Abstract
Leishmaniasis is a parasitic disease that encompasses a range of clinical manifestations affecting people in tropical and subtropical regions of the world. Epidemiological and experimental data indicate that protection from disease can be achieved in most people. In addition, we know how the host immune system must respond to infection in order to control parasite growth. However, there is still no vaccine for use in humans. Here, we review our understanding of host immunity following Leishmania infection and also discuss recent advances in the development of vaccines to prevent leishmaniasis, highlighting a new promising approach that targets the parasite hemoglobin receptor.
Keywords: immunity, leishmania, parasites, vaccines
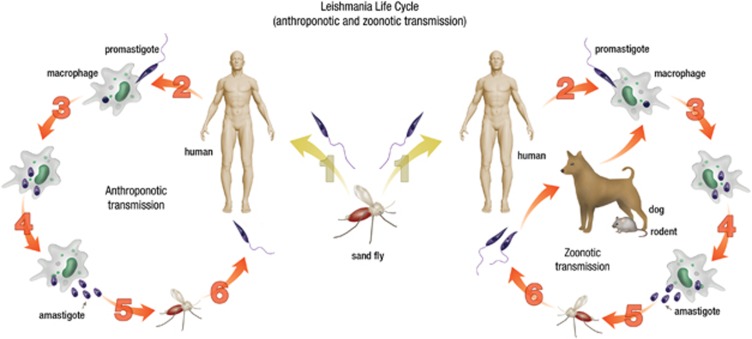
Leishmaniasis is a vector-borne disease caused by obligate, protozoan parasites of the genus Leishmania. These parasites are transmitted by 30 different species of Phlebotomine sand flies as extracellular, flagellated promastigotes and replicate as intracellular, aflagellate amastigotes in mononuclear phagocytes in mammalian hosts.1, 2 Leishmaniasis ranges from self healing, asymptomatic infection to localized skin lesions, and can develop into a life-threatening progressive visceral form of disease. Leishmaniasis is one of the world's most neglected diseases, affecting mainly very poor people in developing countries. It is prevalent throughout the tropical and subtropical regions of Africa, Asia, the Mediterranean, Southern Europe (old world) and South and Central America (new world). The disease is endemic in 88 countries, of which 72 are developing countries. Approximately 350 million people are at risk of contracting leishmaniasis and 1.5–2 million new cases occur annually.3 The transmission of Leishmania parasites is anthroponotic (human to vector to human) in the Indian subcontinent and Asia, while in Africa, Europe and the Americas', it is zoonotic (animal to vector to human), where dogs and rodents act as reservoir (Figure 1).4, 5, 6
Figure 1.
Life cycle and transmission of Leishmania parasites. The promastigote form of Leishmania parasites responsible for human disease (VL, CL and MCL) are injected into the skin as a female sand fly takes a blood meal (1), and are then taken up by host macrophages (2). Promastigotes convert to the non-flagellated, amastigote form inside macrophages (3) and then divide by binary fission (4). The amastigotes are released by the rupture of macrophages (5) and can then be taken up by a female sand fly during another blood meal. The amastigote form then converts in the promastigote form in the midgut of the sand fly and can then again be transmitted to another human (anthroponotic transmission) or to animals that act as reservoirs (zoonotic transmission) (6).
Visceral leishmaniasis (VL), commonly known as kala-azar, is caused by L. donovani and L. infantum in the Old World and L. chagasi in the New World. These parasites preferentially infect macrophages throughout the viscera, and parasites are usually found in the spleen, liver and bone marrow. Clinical features typically include long-term, low-grade fever, hepatosplenomegaly, weight loss, pancytopenia and polyclonal (IgG and IgM) hypergammaglobulinemia.7 Untreated VL will in most cases ultimately lead to death. Approximately 500 000 new cases of VL occur annually.7 Most (90%) VL cases occur in five countries, namely; India, Nepal, Bangladesh, Sudan and Brazil.3 India is one of the most important foci in the world for VL.3, 8 The state of Bihar and neighboring areas of eastern Uttar Pradesh and West Bengal remain particularly badly affected by VL.9 The annual incidence of VL in India is approximately 100 000 cases, and the state of Bihar accounts for more than 90% of these.9 The incidence of VL-human immunodeficiency virusHIV coinfection is now also a serious concern in these endemic regions.10
Cutaneous leishmaniasis (CL) is caused by L. tropica, L. aethiopica and L. major in the Old world, and by L. mexicana, L. guyanensis, L. amazonensis and L. braziliensis in the New world. CL is the most common form of leishmaniasis worldwide, representing 50–75% of all new cases. It can be very difficult to treat, long-lasting and disfiguring. According to the World Health Organization (WHO), the number of CL cases is around 1–1.5 million annually, and 90% of CL cases occur in seven countries; Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia and Syria.3, 6 CL is characterized by the development of an ulcerative skin lesion, which contains numerous parasites. Although the clinical features of CL can vary because of different causative species, a classical lesion starts as a papule or nodule at the site of parasite inoculation and slowly expands.6
Mucocutaneous leishmaniasis (MCL) occurs in the New World and is mainly caused by L. braziliensis and L. panamensis. These species can metastasize to mucosal tissue in the mouth and upper respiratory tract by lymphatic or haematogenous dissemination, and 90% of all MCL cases occur in Bolivia, Brazil and Peru. MCL can present from several months to years after the development of a cutaneous lesion. Although the pathogenesis of VL and CL is relatively well understood, the pathogenesis of MCL is still unclear, although there has been some recent headway in this area. It is believed that host genetic factors are important for the development of disease.
