Abstract
Mesenchymal stem cells (MSCs) are promising cellular suppressor of inflammation. This function of MSCs is partly due to their licensing by inflammatory mediators. In cases with reduced inflammation, MSCs could become immune-enhancer cells. MSCs can suppress the inflammatory response of antigen-challenged lymphocytes from allergic asthma. Although allergic rhinitis (AR) is also an inflammatory response, it is unclear if MSCs can exert similar suppression. This study investigated the immune effects (suppressor vs enhancer) of MSCs on allergen-stimulated lymphocytes from AR subjects (grass or weed allergy). In contrast to subjects with allergic asthma, MSCs caused a significant (P<0.05) increase in the proliferation of antigen-challenged lymphocytes from AR subjects. The increase in lymphocyte proliferation was caused by the MSCs presenting the allergens to CD4+ T cells (antigen-presenting cells (APCs)). This correlated with increased production of inflammatory cytokines from T cells, and increased expressions of major histocompatibility complex (MHC)-II and CD86 on MSCs. The specificity of APC function was demonstrated in APC assay using MSCs that were knocked down for the master regulator of MHC-II transcription, CIITA. The difference in the effects of MSCs on allergic asthma and AR could not be explained by the sensitivity to the allergen, based on skin tests. Thus, we deduced that the contrasting immune effects of MSCs for antigen-challenged lymphocytes on AR and allergic asthma could be disease specific. It is possible that the enhanced inflammation from asthma might be required to license the MSCs to become suppressor cells. This study underscores the need for robust preclinical studies to effectively translate MSCs for any inflammatory disorder.
Keywords: antigen-presenting cells, allergic rhinitis, CIITA, immune enhancement, mesenchymal stem cell
Mesenchymal stem cells (MSCs) differentiate along various lineages to generate specialized cells of all germ layers, such as cartilage, muscle, neurons, and cardiomyocyes.1, 2 MSCs are attractive for cell therapy, mostly due to ease in expansion, reduced ethical concerns and low probability of transformation.2, 3 MSCs are ubiquitous and are referred by different names such as pericytes.4 Regardless of the source, MSCs generally show similarity with respect to immune functions. Phenotypic and functional studies in the literature suggest that MSCs are heterogeneous.5 At present, it is unclear if the heterogeneity is physiological or an artifact of culture methods. A major problem that might contribute to the heterogeneity of MSCs is the lack of consensus on the method and the culture surface to expand MSCs. Another possibility to explain heterogeneity is the co-existence of stem cells and progenitors within the MSC culture. Until reagents are developed that would establish a hierarchy of MSCs, the methods used in a particular study will require detailed documentation for the appropriate interpretation of the data.
Despite the attractiveness of MSCs for cell therapy, one has to be mindful that MSCs can also support cancer growth as well as protect the cancer cells through immune suppression.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The translation of MSCs would need to consider the heterogeneity among cancer cells, as each subset might interact differently with MSCs.16 Although the outcomes on the interaction between MSCs and cancer subsets are studied, research must continue to investigate the therapeutic potential of MSCs. Parallel studies in these two areas will lead to safe and efficient application of MSCs for inflammatory disorders.
The experimental and clinical evidence indicate that MSCs could be effective anti-inflammatory cells for multiple sclerosis, asthma, graft-vs.-host disease, Crohn's disease as well as other inflammatory disorders.17, 18, 19, 20 The milieu of a microenvironment is important to for the licensing of MSCs as immune suppressor or enhancer cells.21, 22 The mechanism by which MSCs exert immune suppression is complex, partly through soluble factors such as cytokines, indoleamine 2,3 dioxygenase, hepatocyte growth factor, prostaglandin E2, and nitric oxide.23, 24, 25, 26 MSCs can alter the functions of T cells, dendritic cells, and natural killer cells by switching the production of cytokines and to alter T-cell response.17, 23 MSCs can respond to chemotactic factors and migrate to areas of inflammation.27
Among the immune-enhancing roles of MSCs is their ability to be antigen-presenting cells (APCs). This function is ‘tightly' controlled by the level of interferon γ (IFN-γ).28, 29 This occurs by the controlled expression of major histocompatibility complex (MHC)-II.30 IFN-γ controls the APC function of MSCs by regulating the expression of the master regulator of MHC-II, CIITA.31 At high levels of IFN-γ, CIITA is retained in the cytosol to prevent the transcription of MHC-II, whereas low levels of IFN-γ caused nuclear retention of CIITA.31 MSCs can also cross present antigen by cross-presentation as another method of their APC function.32
MSCs suppressed the inflammatory responses in animal models of allergic airway inflammation and ragweed-induced asthma.33, 34 Despite the surge of research on MSCs as anti-inflammatory cells, there is a paucity of information on MSCs as immune suppressor. This study investigated the immune effects of MSCs for allergic rhinitis (AR) as AR remains as one of the most prevalent inflammatory conditions.
AR is a common chronic inflammatory disease of the nasal passages, affecting approximately 20% of adults in the United States.35 AR is an allergic disease characterized by an influx of eosinophils and T helper 2 cells, increases in pro-inflammatory cytokines, such as interleukin (IL)-3, IL-4, IL-5, IL-13, influx of eosinophils, and IgE-mediated mast cell degranulation.36 Immune hyper-reactivity to common environmental allergens typically cause chronic nasal congestion, rhinorrhea, sneezing, postnasal drip, and itchy/watery. AR worsens other conditions, such as asthma and sinusitis, and increase health-care cost.37 In an animal model of AR, transplanted MSCs migrated to the nasal mucosa to reduce the symptoms to decrease infiltrating eosinophil and reduced sera IgE.17, 38
The dual immune roles of MSCs suggest a possibility that MSCs could also worsen the inflammatory state of AR.28, 29, 30, 32 To this end, this study investigated a role for MSCs in antigen-challenged peripheral blood mononuclear cells (PBMCs) from patients with AR. Here we report on an enhanced immune effect of MSCs to pollen provocation with PBMCs from AR. The studies showed enhancement in the immune response, because of the APC role of MSCs.
Results
The low expression of MHC-II on MSCs are sufficient to elicit allogeneic responses.22 To account for the contribution of allogeneic differences between the MSCs and the test PBMCs, all of the studies included controls consisting of one-way mixed reactions with MSCs as stimulators and PBMCs as responders (Figures 1, 2, 3, open bars). Studies with γ-irradiated MSCs (2000 Rads) vs non-irradiated MSCs confirmed that the proliferation observed in the mixed cultures was indeed due solely to the PBMCs and not the MSCs (Supplementary Figure S1 online).
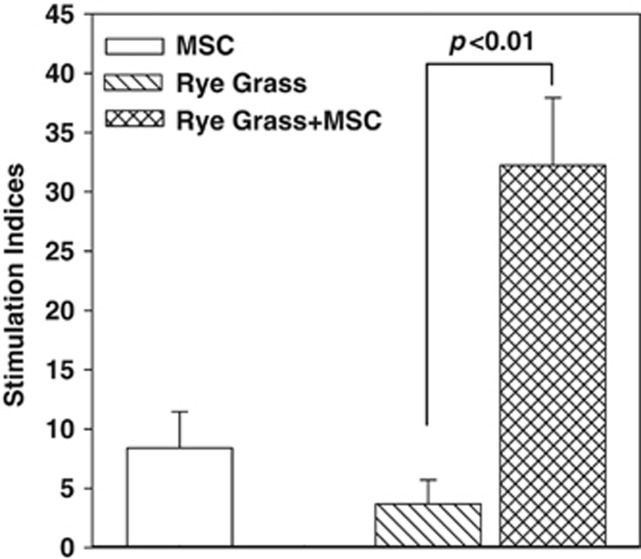
Figure 1.
