Abstract
Multiple sclerosis (MS) is an autoimmune disease of unknown etiology. Infectious agents have been suggested to have a role as environmental factors in MS, but this concept remains controversial. Recently, gastrointestinal commensal bacteria have been implicated in the pathogenesis of autoimmune diseases, but mechanisms underlying the relationship of human systemic autoimmunity with the commensal microbiome have yet to be identified. Consistent with the lack of understanding of pathogenic mechanisms and relevant environmental factors in MS, no blood biomarkers have been identified that distinguish MS patients from healthy individuals. We recently identified a unique gastrointestinal and oral bacteria-derived lipodipeptide, Lipid 654, which is produced by commensal bacteria and functions as a human and mouse Toll-like receptor 2 ligand. Using multiple-reaction-monitoring mass spectrometry, a critical approach in targeted lipidomics, we now report that Lipid 654 can be recovered in the serum of healthy individuals. Most interestingly, we find that Lipid 654 is expressed at significantly lower levels in the serum of patients with MS compared with both healthy individuals and patients with Alzheimer's disease. These results thus identify for the first time a potential mechanism relating the gastrointestinal and oral commensal microbiome to a human systemic autoimmune disease. In addition, these results also identify a potential etiologic environmental factor and novel clinically relevant serum biomarker for MS.
Keywords: autoimmunity, biomarker, commensal bacteria, microbiome, multiple sclerosis, TLR2
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS); it is more common in women than in men and usually with an onset between the ages of 20 and 40 years of age. There are approximately 400 000 individuals diagnosed with MS in the United States, and it is estimated that there are between 1.25 and 1.5 million cases worldwide. Although MS is believed to be caused by an attack of self-reactive T cells on CNS myelin antigens, the pathogenic mechanisms underlying both the onset and progression of the disease remain unknown. Infections have been suggested as having a pathogenic role in MS, although there has been no definitive support for this concept. Recently, based partially on murine models, commensal bacteria populating the gastrointestinal (GI) tract have been implicated in the pathogenesis of autoimmune diseases including MS.1, 2, 3, 4 However, as yet, there is neither definitive evidence for this association in human autoimmunity nor specific mechanisms identified that would explain the putative relationship of human systemic autoimmunity with the microbiome. Because the cause of MS remains unknown, the therapeutic approaches presently available for treating MS involve nonspecific disruption of immune function that are often accompanied by serious adverse side effects.
In addition to a lack of understanding of pathogenic mechanisms and environmental factors in MS, at present there is no blood biomarker identified that clearly distinguishes MS patients from healthy individuals. Such a biomarker would have the potential to function in three important ways: as a diagnostic aid that could be useful before the onset of symptoms; as a marker for predicting disease activity or progression, potentially minimizing the use of the currently available therapeutic agents with significant adverse side effects; and finally as a potential target for therapeutic intervention. In addition, such a biomarker could potentially shed light on the underlying pathogenic mechanisms in MS.
We recently identified a unique bacterially derived serine-containing lipodipeptide, Lipid 654, that is produced by a number of commensal Bacteroidetes species and functions as a ligand for human and mouse Toll-like receptor 2 (TLR2).5 Using multiple-reaction-monitoring (MRM) mass spectrometry, a critical approach in targeted lipidomics, we now report that Lipid 654 is detectable in the serum of all individuals studied. More importantly, we find that Lipid 654 is expressed at significantly lower levels in the serum of patients with MS compared with healthy individuals. These results for the first time identify a potential mechanism relating the GI and oral commensal microbiome to human systemic autoimmunity. In addition, these results also identify a potential etiologic environmental factor and novel clinically relevant serum biomarker for MS.
Results
Lipid 654 is found in serum from normal individuals
Lipid 654 is produced by bacteria commonly found in the human oral cavity and GI tract and demonstrates human and mouse TLR2 agonistic function. To assess whether Lipid 654 gains access to the systemic circulation, we obtained serum samples from 12 healthy individuals. These healthy individuals included eight female subjects and four male subjects ranging in age between 33 and 75 years (Table 1A).
Table 1A. Study subjects: Healthy individuals.
| Age (years) | Gender |
|---|---|
| 36 | Female |
| 56 | Female |
| 33 | Male |
| 46 | Female |
| 75 | Male |
| 72 | Female |
| 61 | Female |
| 34 | Male |
| 54 | Female |
| 68 | Male |
| 58 | Female |
| 53 | Female |
Total lipids were derived from the serum samples by the common phospholipid extraction procedure of Bligh and Dyer6 and MRM-mass spectrometry was used to detect the presence of Lipid 654. MRM-mass spectrometry was chosen as the method of Lipid 654 quantitation because this mode of mass spectrometry offers maximal sensitivity, selectivity and dynamic range and is a critical approach in targeted lipidomics. Three major transition ions of Lipid 654 (as determined by tandem mass spectrometry: Transition 1, m/z 653.5–381.4; Transition 2: m/z 653.5–349.3; and Transition 3: m/z 653.5–131.1) were then used to quantify the recovery of Lipid 654 in human serum samples.
