Abstract
Breastmilk protects infants against infections; however, specific responses of breastmilk immune factors to different infections of either the mother or the infant are not well understood. Here, we examined the baseline range of breastmilk leukocytes and immunomodulatory biomolecules in healthy mother/infant dyads and how they are influenced by infections of the dyad. Consistent with a greater immunological need in the early postpartum period, colostrum contained considerable numbers of leukocytes (13–70% out of total cells) and high levels of immunoglobulins and lactoferrin. Within the first 1–2 weeks postpartum, leukocyte numbers decreased significantly to a low baseline level in mature breastmilk (0–2%) (P<0.001). This baseline level was maintained throughout lactation unless the mother and/or her infant became infected, when leukocyte numbers significantly increased up to 94% leukocytes out of total cells (P<0.001). Upon recovery from the infection, baseline values were restored. The strong leukocyte response to infection was accompanied by a more variable humoral immune response. Exclusive breastfeeding was associated with a greater baseline level of leukocytes in mature breastmilk. Collectively, our results suggest a strong association between the health status of the mother/infant dyad and breastmilk leukocyte levels. This could be used as a diagnostic tool for assessment of the health status of the lactating breast as well as the breastfeeding mother and infant.
Keywords: breastfeeding, breastmilk, immune, immunoglobulin, infection, leukocyte
Through breastfeeding, the transfer of immune factors from the mother to the infant, which had started already in utero, continues postnatally.1, 2 These maternal factors protect the infant from infections and assist in the development of the infant's intestinal mucosa, gut microflora and own defences.3, 4, 5 Indeed, breastfed infants have a lower risk of necrotizing enterocolitis, and reduced susceptibility to gastrointestinal, respiratory and other infections than formula-fed infants.2, 3, 6, 7, 8, 9, 10 The immunomodulatory function of breastmilk is thought to be mediated by both cellular and biochemical components, including maternal leukocytes and biomolecules with antimicrobial, anti-inflammatory, antioxidant and prebiotic activities.6, 9, 11, 12 However, the underlying mechanisms through which these factors act to so consistently confer protection are yet poorly understood.13, 14
In addition to being a well-balanced source of amino acids specifically serving the growth needs of the human infant, breastmilk proteins, such as immunoglobulins and lactoferrin, exert antimicrobial and immunomodulatory activities, enhancing the infant's defence against pathogens.15 Secretory IgA (sIgA), the major human milk immunoglobulin, confers maternal acquired immunity to the infant.16 IgG and IgM antibodies are also present in breastmilk but in much lower concentrations, exerting protective roles in the infant.13 Despite variations observed in the concentrations of immunomodulatory bioactive factors in breastmilk both within and between women,13 the normal baseline levels under healthy conditions and infection-stimulated responses during the course of lactation are not well understood.
The immunoreactive biochemical factors of breastmilk are complemented by maternal leukocytes, which are thought to confer active immunity and influence the development of immunocompetence in the infant, as well as protect the mammary gland from infection.7, 17, 18, 19 Colostrum and mature breastmilk contain various cell types, including mature epithelial cells, progenitor cells, stem cells and leukocytes.20, 21, 22 While some are endogenous to the mammary gland, others (for example, leukocytes) migrate to this site from the lymphatic vessels and systemic circulation.23 Breastmilk leukocytes are thought to be somewhat different from their blood counterparts24, 25, 26 and to exert immunomodulatory functions in the infant via phagocytosis, secretion of antimicrobial factors, such as cytokines and immunoglobulins, or antigen presentation.9, 18, 19, 27 And they are thought to perform these functions not only inside the gastrointestinal tract of the infant, but also in distant tissues where they are transferred via the systemic circulation.1, 9, 24
The concentrations of immune factors in breastmilk are by no means stable. Breastmilk is a complex and dynamic fluid, with a changing composition that responds to infant feeding28 and the stage of lactation.14, 15 Most previous studies on breastmilk leukocytes focused on colostrum/early lactation milk, while very little is known about the leukocyte range in mature breastmilk under healthy conditions and how it changes together with biochemical components in response to infections of the mother, and indeed the infant. Evidence suggests that the health status of both the mother and the infant may influence breastmilk cellular content,29 suggesting an immunological link between the mothers and their breastfeeding infants. This may partly explain the great variability of breastmilk leukocyte content among women reported in the literature. An additional explanation may be provided by the basic microscopy-based leukocyte identification techniques previously utilized, which pose limitations in the context of breastmilk leukocytes, some of which share morphological traits with mammary epithelial subtypes.30 Thus, more accurate and consistent methodology is needed to quantify breastmilk leukocyte content and composition, and establish the baseline levels of immune factors in breastmilk of healthy dyads during lactation and how these may be altered by infection. This is a critical step to improving understanding of viral transmission during the breastfeeding period, and how leukocytes and other breastmilk components protect the infant.31, 32 In addition to the infant, the breast is also thought to be protected by the influx of leukocytes to mammary infection sites.19 Therefore, the leukocyte status of breastmilk may provide a novel means for assessing the health status of the lactating breast, the only metabolically significant organ in the body, without clinical tests to assess its normality. In this context, it is of interest that similar tests are routinely used by the dairy industry to assess the quality of bovine milk and the presence of intramammary infection in the dairy cow.33, 34 To address the question of whether the leukocyte content of breastmilk can be used to assess the health status of the breastfeeding dyad, we examined whether there is a baseline range of leukocytes in mature breastmilk from healthy dyads and how this is influenced by different infections of the mother and/or the infant.
Results
Healthy mother/infant dyads have a low baseline level of leukocytes in breastmilk
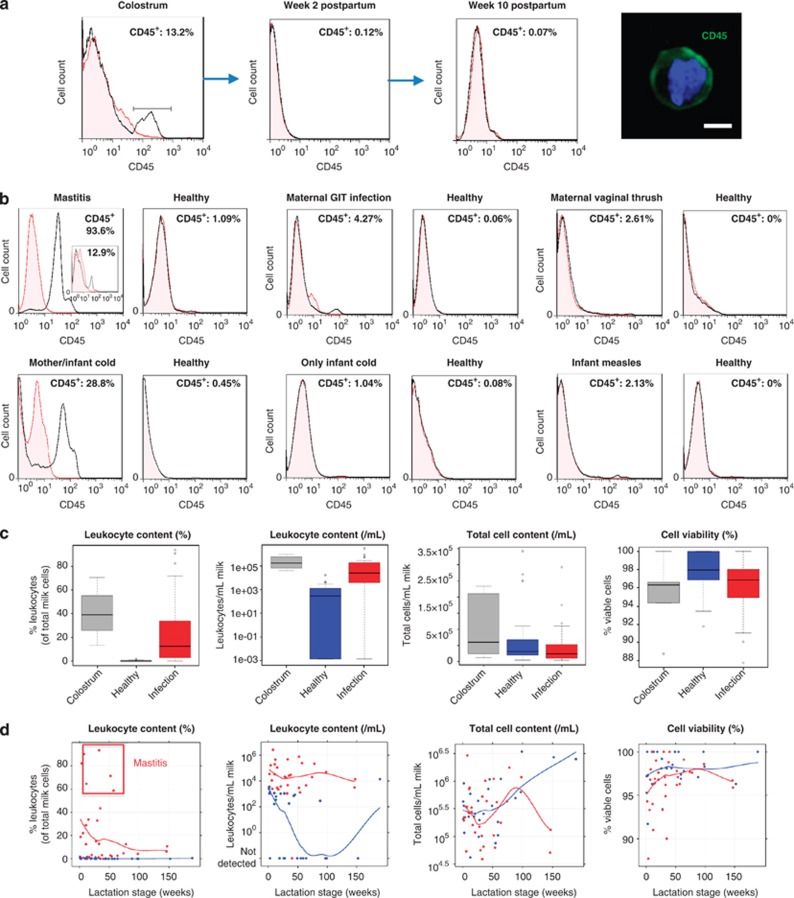
Fluorescence-activated cell sorting (FACS) quantification of CD45 protein expression was done by an analytic gating strategy that excluded interference by dead/dying cells and/or fat globules (Supplementary Figure S1a). Although colostrum always contained considerable levels of leukocytes (13.2–70.4% leukocytes of total milk cells; 32 175–784 080 viable leukocytes per ml colostrum), these rapidly decreased toward the end of the first week postpartum to reach a baseline level in transitional milk (0–1.7% leukocytes of total milk cells; 0–3450 viable leukocytes per ml milk) and mature breastmilk (0–1.5% leukocytes of total milk cells; 0–1151 viable leukocytes per ml milk) when both the breastfeeding mother and infant were healthy (Figure 1a; Supplementary Figures S1a,b; Tables 1 and 2). The low baseline level of mature breastmilk leukocytes under healthy conditions was maintained even at late lactation stages (years 2–4 postpartum) (Table 1) and was found to fluctuate within certain limits (0–2% leukocytes of total milk cells) both between and within individuals.