Proliferative response of peripheral blood mononuclear cells (PBMCs) to rye grass and/or mesenchymal stem cells (MSCs). PBMCs were isolated from the peripheral blood of subjects with allergic rhinitis (AR), and sensitivity to rye grass. The PBMCs were challenged with 5 μl ml−1 rye grass, in the presence or absence of MSCs. After 48 h, the cultures were assessed based on the incorporation of tritiated thymidine. The proliferation for each experimental point is presented as stimulation index (SI). The SI was calculated as the disintegration per minute (d.p.m.) in the experimental point/d.p.m. of unstimulated PBMCs alone. The results (mean±s.d.) are presented for six donors (Table 1: S1–S6). Each AR subject was studied with MSCs from a different donor.
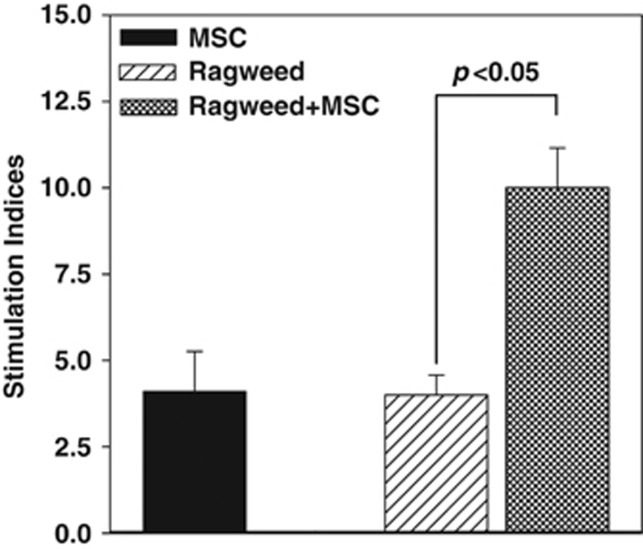
Figure 2.
Proliferative response of peripheral blood mononuclear cells (PBMCs) from allergic rhinitis (AR) to ragweed in the presence or absence of mesenchymal stem cells (MSCs). PBMCs were isolated the peripheral blood of subjects with AR and sensitivity to ragweed. The PBMCs were challenged with 5 μl ml−1 ragweed, in the presence or absence of MSCs. After 48 h, the cultures were assessed for proliferation, based on the incorporation of tritiated thymidine. The proliferation is presented as stimulation index (SI), which was calculated as the disintegration per minute (d.p.m.) of the experimental point/d.p.m. of unstimulated PBMCs alone. The results (mean±s.d.) are presented for six donors (Table 1: S5–S8, S12, S13). Each AR subject was studied with MSCs from a different donor.
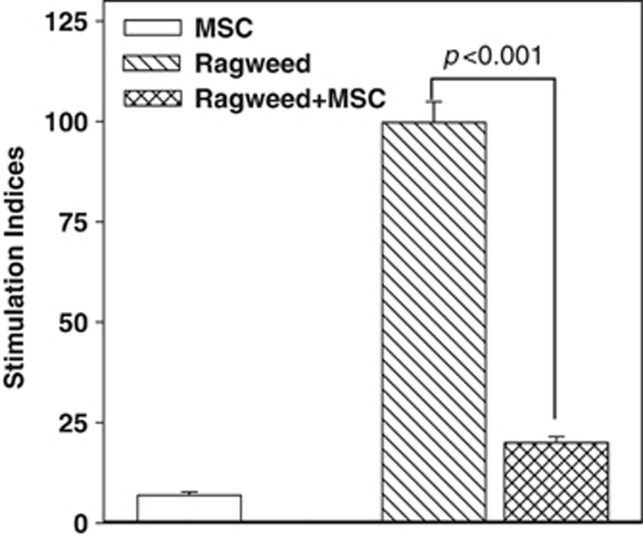
Figure 3.
Proliferation of peripheral blood mononuclear cells (PBMCs) from allergic (ragweed) asthma, in the presence or absence of mesenchymal stem cells (MSCs). PBMCs from subjects with allergy to ragweed and asthma (allergic asthma) were stimulated with ragweed (5 μl ml−1), in the presence or absence of MSCs. After 48 h, the cultures were assessed for proliferation by pulsing with tritiated thymidine. The incorporation of tritiated thymidine was detected with a scintillation counter. The proliferation for each experimental point is presented as stimulation index (SI), which was calculated as the disintegration per minute (d.p.m.) of the experimental point/d.p.m. of PBMCs alone. The results (mean±s.d.) are presented for four donors (Table 1: S18–S21). Each donor was studied in quadruplicates, with MSCs from a different donor.
MSCs increased the proliferation of PBMCs challenged with rye grass
We studied the effects of MSCs on the proliferation of antigen-challenged PBMCs from subjects with rye grass sensitivity. First, we tested for the optimum concentration of rye grass in dose–response studies with antigen alone or with antigen and MSCs. Studies with six donors and rye grass between 1 and 20 μl ml−1 indicated 5 μl ml−1 as the optimum concentration (Supplementary Figure S2 online). This information was based on the effect of PBMCs and MSCs rather than PBMCs alone.
The allogeneic differences between PBMCs with MSCs were verified in mixed cultures with MSCs as the stimulators and PBMCs as the responders.22, 28, 30, 39 The results indicated stimulation indices (SI) of 8.4±3 (±s.d.), which signified allogeneic differences (Figure 1, open bar). PBMCs stimulated with optimal rye grass resulted in SI=3.7±2 (±s.d.; Figure 1, diagonal bar). As the SI was calculated as the proliferation of antigen stimulated PBMCs/unstimulated PBMCs, the increase in SI verified sensitivity to rye grass. Despite the increase, the sensitivity to rye grass was nonetheless relatively low. The addition of MSCs and rye grass antigen showed a significant (P<0.01) increase in the SI to 32±5.7 (±s.d.; Figure 1, hatched bar). This increase was approximately eightfold more than the cultures with antigen alone (Figure 1, diagonal bar). In summary, the data showed an enhancing effect of MSCs in rye-challenged PBMCs from AR subjects.
Effects of MSCs on ragweed-challenged PBMCs from AR subjects
The immune-enhancing effect of MSCs in the rye grass-challenged cultures was unexpected because MSCs can suppress inflammatory responses.22, 40, 41 We therefore examined another allergen to determine whether the enhancing effect was limited to rye grass. We selected ragweed sensitivity from six donors. Dose–response studies with different concentrations of ragweed indicated the optimum concentration to be 5 μl ml−1 (Supplementary Figure S3 online).