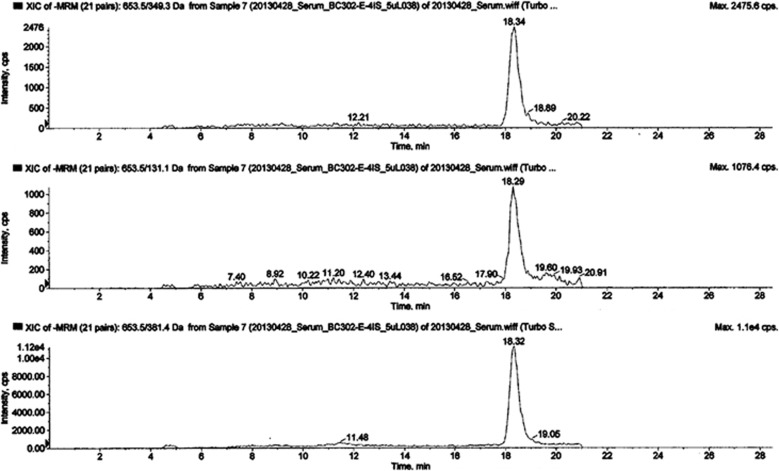
Surprisingly, we detected Lipid 654 in all 12 healthy control-derived lipid serum samples analyzed. An example of Lipid 654 expression in one serum sample derived from a healthy individual is shown in Figure 1. As seen in Figure 1, Lipid 654 was verified by demonstration of all three characteristic MRM transitions that appear at the expected retention time for this lipid. These results represent the first demonstration that Lipid 654, derived from commensal bacteria inhabiting GI or oral sites, routinely gains access to the systemic circulation in healthy humans.
Figure 1.
Serum from healthy individuals contains Lipid 654. Serum was obtained from 12 healthy individuals (see Table 1A). Total lipids were derived from these serum samples and MRM-mass spectrometry was used to analyze the samples, targeting Lipid 654 (m/z 653.5) and quantifying the transitions: Transition 1, m/z 653.5–349.3 (upper panel); Transition 2: m/z 653.5–131.1 (middle panel); and Transition 3: m/z 653.5–381.4 (lower panel). Depicted is an example of the MRM-mass spectrometry analysis of the serum lipid sample of one typical healthy individual.
Lipid 654 is found in significantly lower levels in serum from MS patients versus healthy individuals
In MS, as with most autoimmune diseases, the pathogenesis is believed to involve both genetic and environmental factors. Although no infectious agent has yet been definitively shown to be involved in the pathogenesis of MS, there has been considerable recent interest in the potential role of commensal bacteria in MS and other autoimmune diseases. On the basis of this potential involvement of commensal bacteria in MS, we next asked whether Lipid 654 could be an ‘environmental' factor mediating the effects of mucosal commensal bacteria on the pathogenesis of MS.
To address this question, we obtained serum samples from 17 patients with MS. These MS patients included 12 female subjects and five male subjects ranging in age from18 to 84 years (Table 1B). These patients primarily carried a diagnosis of relapsing–remitting MS (two had a diagnosis of secondary progressive MS and one a diagnosis of progressive relapsing MS) with lengths of disease duration ranging from 3 months to 40 years. This cohort included patients being treated with various drug regimens, patients not treated at the time of the blood sampling and patients never treated for MS (Table 1B). We derived total lipids from each of the 12 healthy control and 17 MS patient serum samples, resuspended 0.5 mg of total lipid from each sample in an equal volume of solvent, and using MRM-mass spectrometry compared the levels of serum Lipid 654 in these samples. These MRM-mass spectrometry analyses were run on three separate occasions (‘Run 1, 2 or 3').