Control measures for leishmaniasis are heavily dependent on chemotherapy. Currently employed drugs are associated with severe toxic side effects and increasing parasite drug resistance.11, 12 This has forced researchers to think about other control measures, and in particular, the development and implementation of an effective vaccine. People cured of Leishmania infections develop lifelong immunity. Therefore, prevention of leishmaniasis through prophylactic vaccination is feasible. Advances in our understanding of Leishmania infection pathogenesis and the generation of host-protective immunity, together with completed Leishmania genome sequences, has opened new avenues for vaccine research. However, major challenges remain, including the translation of ideas from animal models to clinical settings, and the transition of products from the laboratory to the field. This review will highlight recent advancements in the development of vaccines to prevent and/or treat leishmaniasis, and discuss future prospects.
Protective immune responses in the host
A good understanding of immunity generated against pathogens is important for developing an effective vaccine. Our current understanding of host immune responses generated against Leishmania parasites is mainly based on the studies in animal models. Studies in mice show that protective immunity to Leishmania infection requires the development of interleukin-12-dependent, parasite-specific Th1 responses, characterized by interferon-γ and tumor necrosis factor production by CD4+ T cells.13, 14, 15 These inflammatory cytokines are required for the generation of reactive oxygen and nitrogen species by infected macrophages that enables killing of intracellular parasites. Recent advances have also been made in understanding immunoregulatory mechanisms that suppress parasite-specific CD4+ T-cell responses in human VL patients. These include the discovery that interleukin-10 produced by CD4+ T cells is a potent, autocrine inhibitor of interferon-γ production and promotes parasite persistence in spleen tissue from VL patients.16 Thus, interleukin-10 has been identified as a potential therapeutic target for use in combination with drug therapy or to improve therapeutic vaccine efficacy.
The generation of immunological memory is a requirement of effective vaccination. Studies on the generation of effector and central memory CD4+ T cells indicate that central memory T cells mediate long-term immunity to L. major infection, even in the absence of persistent parasites.17 Thus, defining the requirements and understanding the conditions for central memory CD4+ T-cell formation and maintenance will be helpful in vaccine design. Our knowledge that the majority of individuals infected with Leishmania parasites control parasite growth without causing serious disease,18, 19 combined with our understanding about the types of immune responses required for killing parasites and those that suppress this immunity, means that developing vaccines against leishmaniasis is a realistic goal.
Why do we need a vaccine to prevent and/or treat leishmaniasis?
Treatment of leishmaniasis is dependent on chemotherapy. The most commonly used drugs are pentavalent antimonials, oral miltefosine, amphotericin B, liposomal amphotericin B and paramomycin. A major problem is that these drugs are associated with problems of cost, toxicity, length and duration of treatment, route of injection (for example, intravenous infusion) and the development of parasite drug resistance.20 Pentavalent antimonials were the first line of treatment for many years, but increasing parasite resistance in endemic regions has limited their use. In the state of Bihar in India, almost 60% of cases are refractory to treatment with this drug.21 Consequently, amphotericin B is now used as the main drug to treat VL patients. However, this drug is also associated with toxicity and there are reports of drug-resistant parasites.22 Miltefosine was developed as an oral drug and showed an early promise; however, there are now increasing incidences of relapse in patients treated with this drug.23, 24, 25 Recently, a single dose of ambisome (lipid formulation of amphotericin B) was shown to be effective in treating VL patients, with a lower incidence of toxicity, compared with conventional treatment, in a multicentre clinical trial.26 Nevertheless, there are concerns that this type of drug-treatment protocol may promote the development of drug-resistant parasites. Therefore, combination drug therapy is being actively developed for use in endemic regions.27, 28 However, studies in a mouse model suggest that the L. donovani can develop resistance to drugs, even when they are used in combination.29 Therefore, despite advances in chemotherapeutic options, it is unlikely that chemotherapy alone will enable disease elimination, and hence there is an urgent need for an effective vaccine if long-term goals to controlling and eliminating this disease are to be achieved.