PBMCs from the same donors used for the dose–response studies were stimulated with optimum ragweed, in the absence or presence of MSCs. Baseline proliferation was assessed with PBMCs alone. PBMCs and MSCs (allogeneic response) and PBMCs and ragweed showed comparable proliferation (P>0.05; Figure 2, solid and diagonal bars). However, ragweed and MSCs, resulted in significant (P<0.05) increase in the SI as compared with the other two experimental points (Figure 2, hatched bar). In summary, we showed an enhancing effect of MSCs to ragweed-challenged PBMCs. This contrasted the modest in vitro responses to pollen alone.
Effects of MSCs in ragweed-challenged PBMCs from allergic asthma
We previously reported on a suppressive effect of MSCs on allergic asthma using dust mite antigen.20 This was unlike what we observed for the non-asthmatic AR shown in Figures 1 and 2 We therefore selected subjects with asthma who were allergic to ragweed and rye grass to determine whether the immune-enhancing effect of MSCs was caused by the allergen and not the underlying inflammatory condition (AR vs allergic asthma). Furthermore, as we previously studied allergic asthma with dust mite, the section will answer if the effect of MSCs was antigen specific.20
Proliferation studies were established as for Figures 1 and 2 with PBMCs from subjects with allergic asthma and sensitivity to ragweed, in the presence or absence of MSCs. The proliferation (SI) of PBMCs stimulated with ragweed was 103±8 (Figure 3, diagonal bar). The proliferation was reduced (P<0.001) by 4.8-folds in the presence of MSCs (Figure 3, hatched bar). The suppressive role of MSCs for AR subjects contrasted the immune-enhancing effect noted for AR subjects for both rye grass and ragweed (Figures 1 and 2). These results, combined with our demonstration that CIITA knockdown decreased human leukocyte antigen (HLA)-DR expression (Figure 6a), confirmed the essential role of the APC functionality of MSCs in the observed effects on T-cell proliferation in the context of AR.20
Antigen presentation (APC) of antigen-challenged MSCs
MSCs can exert immune enhancer functions such as APCs and cross presentation by MHC-I.29, 30, 32 We proposed similar immune-enhancing effect occurred for the proliferation observed for antigen-challenged PBMCs from AR subjects (Figures 1 and 2). The literature reported on immune-enhancing outcome when the inflammatory milieu cannot adequately license MSCs to become immune-suppressor cells.30 We studied if MSCs can exert APC function when challenged with ragweed or rye grass.
PBMCs from AR subjects were primed with rye grass or ragweed for 5 days. The CD4+ T cells were selected and then added to antigen-pulsed MSCs. Control cultures contained activated CD4+ T cells alone or with unpulsed MSCs. Baseline proliferation contained unactivated CD4+ T cells and pulsed or unpulsed MSCs.
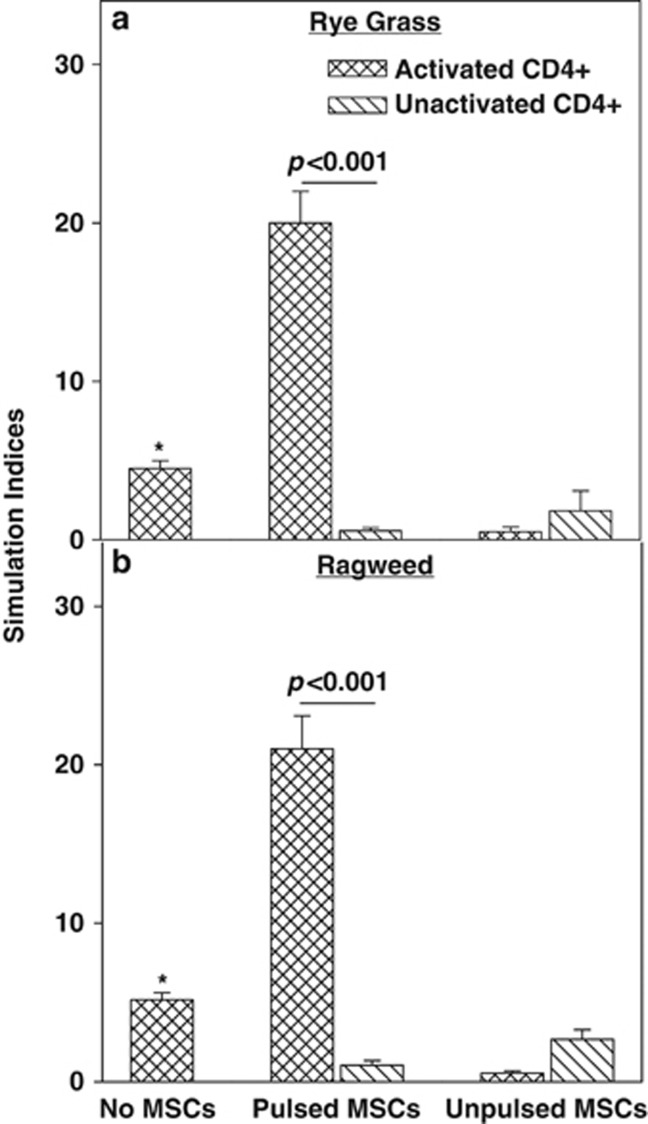
Pre-activated CD4+ T cells added to antigen-pulsed MSCs resulted in significant (P<0.01) proliferation as compared with unprimed CD4+ T cells and pulsed MSCs (Figures 4a and b, middle group). Interestingly, when unpulsed MSCs were added to activated CD4+ T cells, the proliferation was significantly decreased (P<0.05) as compared with unpulsed MSCs/unactivated CD4+ T cells alone (Figures 4a and b, right group). This latter observation indicated that the MSCs acted as immune-suppressor cells to the activated CD4+ T cells, consistent with the immune-suppressive functions of MSCs within an inflammatory milieu.42 The suppressive effect of MSCs on activated CD4+ T cells was specific because similar suppression was not observed for unactivated CD4+ T cell. Also, pulsed MSCs failed to induce the proliferation of unactivated CD4+ T cells. Together, the studies with activated T cells and pulsed MSCs, when combined with the other controls, supported an APC function of MSCs.
Figure 4.
Antigen-presenting cell function of ragweed-challenged mesenchymal stem cells (MSCs). MSCs were studied for APC function with peripheral blood mononuclear cells (PBMCs) from allergic rhinitis subjects with sensitivity to rye grass (a) or ragweed (b). The PBMCs were challenged with the respective antigen for 5 days. The CD4+ cells were selected from the activated PBMCs and then added to 16-h antigen-pulsed MSCs. After 16 h, the proliferation of the CD4+ T cells was studied by tritiated thymidine incorporation. Each antigen was studied with six donors (Table 1: rye grass, S1–S4, S6, S7; ragweed, S9–14, S16). The data are presented as the SI (mean±s.d.), which was calculated by dividing the disintegration per minute (d.p.m.) of each experimental point/d.p.m. of unstimulated PBMCs. *P<0.05 vs activated CD4+ T-cells with unpulsed MSCs.
