Abstract
Interleukin-21 (IL-21) is a common γ-chain cytokine produced by T helper and natural killer T (NKT) cells. It has been shown to regulate the response of various lymphocyte subsets including NK, NKT, T and B cells. Owing to its potent anti-tumor function in preclinical studies and its ability to induce cytotoxicity and interferon-γ (IFN-γ) production in NK and CD8 T cells, recombinant IL-21 (rIL-21) was fast-tracked into early-phase clinical trials of patients with various malignancies. In a phase 2a trial of patients with metastatic melanoma, we analyzed the frequency and function of NKT cells in patients receiving rIL-21. NKT cells were present at a low frequency, but their levels were relatively stable in patients administered rIL-21. Unlike our observations in NK and CD8 T cells, rIL-21 appeared to reduce IFN-γ and TNF production by NKT cells, whereas it enhanced IL-4 production. It also modulated the expression of cell surface markers, specifically on CD4− NKT cells. In addition, an increase in CD3+CD56+ NKT-like cells was observed over the course of rIL-21 administration. These results highlight that IL-21 is a potent regulator of NKT cell function in vivo.
Keywords: NKT, T helper, IL-21, Cancer
Interleukin-21 (IL-21) is part of the family of common γ-chain cytokines, which includes IL-2, IL-4, IL-7, IL-9, and IL-15.1 It is produced by activated CD4 T2 and natural killer T (NKT) cells3, 4 and has potent immune regulatory properties in the context of infection, autoimmunity, and cancer.5, 6 In many preclinical models of cancer, IL-21 has been shown to promote anti-tumor immunity via the induction of CD8 T and NK cell-mediated cytotoxicity and interferon-γ (IFN-γ) production.7 For this reason, phase 1 and phase 2a trials testing the safety and efficacy of recombinant IL-21 (rIL-21) therapy in patients with metastatic melanoma and renal cell carcinoma were previously initiated.7, 8, 9, 10, 11 In these trials, rIL-21 administration displayed limited efficacy as a single agent, but was well tolerated and markedly induced cytotoxic activity and cytokine production by NK and CD8 T cells in vivo.
NKT cells are a potent immunoregulatory cell population heavily implicated in promoting immunity to infection and cancer.12, 13 NKT cells express a semi-variable αβ T-cell receptor (TCR) conserved between mouse and human, which allows them to respond to glycolipid ligands such as  -galactosylceramide (α-GC) in the context of the major histocompatibility complex-I-like molecule, CD1d.14 A hallmark of NKT cells is their ability to rapidly secrete T helper (Th) 1 cytokines such as IFN-γ and TNF as well as Th2 cytokines such as IL-4, concomitantly.15, 16
-galactosylceramide (α-GC) in the context of the major histocompatibility complex-I-like molecule, CD1d.14 A hallmark of NKT cells is their ability to rapidly secrete T helper (Th) 1 cytokines such as IFN-γ and TNF as well as Th2 cytokines such as IL-4, concomitantly.15, 16
Human NKT cells are best defined by α-GC/CD1d tetramer staining, or by the co-expression of Vα24 and Vβ11 TCR chains. Typically, NKT cells comprise approximately 0.1% of total lymphocytes in the blood of healthy individuals, although their numbers vary widely for reasons that are unclear. Human NKT cells can be subdivided into functionally distinct subsets on the basis of CD4 and CD8 expression, including CD4+CD8−, CD4−CD8−, and CD4−CD8+ NKT cells.15, 16, 17 Some, but not all of these cells express the NK cell-associated markers CD56 and CD161,15, 18 whereas T cells expressing CD56 and/or CD161, but not reactive to α-GC/CD1d, may represent non-classical NKT cells, mucosal-associated invariant T (MAIT) cells, or activated T cells with broad TCR reactivity.