Figure 1.
Maternal and/or infant infections stimulate a breastmilk leukocyte response. (a) Reduction of CD45+ leukocyte numbers from colostrum to a low baseline level in transitional and mature breastmilk. A breastmilk CD45+ (green) leukocyte is shown; blue: nucleus; scale bar: 2.5 μm. (b–d) Effect of maternal or infant infections on breastmilk cells in selected examples (b), and in the overall study cohort (N=21) (c), and changes during lactation (d) (blue: healthy dyad; red: under infection). Local regression (loess) smoothers show the overall pattern in the data. In the mastitis example in b, the insert shows the leukocyte content of breastmilk collected on the same expression from the other non-mastitic breast.
Table 1. Total cell, leukocyte, immunoglobulin (sIgA, IgG, IgM) and lactoferrin contents of human breastmilk at different stages of lactation, and responses to infections of the mother/infant dyad.
| Breastmilk component | Health status | Colostruma | Transitional milkb | Months 1–3 | Months 4–6 | Months 7–9 | Months 10–12 | Year 2 | Late lactationc | Involution milkd |
|---|---|---|---|---|---|---|---|---|---|---|
| n=5h | n=6 h, 1i | n=2 h, 7i | n=4 h, 4i | n=4 h, 6i | n=2h, 2i | n=6 h, 7i | n=3h, 3i | n=1 | ||
| Total cellse per ml milk | Healthy | 110 000–2 250 000 | 113 492–883 333 | 228 395–255 769 | 40 000–588 542 | 97 500–433 333 | 706 667–1 066 667 | 75 926–1 075 000 | 633 333–3 357 143 | 2 600 000 |
| Infection | — | 183 333 | 50 000–2 867 383 | 115 278–321 918 | 37 000–504 951 | 437 500–1 000 000 | 60 714–1 708 333 | 49 166–1 166 667 | — | |
| % Leukocytes | Healthy | 13.2–70.4 | 0.0–1.65 | 0.07–0.45 | 0.0–1.52 | 0.0–1.09 | 0.08–0.1 | 0.0–0.06 | 0.0–0.55 | 0.0 |
| Infection | — | 18.8 | 0.72–90.5 | 1.1–33.9 | 1.08–93.6 | > 3 | 2.13–71.7 | 4.27–10.8 | — | |
| Leukocytese per ml milk | Healthy | 32 175–784 080 | 0–3450 | 160–1151 | 0–1025 | 0–1063 | 707–853 | 0–288 | 0–13 750 | 0 |
| Infection | — | 34 467 | 2400–2 594 982 | 2164–109 130 | 1065–472 634 | > 30 000 | 1293–759 834 | 3127–49 817 | — | |
| sIgA (μg ml−1) | Healthy | 1428–2178 | 131–1096 | 534–1276 | 257–960 | 496–1350 | 401–1044 | 137–1243 | 976–1991 | 1761 |
| Infection | — | 922 | 36–1418 | 652–1711 | 611–1509 | 714–789 | 173–2002 | 1657–1906 | — | |
| IgG (μg ml−1) | Healthy | 5.3–12.2 | 2.8–9.7 | 6.4–12.4 | 4.6–10.8 | 4.0–16.4 | 5.0–16.1 | 4.0–10.4 | 4.6–17.8 | 22.9 |
| Infection | — | 13.0 | 6.6–17.1 | 4.8–10.1 | 5.6–14.4 | 7.6–8.8 | 2.3–25.9 | 9.4–13.4 | — | |
| IgM (μg ml−1) | Healthy | 16.2–56.1 | 8.2–29.8 | 10.6–14.9 | 6.5–11.6 | 4.2–23.7 | 8.8–23.3 | 2.9–13.0 | 7.1–23.1 | 100.4 |
| Infection | — | 10.2 | 4.5–19.8 | 10.1–15.4 | 12.6–21.8 | 14.4–19.3 | 5.9–31.1 | 10.5–23.5 | — | |
| Lactoferrin (g l−1) | Healthy | 6.3–7.7 | 2.1–5.2 | 2.5–2.9 | 1.9–3.7 | 1.3–4.0 | 1.2–3.9 | 2.3–4.5 | 3.3–5.8 | 6.2 |
| Infection | — | 4.3 | 2.9–3.7 | 2.0–3.7 | 1.6–3.3 | 1.2–3.6 | 2.1–4.6 | 4.6–5.6 | — |
Abbreviation: sIgA, secretory IgA.
(h=healthy and i=infection).
Colostrum was defined as breastmilk collected between days 0–4 postpartum.
Transitional milk was defined as breastmilk collected between day 5 and week 3 postpartum.
Late lactation was defined as the period between months 25 and 48 postpartum.
Involution milk was collected 5 days after baby weaned off breastmilk (month 38 postpartum).
Total cells per ml and leukocytes per ml refer to total viable cells per ml and viable leukocytes per ml, respectively.
Table 2. Statistical comparison of the levels of measured variables between milk samples grouped by type (colostrum versus mature breastmilk from healthy mother/infant dyads) or health status of the mother/infant dyad (healthy versus under infection), taking into account individual differences.
| Response | Transform | Healthy (mature milk)Value |
Under infection (mature milk) |
Colostrum |
||
|---|---|---|---|---|---|---|
| Diff | P-value | Diff | P-value | |||
| Total cell content (per ml milk) | Loge | 12.8 | −0.3 | 0.271 | 0.5 | 0.326 |
| Viable cell content (per ml milk) | Loge | 12.8 | −0.3 | 0.253 | 0.5 | 0.353 |
| Leukocyte contenta (per ml milk) | Loge(x+0.5) | 3.8 | 5.9 | <0.001 | 7.2 | <0.001 |
| % Total cell viability (of total cells) | None | 97.8 | −1.5 | 0.043 | −2.6 | 0.056 |
| % Leukocytesa (of total cells) | Loge(x+0.5) | −0.25 | 2.5 | <0.001 | 3.6 | <0.001 |
| sIgA (μg ml−1) | None | 860.7 | 169.9 | 0.034 | 1288.5 | <0.001 |
| IgG (μg ml−1) | Loge | 2.01 | 0.22 | 0.048 | 0.07 | 0.710 |
| IgM (μg ml−1) | Loge | 2.49 | 0.064 | 0.606 | 0.864 | <0.001 |
| Lactoferrin (g l−1) | None | 3.41 | −0.08 | 0.658 | 3.88 | <0.001 |
Abbreviation: sIgA, secretory IgA.
