Abstract
Infectious complications remain a leading cause of morbidity and mortality after solid organ transplantation (SOT), and largely depend on the net state of immunosuppression achieved with current regimens. Cytomegalovirus (CMV) is a major opportunistic viral pathogen in this setting. The application of strategies of immunological monitoring in SOT recipients would allow tailoring of immunosuppression and prophylaxis practices according to the individual's actual risk of infection. Immune monitoring may be pathogen-specific or nonspecific. Nonspecific immune monitoring may rely on either the quantification of peripheral blood biomarkers that reflect the status of a given arm of the immune response (serum immunoglobulins and complement factors, lymphocyte sub-populations, soluble form of CD30), or on the functional assessment of T-cell responsiveness (release of intracellular adenosine triphosphate following a mitogenic stimulus). In addition, various methods are currently available for monitoring pathogen-specific responses, such as CMV-specific T-cell-mediated immune response, based on interferon-γ release assays, intracellular cytokine staining or main histocompatibility complex-tetramer technology. This review summarizes the clinical evidence to date supporting the use of these approaches to the post-transplant immune status, as well as their potential limitations. Intervention studies based on validated strategies for immune monitoring still need to be performed.
Keywords: cell-mediated immunity, cytomegalovirus, immune-monitoring strategies, infection, prediction, solid organ transplantation
Despite continued improvement in the clinical management of solid organ transplant (SOT) recipients, infection continues to be one of the leading causes of morbidity and mortality in this population. Two main variables need to be accounted for when evaluating an individual patient's risk for post-transplant infection: the epidemiological exposure (that is, postoperative colonization by multidrug-resistant pathogens) and the ‘net state of immunosuppression'.1 The latter emerges from a complex interaction that encompasses multiple factors, including the type of immunosuppression regimen used, its timing and dosage, the presence of underlying immune defects or viral co-infections, and the evolution of graft function.2 Thus far, clinicians caring for SOT recipients have relied almost exclusively on the therapeutic drug monitoring of immunosuppressive agents to explore the status of immunocompetence of their patients.3 Nevertheless, such an approach appears limited by its unidimensional nature, which does not take into account the large variety of pharmacokinetic, pharmacodynamic and clinical variables that modulate the phenotypic activity of modern immunosuppression protocols.4 Moreover, the evidence supporting the utility of therapeutic drug monitoring of agents other than calcineurin inhibitors and mammalian target of rapamycin inhibitors remains less conclusive.5, 6
As a result of ongoing efforts to fulfill this unmet clinical need, we now have an expanding repertoire of immune-monitoring strategies that may stratify the odds for developing infection in a given SOT recipient and, eventually, provide the basis for tailored immunosuppression and prophylaxis strategies. Of note, these approaches largely differ in their theoretical background, type of event predicted, assay methodology, limitations and feasibility in daily practice. This review summarizes the state of the art in this emerging field.
General rationale for post-transplant immune monitoring
The immune response, either innate or adaptive, is the result of an extremely complex interplay between soluble and membrane-bound signaling mediators and specialized cell populations, eventually leading to the initiation of a number of effector mechanisms.7 The different strategies proposed for immunological monitoring ultimately pursue to reduce the complexity of such a process—or at least a part of it—into an individual or group of parameter(s) or biomarker(s) that, by means of regular measurements, may provide a dynamic insight into the net state of immunosuppression of the subject and the subsequent correlation with the risk for post-transplant infection. Ideally, the assay on which this monitoring is based should be reliable, sensitive and specific enough, highly reproducible, and its results should be available for the clinician within a short turnaround period to allow timely modifications in immunosuppression or prophylaxis.8 The implementation of these strategies must eventually be linked to a feasible intervention that has been proven to provide some clinical benefit.
From a clinical perspective, the approaches to the immune monitoring in SOT recipients could be grouped according to its target into non-pathogen-specific or pathogen-specific. The first category encompasses those strategies aimed at evaluating the functionality of a given arm of the immune system by means of assays (or biological parameters) with no antigen specificity. Therefore, in most of the studies the predicted event is the occurrence of overall infection—with no further classification according to the clinical syndrome or the causal pathogen—or, at the most, a generic type of infection (that is, bacterial or fungal infection). The nature of the biomarker used may be merely quantitative—such as the concentration of serum immunoglobulins—or provide some functional assessment—such as the intra-lymphocytic release of adenosine triphosphate (ATP) under a nonspecific mitogen. In contrast, the pathogen-specific immune-monitoring strategies rely on antigen-specific assays that estimate the magnitude and functionality of adaptive immune responses generated by T cells or B cells against a given pathogen, usually by measuring the production of Th1 effector cytokines (that is, interferon (IFN)-γ or tumor necrosis factor-α) upon stimulation with a known antigen. Although progress has been made in the assessment of different virus-specific cell-mediated immune responses, including Epstein–Barr virus (EBV)9 or BK polyomavirus (BKPyV),10 we will mainly focus on current developments in cytomegalovirus (CMV)-specific T-cell monitoring, in view of its relative state of maturity and wide potential implications for the management of the SOT population.