In preclinical mouse studies, co-administration of rIL-21 and α-GC promotes the rejection of transplanted and spontaneous carcinogen-induced cancers by inducing potent NK cell activity.19 rIL-21 in combination with α-GC also greatly expands the NKT cell population in vitro and regulates NKT cell cytokine production, NK cell-associated receptor expression, and survival.3, 19 For these reasons, we monitored the frequency and phenotype of NKT cells in patients with metastatic melanoma receiving rIL-21. We observed that NKT cell frequencies were stable over 5 days of rIL-21 administration but that NK receptor (CD56 and CD161) expression was dynamic. Furthermore, rIL-21 administration reduced Th1 cytokine production and augmented the production of the Th2 cytokine, IL-4. These results demonstrate that intravenous administration of rIL-21 regulates NKT cell function in patients with stage IV metastatic melanoma.
Results
In a two-stage phase 2a trial of rIL-21 in stage IV malignant melanoma,8 patients were administered 30 μg/kg rIL-21 daily for 5 days followed by 9 days rest over a 6-week period. Patient blood samples were collected on the day of the first rIL-21 administration (day 1) and on days 2 and 5 thereafter. Blood samples were received and processed by flow cytometry on the day of collection except when samples were collected too late to process on the same day. rIL-21 was active in patients enrolled in this trial as measured by enhanced NK and CD8 T cell expression of activation molecules including granzyme B, perforin, and IFN-γ.8, 10
The frequency of NKT cells is stable following rIL-21 administration
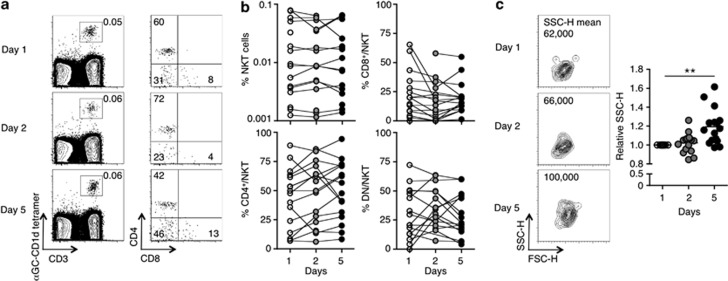
Peripheral blood mononuclear cells were analyzed for the presence of NKT cells by staining with α-GC/CD1d tetramer and CD3. Frequencies of NKT cells (α-GC/CD1d tetramer+CD3+) were low with no more than 0.1% NKT cells detected among lymphocytes in any of the patients (Figures 1a and b). This is in line with studies depicting a reduction in levels of blood NKT cells in patients with various malignancies.20, 21, 22, 23 Nonetheless, NKT cell proportions in the blood appeared relatively stable in all patients over 5 days (Figure 1b). Furthermore, the frequencies of CD4+CD8−, CD8+CD4−, and DN NKT cell subsets among the total NKT cell population were not significantly skewed over time (Figure 1b). Intriguingly, the side-scatter property of NKT cells increased noticeably at day 5 of rIL-21 administration, indicative of an increase in granularity and activation (Figure 1c).
Figure 1.
Natural killer T (NKT) cell frequency and CD4 expression in the peripheral blood remain stable following rIL-21 administration. Blood samples from patients receiving rIL-21 were collected on days 1, 2, and 5 and analyzed for NKT cells (α-GC/CD1d tetramer+ CD3+) and CD4 and CD8 expression on NKT cells. (a) Contour and dot plots of peripheral blood NKT cells (left-hand column) and CD4 vs. CD8 expression (middle) on gated NKT cells from one patient. Numbers depict the percentage of cells in the gate/quadrant. (b) The percentage of NKT cells among total lymphocytes and the percentage of CD4+CD8−, CD4−CD8+, and CD4−CD8− (DN) cells among gated NKT cells is depicted over time in patients receiving rIL-21. (c) Contour plots of Forward scatter-height (FSC-H) vs. Side scatter-height (SSC-H) on gated NKT cells from one patient over time. Numbers depict the mean fluorescence intensity (MFI) of SSC-H. The graph shows the relative MFI of SSC-H on gated peripheral blood NKT cells from patients receiving rIL-21. Results were analyzed using the Wilcoxon signed rank test on the raw data with a Bonferroni correction to account for the number of comparisons being made (**P<0.01).