Table 1B. Study subjects: Multiple sclerosis patients.
| Age (years) | Gender | Durationa | MSb | Treatmentc |
|---|---|---|---|---|
| 18 | Female | 4 mos | RR | Prednisone |
| 49 | Male | 3 mos | RR | None |
| 52 | Female | 11 years | SP | Interferon β-1a |
| 63 | Female | 4 years | RR | Fingolimod |
| 47 | Female | 7 years | RR | Glatiramer acetate |
| 60 | Male | 21 years | RR | Interferon β-1a |
| 70 | Female | 40 years | RR | Interferon β-1a |
| 23 | Male | 5 mos | RR | Prednisone |
| 55 | Female | 13 years | RR | Glatiramer acetate |
| 69 | Female | 17 years | RR | Glatiramer acetate |
| 47 | Female | 2 years | RR | Interferon β-1a |
| 61 | Male | 14 years | RR | Fingolimod |
| 84 | Male | 20 years | RR | Interferon β-1a |
| 65 | Female | 32 years | SP | None |
| 56 | Female | 3 years | RR | Glatiramer acetate |
| 26 | Female | 11 mos | RR | Interferon β-1a |
| 61 | Female | 16 years | PR | Interferon β-1a |
Abbreviations: mos, months; PP, primary progressive; PR, progressive relapsing; RR, relapsing remitting; SP, secondary progressive.
Duration: time since diagnosis.
MS: clinical subtype.
Treatment at the time of donating blood sample.
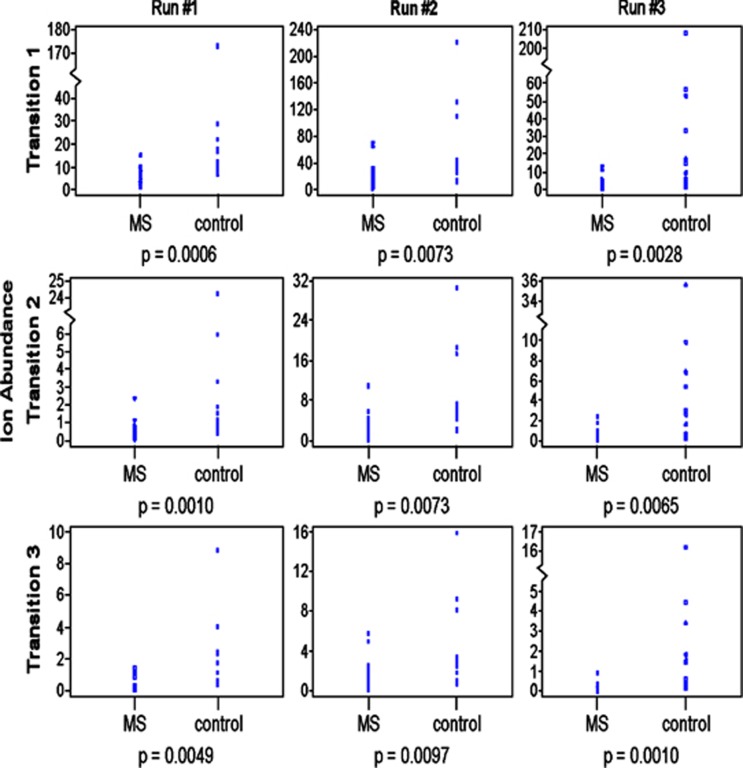
Figure 2 depicts the three separate MRM-mass spectrometry analyses (vertical columns indicating ‘Run 1, 2 or 3') comparing serum levels of Lipid 654 in the 17 MS versus 12 healthy control serum samples. Lipid 654 was identified and quantified in each of these three analyses using three major Lipid 654 daughter ions (Transitions 1–3) as shown in Figure 1. As seen in Figure 2, we found that levels of Lipid 654 were significantly and consistently lower in MS serum samples than in serum samples from healthy individuals. This was true for each of the three transitions and in each of the three runs. Note in Figure 2 that the values for all of the 17 MS patients are represented but are essentially clustered together at the lower levels of ion abundance. In these analyses, the statistical differences between Lipid 654 levels in MS versus healthy control samples ranged from a P-value of 0.0097 to a P-value of 0.0006 (Figure 2).
Figure 2.
Lipid 654 is significantly lower in the serum of MS patients. Serum was obtained from MS patients and healthy individuals (see Tables 1A and B). Total serum lipids were derived from these samples and analyzed using MRM-mass spectrometry to identify and quantify the absolute ion abundance of three transitions of Lipid 654 (Transitions 1, 2 and 3, as in Figure 1). Ion abundance is expressed as 105. MS patients, N=17; healthy individuals, N=12. Wilcoxon's rank-sum test was used to determine statistical significance.