Past and present vaccine candidates
Despite different Leishmania species causing a range of clinical manifestations, genomic analysis indicates a large degree of sequence homology between species, suggesting it may be possible to generate broadly effective vaccines against different clinical diseases. An effective vaccine against leishmaniasis has existed in the past. This involved inoculation with live, virulent parasites, in a process called leishmanization. It was practiced successfully in the former Soviet Union, Middle East and Israel.30, 31 However, it was abandoned in most countries because of logistical problems and safety concerns, due to some individuals developing non-healing lesions and immune suppression.32
Whole-killed (autoclaved) Leishmania promastigotes were also tested as vaccines against CL and VL. Testing of killed parasite vaccines took place in Brazil in the early 1940s, and was then tested either alone or in combination with adjuvant in phase-I, II and III trials.33, 34 Clinical trials with autoclaved Leishmania, adjuvanted with BCG, showed that this approach could reduce the incidence of CL by 18–78%.35, 36 Similar trials were conducted in Iran, Sudan and Ecuador with variable safety and efficacy.37, 38, 39, 40, 41 Unfortunately, the autoclaved parasites showed decreasing potency with time, although studies with thimerosal preserved and non-autoclaved preparations have shown reduced effects of storage.42 However, concerns remain regarding the feasibility of developing killed, whole-parasite vaccines, including the variation in results obtained from different field and clinical trial sites in the past, and potential difficulties in producing such a product to good clinical manufacturing standards.
Various attenuated parasites have also been tested in animal models. These parasites are generally taken up by host cells in a similar way to virulent parasites, and persist for some time without replicating. This allows the host to mount robust immune responses against parasite antigens. Radio-attenuated and biochemically altered parasites have proven to confer good protection in mice and hamsters without adjuvant,43 although concerns regarding conversion back to virulence make the latter option questionable for human use. However, targeted elimination of virulence genes may overcome this problem and could produce attractive vaccine candidates against leishmaniasis. Genetically modified Leishmania parasites lacking essential genes like dyhydrofolate reductase, biopterin reductase or cystein proteases have been shown to stimulate protection against challenge with virulent parasite strains.44, 45, 46 The use of drug-sensitive Leishmania mutants47 alone or with adjuvant has been proposed as a mechanism to induce anti-leishmanial immunity, as has the use of non-pathogenic Leishmania species like L. tarantolae, which can stimulate protection against virulent L. donovani strains.48 However, the main problem with using killed or attenuated parasites are the concerns relating to safety and feasibility for large-scale use in the field.
Other approaches include using immunogenic surface antigens of Leishmania parasites as vaccine candidates. Several of these have been tested in mouse models and canine VL with data suggesting that protection against leishmaniasis can be achieved with defined candidate proteins. A saponin formulation of fucose mannose ligand that is expressed throughout the life cycle of parasite, was found to be safe, protective and immunogenic in an experimental mouse and hamster models.49, 50 This formulation has now become the Leishmune veterinary vaccine, licensed after a series of canine VL field studies.51, 52 Lipid formulations of soluble leishmania antigen from L. donovani were also tested as vaccine candidates in a hamster model of L. donovani infection, and this conferred protection with increased delayed type hypersensitive reactions in response to parasite antigen, enhanced parasite-specific antibody responses and improved parasite-specific T-cell responses.53, 54 Liposomal soluble leishmania antigen (from L. major) incorporated with phosphorothioate CpG ODN (PS CpG) or phosphodiaster CpG ODN (PO CpG) has also been tested in a mouse model of CL, and generated significant levels of protection.55 The excretory/secretory proteins isolated from culture supernatants of L. infantum and adjuvanted with muramyl dipeptide were tested in dogs experimentally infected with L. infantum.56 This vaccine, termed LiESAp-MDP, induced significant, long-lasting protection against canine VL in a field trial in an endemic area of France with naturally infected dogs.57 However, a major hurdle with these fractionated vaccines for human applications is their production to good clinical manufacturing standards, as well as gene variation and polymorphisms in field isolates.