P-values compare each group with the ‘Healthy (mature milk)' group.
For leukocyte content and percentage, the data were transformed using the additive constant 0.5 for both the square root and the log transformations owing to the zeroes in the data.57
Infections of the mother/infant dyad stimulate a leukocyte response in breastmilk
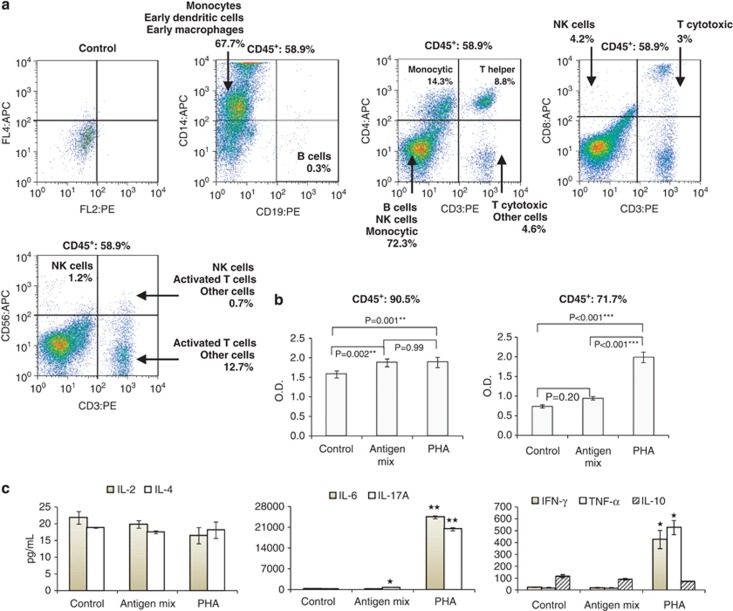
Infections of the breast, other organs or general maternal infections all stimulated a leukocyte response in breastmilk that ranged from 0.7% (sore breast) to 93.6% (mastitis) leukocytes of total milk cells (P<0.001) (Figures 1b–d; Tables 1 and 2). Severe breast infections, such as mastitis, stimulated a greater leukocyte response (P<0.001). The effect of systemic and other organ infections on breastmilk leukocyte content was in agreement with the observed effect of mastitis of one breast on the breastmilk leukocyte content of the other (mastitis-free) breast (Figure 1b). A small increase in breastmilk leukocyte content was also observed when only the infant had an infection, while the mother was asymptomatic (P=0.046) (Figure 1b). No difference was seen between pre- and post-infection baseline leukocyte levels (P=0.48) (Figure 1b; Supplementary Figure S1c). Importantly, the leukocyte response to infection and recovery, as well as transition from colostrum to transitional milk, was rapid (Figures 1a and b). In contrast to leukocytes, milk total cell and viable cell contents did not significantly vary by health status or milk type (Figure 1c; Table 2). However, total cell content significantly increased (P=0.002) with lactation stage (Figure 1d; Table 1). The latter did not significantly influence % cell viability (P=0.09) (Figure 1d; Table 1). Milk leukocyte content did not significantly change after week 1 postpartum as long as the dyad was healthy (P=0.36) (Figure 1d). Leukocytes in breastmilk from women with mastitis contained monocytes, macrophages, dendritic cells, T-helper cells, cytotoxic T cells, natural killer cells and a small population of B lymphocytes (Figure 2a). Infection-stimulated leukocytes contained activated cell subsets that responded to viral antigens and PHA in culture with increased proliferation rate and altered expression of interleukin (IL)-6, IL-17A, interferon-γ and tumor necrosis factor-α (Figures 2b and c).
Figure 2.
Mastitis-specific breastmilk leukocyte subpopulations and properties. (a) Breastmilk collected from women with mastitis contained distinct leukocyte subpopulations, including monocytes, macrophages, T helper cells, cytotoxic T cells, NK (natural killer) cells and B cells. These cells responded to viral antigen and PHA stimulation in vitro via increased proliferation (b) and production of cytokines (c). *P<0.05, **P<0.01.
Infections of the mother/infant dyad stimulate a humoral immune response in breastmilk
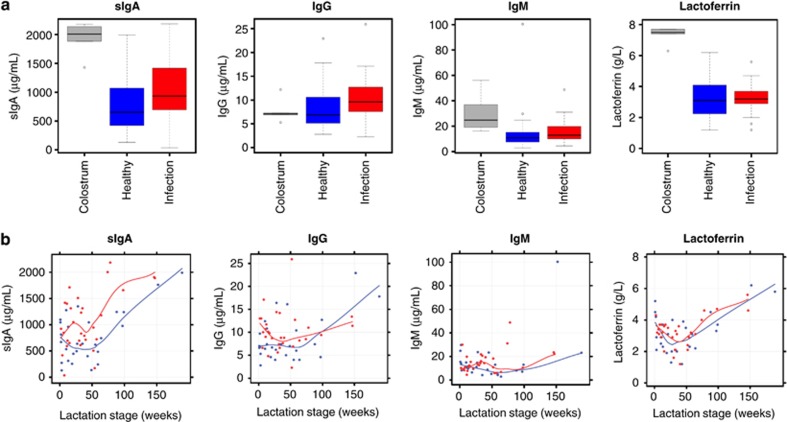
In addition to breastmilk leukocyte response to maternal/infant infection, a less consistent but often significant humoral immune response was observed. sIgA was higher in colostrum compared with mature breastmilk from healthy dyads (P<0.001) (Figure 3a; Tables 1 and 2). In mature breastmilk, sIgA concentration increased only during infection of the mother and/or the infant (P=0.034) (Figure 3a; Tables 1 and 2), and this increase was stronger in organ-specific infections (Table 3). IgG concentration was generally low (2.8–22.9 μg ml−1) (Table 1), with no marked difference between colostrum and mature breastmilk from healthy dyads (P=0.71), and marginally increased with maternal or infant infection (P=0.048) (Figure 3a; Tables 1 and 2). No difference was seen between pre- and post-infection baseline sIgA and IgG levels (P=0.37 and P=0.66, respectively). In few subjects, sIgA and/or IgG concentration was higher in the post-recovery sample, suggesting a potential delayed response to infection (Supplementary Figure S1d). In contrast to sIgA and IgG, no significant changes were seen for IgM or lactoferrin with infections (P=0.61 and P=0.66, respectively), although colostrum and transitional milk concentrations were higher than in mature breastmilk from healthy dyads (P<0.001) (Figure 3a; Tables 1 and 2). Infant age had a profound effect on breastmilk sIgA (P<0.001), IgG (P=0.045) and lactoferrin (P=0.008) concentrations (Figure 3b; Table 1). In the data set of healthy dyads, an initial sIgA decrease from colostrum to mature breastmilk up to around week 25 and a plateau until week 50 was followed by an increase in later lactation (Figure 3b). IgG concentration was constant for the first 60 weeks postpartum, but increased in later lactation (Figure 3b; Table 1). Lactoferrin concentration initially decreased up to around week 25 and then increased as lactation progressed (Figure 3b; Table 1). Involution seemed to influence the biochemical and total cellular, but not the leukocyte, content of breastmilk, with marked increases in these components (Table 1).