MSCs induced the proliferation of PBMCs from AR subjects in the presence of both ragweed and rye grass (Figures 1 and 2). In the case of rye grass and MSCs, the response was synergistic (Figure 1) whereas similar responses to ragweed and MSCs were additive (Figure 2). The observations in Figure 4 indicated that the proliferative effects of ragweed-challenged PBMCs in the presence of MSCs were caused by the APC function of MSCs. In summary, the results indicated APC function of antigen-pulsed MSCs and antigen-activated CD4+ T cells. The results also showed immune suppressive role of unpulsed MSCs when placed in a condition that recapitulated an inflammatory milieu such as preactivated CD4+ T cells.
Increased cytokine production, and expressions of MHC-II and CD86
An APC function of MSCs is expected to cause increases in the production of inflammatory cytokines and enhanced expression of MHC-II and the activation marker, CD86. To determine whether similar changes occurred in the APC assays, we address this with three AR donors with ragweed allergy in APC cultures. We analyzed the media after 48 h with assays containing antigen-pulsed or unpulsed MSCs and activated CD4+ T cells. Baseline cytokine production was assessed in cultures with PBMCs alone.
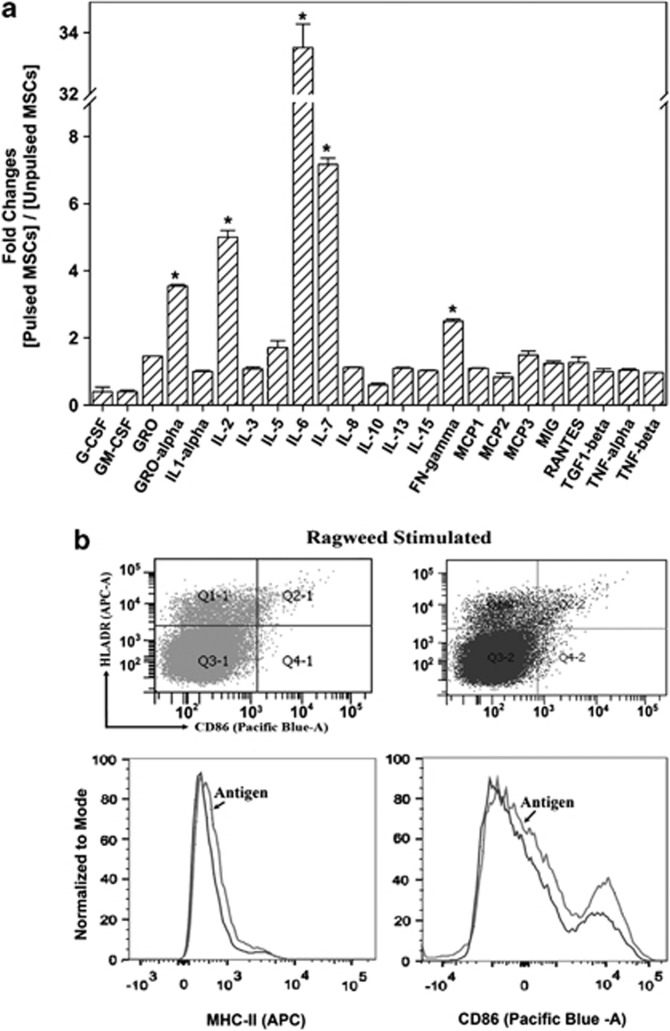
The media were analyzed with cytokine protein arrays in duplicate. The baseline cytokine production was subtracted from the test samples and the resulting densities were normalized to the internal controls. The results were then presented as fold changes of pulsed MSCs/unpulsed MSCs. The results indicated >1.5-fold increases for all cytokines. Particularly, there were significant increases in IL-2, IL-6, and IL-7. IFN-γ was increased slightly above the 1.5-fold level (Figure 5a).
Figure 5.
Cytokine production and expressions of MHC-II and CD86 in mesenchymal stem cells (MSCs). (a) Antigen-presenting cell (APC) assays were established with activated peripheral blood mononuclear cells (PBMCs) and pulsed or unpulsed MSCs. The cells were obtained from three subjects with allergic rhinitis (AR) and ragweed sensitivity (Table 1: S8, S13, S22). After 48 h, the media were analyzed in duplicate with cytokine protein arrays. The densities of the background cytokines (PBMCs alone) were subtracted from the experimental points and then presented as fold change of pulsed MSCs/unpulsed MSCs, ±s.d., n=6. (b) Pulsed and unpulsed MSCs from the APC assays were studied for MHC-II and CD86 by flow cytometry using donors S17, S19, and S22 (Table 1). The analyses were done on CD105+/CD3−/CD25−. *P<0.05 vs other cytokines.
Flow cytometry studied the expressions of MHC-II and CD86 in the antigen-pulsed and unpulsed MSCs from the APC cultures in three independent experiments. We gated the CD105+ cells (MSCs) if they were negative for CD3 and CD25 and then analyzed this subset for MHC-II and CD86. Both MHC-II and CD86 were increased in the antigen-pulsed MSCs as compared with unpulsed MSCs (Figure 5b). The shift shown in the histogram was repeated in the two other experiments. The top panels show representative scatter plots, which were represented by overlays of pulsed and unpulsed MSCs in the lower panels. In summary, there were increases in the proinflammatory cytokines, including the chemokine, GRO-1α, and enhanced expressions of MHC-II and the co-stimulatory CD86 on the antigen-pulsed MSCs.
Blunted APC function in CIITA knockdown MSCs
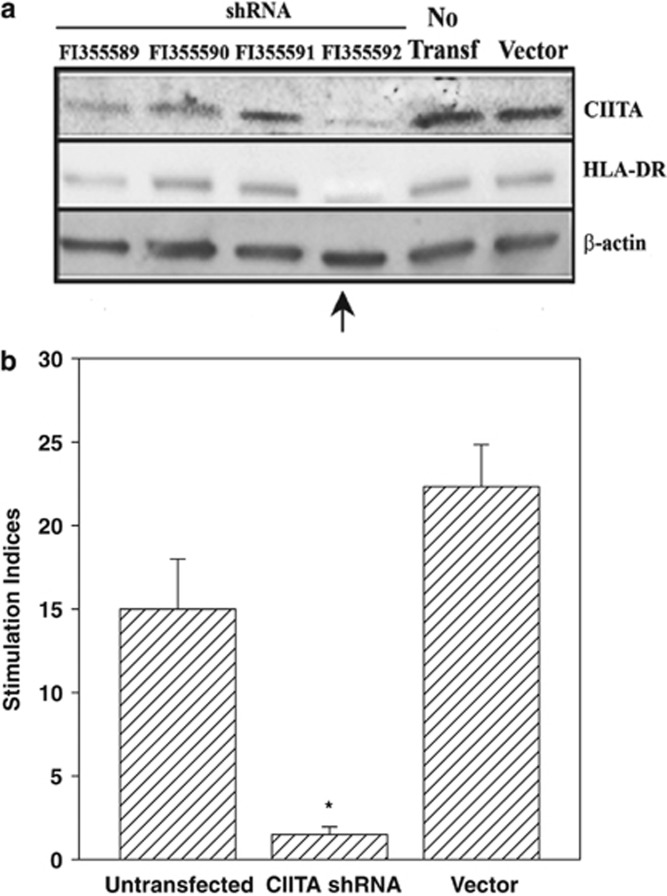
MSCs can express low level of MHC-II and exert APC functions.22, 29, 30 As CIITA is a master regulator of MHC-II transcription,43 we investigated the specificity of APC functions by repeating the APC studies with PBMCs from AR subjects, except with MSCs, knockdown for CIITA. Optimization studies identified clone FI355592 as the most efficient short hairpin RNA construct to knockdown CIITA (Figure 6a, arrow). CIITA knockdown MSCs caused significant (P<0.01) reduction in the proliferation CD4+ T cells within the APC cultures as compared with untransfected MSCs and vector transfants (Figure 6b). These results, combined with our demonstration that CIITA knockdown decreased HLA-DR expression (Figure 6a), confirmed the essential role of MSC APC functionality in the observed effects on T-cell proliferation in the context of AR.