Recombinant proteins, either alone or combined with adjuvant or with bacteria/recombinant virus as a delivery vehicles,58, 59 have also been tested as vaccines in preclinical studies. There have been significant efforts in recent time to identify recombinant antigens that can protect against Leishmania infection in experimental models. Some of these antigens include kinetoplastid membrane protein-11,60, 61 sterol 24-c-methyltranferase,62 amastigote specific protein A2,63 cysteine proteinase B,64 L. braziliensis elongation and initiation factor,65 K26/HASPB,66 Leishmania-activated C kinase,67 promastigote surface antigen 2,68 nucleoside hydrolase69 and surface expressed glycoprotein gp63.70 Although most of these recombinant antigens have been tested in animal models for their immunogenicity and protective efficacy, only a few have progressed to clinical trials in non-human primates, dogs or in preclinical human studies.71, 72 A multisubunit recombinant Leishmania vaccine, Leish-111F, containing a L. major homolog of eukaryotic thiole-specific antioxidant, L. major stress inducible protein-1 and L. braziliensis elongation and initiation factor, in formulation with MPL-SE, has been shown to provide protection in mouse models of CL and VL,73, 74 but failed to prevent canine VL caused by natural L. infantum infection.75 Nevertheless, Leish-111F/MPL-SE is the first defined vaccine candidate to progress to human phase-I and phase-II clinical trials in healthy volunteers in South America, CL and ML patients in Brazil and Peru and patients cured of VL in India.76, 77, 78, 79 As with all subunit vaccines, potential problems include variations in immunogenicity, based on human lymphocyte antigen expression in individuals, gene variation and polymorphisms in parasites, as well as the potential to drive selective pressure of parasites away from the molecules used in vaccines.
Finally, DNA vaccines to prevent leishmaniasis are also undergoing development and testing. This approach is not new,80 but has several advantages, such as low costs of production, stability of materials, sustained expression of relevant antigens and efficient generation of effector and memory immune responses.81 In addition, more than one antigen can be produced by a single construct. The non-methylated CpG motif of bacterial DNA provides the further advantage of activating innate immune cells to produce interleukin-12, which can prime CD4+ T cells to develop into Th1 cells.82 A list of vaccine antigen candidates being tested in DNA vaccines for CL and VL is shown in Table 1.
Table 1. Leishmania vaccine antigens being tested as candidate DNA vaccines.
| Candidate antigen | Models | Disease | Species | Reference |
|---|---|---|---|---|
| LACK | Dog, mice | VL, CL | L. donovani, L. chagasi, L. major, L. infantum | 86, 87, 88, 89, 90 |
| gp63 | Mice, dogs | CL, VL | L. major, L. infantum | 88, 90 |
| KMP11 | Mice | CL, VL | L. major L. donovani | 91, 92, 93 |
| CPB | Dogs, mice | VL, CL | L. infantum, L. major | 94, 95 |
| ORFF | Mice | VL | L. donovani | 96 |
| NH 36 | Mice | VL, CL | L. chagasi, L. maxicana | 97, 98 |
| TRYP | Dogs | VL | L. infantum | 90 |
| PSA-2 | Mice | CL | L. major | 88 |
Abbreviations: CPB, cysteine proteinase B; CL, cutaneous leishmaniasis; gp63, glycoprotein 63; KMP11, kinetoplastid membrane protein-11; LACK, Leishmania-activated C kinase; NH36, nucleoside hydrolase 36; ORFF, open reading frame F; PSA-2, promastigote surface antigen 2; TRYP, tryparedoxin peroxidase; VL, visceral leishmaniasis.
Therefore, despite many years of effort in identifying immunogenic parasite antigens and advances in vaccine technologies, there does not yet appear to be a vaccine candidate capable of delivering the level of protection needed for a disease elimination program. However, a significant advance was recently described in a study by Guha et al.,83 where they targeted the parasite hemoglobin receptor (HbR) using a DNA vaccine approach and tested it in an experimental model of VL. Leishmania parasites require heme for various metabolic activities; however, they lack an endogenous heme synthesis pathway, thus making them dependent on the host. HbR is expressed on the cell surface of the parasite and is conserved among different species. This receptor is not only important for hemoglobin endocytosis,84 but also has hexokinase activity, distinct from host hexokinase,85 which is important for regulating glycolysis. These important properties of parasite HbR led Guha and colleagues to test this molecule as a DNA vaccine candidate.