Figure 3.
Maternal and/or infant infections stimulate a breastmilk humoral response. (a) Effect of maternal or infant infections on breastmilk biochemical content (sIgA, IgG, IgM and lactoferrin) in the overall study cohort (N=21). (b) Changes of the breastmilk biochemical content during lactation under healthy conditions (blue) and under infection (red). Local regression (loess) smoothers show the overall pattern in the data.
Table 3. Effects of different types of infection on breastmilk cellular and biochemical composition.
| Response | HealthyValue |
Infant only |
Breast-related |
Cold |
Other infections |
||||
|---|---|---|---|---|---|---|---|---|---|
| Diff | P-value | Diff | P-value | Diff | P-value | Diff | P-value | ||
| Total cell content (per ml milk) (loge) | 12.8 | −0.9 | 0.127 | 0.6 | 0.133 | −0.6 | 0.094 | −0.4 | 0.348 |
| Viable cell content (per ml milk) (loge) | 12.8 | −0.9 | 0.123 | 0.6 | 0.143 | −0.6 | 0.085 | −0.4 | 0.345 |
| Leukocyte contenta (per ml milk) (loge(x+0.5)) | 3.7 | 4.3 | 0.046 | 6.7 | <0.001 | 6.1 | <0.001 | 5.9 | 0.0004 |
| % Total cell viability (of total cells) | 97.8 | −0.9 | 0.571 | −1.7 | 0.093 | −2.1 | 0.025 | −0.2 | 0.834 |
| % Leukocytesa (of total cells) (loge(x+0.5)) | −0.3 | 1.1 | 0.064 | 3.2 | <0.001 | 2.8 | <0.001 | 2.1 | <0.001 |
| sIgA | 858 | 88 | 0.632 | 174 | 0.155 | 144 | 0.197 | 302 | 0.042 |
| IgG (loge) | 2.02 | 0.07 | 0.738 | 0.58 | 0.0003 | 0.04 | 0.758 | 0.12 | 0.481 |
| IgM (loge) | 2.49 | 0.21 | 0.474 | 0.05 | 0.796 | 0.02 | 0.913 | 0.16 | 0.493 |
| Lactoferrin | 3.4 | −0.2 | 0.638 | −0.1 | 0.787 | −0.1 | 0.654 | 0.1 | 0.766 |
Abbreviation: sIgA, secretory IgA.
Groups include: infant-only infection (N=3), breast-related infection (N=9), cold (N=12), other organ-specific infections (eye, ear, vaginal, urinary tract and gastrointestinal infections; N=6) and no infection/healthy (N=28). P-values compare infection groups with the ‘Healthy' group.
For leukocyte content and percentage, the data were transformed using the additive constant 0.5 for both the square root and the log transformations owing to the zeroes obtained.57
Breastmilk immune response differs between infection types
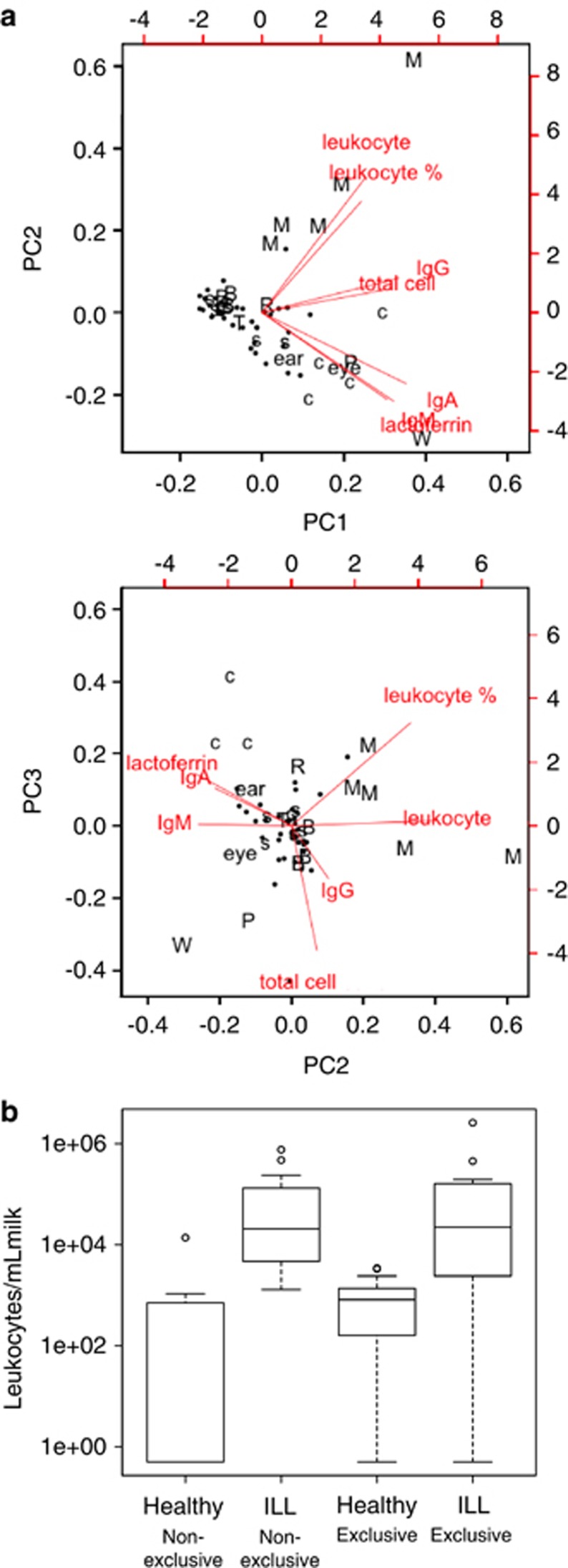
Breastmilk leukocyte content was significantly higher for all infection types compared with the healthy baseline, with the weakest response seen for infant infections (P=0.046), and the strongest response for breast infections (P<0.001), particularly mastitis (Table 3). A decrease in % cell viability with infection was observed only for maternal colds (P=0.025). Total breastmilk cell content increased during breast-related infections, being associated with a stronger leukocyte response in mastitis compared with less severe breast infections (Table 3). Principal component analysis (PCA) demonstrated distinctive response patterns for specific sample types. Mastitis (N=5) clustered separately from other infections, being strongly associated with breastmilk leukocyte content (Figure 4a). Weaning (N=1) and menstruation (N=1) were separate from the rest of the healthy data set. Colostrum also tended to cluster differently from the healthy data set (Figure 4a).
Figure 4.
Interactions between breastmilk components in relation to specific infections, stage of lactation and exclusivity of breastfeeding. (a) Biplots illustrating the relationships between seven breastmilk immune markers as determined from a PCA. The first two components (PC1 and PC2) are shown in the top plot, and the second and third components (PC2 and PC3) are seen in the middle plot. The combination of these two plots provides a three-dimensional representation of the multivariate data. Dots illustrate the individual records, and the vectors indicate the relative weights of the markers on each of the principal components. Vector labels include: ‘Leukocyte': leukocytes per ml milk, ‘total cell': total cells per ml milk, ‘c': colostrum, ‘•': healthy, ‘M': mastitis, ‘R': recovering mastitis, ‘B': other breast conditions, ‘eye' and ‘ear': maternal eye and ear infections, respectively, ‘W': weaning, ‘P': periods, ‘T': vaginal thrush, ‘s': other infections of the mother or baby. Red lines show the relative weights of each of the measures on each of the components. (b) Effect of breastfeeding status (exclusive, non-exclusive) on milk leukocyte content under healthy and infection conditions.