rIL-21 administration specifically regulates CD4− NKT cell surface receptor expression
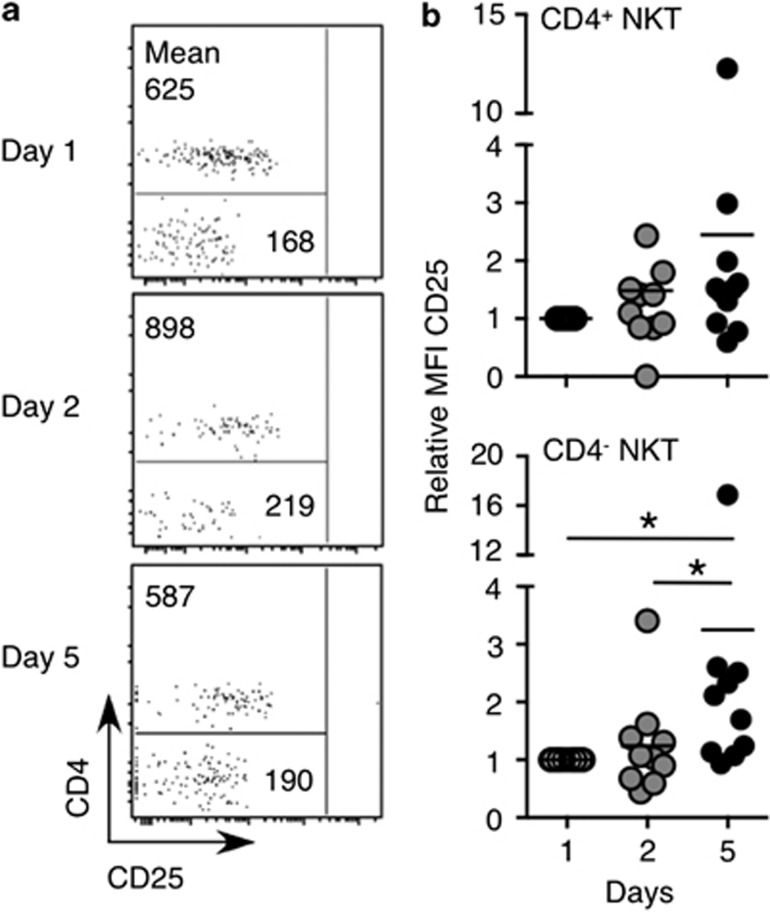
Given that IL-21 regulated activation of mouse NKT cells and altered expression of NK cell-associated receptors,3, 24 NKT cells from patients receiving rIL-21 were analyzed for changes in the expression of activation or differentiation markers including CD25, CD161, and CD56. The expression of CD25 is higher on CD4+ than CD4− NKT cells, whereas CD161 and CD56 are more highly expressed by CD4− NKT cells.15, 18 For this purpose, we examined the expression of these molecules in individual subsets of NKT cells. Because of the low frequency of NKT cells in some patients, surface marker expression on NKT cells could not be reliably assessed in all patients. CD25 expression was generally higher on CD4+ compared with CD4− NKT cells (Figure 2a) before the onset of rIL-21 administration, as expected. Following rIL-21 administration, CD25 expression was significantly upregulated by CD4− NKT cells at day 5 (Figure 2b). CD25 also appeared to be upregulated on CD4+ NKT cells from some patients at this time point, although this was not statistically significant at the group level.
Figure 2.
CD25 expression on CD4+ and CD4− natural killer T (NKT) cells following rIL-21 administration. Blood samples were collected and CD25 expression was analyzed on gated NKT cells. (a) Dot plots of gated NKT cells from one patient depicting CD25 vs. CD4 expression. Numbers show the MFI of cells in the gated region. (b) The relative MFI of CD25 expression on gated peripheral blood CD4+ and CD4− NKT cells from patients receiving rIL-21. Note that for some patients, NKT cell proportions were too low to reliably depict CD25 expression, and hence, these patients were omitted from the analysis. Results were analyzed using the Wilcoxon signed rank test on the raw data with a Bonferroni correction to account for the number of comparisons being made (*P<0.05).