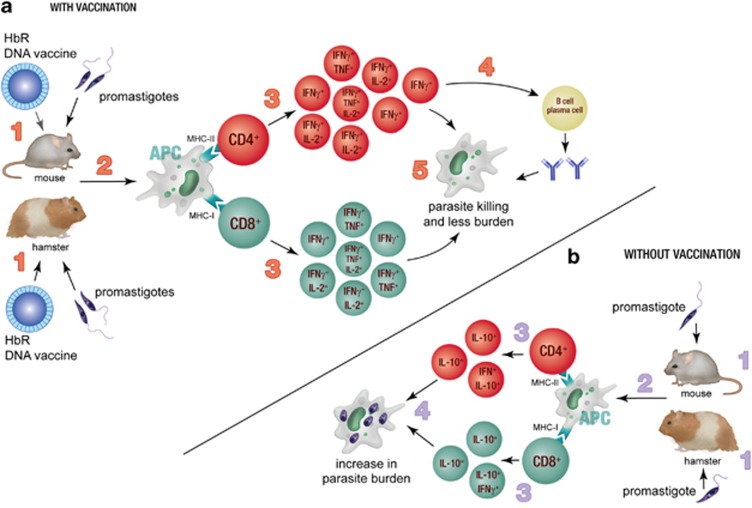
The success of any vaccine depends on many factors, including the generation of effective antigen-specific antibody responses, the priming and maintenance of parasite-specific T-cell responses and the generation of T cells with appropriate effector functions. Guha et al. found that patients with active VL produced reactive antibodies against HbR, and that these antibodies were able to inhibit parasite growth in a complement dependent manner in vitro. They also showed that HbR-DNA vaccination of mice stimulated the production of antigen-specific IgG2a antibodies and promoted the generation of antigen-specific T-cell responses that were able to produce multiple Th1-related cytokines simultaneously (that is, a polyfunctional T-cell response). Moreover, immunization with this DNA vaccine enabled sterile cure in hamsters and mice following challenge with virulent L. donovani (Figure 2). This is remarkable. These results were obtained in the absence of adjuvant, and thus highlight the potential of HbR as a DNA vaccine candidate for human use. However, further testing, including independent validation of efficacy, must be performed. In addition, and as mentioned earlier, DNA vaccines have shown great promise in animal models, but have not yet proven their utility in humans. There have been no clinical trials beyond phase-II to test DNA vaccines in humans. Thus, a major challenge for DNA vaccine candidates, such as parasite HbR, remains the demonstration of safety and efficacy in humans in both clinical trial and field settings.
Figure 2.
The effect of HbR-DNA vaccination. (a) Mice and hamsters were vaccinated with the HbR-DNA vaccine before infection with L. donovani promastigotes (1). The antigens were presented by antigen-presenting cells (APC) to CD4+ and CD8+ T cells in context of MHC-II and MHC-I molecules, respectively (2). This led to the enhanced proliferation of both CD4+ and CD8+ T cells (3) and generation of multifunctional CD4+ and CD8+ T cells. CD4+ T cells enhanced antibody generation by B cells/plasma cells (4). The combined effect of increased multifunctional CD4+ and CD8+ T cells and antibody resulted in complete clearance of the parasite (5). (b) Non-vaccinated mice and hamsters were infected with L. donovani promastigotes (1). Antigens were presented by APC to CD4+ and CD8+ T cells, (2) which resulted in limited T-cell proliferation and the generation of interleukin-10 producing cells (3) leading to enhanced parasite growth (4).
Concluding remarks: problems and future direction
Vaccination is the most cost-effective way of controlling infectious diseases. The success of vaccine development depends upon understanding the immunobiology of pathogen/host interactions, selection of appropriate vaccine candidates and choosing the right adjuvant or delivery vehicle. In addition, the vaccine must be able to generate long-lasting immunity, the best immune correlates of protection must be identified so vaccine efficacy can be efficiently evaluated and it must be able to transition from preclinical testing to human trials. However, despite a better understanding of immune regulatory pathways established following infection or vaccination, we still have a limited capacity to modulate these to clinical advantage with available adjuvants or drugs. Ideally, the vaccine should also be effective against all causative agents for a particular disease. This would allow significant saving in product development and testing, which will be an important consideration in future vaccine development programs. The development of a vaccine against leishmaniasis has been slow. However, increased knowledge gained in recent years in all of the above areas is paving the way for renewed efforts to make and test new vaccines aimed at preventing and/or treating leishmaniasis. If funding sources can be identified and commit to the long road of vaccine development, we are confident this is one parasitic disease that can ultimately be controlled.
Acknowledgments
Research in the authors' laboratory is funded by the Australian NHMRC and the Australia-India Strategic Research Fund. We thank Madeleine Flynn for help in preparing both figures.