Non-exclusive breastfeeding is associated with lower baseline milk leukocyte levels
Breastfeeding status significantly related to milk leukocyte content. Under healthy conditions, the baseline breastmilk leukocyte content was lower in non-exclusively breastfed infants (P=0.024), the majority of whom received breastmilk with no detectable leukocytes (Figure 4b). The infection response in both exclusive and non-exclusive breastfeeding dyads was such that similar leukocyte levels were seen during infection (Figure 4b).
Discussion
Breastmilk has long been known to contain leukocytes,30 but their regulation and variation between individuals and stages of lactation are not well established. Here, we show that mature breastmilk from healthy mother/infant dyads retains a low baseline level of 0–2% leukocytes during lactation. Infections of the breast, other organs or general infections of the mother or the infant stimulate a rapid breastmilk leukocyte response, with restoration of the baseline levels upon recovery. We suggest that the general health status of the dyad, recent health history, environmental factors that they are exposed to and/or the severity of an infection determine the variation in breastmilk leukocyte content during the healthy and infection statuses. Other factors not considered in the present study, such as the menstrual cycle, may also influence breastmilk leukocyte content and warrant further investigation.
The humoral breastmilk response to infection was less consistent and more variable than the cellular response, with sIgA and IgG showing the most consistent humoral response. The most abundant immunoglobulin in breastmilk was sIgA, followed by IgM and IgG, consistent with previous literature.35, 36, 37, 38, 39 The variation observed in humoral breastmilk responses to infection could be explained by a differential response of each immunological biochemical factor to different infections, a delayed response in some dyads/infection types, or an underlying health condition of the mother and/or the infant during collection of the post-recovery samples. The latter is unlikely as no evidence for a leukocyte response was found in these post-recovery samples. Of note, the most consistent humoral immune response was observed during mastitis, which represents a severe infection of the breast, and is in agreement with the strong leukocyte response.
Indeed, mastitis recorded the highest milk leukocyte contents, which sometimes delayed to return to baseline levels, particularly when there was a delay in diagnosis and treatment. Mastitis is common among breastfeeding women in early stages of lactation, being associated with inadequate emptying of the breast.40, 41, 42 This can stimulate leukocyte trafficking to the breast, explaining the high levels of milk leukocytes detected, with distinct profiles including activated T cells that responded to antigen stimulation via proliferation and cytokine production. Milder breast infections showed contrasting breastmilk responses (Table 3). Thus, the distinct changes in breastmilk leukocyte levels and properties during mastitis could be used as novel diagnostic tools for this condition, in which early diagnosis is important for a successful treatment and the maintenance of breastfeeding for longer periods.40, 41, 42 This is often not possible due to limited understanding of its causes, the variability in associated microorganisms40, 41, 42 and lack of tests that assess the health status of the lactating breast. The measurement of leukocyte content and subtypes in breastmilk using FACS, together with assessment of milk cytokine profile and dominant microorganisms, could be used as novel diagnostic tools to allow fast treatment of mastitis and other breast conditions during lactation.
Different infections showed differential effects on breastmilk composition. Some infections related to a stronger humoral response, while others to a stronger cellular response (Figure 4b). The latter was particularly seen in breast infections, which in contrast to other infections, were accompanied by an increase in total cell content. Although in milder breast infections (sore breast and blocked duct), non-immune cell populations contributed to the increase in total cell content, in mastitis this increase was significantly associated with a leukocyte response (Table 3).
In addition to maternal infection, a small but significant breastmilk leukocyte response was observed when the infant had an infection, but the mother was asymptomatic. This finding is supported by a recent study from Riskin et al.43 that also reported a response of breastmilk leukocytes to active infection of nursing infants. This further reinforces the potential of breastmilk leukocyte measurement by FACS to be used in assessment of the health status of both the breastfeeding mother and infant. While the mechanism behind the leukocyte movement into the breast during an infection of the infant is still unclear, exposure of the mother to the infant's infection may stimulate an immunological response in the mother that is manifested without evident symptomatology, but which influences breastmilk leukocyte content. A potential way for this to happen is during breastfeeding. During a milk ejection, duct pressure increases, milk ducts dilate and milk flows toward the nipple/baby's mouth. As oxytocin wears off, duct pressure decreases, milk ducts reduce in size and milk flows backwards,44 likely together with saliva from the baby's mouth. This is a time when it is possible that microorganisms from the infant could be transferred back into the breast, most likely during a pause in suckling, stimulating a local immune response.
In addition to infection, the stage of lactation influenced breastmilk composition. With the exception of IgG concentration, significant decreases were seen in immunomodulatory biomolecules and leukocyte content from colostrum to mature breastmilk, consistent with previous reports.35, 36, 37, 38, 39 From week 2 postpartum onwards, total cell content increased with lactation stage, but leukocyte content was maintained at a low baseline level across lactation under healthy conditions. This is in accordance with previous findings showing a leukocyte change early on from colostrum to mature breastmilk,14, 45, 46, 47 and with a recent study showing low levels of leukocytes in mature breastmilk under healthy conditions in a Chinese population.48 Percentage cell viability did not change significantly across lactation, in contrast to immunoglobulins and lactoferrin, which were differentially affected during the course of lactation, in accordance with previous studies.35, 36, 37, 38, 39 Involution milk showed marked increases in total cell content and biochemical immune factor concentrations, suggesting that involution influences the biochemical and total cellular, but not the leukocyte, content of breastmilk. This is in support of the notion that cells from the epithelial compartment mediate clearing of apoptotic cells via phagocytosis in the involuting gland.49
Exclusive breastfeeding was associated with a higher baseline level of leukocytes in breastmilk under healthy conditions. This may be associated with overall suckling time on the breast, and suggests that infants that are non-exclusively breastfed receive not only lower breastmilk volumes, but also breastmilk that contains fewer leukocytes. The higher breastmilk baseline leukocyte levels in exclusively breastfeeding dyads may contribute to the lower incidence of infections observed in this group, further reinforcing the protective role of breastmilk leukocytes for the infant.3, 6, 9
The rapid response of breastmilk leukocytes to infections of the mother and the infant suggests that this is a tightly regulated process aimed at conferring additional immunological support to the infant. In situations where individuals do not have ready access to medicine, particularly in developing countries, breastfeeding-mediated protection may be a determining factor in infant recovery and survival. Formula does not offer this protection and the ability to adjust to infant needs. Thus, these findings present new information that is relevant to updating public policy on early infant nutrition that maximizes immunological development and protection against infections. At the same time, they offer new grounds for examining the mechanisms behind the very low rates of symptomatic HIV and cytomegalovirus disease in infants exclusively breastfed by infected mothers despite exposure to virus-infected breastmilk leukocytes.31, 32 This suggests that the breast is a mucosal site preferentially sheltered from viral replication and/or the presence of anti-viral transmission agents in breastmilk that confer enhanced local virologic control and protection to the infant.25 Future examination of these hypotheses and the immunological response of the cellular and biochemical components of breastmilk to mother HIV are warranted.