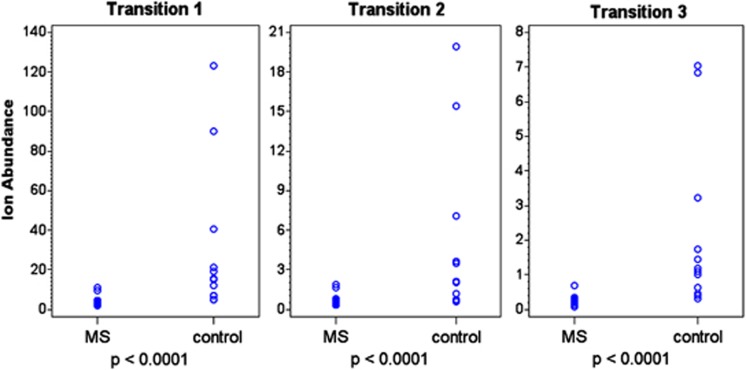
To correct for potential variations arising from slight chromatographic and mass spectrometric alterations occurring during the analysis, an internal standard was added to each sample for Run no. 3. This internal standard permitted quantification of Lipid 654 by correcting, for example, for diminished instrument sensitivity with increasing sample number or variations in lipid infusion by the automatic sampler. As seen in Figure 3, adjusting for the efficiency of Lipid 654 detection using the internal standard yielded essentially identical results as those depicted in Figure 2, that is, a very significant difference in serum levels of Lipid 654 between MS patients and healthy controls was found again. Note again in Figure 3 that the values for all of the 17 MS patients are clustered together at the lower levels of ion abundance.
Figure 3.
Addition of an internal standard to the MRM-mass spectrometry analysis confirms that Lipid 654 is significantly lower in the serum of MS patients. Serum was obtained from MS patients and healthy individuals (Tables 1A and B) and total serum lipids were analyzed by MRM-mass spectrometry for expression of Lipid 654 using Transitions 1, 2 and 3 as in Figure 2. As an additional control for MRM-mass spectrometry efficiency, a defined quantity of 13C-labeled total lipids derived from P. gingivalis was added to each sample. The level of recovery of 13C-labeled Lipid 654 was then used to adjust each value based on the efficiency of the analysis of that sample. Ion abundance is expressed as 105. MS patients, N=17; healthy individuals, N=12. Means: Transition1, MS patients 414 891; control patients 3 003 525; Transition 2: MS patients 66 482; control patients 497 055; Transition 3: MS patients 26 762; control patients 211 245. Wilcoxon's rank-sum test was used to determine statistical significance.
The age and gender distribution of our MS and healthy populations in this study were generally similar. In the population of MS patients studied to this point, we have found no significant correlation of serum Lipid 654 levels with the subtype of MS, duration of disease, gender, age or treatment modality. However, as more MS patients are studied, it is possible that serum Lipid 654 levels will correlate with some or all of these disease parameters. We next generated receiver operating characteristic curves using the results depicted in Figure 3. The internal standard adjusted data shown in Figure 3 revealed that at an appropriate ion abundance cutoff value for each transition, the specificity of low serum Lipid 654 levels for MS patients ranged from 83 to 99% and the sensitivity associated with these specificity values ranged from 82 to 94%. These results suggest for the first time that a unique bacterially derived serine containing lipodipeptide is differentially recovered in the serum of MS patients compared with healthy individuals and that serum levels of Lipid 654 may prove to be a clinically relevant serum biomarker for MS.
Lipid 654 is found in significantly lower levels in serum from MS patients compared with Alzheimer's patients
We next asked if the levels of serum Lipid 654 in MS patients would also be lower than those in patients with other neurological diseases. For this second study, we obtained frozen serum samples from MS patients and from patients with Alzheimer's disease from the Human Brain and Spinal Fluid Resource Center at UCLA. In contrast to the studies described above in which our laboratory handled all of the serum samples using technical approaches that allowed for optimal lipid recovery and analysis, the UCLA specimens were handled utilizing universal and standard methods for obtaining serum samples. Moreover, the UCLA serum bank samples included those derived as post-mortem specimens, whereas the studies described above included no post-mortem specimens.
From the UCLA serum bank, the MS patient samples included those from 13 male subjects, all carrying a diagnosis of primary progressive MS. These MS patients had an age range of 45–81 years. No treatment information was available for these MS patients. One of the 13 MS patient samples was a post-mortem specimen. The Alzheimer's patient samples included those from eight female subjects and seven male subjects with an age range of 59–94 years. All of the Alzheimer's samples were post-mortem samples.