Figure 6.
Effects of CIITA knockout mesenchymal stem cells (MSCs) in the antigen-presenting cell (APC) response to ragweed. (a) MSCs were transfected with different CIITA short hairpin RNA (shRNA) constructs. MSCs were transfected with different shRNA constructs in an effort to knockdown expression of CIITA. Western blots were performed for CIITA and MHC-II (HLA-DR) and normalized with anti-β-actin. The membrane was stripped and reprobed after blotting with each antibody. (b) APC assay was performed as for Figure 4 with MSCs, untransfected, transfected with vector alone, or transfected with the vector encoding the shRNA construct exhibiting the optimal knockdown of CIITA. The MSCs were co-cultured with peripheral blood mononuclear cells (PBMCs) from allergic rhinitis subjects with sensitivity to rye grass (Table 1: S5, S7, S8) or ragweed (Table 1: S14, S15, S17). CD4+ cells were selected from 5-day ragweed (5 μl ml−1)-challenged PBMCs and then incubated with 16-h pulsed MSCs. After 16 h, the proliferation of the CD4+ T cells was assessed, based on tritiated thymidine incorporation. The results for ragweed and rye grass were similar and are plotted together as SI, mean±s.d., n=6. The SI was calculated by the disintegration per minute (d.p.m.) of each experimental point/d.p.m. of PBMCs alone. *P<0.05 vs untransfected and vector transfectant.
Discussion
We previously reported that MSCs suppressed the proliferation of PBMCs from patients with allergic asthma and also blunted the maturation of dendritic cells.20 We also showed evidence of immune tolerance with repeated exposure of MSCs and allergen to the lymphocytes from subjects with allergic asthma.20 Thus, we began this study by assuming that MSCs will suppress the antigen-challenged lymphocytes from AR subjects, without asthma. Our assumption was based on MSCs functioning as immune suppressor cells in an inflammatory milieu as noted for allergic asthma.20, 22 However, we observed the opposite effects with enhanced proliferation of T cells when MSCs were added to PBMCs from AR patients (Figures 1 and 2). Although Figures 1 and 2 showed the response to ragweed and rye grass, we also showed similar effects for other allergens: Timothy, Bermuda, and Meadow Fescue (Supplementary Figure S4 online). Interestingly, the PBMCs alone did not show significant differences when challenged with different concentrations of the antigens (Supplementary Figures S2 and S3 online). However, dose–response effects were noted when the antigens were added together with MSCs (Supplementary Figures S2 and S3 online). This further confirmed that the increase in proliferation was due to the APC function of MSCs rather than direct interaction with the PBMCs.
In contrast to the studies with AR alone, we tested four subjects with AR and asthma and found that MSCs acted as immune suppressor (Figure 3). The observations noted for AR with asthma was consistent with the immune suppressor function of MSCs in inflammatory processes.20, 41 Although we do not have an explanation for the differences between AR with or without asthma, we propose that the response by antigen-challenged PBMCs from AR with asthma was more efficient to license the MSCs to be immune suppressor. It is unclear if this could be due to the development of an exacerbated inflammatory milieu. Although speculative, the response might be due to the subject's intrinsic sensitivity to allergen, the duration of allergen exposure, and the underlying inflammation.
Rye grass stimulation did not show an additive response when PBMCs from AR subjects were stimulated with allogeneic MSCs and antigen stimulation (Figure 1). Rye grass stimulation in the presence of MSCs resulted in SI of ∼35, whereas MSCs alone was ∼10 and rye grass alone was ∼3. Similar studies with ragweed can argue for additive effect (Figure 2). However, the studies on the APC functions of MSCs with ragweed supported APC effects (Figures 4 and 6).
The enhanced proliferation by MSCs was not expected as allogeneic MSCs can exert veto properties.22, 30 The increased proliferation of the CD4+ T cells was caused by the APC function of MSC (Figure 4). This was supported by increases in cytokines, and the expressions of MHC-II and the co-stimulatory CD86 (Figure 5). The expression of MHC-II requires the expression of the master regulator of transcription, CIITA.43 The relationship between CIITA and MHC-II was also needed for MSCs to be APCs (Figure 6). Although the levels of IFN-γ were increased in the APC assay, the increase was not at the same level as IL-2 (Figure 5). We believe that this relatively modest increase in IFN-γ was sufficient to induce T-cell proliferation and differentiation while facilitating sustained MHC-II for APC function.31
MSCs presented ragweed to the lymphocytes from AR subjects. However, unpulsed MSCs were able to suppress the proliferation of pre-activated CD4+ cells. This contrasted the APC response when pulsed MSCs were added to preactivated PBMCs (Figure 4, right vs middle groups, hatched bars). These observations require an expanded discussion because it underscores the possibility that MSCs might be capable of exerting dual immune function within the same milieu. The reason why MSCs can suppress activated CD4+ T cells is because it gets an opportunity to exert veto function where the activated T cells mimic a graft-vs.-host type response.22 In contrast, antigen-pulsed MSCs are able to present the antigen to activated T cells.
Qualitative assessment for the sensitivity of the study subjects to the allergens showed some marked differences (Table 1). However, we cannot make an assessment regarding a correlation between sensitivity to the allergen and the responsiveness of MSCs. Consistently, MSCs could increase the proliferation of CD4+ T cells, indicating APC function.
Table 1. Demographics of enrolled subjects.
| Subject | Age (years) | Ethnicity | Skin prick test |
|---|---|---|---|
| S1–S4 | 30–35 | Hispanic | Rye grass (2+); positive grasses, trees, mold |
| S5–S8 | 37–40 | Asian | Rye grass (2+); positive to trees, dust mite |
| S9–S12 | 20–25 | Hispanic | Ragweed (4+); positive to trees, cockroach, cat pelt |
| S13–S16 | 23–30 | African American | Ragweed (4+) |
| S17 | 27 | Asian | Ragweed (4+), Bermuda grass (+) |
| S18 S19–21 | 30 27–30 | Hispanic African American | Asthma, rye grass ( 4+); ragweed (4+); positive to grasses and other weeds, dust mite, and cat Asthma, rye grass (2+); ragweed (4+); dustmite (−) |
| S22 | 30 | Hispanic | Ragweed 3+ positive to trees, grass (−) |
In an experimental model of AR, MSCs can suppress the immune response of AR.17, 33 However, the mechanism by which this occurs is yet to be determined. Our in vitro findings, which studied human PBMCs, indicated that MSCs cannot be licensed by AR to become immunosuppressor cells, and such outcome might be dependent on the type of inflammatory disorder. Because of the small number of sample size, we cannot make an assumption on a link between acute sensitivity to the allergens and response of MSCs. Further studies with careful quantitation of the response to skin test are needed to determine whether there could be a subset of subjects with AR whom might respond to MSC therapy.