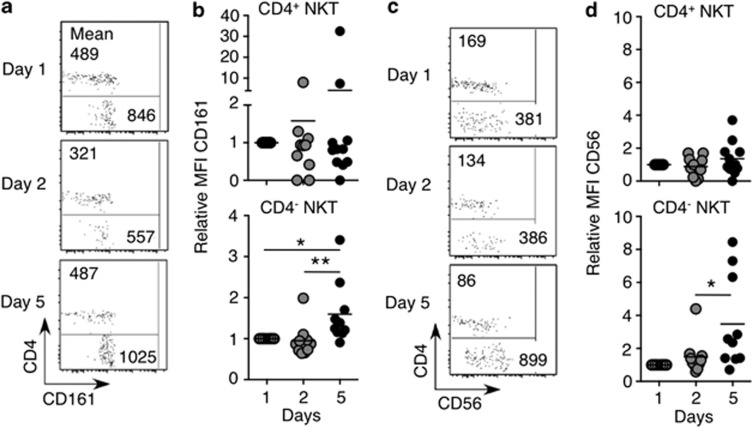
CD161 and CD56 expression is higher in CD4− than in CD4+ human NKT cells.15, 18 Over the course of rIL-21 administration, expression of these markers was moderately, albeit, significantly enhanced specifically on the CD4− fraction of NKT cells, but not on CD4+ NKT cells (Figure 3a–d). These results suggest that rIL-21 specifically regulates the expression of NK cell-associated receptors on CD4− but not CD4+ NKT cells in these patients.
Figure 3.
CD161 and CD56 expression on CD4+ and CD4− natural killer T (NKT) cells following rIL-21 administration. Blood samples were collected and CD161 and CD56 expression was analyzed on gated NKT cells. (a, c) Dot plots of gated NKT cells from one patient depicting CD161 and CD56 expression. Numbers show the MFI of cells in the gated region. (b, d) The relative MFI of CD161 and CD56 expression on gated peripheral blood CD4+ and CD4− NKT cells from patients receiving rIL-21. Note that for some patients, NKT cell proportions were too low to reliably depict CD161 or CD56 expression, and hence, these patients were omitted from the analysis. Results were analyzed using the Wilcoxon signed rank test on the raw data with a Bonferroni correction to account for the number of comparisons being made (*P<0.05, **P<0.01).
rIL-21 administration augments the ratio of Th2:Th1 cytokines produced by NKT cells
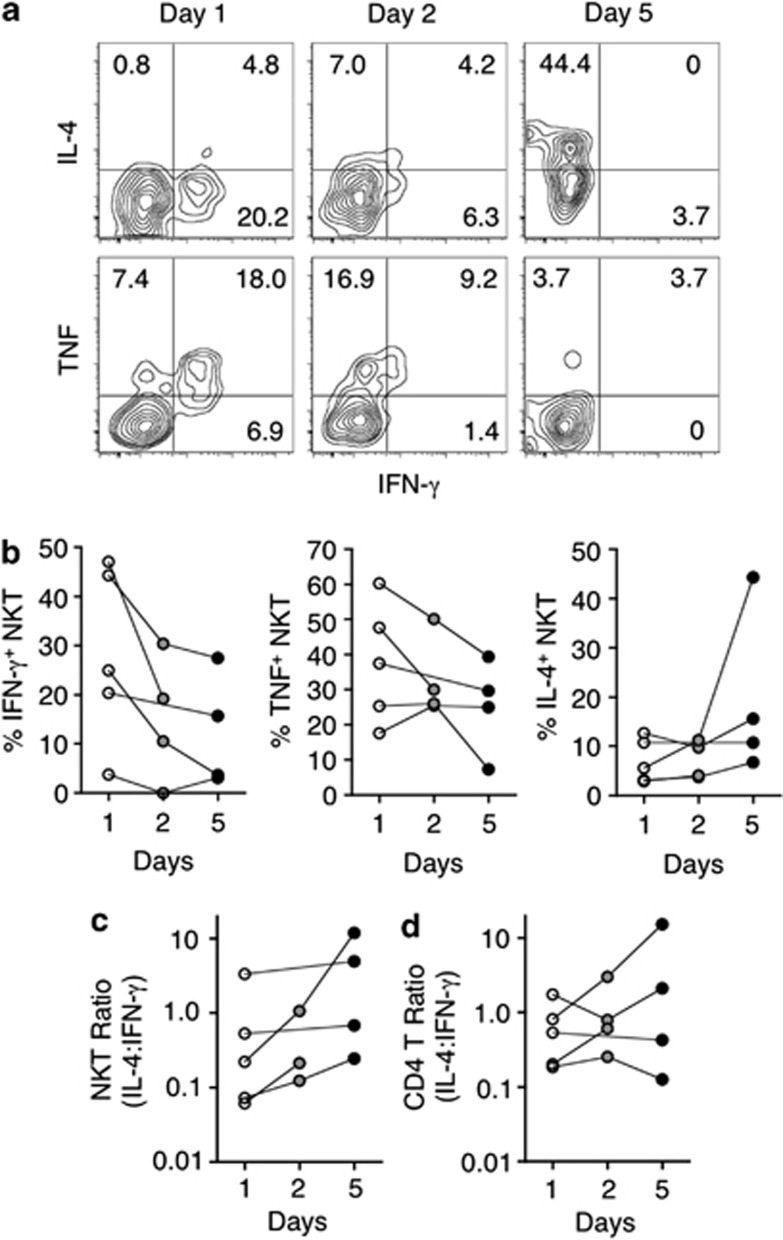
NKT cell IFN-γ, TNF, and IL-4 production was analyzed by intracellular staining after administration of rIL-21. This analysis was limited to patient samples in which NKT cells were the most prevalent to ensure that sufficient cells could be analyzed. For this experiment, samples were stored frozen until they could all be tested side by side at the end of the study. All samples were thawed simultaneously and