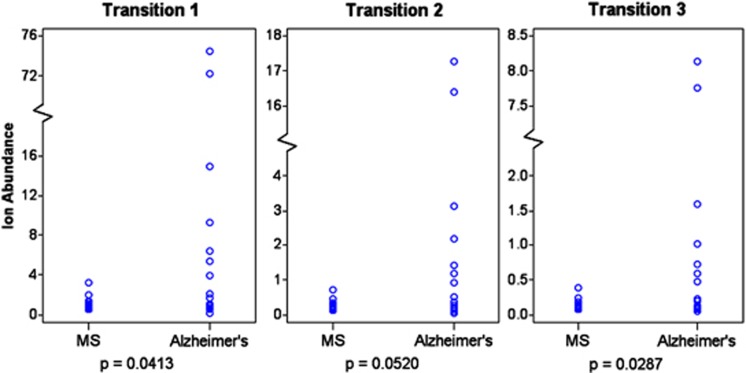
Total lipids were derived from these serum samples and analyzed for levels of Lipid 654 using MRM-mass spectrometry as described above. As with the samples that were derived in our laboratory, we quantified levels of expression of Lipid 654 using Transitions 1, 2 and 3 (see Figures 2 and 3). Strikingly, although these were banked frozen samples obtained from another institution, we nevertheless found that the levels of Lipid 654 in these MS serum samples were significantly lower than those of the Alzheimer's patients (Figure 4). Two of the three transitions demonstrated statistically significant differences between the MS and Alzheimer's samples, whereas the other transition yielded a P-value of 0.052. As seen in Figure 4, two Alzheimer's samples demonstrated extremely high serum expression of Lipid 654. These values are consistent with our overall findings that MS serum samples demonstrate low Lipid 654 expression. However, dropping these two high expressors from the data resulted in the P-values becoming slightly nonsignificant (not shown), but the interpretation of the data remains unchanged.
Figure 4.
Lipid 654 expression is lower in the serum of MS patients versus Alzheimer's patients. Frozen banked serum samples from MS and Alzheimer's patients were obtained from the UCLA Human Brain and Spinal Fluid Resource Center. Total serum lipids were derived and analyzed using MRM-mass spectrometry to identify and quantify the absolute ion abundance of Lipid 654 using three transitions of Lipid 654 (Transitions 1, 2 and 3, as in Figure 2). As in Figure 3, a defined quantity of 13C-labeled total lipids derived from P. gingivalis was added to each sample and used to adjust each value based on the efficiency of the analysis of that sample. Ion abundance is expressed as 105. MS patients, N=13; Alzheimer's patients, N=15. Means: Transition 1, MS patients 112 139; Alzheimer's patients 1 297 909; Transition 2, MS patients 26 333; Alzheimer's patients 295 680; Transition 3: MS patients 13 786; Alzheimer's patients 142 076. Wilcoxon's rank-sum test was used to determine statistical significance.
Thus, overall, we found that MS patients have significantly lower levels of serum Lipid 654 when compared with either healthy controls or patients with Alzheimer's disease. Likely as a result of the special serum handling procedures for optimizing lipid recovery used in our laboratory, the overall levels of recovery of Lipid 654 were lower in samples derived from the UCLA serum bank when compared with samples derived in our laboratory. This made direct comparison of the samples derived in our laboratory and the banked samples difficult to interpret. However, in the UCLA samples, all of the MS patients had a diagnosis of primary progressive MS, whereas the majority of the MS patients studied in our laboratory had a diagnosis of relapsing–remitting MS. Thus, it is possible that primary progressive MS patients may indeed have lower Lipid 654 levels than relapsing–remitting MS patients. Future studies will examine this issue in greater depth. Nevertheless, these results further suggest that low expression of serum Lipid 654 may be relevant in understanding pathogenic mechanisms in MS, particularly as they relate to the human mucosal microbiome, and may also represent a new and clinically relevant biomarker in patients with MS.
Discussion
The pathogenic mechanisms underlying both the onset and progression of MS remain unknown. Recently, the relationship of the commensal microbiota to autoimmune disease has become a topic of great interest. This focus involves not only the relationship of the GI microbiome to inflammatory diseases of the GI tract but also the association of the microbiome to systemic autoimmune diseases. To date, however, very little is understood mechanistically about how changes in commensal flora can influence systemic autoimmunity. Studies in mice have revealed that certain GI organisms such as segmented filamentous bacteria can regulate the generation of local and systemic Th17 responses and thus affect the incidence of autoimmune disease models.4, 7, 8 Although the precise mechanism by which segmented filamentous bacteria mediate this affect remains unknown, recently it has been reported that specific gut commensals, such as segmented filamentous bacteria, have the ability to modulate the activation threshold of self-reactive T cells.9 However, segmented filamentous bacteria have not yet been identified in humans. Although a number of murine studies have documented the effect of GI flora on intestinal immunoregulation and regulatory T cells,10, 11, 12, 13, 14 few murine studies have reported an association between GI flora and systemic immunoregulation. Most importantly in terms of our present results, little is known about mechanisms associating systemic autoimmunity in humans and commensal flora.