The exacerbated response by MSCs in antigen-challenged PBMCs was a surprise. The clinical translation of such a finding is very important, and suggested that the subjects must be carefully selected. Our studies suggested that the type of response seen, i.e., clinical benefit or worsening of symptoms, following infusion of MSCs might depend on the timing of antigen exposure, perhaps the patient's in vitro sensitivity to the allergen, and mostly if the patients are asthmatic. Given the clinical and economic consequences of poorly controlled AR, it is critical to understand how MSCs will affect a patient's allergic condition. Further investigation in this subject is warranted to understand the underlying mechanisms, and predict clinical response in individuals receiving stem cell therapy.
MSCs exhibited plasticity with regards to the immune response during pollen provacation. Comparing the findings in this study with the veto property of MSCs,22 one asks if the particular response of MSCs depends on the level of activation of PBMCs and, perhaps, the in vitro sensitivity to allergen? The findings in this study underscores that one cannot broadly assumes that MSCs will be immune suppressor for inflammatory conditions. Further investigations are needed to predict the clinical response of individuals receiving stem cell therapy.
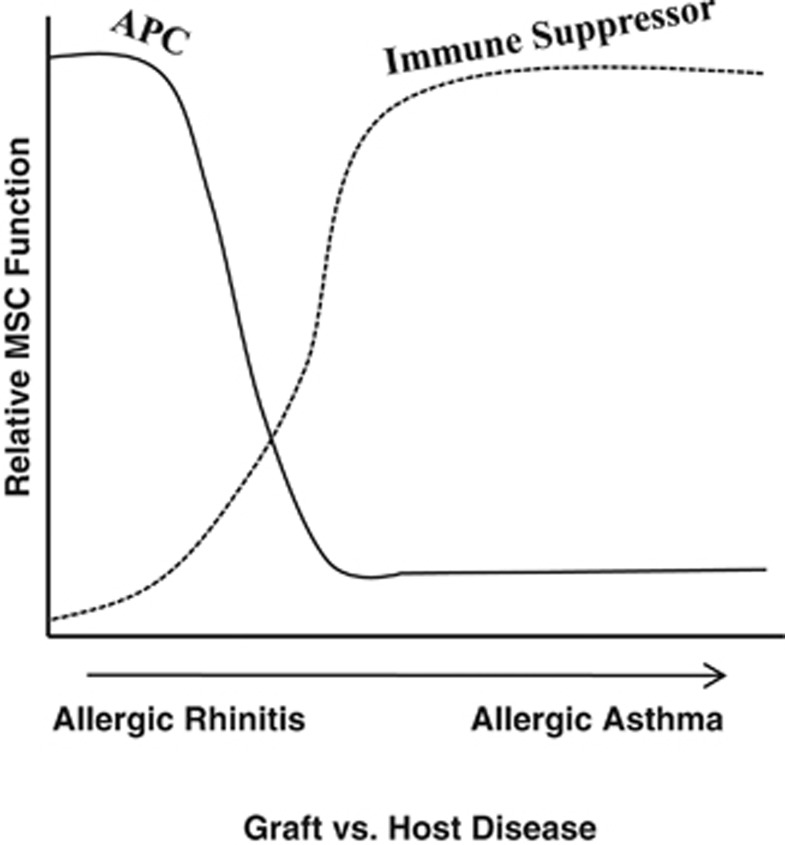
Figure 7 summarizes the different effects of MSCs in varied inflammatory conditions. The published studies indicate that inflammatory conditions such as allergic asthma and graft-vs.-host disease can be suppressed by MSCs. In contrast, this study shows that similar suppression does not occur for AR. The inflammatory response of allergens to rhinitis and asthma are similar. However, rhinitis could be limited to the lungs, whereas asthma might be systemic. Future studies with animal models will be able to address these questions. Also, the degree of inflammation could be important because other studies have shown that if there are inadequate level of cytokines to induce nitric oxide, this could result in inadequate ability of MSCs to be immune-suppressor cells.44
Figure 7.
A cartoon depicts the relative proliferation of peripheral blood mononuclear cells (PBMCs) to antigen as a function of allergic rhinitis and AA. The cartoon combines the findings of this study and our previous report, which showed a suppressive effect of mesenchymal stem cells (MSCs) for allergic asthma.20 APC, antigen-presenting cell.
We expect that CIITA will be within the cytosol of patients with allergic asthma as MSCs suppressed the inflammatory response.20 This needs to be studied in future analyses in parallel with the model shown in this report. If CIITA is cytosolic, studies are needed to reduce the inflammation and then investigate if this switches the suppressive role of MSCs similar to what is observed for AR. If so, this will be highly significant to the translation of MSCs in patients not only with allergic inflammatory diseases but also others as MSCs could be suppressive and then switch to immune enhancement. Overall, this report underscores an important issue in the immunology of MSCs. We propose that this area needs to be dissected for safe and effective treatment with MSCs.
Methods
Reagents
Phosphate-buffered saline (PBS), pH 7.4, was purchased from Life Technologies (Carlsbad, CA, USA), Ficoll Hypaque, Dulbecco's modified Eagle's medium, and RPMI 1640 from Sigma (St Louis, MO, USA), defined Hyclone fetal calf sera (FCS) from Thermo Fisher Scientific (Waltham, MA, USA), and allergen extracts from Hollister-Stier Laboratories (Spokane, WA, USA).
Study subjects
Study subjects who met the inclusion criteria and with known AR because of grass or ragweed allergy were included in the study between April and December. The demographics and allergic state of patients are shown in Table 1 The studies used rye grass and ragweed as they are standardized allergens. The Institutional Review Board of University of Medicine and Dentistry of New Jersey, Newark Campus, approved the use of blood from human subjects.
The patient's physician diagnosed AR, based on the following clinical symptoms: nasal congestion, rhinorrhea, sneezing, nasal itching and itchy, watery eyes, and skin reactivity to pollen. A positive response on skin prick testing to standardized pollen of grass and ragweed was defined as a wheal response of >3 mm greater as compared with the negative saline control. Subjects were included if skin prick testing was done within 1 year of enrollment. In addition, patients were enrolled if they were off antihistamine for at least 5 days. Subjects with significant co-morbid conditions, such as heart disease, atopic dermatitis, immunodeficiency, diabetes, cancer, HIV, and pregnancy were excluded. The following exclusion criteria were used: immunotherapy treatment, oral corticosteroids, and immunosuppressants such as methotrexate and azathioprine within 6 months of the date of study. Patients who were using nasal corticosteroid were included in the study. Informed consents were obtained from all study subjects.