The lactating breast, being the only metabolically significant organ of the body, for which a medical test does not exist, is often subjected to infections, which are diagnosed late, misdiagnosed or remain untreated. This contributes to an early cessation of breastfeeding with adverse effects for both the mother and the infant. The detection of breastmilk leukocyte levels may be useful in the assessment of the health status of the lactating breast, and the breastfeeding woman and infant. Similar tests are routinely used by the dairy industry to assess the quality of bovine milk based on the detection of an increase in somatic cell count being indicative of intramammary infection (California mastitis test).33, 34 Our studies have further refined and extended this test to human lactation by demonstrating a specific increase in breastmilk leukocytes with infection of the breastfeeding mother and/or infant. The diagnostic potential of this method will be reinforced by establishment of associations between specific infections and milk leukocyte/biochemical composition. This could prove particularly useful in early, rapid and successful diagnosis of these infections and the underlying microorganism(s) as well as in assessing the effectiveness of a treatment. In addition, our data provide the basis for investigating the effect of other factors, such as the menstrual cycle50 or nipple damage/duct obstruction associated with nipple piercing,51 on the health status of the breast via measurement of breastmilk leukocyte content.
Methods
Study design
The study was approved by the Human Research Ethics Committee of The University of Western Australia. Twenty-one breastfeeding dyads were recruited for the study and provided written informed consent. The lactation spectrum ranged from day 1 to year 4 postpartum. Breastmilk samples (5–50 ml) were collected at the following stages of lactation: colostrum (days 1–4 postpartum, N=5), transitional milk (day 5 to week 3 postpartum, N=7) or mature breastmilk (week 4 postpartum and beyond, N=51), and were collected during any infection of the mother or the infant as well as prior and/or after recovery (Supplementary Figure S2). The participants provided detailed description of the symptoms of the infections, which included organ-specific and general infections of the mother and/or the infant (Supplementary Table S1). Infections were clinically diagnosed by the participant's general practitioner, and managed with appropriate treatment. Milk samples were aseptically collected in the morning using an electric breast pump (Medela AG, Baar, Switzerland) and kept in sterile polypropylene vials at ∼20 °C in the dark during transportation to the laboratory. Cellular analyses were conducted within 1–2 h of expression, while all other analyses were done in frozen (−80 °C) sample aliquots as described below. Total cells were isolated from breastmilk as described previously.22 Total cell content and viability were determined with a Neubauer hemocytometer (BRAND, Wertheim, Germany) by Trypan Blue exclusion. Subsequently, cells were analyzed using FACS, immunofluorescence staining and immunoreactivity physiological tests.
Flow cytometry
The cell suspension was distributed in vials and cells were incubated for 30 min at 4 °C with fluorescent-labeled anti-human CD45 (Supplementary Table S2). For leukocyte subset analysis, fluorescent-labeled antibodies against CD45, CD3, CD4, CD8, CD19, CD56 and CD14 were combined (Supplementary Table S2). Cells were washed twice in 3% fetal bovine serum in phosphate-buffered saline (PBS) and incubated for 30 min at 4 °C with live/dead fixable far-red dead cell stain (Life Technologies, Mulgrave, VIC, Australia) as per manufacturer's instructions. Cells were fixed in 1% paraformaraldehyde/0.7% sucrose solution in PBS and analyzed with a FACS Calibur Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Further data analyses were done using FlowJo (http://www.flowjo.com). Respective isotype controls were used to standardize background fluorescence. A completely unstained sample was always prepared in which propidium iodine (Sigma-Aldrich, Castle Hill, NSW, Australia) was added to check for the cellular uniformity of the cell suspension. In cases where this sample contained non-cellular particles (such as cellular fragments or fat globules), propidium iodine was added in all other samples (stained and controls) of the same cell suspension and only the cellular component was considered in the analyses.
Immunostaining
The cell suspension was fixed in 1% paraformaraldehyde/0.7% sucrose solution in PBS. Cytospins on glass slides were generated using Shandon Cytospin 3 (Shanton) centrifuge at 40.7 g for 4 min, and incubated with fluorescent-labeled anti-human CD45 antibody (Supplementary Table S2) for 1 h at room temperature under humid conditions. Cytospins were washed in PBS, incubated with 4′6-diamidino-2-phenylindole (1:100) for 15 min, mounted in Cytomation mounting medium (Dako Australia, Campbellfield, VIC, Australia) and observed using a Nikon A1Si confocal microscope. Data were collected using the Nikon NIS Elements software package (Melville, NY, USA).
Immunoreactivity of milk leukocytes
In cases of very high breastmilk leukocyte content (60–90% of total cells), typically when the mother was diagnosed with mastitis, a subsample of the isolated live breastmilk leukocytes was cultured in U-bottom cell culture 96-well plates (Sarstedt, Numbrecht, Germany) at 500 000 cells per well in RPMI basic medium (Gibco, Grand Island, NY, USA) supplemented with 5% human serum and 5% antibiotic/antimycotic (Invitrogen). Six to eight statistical replicates (wells) were created per condition, which included (1) control (cell suspension in the above medium), (2) antigen effect (addition of 1 μl per well of CEF peptide pool, Cat No. 3615-1, Mabtech, Nacka Strand, Sweden) and (3) PHA effect (addition of 5 μl per well of Leucoagglutinin PHA-L, Cat No. L-4144, Sigma-Aldrich). Cells were incubated at 37 °C and 5% CO2 for 5 days before (a) removal of a 50-μl aliquot of culture supernatant and storage at −20 °C for cytokine analyses, and (b) addition of BrdU-labeling solution for measurement of cell proliferation using a BrdU Cell Proliferation enzyme-linked immunosorbent assay kit (Colorimetric, Roche, Dee Why, NSW, Australia, Cat. No. 11 647 229 001) according to the manufacturer's instructions.
Cytokine measurements
Cytokines were determined in culture supernatants obtained from the immunoreactivity tests using the BD Cytometric Bead Array Cytokine Kit (Becton Dickinson) for seven cytokines (IL-2, IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor-α and interferon-γ), following the manufacturer's instructions and assaying samples in duplicate in 1:1 dilution. A five-parameter logistic model was used to convert the bead fluorescence intensities to analyte concentrations.
Enzyme-linked immunosorbent assay
Immunoglobulin (sIgA, IgG and IgM) and lactoferrin breastmilk concentrations were measured by enzyme-linked immunosorbent assay in defatted acellular sample aliquots stored at −80 °C, as described previously.52 The 96-well microtitre plates were coated with 250 μl per well of anti-human sIgA (37 °C, 1 h), IgG or IgM (4 °C, overnight) (Supplementary Table S2). Subsequently, 200 μl per well of blocking solution (0.05% Tween-20/PBS containing 1 mg ml−1 BSA for sIgA/lactoferrin; skim bovine milk for IgG/IgM) was added for 30 min. The stored breastmilk samples were thawed to room temperature and diluted in 0.05% Tween-20/PBS 5000 times for sIgA, 600 times for IgG and IgM, and 50 000 times for lactoferrin. After washing (0.05% Tween-20/PBS) three times, 200 μl per well of standard, sample or control were added in duplicates for an 1-h incubation at 37 °C. Washing was followed by addition of 200 μl per well of secondary antibody and incubation for 1 h at 37 °C (Supplementary Table S2). The dilution of the secondary antibody for sIgA/lactoferrin was done in 0.05% Tween-20/PBS containing 1 mg ml−1 BSA, whereas for IgG/IgM in 0.05% Tween-20/PBS. In the IgM assay, incubation with the secondary antibody was followed by three washes and subsequent incubation with 200 μl per well of NeutrAvidin-HRP for 1 h at 37 °C (Supplementary Table S2). After secondary/tertiary antibody incubation, washing and rinsing with deionised water, 200 μl per well of color reagent were added and incubated in the dark at room temperature for 10–30 min. The color reaction was stopped by addition of 100 μl per well of 3 M sulfuric acid and the plate was read at 405 nm using a BioTek PowerWave X5 plate reader (BioTek, Winooski, VT, USA). The recovery of a known amount of sIgA, IgG, IgM or lactoferrin added to milk samples was on average 100.6±2.9%. The inter-assay coefficient of variation was on average 3.6%.