We recently identified and characterized a unique bacterially derived serine-containing lipodipeptide, Lipid 654, that is produced by a number of Bacteroidetes species and functions as a ligand for human and mouse TLR2.5 Using MRM-mass spectrometry, a critical approach in targeted lipidomics, we now report that Lipid 654 can be recovered in the serum of all individuals. This suggests that Lipid 654 derived from commensal bacteria inhabiting the GI tract or oral cavity routinely gains access to the systemic circulation. Although it is unknown at this time whether Lipid 654 is secreted by these organisms or is released upon death or phagocytosis of the bacteria, these results suggest that one mechanism by which the microbiome may interact with the systemic immune system is via circulating Lipid 654.
Potentially even more relevant to human autoimmunity is our finding that Lipid 654 is expressed at significantly lower levels in the serum of patients with MS compared with both healthy individuals and patients with Alzheimer's disease. In the population of MS patients studied to this point, we have found no significant correlation of serum Lipid 654 levels with subtype of MS, duration of disease, gender, age or treatment modality. However, as more MS patients are studied, it is very possible that serum Lipid 654 levels will correlate with some or all of these disease parameters. Overall, however, our previous characterization of Lipid 654, together with our presently reported results, suggests that bacterially derived Lipid 654 may be one mechanism connecting the commensal microbiome to human systemic autoimmune disease.
How can our present findings be understood in the context of both the microbiome and potential disease mechanisms in MS? In murine models of autoimmune diseases, TLR ligation can be disease inhibiting as well as disease enhancing. Touil et al.15 has demonstrated that the TLR3 ligand poly I:C leads to inhibition of experimental autoimmune encephalomyelitis when given to SJL mice 3 times after induction of experimental autoimmune encephalomyelitis. In these studies, the experimental autoimmune encephalomyelitis inhibition was mediated by TLR3-induced interferon-β and chemokine (C–C motif) ligand 2 production. Moreover, it has been documented that specific bacterial products derived from intestinal bacteria do indeed gain access to the systemic circulation.16 Thus, it is possible that Lipid 654 chronically enters the systemic circulation from commensal bacteria residing in the GI tract and oral cavity, and, as a TLR2 ligand, serves as an immunoregulatory factor normally dampening and regulating immune responses in healthy individuals.
One mechanism for this putative immunoregulatory function of serum Lipid 654 may involve maintenance of a certain level of both systemic tonic TLR2 signaling and interferon-β. The relevance of this mechanism is suggested by several recently reported studies. Artis and coworkers17 have reported evidence for an interplay between commensal bacteria and the ‘set-point' of systemic macrophage interferon signaling pathways. These authors suggest that this interplay involves low-level tonic signaling by commensal bacteria or commensal bacterial products that regulates the steady-state status of anti-viral type 1 interferon pathways in macrophages. Importantly, however, the components of the intestinal microbiota that mediate this macrophage ‘set-point' remain unknown. Most recently, Vogel and coworkers18 have documented that prior ligation of TLR2, both in vitro and in vivo, strongly ‘primes' macrophages for both increased interferon-β production and suppression of proinflammatory genes upon restimulation with TLR/Rig-I-like receptor ligands. Given these findings, our present results may suggest that the higher serum levels of commensal bacteria-derived Lipid 654, as we found in healthy individuals, normally function to regulate innate immune responses via maintaining such tonic ‘TLR2 priming'. Moreover, any lowering of serum lipid 654 levels, as we found in our MS patients, could be associated with a deficiency in this normal innate immune regulation and a resultant hyper-reactive ‘escape' of autoreactive immune responses. As such, lowered serum levels of Lipid 654 could represent a mechanism linking MS and the human microbiome.
In addition to alterations in the GI or oral microbiome, low levels of expression of Lipid 654 in the serum of MS patients could result from other potential mechanisms. These include an increase in the degradation of Lipid 654 (e.g., in the GI tract or in the serum) or an alteration in the normal pattern of trafficking of Lipid 654 in or out of the serum. These possibilities are presently under investigation in our laboratory.