Non-pathogen-specific monitoring
As summarized in Table 1, the proposed strategies for nonspecific immune monitoring after transplantation are notably heterogeneous in terms of complexity, capacity for functional assessment and technical requirements.
Table 1. Summary of proposed methods for non-pathogen-specific immune monitoring in SOT recipients.
| Characteristic | Serum immunoglobulins | Serum complement factors (C3, C4, MBL) | Peripheral blood lymphocyte sub-populations | Soluble CD30 | iATP in CD4+ T cells (ImmuKnow assay) |
|---|---|---|---|---|---|
| Required sample | Serum | Serum | Whole blood | Serum | Whole blood |
| Assay | Nephelometry | Nephelometry or ELISA | Flow cytometry | ELISA | Quantification of iATP release in PHA-stimulated CD4+ T cells |
| Functional analysis | No | No | No | Yes | Yes |
| Advantages | Economical and easy to perform. Potential for replacement therapy with IVIGs | Economical and easy to perform. Potential for genotyping of mbl2 gene variants | Easy to perform (automatized methods) | Easy to perform. Commercial assay. Low volume of serum required (25 μl) | Only FDA-approved commercial assay. Highly standardized. Large volume of studies |
| Limitations | Lack of standardized cutoff values. No information on the functionality of the humoral response | Lack of standardized cutoff values. No information on the functionality of the complement system | Lack of standardized cutoff values. No information on the functionality of the cellular response | Only few studies on predicting infection with discordant findings | Only modest PPV and NPV in studies to date. Relatively high cost. Potentially biased by sample storage time |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; FDA, Food and Drug Administration; iATP, intracellular adenosine triphosphate; IVIGs, intravenous immunoglobulins; MBL, mannose-binding lectin; NPV, negative predictive value; PHA, phytohemagglutinin; PPV, positive predictive value; SOT, solid organ transplantation.
Serum immunoglobulin levels
For some years, the occurrence of secondary de novo hypogammaglobulinemia (HGG) was a somewhat neglected immunosuppression-related complication in SOT recipients. Nevertheless, a recent meta-analysis reported that mild (serum immunoglobulin G (IgG) levels 400–700 mg dl−1) and severe (IgG <400 mg dl−1) HGG occur in as many as 39% and 15% of patients during the first year post-transplant, respectively.11 The incidence and clinical implications of HGG have been assessed in kidney,12, 13, 14, 15 liver,16 lung,17, 18, 19 heart20 and intestinal21 transplant recipients. The mechanisms leading to post-transplant HGG are not fully clarified and are likely multifactorial, including the decrease in CD4+ T-cell numbers and its subsequent impact on B-cell activation.22 The use of mycophenolate mofetil has been also shown to increase the incidence of HGG in some studies,12 presumably through a direct detrimental effect on B-cell function.23 In addition, certain graft-specific risk factors have been identified, such as the presence of bronchiolitis obliterans syndrome for lung transplantation18, 19 or the administration of steroid pulses for heart transplantation.20
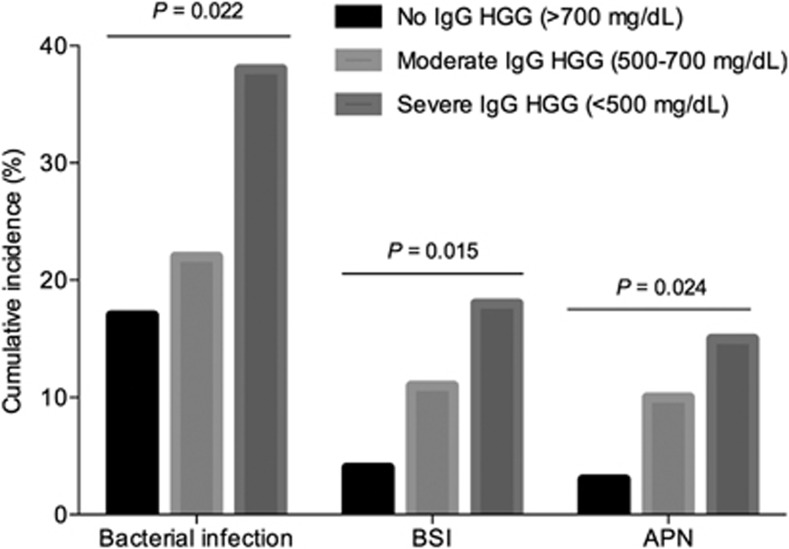
The humoral arm of the immune response is primarily responsible for the clearance of encapsulated bacteria (that is, Streptococcus pneumoniae or Haemophilus influenzae type b) by opsonization, antigen neutralization and complement activation.24 Post-transplant HGG, specifically of IgG, acts therefore as a good predictor for bacterial infection.11 A recent prospective study in kidney transplant recipients found a ‘dose-effect' in the occurrence of infection according to the post-transplant IgG levels, with a clear gradient from mild or moderate to severe HGG (Figure 1).15 The impact of IgG HGG on the incidence