Statistical analyses
Statistical analyses were performed in R 2.9.0 153 using the base packages and libraries lattice,54 nlme55 and multcomp.56 Results are presented as mean±s.e.m. unless otherwise specified, and P<0.05 was considered significant. Linear mixed effects models were used to determine which of the response variables (leukocyte content (% of total cells), leukocyte content (per ml milk), total and viable cell contents (per ml milk), cell viability (% viable cells of total cells), sIgA, IgG, IgM and lactoferrin) were affected by milk type, lactation stage, health status of the mother/infant dyad and breastfeeding status (exclusive versus non-exclusive). All models included a grouping factor of dyad and a random effect of different baseline levels for each dyad. Where health status was included in a model, ‘healthy' was considered to be the reference level. To determine whether data transformation was required, comparisons were made between raw data, square root-transformed data and natural log-transformed data models. After fitting the univariate model for each predictor, diagnostic plots (fitted values versus standardised residuals; qq-plot of residuals) were examined. In cases where a transformation was obviously superior, this was used for all covariate models. Owing to the measured zeroes obtained for leukocyte content (% and per ml milk) of some samples, these variables were transformed using the additive constant 0.5 for both the square root and the log transformations.57 Differences between colostrum, ‘healthy' and ‘infection' samples were tested for with a three-level categorical predictor of milk type. Differences between ‘healthy' samples and each of the infection types were tested using a five-level factor (‘healthy', ‘breast', ‘baby', ‘cold' and ‘other'), with the colostrum samples omitted. Differences between pre- and post-infection ‘healthy' samples were tested where data were available (n=7) by classifying ‘healthy' as a two-level factor (‘pre' and ‘post). To determine whether there were interactions between the health status of the dyad and either lactation stage (age in weeks) or exclusive breastfeeding (yes/no) in mature breastmilk, interaction models were considered for each of these covariates separately. Infection status was considered as a two-level factor (‘healthy' and ‘infection'). Models were selected using backwards stepwise methods, starting with the main effects+interaction model. Where the interaction was significant, all main effects were retained in the model.
For the lactation stage model, analysis was restricted to records where the baby was under 60 weeks of age (N=46 records, for 15 participants), due to the scarcity of data beyond this point. The effect of viral antigens and PHA on proliferation rate in leukocyte cultures generated from two samples of mastitic breastmilk was also assessed using linear mixed effects models. Separate models were used for each response variable, with replicate as the grouping variable and a random effect of different baseline effects per replicate. Tukey's HSD test was used to test all pairwise comparisons between the three treatments (control, viral antigen and PHA). Multivariate patterns in the immune markers (sIgA, IgG, IgM and lactoferrin) and three cellular measures (total cell and leukocyte contents, % leukocytes) were investigated analytically using singular value decomposition principal components analysis on scaled and centered data and graphically using biplots. This enabled comparisons between the conditions that were seen infrequently in this study.
Acknowledgments
This work was supported by an unrestricted research grant from Medela AG (Switzerland) to PEH, ARH, CTL, NT and FH, and a Women and Infants Research Foundation Scholarship to FH. Many thanks are extended to all participating mothers and the Australian Breastfeeding Association for support in recruitment of participants. The authors acknowledge the facilities, scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterisation and Analysis, The University of Western Australia. FH designed and performed the study, and wrote the manuscript; ARH did statistical analyses and reviewed the manuscript; PM conducted immunoglobulin measurements and reviewed the manuscript; CTL and NT provided advice and reviewed the manuscript; and PEH and LF supervised the study and reviewed the manuscript.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunol. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells. Pediatrics. 2007;119:e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- Le Huerou-Luron I, Blat S, Boudry G. Breast-v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–36. doi: 10.1017/S0954422410000065. [DOI] [PubMed] [Google Scholar]
- Slade HB, Schwartz SA. Mucosal immunity: the immunology of breast milk. J Aller Clin Immunol. 1987;80:348–358. doi: 10.1016/0091-6749(87)90041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WA. The dynamic effects of breastfeeding on intestinal development and host defense. Adv Exp Med Biol. 2004;554:155–170. doi: 10.1007/978-1-4757-4242-8_15. [DOI] [PubMed] [Google Scholar]
- Kramer MS. ‘Breast is best': the evidence. Early Hum Dev. 2010;86:729–732. doi: 10.1016/j.earlhumdev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Winberg J. Breast milk and defence against infection in the newborn. Arch Dis Child. 1972;47:845–848. doi: 10.1136/adc.47.256.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CdV. Protective effect of breast feeding against infection. Br Med J. 1990;300:11–16. doi: 10.1136/bmj.300.6716.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson LA. Breastfeeding stimulates the infant immune system. Sci Med. 1997;4:12–21. [Google Scholar]
- Cesar JA, Victora CG, Barros FC, Santos IS, Flores JA. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case-control study. BMJ. 1999;318:1316–1320. doi: 10.1136/bmj.318.7194.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Hickler W, Sprenger R. Demonstration that milk cells invade the suckling neonatal mouse. Am J Repr Immunol. 1983;4:95–98. doi: 10.1111/j.1600-0897.1983.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Kmetz M, Dunne HW, Schultz RD. Leukocytes as carriers in the transmission of bovine leukemia: invasion of the digestive tract of the newborn by ingested, cultured, leukocytes. Am J Vet Res. 1970;31:637–641. [PubMed] [Google Scholar]
- Agarwal S, Karmaus W, Davis S, Gangur V. Immune markers in breast milk and fetal and maternal body fluids: a systematic review of perinatal concentrations. J Hum Lact. 2011;27:171–186. doi: 10.1177/0890334410395761. [DOI] [PubMed] [Google Scholar]
- Franca EL, Reis Nicomedes T, Mattos Paranhos Calderon I, Franca ACH. Time-dependent alterations of soluble and cellular components in human milk. Biol Rhythm Res. 2010;41:333–347. [Google Scholar]
- Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- Telemo E, Hanson LA. Antibodies in milk. J Mammary Gland Biol Neoplasia. 1996;1:243–249. doi: 10.1007/BF02018077. [DOI] [PubMed] [Google Scholar]
- Head JR, Beer AE, Billingham RE. Significance of the cellular component of the maternal immunologic endowment in milk. Transplant Proc. 1977;9:1465–1471. [PubMed] [Google Scholar]
- Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child. 1989;64:930–933. doi: 10.1136/adc.64.7_spec_no.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirt DP, Adkins LT, Palkowetz KH, Schmalstieg FC, Goldman AS. Activated and memory T lymphocytes in human milk. Cytometry. 1992;13:282–290. doi: 10.1002/cyto.990130310. [DOI] [PubMed] [Google Scholar]
- Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007;329:129–136. doi: 10.1007/s00441-007-0390-x. [DOI] [PubMed] [Google Scholar]
- Thomas E, Zeps N, Cregan M, Hartmann P, Martin T. 14-3-3sigma (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle. 2011;10:278–284. doi: 10.4161/cc.10.2.14470. [DOI] [PubMed] [Google Scholar]
- Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30:2164–2174. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AS, Goldblum RM. Transfer of maternal leukocytes to the infant by human milk. Curr Top Microb Immunol. 1997;222:205–213. doi: 10.1007/978-3-642-60614-4_10. [DOI] [PubMed] [Google Scholar]
- Michie CA. The long term effects of breastfeeding: a role for the cells in breast milk. J Trop Ped. 1998;44:2–3. doi: 10.1093/tropej/44.1.2. [DOI] [PubMed] [Google Scholar]
- Sabbaj S, Ghosh MK, Edwards BH, Leeth R, Decker WD, Goepfert PA, et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol. 2005;174:2951–2956. doi: 10.4049/jimmunol.174.5.2951. [DOI] [PubMed] [Google Scholar]
- Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K, et al. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol. 2009;182:7155–7162. doi: 10.4049/jimmunol.0803107. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeeds and fat content of breastmilk throughout the day. Pediatrics. 2006;117:e387–e395. doi: 10.1542/peds.2005-1417. [DOI] [PubMed] [Google Scholar]
- Bryan DL, Hart PH, Forsyth KD, Gibson RA. Immunomodulatory constituents of human milk change in response to infant bronchiolitis. Ped Aller Immunol. 2007;18:495–502. doi: 10.1111/j.1399-3038.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- Engel S. An investigation of the origin of the colostrum cells. J Anat. 1953;87:362–366. [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Ibegbu CC, Theiler R, Xu YX, Bansil P, Jamieson DJ, et al. Breast milk CD4+ T cells express high levels of C chemokine receptor 5 and CXC chemokine receptor 4 and are preserved in HIV-infected mothers receiving highly active antiretroviral therapy. J Infect Dis. 2007;195:965–972. doi: 10.1086/512082. [DOI] [PubMed] [Google Scholar]
- Kurath S, Halwachs-Baumann G, Muller W, Resch B. Trasmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010;16:1172–1178. doi: 10.1111/j.1469-0691.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J Dairy Sci. 2001;84:2018–2024. doi: 10.3168/jds.S0022-0302(01)74645-0. [DOI] [PubMed] [Google Scholar]
- Sharma N, Singh NK, Bhadwal MS. Relationship of somatic cell count and mastitis: an overview. Asian-Aust J Anim Sci. 2011;24:429–438. [Google Scholar]
- Mata L, Wyatt RG. Host resistance to infection. Am J Clin Nutr. 1971;1971:976–986. doi: 10.1093/ajcn/24.8.976. [DOI] [PubMed] [Google Scholar]
- Peitersen B, Bohn L, Andersen H. Quantitative determination of immunoglobulins, lysozyme, and certain electrolytes in breast milk during the entire period of lactation, during a 24-hour period, and in milk from the individual mammary gland. Acta Paed Sca. 1975;64:709–717. doi: 10.1111/j.1651-2227.1975.tb03909.x. [DOI] [PubMed] [Google Scholar]
- Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunologic factors in human milk during the first year of lactation. J Ped. 1982;100:563–567. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- Jatsyk GV, Kuvaeva IB, Gribakin SG. Immunological protection of the neonatal gastrointestinal tract: the importance of breast feeding. Acta Paed Sca. 1985;74:246–249. doi: 10.1111/j.1651-2227.1985.tb10958.x. [DOI] [PubMed] [Google Scholar]
- Keller MA, Gendreau-Reid L, Heiner DC, Rodriguez A, Short JA. IgG4 in human colostrum and human milk: continued local production or selective transport from serum. Acta Paed Sca. 1988;77:24–29. doi: 10.1111/j.1651-2227.1988.tb10592.x. [DOI] [PubMed] [Google Scholar]
- Fetherston C. Risk factors for lactation mastitis. J Hum Lact. 1998;14:101–109. doi: 10.1177/089033449801400209. [DOI] [PubMed] [Google Scholar]
- Amir LH, Forster DA, Lumley J, McLachlan H. A descriptive study of mastitis in Australian breastfeeding women: incidence and determinants. BMC Public Health. 2007;7:62. doi: 10.1186/1471-2458-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Academy of Breastfeeding Medicine Protocol Committee ABM clinical protocol #4: mastitis. Revision, May 2008. Breastfeed Med. 2008;3:177–180. doi: 10.1089/bfm.2008.9993. [DOI] [PubMed] [Google Scholar]
- Riskin A, Almog M, Peri R, Halasz K, Srugo I, Kessel A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Ped Res. 2012;71:220–225. doi: 10.1038/pr.2011.34. [DOI] [PubMed] [Google Scholar]
- Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004;113:361–367. doi: 10.1542/peds.113.2.361. [DOI] [PubMed] [Google Scholar]
- Ho FC, Wong RL, Lawton JW. Human colostral and breast milk cells. A light and electron microscopic study. Acta Paed Sca. 1979;68:389–396. doi: 10.1111/j.1651-2227.1979.tb05025.x. [DOI] [PubMed] [Google Scholar]
- Pitt J. The milk mononuclear phagocyte. Pediatrics. 1979;64:745–749. [PubMed] [Google Scholar]
- Buescher ES, Pickering LK.Polymorphonuclear leukocytes in human colostrum and milkIn: Howell RR, Morriss FH, Pickering LK, (eds). Human Milk in Infant Nutrition and Health. Charles C Thomas Publisher: Springfield, USA; 1986160–173. [Google Scholar]
- Jin Y-Y, Zhao W, Cao R-M, Wang X, Wu S-M, Chen T-X. Characterization of immunocompetent cells in human milk of Han Chinese. J Hum Lact. 2011;27:155–162. doi: 10.1177/0890334410392041. [DOI] [PubMed] [Google Scholar]
- Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Rep. 2008;78:586–594. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- Hartmann PE, Prosser CG. Acute changes in the composition of milk during the ovulatory menstrual cycle in lactating women. J Physiol. 1982;324:21–30. doi: 10.1113/jphysiol.1982.sp014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin CP, Deacon JP, Rowan MK, Hartmann PE, Geddes DT. Association of nipple piercing with abnormal milk production and breastfeeding. JAMA. 2009;301:2550–2551. doi: 10.1001/jama.2009.877. [DOI] [PubMed] [Google Scholar]
- Fetherston CM, Lai CT, Hartmann PE. Relationships between symptoms and changes in breast physiology during lactation mastitis. Breastfeed Med. 2006;1:136–145. doi: 10.1089/bfm.2006.1.136. [DOI] [PubMed] [Google Scholar]
- R DCT . R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2009. [Google Scholar]
- Sarkar D.Lattice: Lattice Graphics. R package version 0.17-22 ed2009
- Pinheiro J, Bates D, DebRoy S, Sarkar D.Core team atR. nlme: Linear and nonlinear mixed effects models. R package version 3.1-89 ed2008
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Yamamura K. Transformation using (x+0.5) to stabilize the variance of populations. Res Popul Ecol. 1999;41:229–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.