc (17.78 ± 1.91 s) also approximated the mean PWL of group CON (17.46 ± 1.24 s). What is more, MK-801 had no effects on pain behaviours in normal rats (p > 0.05, group MK-801 compared with group CON, n = 6 per group; Fig. 4A and B).
4. Discussion and conclusions
This study demonstrated that ephrinB/EphB signalling is involved in RIH, and ephrinB/EphB signalling might be the upstream of NMDA receptor. And we displayed the following findings: (1) continuing infusion of remifentanil produced a thermal hyperalgesia and mechanical allodynia, which was accompanied with increased protein expression of EphB1 receptor, ephrinB1 ligand and Fos in spinal dorsal horn cord. (2) What appeared above was suppressed by pretreatment with EphB1-Fc or MK-801. (3) Increased pain behaviours induced by intrathecal injection of ephrinB1-Fc were suppressed by MK-801.
In 1970s, researchers found that in animal experiment, opioids can cause pain hypersensitivity, increasing the previous pain intensity during treatment, which is OIH; the succeeding clinical cases and experimental studies also produced similar conclusions. Remifentanil is a super short-acting agonist of μ-opioid receptor, which was approved for clinical use by the Food and Drug Administration in 1996. The chemical structure of remifentanil contains methyl ester bond, leading that it is metabolized by non-specificity plasma esterase, independent of liver and kidney (Feldman et al., 1991). Therefore, remifentanil is a befitting drug for old patients and patients with liver or kidney dysfunction without worrying about delayed anaesthesia recovery. Another advantage of remifentanil is that it has constant half-life (Trescot et al., 2008), causing no accumulation phenomenon even in long-term continuous infusion compared with other opioids. Consequently, remifentanil has been widely used in general anaesthesia, post-operative analgesia and labour analgesia in that it has a fast metabolism and improved intravenous anaesthesia controllability (Thompson and Rowbotham, 1996). The current study has proven that continuing infusion of remifentanil for intraoperative analgesia causes pain hypersensitivity indispensably at the same time. OIH has a close relationship with pharmacokinetics (Derrode et al., 2003), remifentanil works faster and analgesia function disappears faster after stopping drug, too, resulting in a faster and more obvious OIH compared with other long-acting opiates. In our research, measuring PWT and PWL of incision pain model at multiple time points imitated the perioperative hyperalgesia. We compared incisional surgery combined remifentanil infusion with incisional surgery received saline infusion, which only change one variable – with or without infusion of remifentanil, and confirmed that RIH after surgery that lasted at least 5 days.
Our previous study (Yu et al., 2012) and other research (Battaglia et al., 2003; Song et al., 2008) have demonstrated that activated ephrinB/EphB signalling in spinal level participates in the nociceptive process and central sensitization. In the present study, we showed that the protein expression of ephrinB1 ligand and EphB1 receptor in spinal dorsal horn cord increased significantly in RIH rats; it did affirm that this signal plays an essential role in the production and persistence of RIH. The possible mechanisms underlying such a role of ephrinB/EphB signalling in regulating RIH have not been evaluated and remained unknown. We hypothesize that the ephrinB/EphB signalling may alert RIH rat pain threshold by triggering the following pathways: ephrinB activates postsynaptic EphB receptors on spinal dorsal horn neurons (Battaglia et al., 2003), then the EphB receptors interact with NMDA receptors and regulate excitatory synapse formation (Dalva et al., 2000). This capacity regulating the development of normal function and plasticity at glutamatergic synapses in spinal cord neurons (Calo et al., 2006) leads to synaptic LTP between C fibres and spinal dorsal horn (Ruan et al., 2010), which is the direct pathologic basis of hyperalgesia (Sandkuhler, 2009). Current research (Liu et al., 2002) have shown that high-frequency stimulation induces LTP and up-regulates Fos expression in wild mice, but in EphB1−/− mice, high-frequency stimulation neither induces LTP nor up-regulates Fos expression in spinal cord; this study is consistent with our hypothesis. In particular, the immunohistochemical staining of Fos protein of laminae I and II neurons in spinal dorsal horn cord, which is the terminal region of manifold nociceptive fibres such as C fibres and Aδ fibres, showed that RIH rats had a significantly increased Fos protein expression compared with saline infusion rats. In addition, blocking of EphB receptor or NMDA receptor suppressed RIH; it indicated that both ephrinB/EphB signalling and NMDA receptor participate in the RIH process, as our hypothesis described.
To further confirm the manner in which ephrinB/EphB signalling acts with NMDA receptor to produce RIH, we conducted experiment 3 described in section 2.8. We found increasing pain behaviours after intrathecal injection of ephrinB1-Fc, EphB receptor activator, which was suppressed by pretreatment of NMDA receptor antagonist MK-801, indicating that activation of NMDA receptor may be the downstream event of activation of ephrinB/EphB.