stimulated with phorbol 12-myristate 13-acetate and ionomycin. The proportion of cytokine-producing cells varied considerably between patients (Figure 4a, b). Intriguingly, over the course of rIL-21 treatment, NKT cell IFN-γ (5/5 patients) and TNF (4/5 patients) production decreased (Figure 4b). In contrast, IL-4 production by NKT cells was increased in four out of five patients and the ratio of IL-4/IFN-γ production in NKT cells increased in all patients over 5 days (Figure 4c). In comparison, the ratio of IL-4/IFN-γ production in conventional CD4 T cells followed no clear trend over 5 days (Figure 4d). This suggests that rIL-21 administration specifically skews NKT cell cytokine production toward Th2 and away from Th1 cytokines.
Figure 4.
RIL-21 administration alters the production of T helper 1 (Th1) and Th2 cytokines by natural killer T (NKT) cells. On the days of sample acquisition, an aliquot of cells was frozen for subsequent analysis at a later time. At this time, peripheral blood cells from patients in which NKT cells were most prevalent were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 2.5 h in the presence of GolgiStop. Subsequently, cells were stained for α-GC/CD1d tetramer, CD3, CD4, intracellular interferon-γ (IFN-γ), tumor necrosis factor (TNF), and interleukin-4 (IL-4) and analyzed by flow cytometry. Vehicle-loaded tetramer was also added to the staining cocktail to gate out nonspecific events. (a) Shown are contour plots of NKT cells from one patient over the course of rIL-21 administration. Plots represent IFN-γ vs. IL-4 or TNF on gated NKT cells. (b–d) Graphs depict the total frequency of cytokine-producing cells among NKT cells (b), the ratio of IL-4/IFN-γ production in NKT cells (c) and the ratio of IL-4/IFN-γ production in conventional CD4 T cells (d) from five individual patients.