At present, there is no blood biomarker that clearly distinguishes healthy individuals from those with MS. Such a biomarker could be critical in three important ways. First, a biomarker for MS could aid in the diagnosis of MS. At present, the diagnosis of MS is made with a combination of clinical symptoms and signs and MRI results and there is no single test that can make a definite diagnosis of multiple sclerosis. Because these parameters are less than diagnostically specific, a serum biomarker could function as a critical additional factor in making the diagnosis of MS. A more accurate diagnostic approach could allow for an earlier initiation of therapy, thus potentially decreasing long-term disability and disease progression. Equally important, given that both genetic and environmental factors are involved in the etiology of MS, such a diagnostic biomarker could potentially be used to screen family members of patients with MS to predict who might be at highest risk for the development of MS. The second way in which a biomarker could have a significant role is as a presymptom predictor of an impending exacerbation or progression of disease activity. The therapeutic approaches presently available for treating MS patients are fraught with serious adverse side effects. This use of a biomarker might then allow for a tailoring of the treatments on an ‘as needed' basis rather than on an everyday chronic regimen as is presently the case. This could minimize adverse side effects. Finally, a blood biomarker might shed significant light on the underlying pathogenic mechanisms in MS and subsequently represent a potential target for therapeutic intervention.
In the present study, we have identified that the level of expression of serum Lipid 654 may potentially represent such a serum biomarker for MS. Specifically, we have found that levels of serum Lipid 654 in MS patients are significantly lower than those of healthy individuals. The age and gender distribution of the MS and healthy populations studied were generally similar. As yet, we have found no significant correlation of serum Lipid 654 levels with the subtype of MS, duration of disease, gender, age or treatment modality. However, as more MS patients are studied, it is very possible that serum Lipid 654 levels will correlate with some or all of these disease parameters. The true specificity of lower levels of serum Lipid 654 for MS will require studying larger numbers of subjects, including those with other neurological diseases. Regarding other neurological diseases, in the present study, using samples derived in another institution, we found that MS serum Lipid 654 levels are significantly lower than those of Alzheimer's patients. Future studies in our laboratory will focus on serum Lipid 654 expression in additional patients with non-MS neurological diseases and in individual MS patients followed longitudinally over time.
Finally, given that our findings are based on MRM-mass spectrometry, any practical use of serum levels of Lipid 654 as a biomarker will require another approach for quantification. Our laboratory is presently generating monoclonal anti-Lipid 654 antibodies for this purpose. Nevertheless, our present results indicating a specificity of low serum Lipid 654 levels for MS patients ranging from 83 to 99% with an associated sensitivity ranging from 82 to 94% suggest that serum levels of Lipid 654 may prove to be a practical and clinically relevant serum biomarker for MS.
In summary, we have found that Lipid 654, a unique bacterial lipodipeptide derived from commensal bacteria, is found in significantly lower levels in the serum of patients with MS. These results thus identify a potential mechanism relating the mucosal microbiome to human systemic autoimmunity and, as such, a potential etiologic environmental factor and serum biomarker for MS.
Methods
Patient samples
All studies were performed using protocols approved by the IRB at the University of Connecticut Health Center (UCHC). Healthy controls were recruited from volunteer donors at UCHC. Patients with MS were recruited both from the MS clinic at UCHC as well as from other physicians in the state of Connecticut. Samples were only drawn from patients who had not eaten for at least 2 h. Blood samples in clotting tubes remained at room temperature for exactly 1 h. After centrifugation to separate the serum, the serum was pipetted into glass tubes using glass pipettes and frozen immediately at −80 °C. Only glass pipettes were used in all subsequent handling of the serum samples to avoid adsorption of lipids to plastic. Banked frozen serum samples were obtained from the Human Brain and Spinal Fluid Resource Center at UCLA.
Derivation of serum lipid samples
All blood samples were stored frozen until processing. For lipid extraction, the serum samples were thawed and lipids were extracted from 0.5 ml of each sample in organic solvent according to the method of Bligh and Dyer.6 After drying the organic solvent extracts under nitrogen, the lipid extracts were reconstituted in hexane:isopropanol:water (high-performance liquid chromatography (HPLC) solvent, 6:8:0.75, v/v/v) and vortexed. A small amount of each sample was dried and weighed, and a defined amount of each sample (500 μg) was transferred to a clean glass vial for either further processing or for MRM-mass spectrometry analysis.