Ephs is the largest subfamily of RTKs in the human genome; Eph receptors divided into EphA receptors (EphA1- EphA8) and EphB receptors (EphB1- EphB4, EphB6), so did their ligands that divided into two subtypes: ephrinA1-ephrinA5 and ephrinB1–ephrinB3 (Kullander and Klein, 2002). The available reagents we used did not allow us to identify the specific Eph receptors and ephrin ligands activated in our experiments, although the specificity of the antibodies used for detection of ephrinB1 ligand and EphB1 receptor made us to believe that these specific proteins were up-regulated after remifentanil infusion. The possibility of other proteins of EphA and EphB family involved in the RIH cannot be excluded.
Our findings indicated that ephrinB/EphB signalling is involved in RIH. EphrinB/EphB signalling might be the upstream of NMDA receptor.
Author contributions
W.S.X. and Y.N.P. conceived and designed the experiments. W.S.X., Y.N.P., L.H.T., L.S.J., L.N.Y., X.L.Z. and F.J.Z. performed the experiments. W.S.X., Y.N.P. and L.H.T. analysed the data. W.S.X., Y.N.P., L.S.J., L.N.Y. and M.Y. contributed to the reagents/materials/analysis tools. W.S.X., Y.N.P. and M.Y. wrote the paper. All authors discussed the results and commented on the manuscript.
Acknowledgments
We thank Dr Xing Zhang for her advice and assistance in laboratory management.
References
- Battaglia AA, Sehayek K, Grist J, McMahon SB, Gavazzi I. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003;6:339–340. doi: 10.1038/nn1034. [DOI] [PubMed] [Google Scholar]
- Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17:498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Calo L, Cinque C, Patane M, Schillaci D, Battaglia G, Melchiorri D, Nicoletti F, Bruno V. Interaction between ephrins/Eph receptors and excitatory amino acid receptors: Possible relevance in the regulation of synaptic plasticity and in the pathophysiology of neuronal degeneration. J Neurochem. 2006;98:1–10. doi: 10.1111/j.1471-4159.2006.03844.x. [DOI] [PubMed] [Google Scholar]
- Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis. 2011;30:258–270. doi: 10.1080/10550887.2011.581985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Criado AB, Gomez e Segura IA. Reduction of isoflurane MAC by fentanyl or remifentanil in rats. Vet Anaesth Analg. 2003;30:250–256. doi: 10.1046/j.1467-2995.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Derrode N, Lebrun F, Levron JC, Chauvin M, Debaene B. Influence of peroperative opioid on postoperative pain after major abdominal surgery: Sufentanil TCI versus remifentanil TCI. A randomized, controlled study. Br J Anaesth. 2003;91:842–849. doi: 10.1093/bja/aeg263. [DOI] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, Shafer SL. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology. 1993;79:881–892. doi: 10.1097/00000542-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Elhabazi K, Humbert JP, Bertin I, Schmitt M, Bihel F, Bourguignon JJ, Bucher B, Becker JA, Sorg T, Meziane H, Petit-Demouliere B, Ilien B, Simonin F. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology. 2013;75:164–171. doi: 10.1016/j.neuropharm.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, Lutz MW, Johnson MR, Leighton HJ. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgetics. J Med Chem. 1991;34:2202–2208. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Grandy DK. Opiate modulating properties of nociceptin/orphanin FQ. Peptides. 2000;21:151–172. doi: 10.1016/s0196-9781(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Liu WT, Han Y, Li HC, Adams B, Zheng JH, Wu YP, Henkemeyer M, Song XJ. An in vivo mouse model of long-term potentiation at synapses between primary afferent C-fibers and spinal dorsal horn neurons: Essential role of EphB1 receptor. Molecular Pain. 2009;5:29. doi: 10.1186/1744-8069-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Watkins LR. Endogenous pain facilitatory systems: Antianalgesia and hyperalgesia. J Pain. 1992;1:191–198. [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Ruan JP, Zhang HX, Lu XF, Liu YP, Cao JL. EphrinBs/EphBs signaling is involved in modulation of spinal nociceptive processing through a mitogen-activated protein kinases-dependent mechanism. Anesthesiology. 2010;112:1234–1249. doi: 10.1097/ALN.0b013e3181d3e0df. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Simonnet G, Rivat C. Opioid-induced hyperalgesia: Abnormal or normal pain? Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- Song XJ, Zheng JH, Cao JL, Liu WT, Song XS, Huang ZJ. EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain. 2008;139:168–180. doi: 10.1016/j.pain.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Thompson JP, Rowbotham DJ. Remifentanil – An opioid for the 21st century. Br J Anaesth. 1996;76:341–343. doi: 10.1093/bja/76.3.341. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: Responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–S153. [PubMed] [Google Scholar]
- Yu LN, Zhou XL, Yu J, Huang H, Jiang LS, Zhang FJ, Cao JL, Yan M. PI3K contributed to modulation of spinal nociceptive information related to ephrinBs/EphBs. PLoS ONE. 2012;7:e40930. doi: 10.1371/journal.pone.0040930. [DOI] [PMC free article] [PubMed] [Google Scholar]