References
- Pearson RD, Sousa AQ. Clinical spectrum of Leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector–host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Canavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Advances in parasitology. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. I J Antimicrob Agents. 2010;36 (Suppl 1:S62–S65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control. Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- Desjeux P. Global control and leishmania HIV co-infection. Clin Dermatol. 1999;17:317–325. doi: 10.1016/s0738-081x(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6:849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol. 2004;165:2123–2133. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbach W, Laskay T. The host response to Leishmania infection. Adv Immunol. 2000;74:275–317. doi: 10.1016/s0065-2776(08)60912-8. [DOI] [PubMed] [Google Scholar]
- Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, et al. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204:1134–1137. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, et al. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis. 2010;2:167–176. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Rai M, Sundar S. Management of visceral leishmaniasis: Indian perspective. J Postgrad Med. 2005;51 (Suppl 1:S53–S57. [PubMed] [Google Scholar]
- Srivastava P, Prajapati VK, Rai M, Sundar S. Unusual case of resistance to amphotericin B in visceral leishmaniasis in a region in India where leishmaniasis is not endemic. J Clin Microbiol. 2011;49:3088–3091. doi: 10.1128/JCM.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, et al. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis. 2012;6:e1657. doi: 10.1371/journal.pntd.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, et al. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis. 2013;56:1530–1538. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- Sundar S, Singh A. What steps can be taken to counter the increasing failure of miltefosine to treat visceral leishmaniasis. Expert Rev Anti Infect Ther. 2013;11:117–119. doi: 10.1586/eri.12.170. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expert Opin Pharmacother. 2013;14:53–63. doi: 10.1517/14656566.2013.755515. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez R, Manzano JI, Castanys S, Gamarro F. Leishmania donovani develops resistance to drug combinations. PLoS Negl Trop Dis. 2012;6:e1974. doi: 10.1371/journal.pntd.0001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt CL. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983;76:377–383. [PubMed] [Google Scholar]
- Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi RL, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17 (5:466–472. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- Velez ID, del Pilar Agudelo S, Arbelaez MP, Gilchrist K, Robledo SM, Puerta JA, et al. Safety and immunogenicity of a killed Leishmania (L.) amazonensis vaccine against cutaneous leishmaniasis in Colombia: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2000;94:698–703. doi: 10.1016/s0035-9203(00)90239-6. [DOI] [PubMed] [Google Scholar]
- Antunes CM, Mayrink W, Magalhaes PA, Costa CA, Melo MN, Dias M, et al. Controlled field trials of a vaccine against New World cutaneous leishmaniasis. Int J Epidemiol. 1986;15:572–580. doi: 10.1093/ije/15.4.572. [DOI] [PubMed] [Google Scholar]
- Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540–1543. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Ehsasi S, Shidani B, Bahar K. Stepwise safety trial of a killed Leishmania vaccine in Iran. Clin Dermatol. 1996;14:497–502. doi: 10.1016/0738-081x(96)00072-7. [DOI] [PubMed] [Google Scholar]
- Bahar K, Dowlati Y, Shidani B, Alimohammadian MH, Khamesipour A, Ehsasi S, et al. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol. 1996;14:489–495. doi: 10.1016/0738-081x(96)00071-5. [DOI] [PubMed] [Google Scholar]
- Armijos RX, Weigel MM, Aviles H, Maldonado R, Racines J. Field trial of a vaccine against New World cutaneous leishmaniasis in an at-risk child population: safety, immunogenicity, and efficacy during the first 12 months of follow-up. J Infect Dis. 1998;177:1352–1357. doi: 10.1086/515265. [DOI] [PubMed] [Google Scholar]
- Khalil EA, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, Musa B, et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356:1565–1569. doi: 10.1016/s0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- Kamil AA, Khalil EA, Musa AM, Modabber F, Mukhtar MM, Ibrahim ME, et al. Alum-precipitated autoclaved Leishmania major plus bacille Calmette-Guerrin, a candidate vaccine for visceral leishmaniasis: safety, skin-delayed type hypersensitivity response and dose finding in healthy volunteers. Trans R Soc Trop Med Hyg. 2003;97:365–368. doi: 10.1016/s0035-9203(03)90171-4. [DOI] [PubMed] [Google Scholar]
- De Luca PM, Mayrink W, Alves CR, Coutinho SG, Oliveira MP, Bertho AL, et al. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine. 1999;17:1179–1185. doi: 10.1016/s0264-410x(98)00338-7. [DOI] [PubMed] [Google Scholar]