of other bacterial infections has been also demonstrated for bacteremia15, 17 and Clostridium difficile-associated diarrhea.25 Less intuitively, some authors have suggested that the risk of CMV disease20, 26 or invasive fungal infection17 is increased in patients with post-transplant HGG. The meta-analysis by Florescu et al.11 reported that the odds of CMV and fungal infection for severe HGG were 2.89 and 3.69 times higher, respectively, than those for mild HGG. Although the role in the control of CMV replication of neutralizing antibodies targeting the viral glycoprotein B is increasingly recognized,27, 28 it could be argued that the detection of low immunoglobulin levels simply represents a surrogate for a higher degree of immunosuppression or poorer clinical status.29 In that line, Doron et al.16 found a lower long-term survival in liver transplant recipients with HGG, even in the absence of a discernible effect on the incidence of infection. Despite this and other limitations (the heterogeneity of studies and the lack of common definitions for the different categories of HGG), this approach to the monitoring of humoral response offers two additional advantages. First, the measurement of serum immunoglobulins by nephelometry is a widely available technique and economically affordable (≈13 US Dollars per determination).15 Second, as opposed to other immune defects in transplant recipients, HGG is potentially reversible without increasing the risk of graft rejection, and some studies have already evaluated the preemptive replacement therapy with intravenous immunoglobulin, with promising results.30, 31
Figure 1.
Cumulative incidences at month 6 post-transplant for overall bacterial infection, bloodstream infection (BSI) and acute pyelonephritis (APN) according to the serum IgG levels at month 1 in a prospective cohort of 271 kidney transplant recipients (modified from reference Fernández-Ruiz et al.15 plus personal data (Fernández-Ruiz M, 2013, unpublished data)).
Serum complement factors
The complement system constitutes another target for monitoring strategies in view of the relevance of its effector functions (opsonophagocytosis, induction of acute inflammation and cellular lysis) in innate and adaptive humoral immune responses.32 The relative contribution of complement is further highlighted in the setting of post-transplant immunosuppression, which is primarily directed against adaptive cellular immunity.33 The assessment of complement functionality has classically relied on in vitro hemolytic assays (CH50 and AP50 for the classical and alternative pathways, respectively).34 However, the complexity and time-consuming nature of these methods preclude their daily clinical application. The measurement of serum levels of certain components by a more feasible method (nephelometry) represents a convenient proxy for the complement activity. The three activation cascades converge on the third component of the complement to form the C5 convertase (C4bC2aC3b for the classical and lectin pathways and [C3b]2Bb for the alternative pathway) and, ultimately, to assemble the membrane attack complex (C5b-C9).32 Therefore, this pivotal role played by C3 makes it a good candidate for monitoring. The utility of this strategy has been shown in a prospective study of 270 kidney transplant recipients, in which the presence of C3 hypocomplementemia (serum levels <83.0 mg dl−1) at month 1 was as an independent risk factor for the subsequent occurrence of overall and bacterial infection (hazard ratios of 1.9 and 2.1, respectively).35 Comparable findings have been reported for liver36 and heart transplant recipients.37 Unfortunately, the only intervention that seems feasible in a patient with low complement levels consists of decreasing immunosuppression, which in turn could increase the risk of graft rejection.