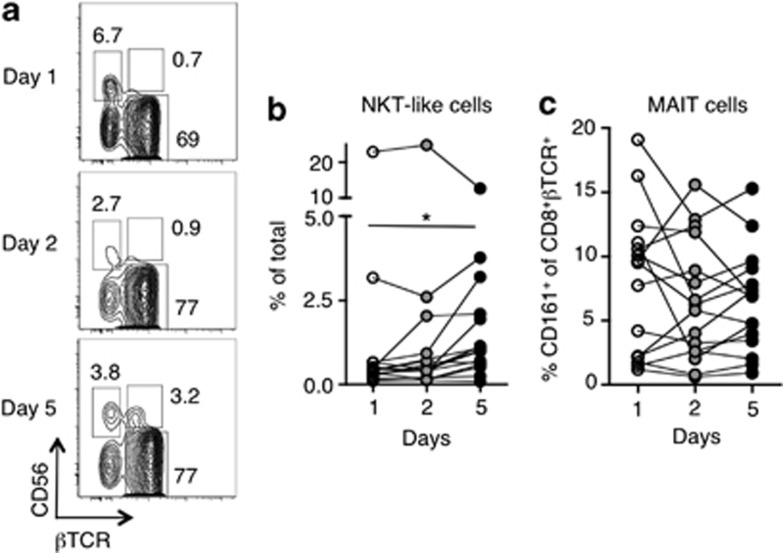
NKT-like cells (βTCR+CD56+α-GC/CD1d tetramer−) are increased following rIL-21 administration
In addition to α-GC-reactive NKT cells, we also assessed the prevalence of βTCR+CD56+α-GC/CD1d tetramer− ‘NKT-like' cells in peripheral blood mononuclear cells. This population of NKT-like cells was substantially increased in frequency at day 5 of rIL-21 administration (Figures 5a and b). As no such increase in α-GC-CD1d reactive NKT cells was observed over this time course, it was possible that these cells represented other types of innate-like T cells such as Type-2 NKT cells or MAIT cells. We analyzed the frequency of MAIT cells as defined by the surrogate phenotype of CD161+βTCR+CD8+CD4−α-GC/CD1d tetramer− cells, however, these cells were not increased in frequency over the course of rIL-21 administration (Figure 5c).
Figure 5.
Natural killer T (NKT)-like (CD56+βTCR+) cells become more prevalent over the course of rIL-21 administration. (a) Representative contour plots of β T-cell receptor (βTCR) vs. CD56 expression on non-α-GC/CD1d-restricted cells. Numbers represent the percentage of cells in the gated region. (b) The frequency of NKT-like cells in patients administered rIL-21. (c) The frequency of mucosal-associated invariant T (MAIT) cells (CD161+ among βTCR+CD8+CD4−α-GC/CD1d tetramer−) was analyzed over the course of rIL-21 administration. Results were analyzed using the Wilcoxon signed rank test with a Bonferroni correction to account for the number of comparisons being made (*P<0.05).
Taken together, these results demonstrate that in vivo administration of rIL-21 in stage IV malignant melanoma patients modulates NKT cell surface marker expression and skews the balance of Th cytokines produced by NKT cells. In addition, it enhances the frequency of βTCR+CD56+α-GC/CD1d tetramer− ‘NKT-like' cells in the peripheral blood that are not classical NKT cells or MAIT cells.
Discussion
In this phase 2a trial in malignant melanoma patients, two confirmed responses were observed in the months following rIL-21 administration.8 All patients showed signs of rIL-21 activity, be it by fluctuations in blood lymphocyte counts, activation of STAT-3, or through enhanced IFN-γ or granzyme B expression by NK and CD8 T cells.8, 10 We have previously shown that murine rIL-21 induces NKT cell granzyme B expression and an increase in cell granularity, as distinguished by flow cytometric side-scatter properties.3 Because of the low frequency of NKT cells in the patients analyzed, it was not possible to directly assess granzyme B expression. However, the increased side-scatter properties of NKT cells allude to enhanced cytotoxic function. Although the upregulation of STAT-3 promptly after rIL-21 administration demonstrates the direct impact of this cytokine,10 IL-21 is also known to make responding T cells impervious to regulatory T (Treg) cell-mediated suppression and can alter Treg homeostasis.25, 26, 27 It remains to be shown whether the functional changes in NK, CD8 T, and NKT cells were in part mediated via an indirect action of IL-21 on Treg.
The effects of IL-21 on cytokine production by various cell types are complex. In general, the effects observed in the patients studied reflect the findings of numerous studies in mice. rIL-21 substantially enhanced IFN-γ production in both NK and CD8 T cells in these patients, in line with murine studies.7 Yet, in NKT cells, which are normally adept at producing IFN-γ, rIL-21 substantially diminished IFN-γ production and slightly enhanced IL-4 production, skewing the Th1/Th2 balance, an observation we previously made in mice.3 This skewing was not observed in conventional CD4 T cells despite reports that rIL-21 can enhance Th2 development28, 29 implying that IL-21 mediates unique effects on human NKT cells. Unfortunately, IL-17 production by NKT cells was not assessed in this trial but given the ability of IL-21 to enhance IL-17 production by human CD4 T cells,30 it would be intriguing to investigate if this cytokine performs the same function for NKT cells.