Blood samples were drawn at a time when most subjects were asymptomatic or slightly symptomatic and were considered healthy. One subject with asthma was included as a control to evaluate the MSCs, which should exert suppressive effects on the lymphocyte proliferation.20 Due to the need to adhere to the approved human subject protocol, e.g., limit on the total amount of blood that was taken for the studies, not all subjects were studied in each assay. The subjects who were used in each assay are stated in the figure legend.
Isolation of PBMCs
Approximately 10 ml of blood was obtained from study subjects in heparinized tubes. The PBMCs were immediately isolated by Ficoll-Hypaque gradient separation. The blood was diluted with equal volume of sterile PBS and then added to an equal volume of Histopaque. The buffy coat containing PBMCs was collected and then washed three times in PBS. After the final wash, PBMCs were resuspended at 106 ml−1 RPMI 1640 with 10% FCS.
Culture of human MSCs
MSCs were expanded from bone marrow aspirates of healthy volunteers, aged 20–30 years. The use of aspirates was approved by the Institutional Review Board. Each volunteer signed informed consent. The method was previously described.22 Briefly, unfractionated aspirates were diluted in Dulbecco's modified Eagle's medium containing 10% FCS and then placed in vacuum gas plasma-treated plates (BD Falcon; Franklin Lakes, NJ, USA). The initial plating of whole bone marrow (BM) aspirates prevented the loss of endogenous MSCs. After 3 days of incubation, Ficoll Hypaque density gradient was used to separate the mononuclear fraction from the red blood cells and neutrophils. The reason for removing the red blood cells was to avoid toxicity to the adherent MSCs, caused by red cell lysis. The mononuclear cells were replaced in the original culture dishes. At weekly intervals, 50% of the media was replaced with fresh media. The adherent cells were serially passaged and after four cell passages, the adherent cells were symmetric, CD14−, CD29+, CD44+, CD34−, CD45−, CD105+, prolyl-4-hydroxylase−.
The method described above selected a population of pluripotent MSCs that expressed low level of MHC-II. The expanded MSCs formed functional peptidergic and dopamingeric neurons as well as differentiation along adipogenic, osteogenic, and chondrogenic lineages.22, 45 We compared different tissue culture surfaces and obtained high efficiency of pluripotent MSCs with plasma-treated surfaces.
In our lab, we have determined that the surface and the type of culture dish (flask vs Petri dish) are important in the doubling times for the MSCs. Some laboratories used platelet-rich plasma to expand MSCs. As platelets are a rich source of transforming growth factor-β1, we did not use this method because this might include a bias into MSCs with immune-suppressive functions. Nonetheless, we compared MSCs cultured in Petri dishes (Falcon 3003) and corning tissue culture flask with the same media. The MSCs in the Petri dishes showed a longer doubling time, but both sources showed similar responses with respect to the functional studies.
Proliferation assays
Cell proliferation was based on 3H-TdR incorporation as described.22 PBMCs were resuspended at 106 ml−1 in RPMI 1640 containing 10% FCS and then stimulated with allergen. The allergen was selected from the subject's known allergies (Table 1). Pollen (Hollister-Stier) was added at 5 μl ml−1 using the following: standardized Rye Grass (Lot # E08L1341); Timothy Grass (Lot #F08L1362); Bermuda (Lot #G09L7298); Meadow Fescue (Lot #C08J0534); short Ragweed (Lot #E10F0143). Except for ragweed, the stock concentrations of all allergens were 10 000 BAU ml−1. The stock concentration of ragweed was 1:20 (weight/volume) with an Amba1 content of 191. The optimal concentrations for each allergen were determined in titration assays for each subject in dose–response curves. The concentrations, at peak proliferation, were selected for all studies. The concentrations of all allergens except ragweed, presented as μl ml−1, are equivalent to 50 BAU ml−1.
PBMCs and MSCs were added at 50:1 ratios in wells of 96-well tissue culture plates. This was achieved by diluting the PBMCs at 106 ml−1 and MSCs at 2 × 104 ml−1. The MSCs were irradiated with 2000 Rads or non-irradiated. The radiation was delivered with a cesium source as described.22 The cell mixture contained the respective antigen at the concentration, stated above. The following parallel cultures were performed: PBMCs with antigen in the absence of MSCs; PBMCs and MSCs without antigen. After 48 h, each well was pulsed with 1 μCi ml−1 of 3H-TdR. After 16 h, the cells were harvested on glass fiber filters to study radioactive incorporation in a liquid scintillation counter (Beckman; Fullerton, CA, USA). The SI were calculated as disintegration per minute (d.p.m.) of experimental points/d.p.m. of unstimulated PBMCs. We used d.p.m. instead of counts per minute (c.p.m.) to account for the efficiency of the scintillation counters used to count the incorporation of 3H-TdR.
Immunoprecipitation/western blot
Immunoprecipitation of Class II, MHC, transactivator (CIITA) was performed with cytoplasmic and nuclear cell extracts using the Protein G-Agarose kit (Roche Applied Bioscience, Indianapolis, IN, USA). Briefly, the extracts were incubated with anti-CIITA (Santa Cruz, Santa Cruz, CA, USA) at 1:500 final dilution at 4 °C overnight. After this, the reactions were incubated with protein-G agarose (1:50) at 4 °C for 4 h on a rocking platform. The reactions were centrifuged at 4 °C, 12 000 g for 15 min, and the pellets were washed once with 1 × PBS and then resuspended in 1 × sample buffer containing 0.5% β-2-mercaptoethanol (ME). The extracts (20 μg of total protein) were analyzed by western blots.
The samples were electrophoresed on a 12% Mini-PROTEAN Precast Gel (Bio-Rad, Hercules, CA, USA) and then transferred to polyvinylidene difluoride membranes (Perkin-Elmer, Whaltham, MA, USA). The membranes were incubated with anti-CIITA at 1:500 dilution, 4 °C overnight; washed; incubated with HRP-conjugated goat anti-mouse IgG (1:1000) for 2 h at 4 °C. Horse radish peroxidase (HRP) was developed with chemiluminescence detection reagents (Thermo Scientific). The molecular weight was determined with SeeBlueM plus 2 Pre-stained standards (Life Technology).
Western blot analysis for HLA-DR (Santa Cruz) used rabbit polyclonal anti-HLA-DRa; ribosomal protein (Upstate, Lake Placid, NY, USA) used goat polyclonal antibody; Acetyl-Histone H3 (Upstate) used rabbit polyclonal IgG. β-actin used murine monoclonal IgG (Sigma).
CIITA knockdown
CIITA short hairpin RNA were purchased from Origene (Rockville, MD, USA). Four different inserts were tested to identify the insert that could efficiently knockdown CIITA. The inserts were ligated in pRFP-C-RS under the control of U6 promoter and were assigned the following by Origene: FI355589, FI355590, FI355591, and FI355592. The plasmid and vector without insert were transfected in MSCs as described using Effectene Transfection Reagent Kit (Qiagen, Valencia, CA, USA).30 At 72 h and 1 week after transfection, western blots for CIITA indicated efficient knockdown by the FI355592 plasmid. All assays were done with MSCs transfected with FI355592.