Internal standard
The serine lipid internal standard was prepared by culturing Porphyromonas gingivalis in the brain–heart infusion broth19 supplemented with 13C(1)-sodium acetate (0.5 g l−1). Bacteria were harvested by centrifugation and total lipids were extracted and fractionated as described previously. 5, 19 The lipid fraction with an ion mass of m/z 660.7 coeluted with Lipid 654 using the HPLC chromatographic conditions described above. Identity of the internal standard was further confirmed by tandem mass spectrometry of its fragment ions. The background level of m/z 653.5 lipid in this internal standard lipid preparation was only 1.5% of the abundance of the m/z 660.7 species, and the Lipid 654 recovered in serum samples was corrected for carryover of m/z 654.5 in the internal standard. This 13C-labeled lipid fraction was used as the internal standard for quantifying Lipid 654 in serum samples. A calibration curve was generated using serially diluted lipid 660.7 standard added to vials containing serum lipids. The detection limit of lipid 660.7 was determined to be 50 fmol ml−1 of serum sample. The upper limit of dynamic range for quantitation was 10 nmol ml−1 of serum matrix. Linearity of quantitation was observed with the regression coefficient of R2>0.998.
Mass spectrometry
The serum lipid samples were injected using a Shimadzu (10ADVP) HPLC system interfaced with a QTrap 4000 mass spectrometer (AB Sciex, Framingham, MA, USA). Samples were introduced with a Shimadzu SIL-10A automatic sampler (Shimadzu North America, Columbia, MD, USA) and were eluted over a normal phase silica gel column (2.1 mm × 10 cm, 5 μm; Ascentis; Supelco; Sigma-Aldrich, St Louis, MO, USA) using isocratic separation with HPLC solvent as described above and the column temperature maintained at 40 °C. A lipid profiling analysis was performed under single quadrupole mass spectrometry mode to screen the lipid constituents in the serum samples, and the chromatographic parameters were set accordingly. The flow rate of HPLC separation was 0.15 ml min−1, for a period of 21 min, after which the solvent flow was increased to 0.25 ml min−1 for 9 min and a 10-port switching valve diverted residual lipid products to waste. The flow rate was then returned to 0.15 μl min−1 to stabilize for the next sample injection.
When both healthy control individual and MS patient samples were analyzed, the injection of MS and control serum lipid samples were alternated during MRM analyses. For every 4–5 samples analyzed, blank samples and internal standard samples were introduced to verify minimal carryover of serum Lipid 654 between samples. The optimal ion transitions, unique to Lipid 654, were chosen from previously acquired tandem mass spectra using product ion scan mode. The MRM collision energy and declustering energy were optimized for three selected product ion transitions using ramp scanning of the potentials while directly infusing the highly enriched Lipid 654. The optimal collision energy and declustering energy potentials for Lipid 654 and internal standard lipids were −52 and −90 V, respectively. Both entrance and collision cell exit potentials were set to −10 V. Lipid ion transition peaks were integrated using the Analyst software feature, and the percentage abundance of each lipid class was calculated from the integrated ion transition peaks.
Statistics
Wilcoxon's rank-sum test was used to determine significant differences between sample categories.
Acknowledgments
This work was supported by grants from the National MS Society—RG4070-A-6 (RBC), the National Institutes of Health—NIH 1 R01 DE021055–01A1 (FCN) and the Innovative Molecular Analysis Technologies program of the National Cancer Institute—1R21CA155536-01 (XY). We thank the University of Connecticut Clinical Research Center for their assistance in drawing the blood samples in this study, Kenneth Clark for his assistance in preparing the figures for this manuscript and the Human Brain and Spinal Fluid Resource Center at UCLA for making available serum samples from MS and Alzheimer's patients.
The authors declare no conflict of interest. An application is pending with the US Patent and Trademark Office, patent number PCT/US2011/058551 (RBC and FCN).
References
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Cervantes JL, Maciejewski MW, Farrokhi V, Nemati R, Yao X, et al. Serine lipids of Porphyromonas gingivalis are human and mouse Toll-like receptor 2 ligands. Infect Immun. 2013;81:3479–3489. doi: 10.1128/IAI.00803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108 (Suppl 1:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappert P, Bouladoux N, Naik S, Schwartz RH. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity. 2013;38:1198–1210. doi: 10.1016/j.immuni.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity. 2013;38:1187–1197. doi: 10.1016/j.immuni.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Umesaki Y, Honda K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Benef Microbes. 2010;1:327–334. doi: 10.3920/BM2010.0026. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly Y, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touil T, Fitzgerald D, Zhang GX, Rostami A, Gran B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol. 2006;177:7505–7509. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt M.C, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg G, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DJ, Polumuri SK, Pennini ME, Lai W, Xie P, Vogel SN. Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production. PLoS Pathogen. 2013;9:e1003479. doi: 10.1371/journal.ppat.1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols FC, Riep B, Mun J, Morton MD, Bojarski MT, Dewhirst FE, et al. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J Lipid Res. 2004;45:2317–2330. doi: 10.1194/jlr.M400278-JLR200. [DOI] [PubMed] [Google Scholar]