CD4+ and CD4− NKT cell subsets are functionally distinct in mice31 and humans15, 16 and the participation of one subset over another has the potential to greatly skew immunity. For this reason, the elucidation of factors that influence the function of one subset over another may prove useful. Over the course of rIL-21 administration, there was an apparent bias in the upregulation of several markers on CD4−, but not CD4+ NKT cells. Whether this is due to a difference in subset expression of the IL-21 receptor in these patients or an altered responsiveness for another reason remains to be shown. It should be noted, however, that the reduction in IFN-γ and TNF production after rIL-21 administration appeared to occur in both CD4+ and CD4− NKT cells (data not shown), as did the enhancement in NKT cell granularity. Further, CD4+ NKT cells also appeared to moderately upregulate CD25 expression. This implies that CD4+ NKT cells are indeed IL-21-responsive, yet do not modulate their expression of certain NK cell-associated receptors following IL-21 stimulation.
In addition to our analysis of α-GC-reactive NKT cells, rIL-21 appeared to expand a population of NKT-like (βTCR+CD56+α-GC/CD1d tetramer−) cells. Our analysis ruled out that this population represented classical NKT cells and no expansion was observed in MAIT-like cells over the course of rIL-21 administration. It would be interesting to investigate whether this population expresses a restricted set of TCRs that might reflect a different innate-like T-cell population, such as CD1b-reactive T cells,32 or whether it is an IL-21-responsive conventional T-cell population expressing a diverse range of TCRs. A more extensive analysis of cytokine production and IL-21 receptor expression in this subset may also help to identify cells that are particularly responsive to IL-21 in humans and identify potentially novel cellular targets of this cytokine.
Taken together, our data show that NKT cells are responsive to rIL-21 administration in patients with stage IV malignant melanoma and that studying the effect of IL-21 on other T-cell populations may be a useful avenue for future research.
Methods
Recombinant IL-21
rIL-21 was provided by Novo Nordisk (Bagsvaerd, Denmark). rIL-21 was expressed in Escherichia coli as the NH2-terminal methionylated form. Zymogenetics in Seattle (WA) developed processes for production, refolding, and purification of the molecule and analytic methods for assessment of purity and potency. Manufacturing was done in the good manufacturing practice facilities of Avecia (Milford, MA, USA). Each vial contained 0.8 ml of rIL-21 at a concentration of 10 mg/ml and diluted for administration using sterile saline for injection (0.9% (w/v) sodium chloride).
Trial design
The phase 2a component of trial NN028-1614 was an open-label, nonrandomized two-stage design. The primary objective of the trial was to determine the effect of rIL-21 on tumor size in subjects with metastatic melanoma. Data on overall survival, inclusion criteria, adverse effects, and lymphocyte responses to rIL-21 were previously reported by Davis et al.8 rIL-21was administered by intravenous bolus injection at a dose of 30 μg/kg, in a dosing regimen of daily treatment for 5 days followed by 9 days rest (‘5+9') for 6 weeks, followed by 2 weeks without dosing, for a total treatment cycle length of 8 weeks. The study was conducted between May 2006 and January 2008 at Austin Health, Peter MacCallum Cancer Centre, Cabrini Health, Westmead Hospital, and Sir Charles Gairdner Hospital. All participants provided written informed consent before any study-specific procedures. The trial protocol was approved by the Human Research Ethics Committees of the participating hospitals and was conducted under the Australian Therapeutic Goods Administration Clinical Trials Notification scheme (ClinicalTrials.gov identifier: NCT00336986).