APC assay
Day 1, Cell activation: PBMCs (5 × 106 ml−1) were incubated with optimum dose of ragweed or rye grass (5 μl ml−1). Unactivated cells contained only media. Day 4, Pulsing: MSCs (2 × 104 ml−1) were incubated for 24 h with the same amount of the allergens. Unpulsed MSCs omitted antigen. Day 5, Enrichment of CD4± T cells: CD4+ cells were enriched by negative selection of other immune cell subsets. PBMCs (106 ml−1) were incubated with a cocktail of antibodies: CD3, CD8, CD11, CD56, CD20, each at 1:200 final dilution. After 1 h of incubation on ice, cells were washed with PBS and resuspended in 0.5 ml of PBS and 100 μl of Dynabead goat anti-mouse IgG (Invitrogen, Grand Island, NY, USA). The Dynabead-coupled cells were removed with a magnetic separator. The negative population was analyzed for CD4 by flow cytometry and the result indicated >90% labeling with anti-CD4. Day 5, Assay: Pulsed MSCs were resuspended in Dulbecco's modified Eagle's medium with 10% FCS at 106 ml−1 and then subjected to 2000 Rads of γ-irradiation. Irradiation rendered the cells in cycling quiescence, but metabolically active. CD4+-enriched cells (4 × 104 ml−1) were added to 50, 102, 103, or 104 ml−1 to the γ-irradiated MSCs. After 24 h, cells were pulsed with 1 μCi of [methyl-3H]-TdR/well. After 16 h, cells were harvested, analyzed for radioactive incorporation, and the simulation indices were calculated by dividing the d.p.m. of experimental points by d.p.m. of unactivated CD4+ T cells.
Flow cytometry
Flow cytometry for MHC-II and CD86 was performed with multi-color flow cytometry and the following antibodies: V450-CD86 and CD105 (Becton Dickinson, Franklin Lakes, NJ, USA), phycoerythrin-CD14 (Becton Dickinson), allophycocyanin-HLA-DR (Caltag Laboratories, Burlingame, CA, USA), CD3 and CD25 from a Human Regulatory T-Cell Staining Kit from e-Bioscience (San Diego, CA, USA). Nonspecific labelings used fluorochrome-conjugated isotype from Becton Dickinson. Positive control BD Compbeads (BD Biosciences, San Jose, CA, USA) were labeled with the all fluorochrome-tagged antibodies. After labeling, cells were analyzed with the LSRII system (BD Biosciences). The cells were gated on those positive for CD105 and negative for CD3 and CD25. The data were analyzed with the FACSCalibur system (BD Biosciences).
Cytokine array
Cytokine production by PBMCs, with pulsed and unpulsed MSCs, was assessed using the Human Cytokine Antibody Array 1 (RayBiotech, Norcross, GA, USA), as per manufacturer's instruction, also previously described.22 Briefly, the media were collected after 48 h for cytokine determination. The media from cultures containing PBMCs alone were assessed for background cytokine. The values were subtracted from the experimental. The densities of spots were quantitated with UN-SCAN-IT densitometry software (Silk Scientific, Orem, UT, USA). Cytokines were normalized to internal positive controls and presented as fold change relative to an internal control, arbitrarily assigned a value of 1. After this, the data were presented as fold changes of cultures with pulsed MSCs/unpulsed MSCs.
Statistical analysis
Data were analyzed using analysis of variance and Tukey–Kramer multiple comparisons test. A P-value of <0.05 was considered significant.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010;1:693–705. doi: 10.4155/tde.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Rameshwar P. Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Ther Deliv. 2012;3:997–1004. doi: 10.4155/tde.12.69. [DOI] [PubMed] [Google Scholar]
- Caplan AI. All MSCs are pericytes. Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Boregowda SV, Phinney DG. Therapeutic applications of mesenchymal stem cells: current outlook. BioDrugs. 2012;26:201–208. doi: 10.1007/BF03261879. [DOI] [PubMed] [Google Scholar]
- Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-{beta} J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- Rameshwar P. Breast cancer cell dormancy in bone marrow: potential therapeutic targets within the marrow microenvironment. Expert Rev Anticancer Ther. 2010;10:129–132. doi: 10.1586/era.10.3. [DOI] [PubMed] [Google Scholar]
- Comsa S, Ciuculescu F, Raica M. Mesenchymal stem cell-tumor cell cooperation in breast cancer vasculogenesis. Mol Med Report. 2012;5:1175–1180. doi: 10.3892/mmr.2012.796. [DOI] [PubMed] [Google Scholar]
- Momin EN, Vela G, Zaidi HA, Quinones-Hinojosa A. The oncogenic potential of mesenchymal stem cells in the treatment of cancer: directions for future research. Curr Immunol Rev. 2010;6:137–148. doi: 10.2174/157339510791111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator. Cancer Biother Radiopharm. 2009;24:717–721. doi: 10.1089/cbr.2009.0652. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Rameshwar P. Microenvironmental considerations in the application of human mesenchymal stem cells in regenerative therapies. Biologics. 2008;2:699–705. doi: 10.2147/btt.s2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggi N, Suva ML, De VC, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BA, Narine K, De NW, Mareel M, Bracke M, De WO. Resident and bone marrow-derived mesenchymal stem cells in head and neck squamous cell carcinoma. Oral Oncol. 2010;46:336–342. doi: 10.1016/j.oraloncology.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Corcoran KE, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Ramkissoon SH, Bryan M, Pliner LF, Dontu G, Patel PS, et al. Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Sci Rep. 2012;2:906. doi: 10.1038/srep00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, et al. IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- Vaes B, Van't Hof W, Deans R, Pinxteren J. Application of MultiStem((R)) allogeneic cells for immunomodulatory therapy: clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Front Immunol. 2012;3:345. doi: 10.3389/fimmu.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51:5–16. [PubMed] [Google Scholar]
- Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergyΓÇôinduced asthma. J Allergy Clin Immunol. 2012;129:1094–1101. doi: 10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp. 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Sem Immunopathol. 2011;33:593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Galipeau J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. Handb Exp Pharmacol. 2007;180:45–66. doi: 10.1007/978-3-540-68976-8_3. [DOI] [PubMed] [Google Scholar]
- Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KC, Trzaska KA, Smirnov SV, Kotenko SV, Schwander SK, Ellner JJ, et al. Down-regulation of MHC II in mesenchymal stem cells at high IFN-gamma can be partly explained by cytoplasmic retention of CIITA. J Immunol. 2008;180:1826–1833. doi: 10.4049/jimmunol.180.3.1826. [DOI] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, et al. Bone Marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001;22:185–189. [PubMed] [Google Scholar]
- Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–S16. doi: 10.1016/j.anai.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in ‘innate tolerance'. Immunology. 2012;137:206–213. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crop MJ, Baan CC, Korevaar SS, Ijzermans JNM, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19:1843–1853. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression. Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- Wright KL, Ting JPY. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 2006;27:405–412. doi: 10.1016/j.it.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaska K, Rameshwar P.Dopaminergic neuronal differentiation protocol for human mesenchymal stem cellsIn: Vemuri M, Chase LG, Rao MS, (eds). Mesenchymal Stem Cell Assays and Applications. Methods in Molecular Biology Humana Press: Totowa, NJ; 2011295–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.