- Rivier D, Bovay P, Shah R, Didisheim S, Mauel J. Vaccination against Leishmania major in a CBA mouse model of infection: role of adjuvants and mechanism of protection. Parasite Immunol. 1999;21:461–473. doi: 10.1046/j.1365-3024.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- Souza AE, Bates PA, Coombs GH, Mottram JC. Null mutants for the lmcpa cysteine proteinase gene in Leishmania mexicana. Mol Biochem Parasitol. 1994;63:213–220. doi: 10.1016/0166-6851(94)90057-4. [DOI] [PubMed] [Google Scholar]
- El Fadili A, Kundig C, Roy G, Ouellette M. Inactivation of the Leishmania tarentolae pterin transporter (BT1) and reductase (PTR1) genes leads to viable parasites with changes in folate metabolism and hypersensitivity to the antifolate methotrexate. J Biol Chem. 2004;279:18575–18582. doi: 10.1074/jbc.M400652200. [DOI] [PubMed] [Google Scholar]
- Veras P, Brodskyn C, Balestieri F, Freitas L, Ramos A, Queiroz A, et al. A dhfr-ts- Leishmania major knockout mutant cross-protects against Leishmania amazonensis. Mem Inst Oswaldo Cruz. 1999;94:491–496. doi: 10.1590/s0074-02761999000400011. [DOI] [PubMed] [Google Scholar]
- Muyombwe A, Olivier M, Ouellette M, Papadopoulou B. Selective killing of Leishmania amastigotes expressing a thymidine kinase suicide gene. Exp Parasitol. 1997;85:35–42. doi: 10.1006/expr.1996.4115. [DOI] [PubMed] [Google Scholar]
- Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun. 2005;73:6372–6382. doi: 10.1128/IAI.73.10.6372-6382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik-de-Sousa CB, Paraguai-de-Souza E, Gomes EM, Borojevic R. Experimental murine Leishmania donovani infection: immunoprotection by the fucose-mannose ligand (FML) Braz J Med Biol Res. 1994;27:547–551. [PubMed] [Google Scholar]
- Santos WR, Aguiar IA, Paraguai de Souza E, de Lima VM, Palatnik M, Palatnik-de-Sousa CB. Immunotherapy against murine experimental visceral leishmaniasis with the FML-vaccine. Vaccine. 2003;21:4668–4676. doi: 10.1016/s0264-410x(03)00527-9. [DOI] [PubMed] [Google Scholar]
- da Silva VO, Borja-Cabrera GP, Correia Pontes NN, de Souza EP, Luz KG, Palatnik M, et al. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (Sao Goncalo do Amaranto, RN) Vaccine. 2000;19:1082–1092. doi: 10.1016/s0264-410x(00)00339-x. [DOI] [PubMed] [Google Scholar]
- Parra LE, Borja-Cabrera GP, Santos FN, Souza LO, Palatnik-de-Sousa CB, Menz I. Safety trial using the Leishmune vaccine against canine visceral leishmaniasis in Brazil. Vaccine. 2007;25:2180–2186. doi: 10.1016/j.vaccine.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65:2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Dube A, Nadeem A, Khan S, Saleem I, Garg R, et al. Non PC liposome entrapped promastigote antigens elicit parasite specific CD8+ and CD4+ T-cell immune response and protect hamsters against visceral leishmaniasis. Vaccine. 2006;24:1800–1810. doi: 10.1016/j.vaccine.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Shargh VH, Jaafari MR, Khamesipour A, Jaafari I, Jalali SA, Abbasi A, et al. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine. 2012;30:3957–3964. doi: 10.1016/j.vaccine.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Lemesre JL, Holzmuller P, Cavaleyra M, Goncalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23:2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Lemesre JL, Holzmuller P, Goncalves RB, Bourdoiseau G, Hugnet C, Cavaleyra M, et al. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine. 2007;25:4223–4234. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- Xu D, McSorley SJ, Chatfield SN, Dougan G, Liew FY. Protection against Leishmania major infection in genetically susceptible BALB/c mice by gp63 delivered orally in attenuated Salmonella typhimurium (AroA- AroD-) Immunology. 1995;85:1–7. [PMC free article] [PubMed] [Google Scholar]
- Maroof A, Brown N, Smith B, Hodgkinson MR, Maxwell A, Losch FO, et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis. 2012;205:853–863. doi: 10.1093/infdis/jir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agallou M, Margaroni M, Karagouni E. Cellular vaccination with bone marrow-derived dendritic cells pulsed with a peptide of Leishmania infantum KMP-11 and CpG oligonucleotides induces protection in a murine model of visceral leishmaniasis. Vaccine. 2011;29:5053–5064. doi: 10.1016/j.vaccine.2011.04.089. [DOI] [PubMed] [Google Scholar]
- Carrillo E, Crusat M, Nieto J, Chicharro C, Thomas Mdel C, Martinez E, et al. Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine. 2008;26:1902–1911. doi: 10.1016/j.vaccine.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25:7450–7458. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001;20:59–66. doi: 10.1016/s0264-410x(01)00322-x. [DOI] [PubMed] [Google Scholar]
- Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24:2169–2175. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Skeiky YA, Kennedy M, Kaufman D, Borges MM, Guderian JA, Scholler JK, et al. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;161:6171–6179. [PubMed] [Google Scholar]
- Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–7071. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- Benhnini F, Chenik M, Laouini D, Louzir H, Cazenave PA, Dellagi K. Comparative evaluation of two vaccine candidates against experimental leishmaniasis due to Leishmania major infection in four inbred mouse strains. Clin Vaccine Immunol. 2009;16:1529–1537. doi: 10.1128/CVI.00153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E, Symons FM, Baldwin TM, Curtis JM, Scheerlinck JP. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect Immun. 1995;63:4261–4267. doi: 10.1128/iai.63.11.4261-4267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wabel MA, Tonui WK, Cui L, Martin SK, Titus RG. Protection of susceptible BALB/c mice from challenge with Leishmania major by nucleoside hydrolase, a soluble exo-antigen of Leishmania. Am J Trop Med Hyg. 2007;77:1060–1065. [PubMed] [Google Scholar]