Peripheral blood isolation and analysis
For immunophenotyping of NKT cells, whole blood was collected in 6-ml ACD vacutainer tubes. Samples were delivered at ambient temperature and analyzed within 24 h of receipt. The following antibodies from BD Biosciences (Franklin Lakes, NJ, USA) were used: CD3 PE-CY7 (SK7), αβ TCR FITC (T1OB9.1A-31), CD161 APC (DX12), CD56 APC (B159), CD25 PE-CY7 (M-A251), CD4 APC-CY7 (RPA-T4), CD8 FITC (G42-8), IFN-γ FITC (4S.B3), IL-4 APC (MP4-25D2), TNF Alexa fluor 700 (MAb11). Mouse CD1d tetramer loaded with α-GC was produced in house using recombinant baculovirus encoding his-tagged mouse CD1d and mouse β2 microglobulin, originally provided by Professor M Kronenberg's laboratory (La Jolla Institute for Allergy and Immunology, San Diego, CA, USA). α-GC/CD1d tetramer also recognizes the human NKT cell TCR. Flow cytometry was performed on the LSR-II (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR, USA) software. Human samples were always fixed before flow cytometric acquisition.
Peripheral blood cell stimulations and intracellular staining
Cells were stimulated for 3 h in the presence of 0.05 μg/ml phorbol 12-myristate 13-acetate, 0.75 μg/ml ionomycin (Sigma-Aldrich, St Louis, MO, USA), and GolgiStop (BD Biosciences). Cells were stained for surface markers, then fixed and permeabilized using the BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit. Cells were cultured in media containing RPMI-1640 (Gibco BRL, Life technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal calf serum (JRH, Lenexa, KS, USA), 100 U/ml penicillin (Gibco BRL, Life technologies), 1 mM sodium pyruvate (Invitrogen Life Technologies, Carlsbad, CA, USA), 0.1 mM nonessential amino acids (Invitrogen Life Technologies), 100 μg/ml streptomycin (Gibco BRL, life technologies), 15 mM HEPES (Gibco BRL, Life technologies), 2 mM glutamax (Gibco BRL, Life technologies), and 50 μM 2-ME (Sigma-Aldrich) in six-well plates (Nalge NunC, Rochester, NY, USA).
Statistical analysis
Data were analyzed using the non-parametric Wilcoxon signed rank test. The non-normalized data were used to calculate statistical significance even where relative mean fluorescence intensity is shown in Figures 2 and 3. A Bonferroni correction was made to account for multiple comparisons.
Acknowledgments
We thank Stuart Berzins for his insights into analysis of human NKT cells, Konstantinos Kyparissoudis for technical expertise, and Kenneth Field for assistance with flow cytometry (University of Melbourne). This work was supported by a NHMRC program grant, J.M.C. was supported by a Cancer Research Institute Predoctoral Emphasis Pathways in Tumor Immunology grant, D.I.G. is supported by a NHMRC Senior Research Principal Research Fellowship, M.J.S. is supported by a NHMRC Australia Fellowship.
References
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- Harada M, Magara-Koyanagi K, Watarai H, Nagata Y, Ishii Y, Kojo S, et al. IL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. 2006;203:2929–2937. doi: 10.1084/jem.20062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. T helper cell differentiation: IL-21 and T helper cell differentiation: Jack of all trades. Immunol Cell Biol. 2008;86:554–556. doi: 10.1038/icb.2008.51. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- Swann JB, Coquet JM, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Wilson SB. CD1d-restricted T-cell subsets and dendritic cell function in autoimmunity. Immunol Cell Biol. 2004;82:307–314. doi: 10.1111/j.0818-9641.2004.01253.x. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Cochrane AD, Pellicci DG, Smyth MJ, Godfrey DI. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur J Immunol. 2005;35:1399–1407. doi: 10.1002/eji.200425958. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi S, Kobayashi S, Ito T, Magara KK, Mikuni O, Kamada N, et al. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102:159–165. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- Konishi J, Yamazaki K, Yokouchi H, Shinagawa N, Iwabuchi K, Nishimura M. The characteristics of human NKT cells in lung cancer--CD1d independent cytotoxicity against lung cancer cells by NKT cells and decreased human NKT cell response in lung cancer patients. Hum Immunol. 2004;65:1377–1388. doi: 10.1016/j.humimm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, et al. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, et al. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119:4656–4664. doi: 10.1182/blood-2011-10-388546. [DOI] [PubMed] [Google Scholar]
- Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich A, Marsland BJ, Sondregger I, Kurrer M, Hodge MR, Harris NL, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]