- Connell ND, Medina-Acosta E, McMaster WR, Bloom BR, Russell DG. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Goto Y, Gidwani K, Cowgill KD, Sundar S, Reed SG. Evaluation of ex vivo human immune response against candidate antigens for a visceral leishmaniasis vaccine. Am J Trop Med Hyg. 2010;82:808–813. doi: 10.4269/ajtmh.2010.09-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh OP, Stober CB, Singh AK, Blackwell JM, Sundar S. Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis. PLoS Negl Trop Dis. 2012;6:e1874. doi: 10.1371/journal.pntd.0001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeiky YA, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20:3292–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23:5245–5251. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- Llanos-Cuentas A, Calderon W, Cruz M, Ashman JA, Alves FP, Coler RN, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine. 2010;28:7427–7435. doi: 10.1016/j.vaccine.2010.08.092. [DOI] [PubMed] [Google Scholar]
- Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29:3531–3537. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- Guha R, Gupta D, Rastogi R, Vikram R, Krishnamurthy G, Bimal S, et al. Vaccination with leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci Transl Med. 2013;5:202ra121. doi: 10.1126/scitranslmed.3006406. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Tripathi J, Tandon R, Raje M, Roy RP, Basu SK, et al. Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem. 1999;274:2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G, Vikram R, Singh SB, Patel N, Agarwal S, Mukhopadhyay G, et al. Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J Biol Chem. 2005;280:5884–5891. doi: 10.1074/jbc.M411845200. [DOI] [PubMed] [Google Scholar]
- Melby PC, Yang J, Zhao W, Perez LE, Cheng J. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect Immun. 2001;69:4719–4725. doi: 10.1128/IAI.69.8.4719-4725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro MJ, Zarate JJ, Hanke T, Rodriguez D, Rodriguez JR, Esteban M, et al. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine. 2003;21:2474–2484. doi: 10.1016/s0264-410x(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi K. A comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine. 2004;22:1631–1639. doi: 10.1016/j.vaccine.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Marques-da-Silva EA, Coelho EA, Gomes DC, Vilela MC, Masioli CZ, Tavares CA, et al. Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-gamma production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge. Parasitol Res. 2005;98:67–74. doi: 10.1007/s00436-005-0008-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cortes A, Ojeda A, Lopez-Fuertes L, Timon M, Altet L, Solano-Gallego L, et al. Vaccination with plasmid DNA encoding KMPII, TRYP, LACK and GP63 does not protect dogs against Leishmania infantum experimental challenge. Vaccine. 2007;25:7962–7971. doi: 10.1016/j.vaccine.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–7171. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- Bhaumik S, Basu R, Sen S, Naskar K, Roy S, KMP-11 DNA. immunization significantly protects against L. donovani infection but requires exogenous IL-12as an adjuvant for comparable protection against L. major. Vaccine. 2009;27:1306–1316. doi: 10.1016/j.vaccine.2008.12.053. [DOI] [PubMed] [Google Scholar]
- Guha R, Das S, Ghosh J, Naskar K, Mandala A, Sundar S, et al. Heterologous priming-boosting with DNA and vaccinia virus expressing kinetoplastid membrane protein-11 induces potent cellular immune response and confers protection against infection with antimony resistant and sensitive strains of Leishmania (Leishmania) donovani. Vaccine. 2013;31:1905–1915. doi: 10.1016/j.vaccine.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Rafati S, Salmanian AH, Taheri T, Vafa M, Fasel N. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine. 2001;19:3369–3375. doi: 10.1016/s0264-410x(01)00081-0. [DOI] [PubMed] [Google Scholar]
- Rafati S, Nakhaee A, Taheri T, Taslimi Y, Darabi H, Eravani D, et al. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23:3716–3725. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sharma A, Madhubala R. Ubiquitin conjugation of open reading frame F DNA vaccine leads to enhanced cell-mediated immune response and induces protection against both antimony-susceptible and -resistant strains of Leishmania donovani. J Immunol. 2009;183:7719–7731. doi: 10.4049/jimmunol.0900132. [DOI] [PubMed] [Google Scholar]
- Gamboa-Leon R, Paraguai de Souza E, Borja-Cabrera GP, Santos FN, Myashiro LM, Pinheiro RO, et al. Immunotherapy against visceral leishmaniasis with the nucleoside hydrolase-DNA vaccine of Leishmania donovani. Vaccine. 2006;24:4863–4873. doi: 10.1016/j.vaccine.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73:812–819. doi: 10.1128/IAI.73.2.812-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]