The functional status of the lectin activation pathway may be specifically explored by assessing the serum concentrations of mannose-binding lectin (MBL), which in turn are largely determined by various polymorphisms occurring in the mbl2 gene or its promoter region.38 Structurally related to the C1q component of the classical pathway, serum MBL can also be easily measured by nephelometry or enzyme-linked immunosorbent assay. In a cohort of 102 liver transplant recipients, the presence of low MBL levels were associated with a higher incidence of clinically significant infection (52% vs 20% P-value=0.003). Donor mbl2 genotype was the strongest determinant of post-transplant circulating MBL levels, a not surprising finding considering that this pattern recognition molecule is produced primarily by the liver.39 MBL deficiency has been also linked to the development of sepsis in kidney or pancreas–kidney transplant recipients14, 40 or CMV infection after discontinuing valganciclovir prophylaxis.41 More studies are needed to determine the optimal cutoff levels and timing for the monitoring of this biomarker. In addition, it remains to be clarified whether the demonstration of MBL-deficient genotypes of mbl2 or related genes (MBL-associated serine protease or ficolin-2 genes) could avoid the need for post-transplant monitoring of serum levels, as suggested by some authors.42, 43
Peripheral blood lymphocyte sub-populations
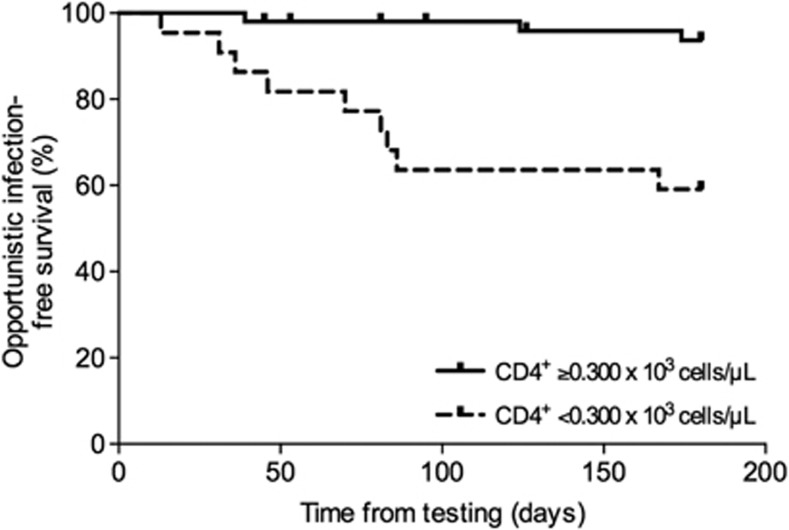
The administration of lymphocyte-depleting agents (that is, rabbit polyclonal antithymocyte globulin or anti-CD52 (alemtuzumab) monoclonal antibody) for induction therapy or treatment of rejection is a well-established risk factor for the occurrence of post-transplant infection.44 Similar to monitoring of patients with human immunodeficiency virus infection, the kinetics of certain peripheral blood lymphocyte sub-populations have been explored as the basis for post-transplant immune monitoring. Calarota et al.45 regularly assessed the CD4+ and CD8+ T-cell numbers during the first 8 months after kidney and heart transplantation and reported that those patients who developed opportunistic infections—because of CMV in most of cases—had lower counts as compared with those without. In the specific setting of human immunodeficiency virus patients undergoing kidney transplantation, the presence of a CD4+ T-cell count <200 cells μl−1 was associated with the occurrence of opportunistic or severe infection.46 Various authors have consistently shown that the risk of Pneumocystis jiroveci pneumonia after kidney transplantation is increased in recipients with low CD4+ T-cell counts,47, 48, 49 and it has been suggested that the dynamics of peripheral blood lymphocyte sub-population may help to guide the duration of prophylaxis with trimethoprim-sulfamethoxazole, similarly to human immunodeficiency virus patients.48 In a recent prospective study with 82 liver transplant recipients, having a CD4+ T-cell count 300 cells μl−1 at month 1 increased significantly the risk of subsequent opportunist infection (Fernández-Ruiz M, 2013, unpublished data; Figure 2). Similarly, the depletion of the CD4+ T-cell subset is also useful to predict de novo post-transplant malignancy—another complication clearly related to over-immunosuppression—in the long term.50, 51, 52 The enumeration of peripheral blood lymphocyte sub-populations is technically simple, has a short turnaround time and may be performed in a fully automated way. In addition, the interpretation of its results appears easily intuitive to the clinician. However, we still need more studies to validate the prognostic accuracy of this strategy in different types of transplant recipients who have or have not received lymphocyte-depleting antibodies.
Figure 2.
Opportunistic infection-free survival in 82 liver transplant recipients according to the CD4+ T-cell count at month 1 post-transplant (P-value=0.0001; log-rank test) (Fernández-Ruiz M, 2013, unpublished data).
Soluble CD30
CD30 is a 120 kDa transmembrane glycoprotein belonging to the tumor necrosis factor/nerve growth factor receptor superfamily.53 As well as being a classic marker for malignant cells of Hodgkin's lymphoma, CD30 has been recently implied in the regulation of the balance between Th1 and Th2 responses and the generation of T-cell memory.54, 55 A soluble form of CD30 (sCD30) of 85 kDa is cleaved in the bloodstream from the surface of activated T cells56 and its serum concentrations may be used as a functional marker for T-cell responsiveness.
In a seminal study based on a multicenter cohort of kidney transplant recipients, the long-term graft survival was significantly diminished in those with high pre-transplant serum levels of sCD30, with most of graft losses because of acute rejection.57 The kinetics during the post-transplant period have been also found to be useful in predicting alloreactivity, as recipients with a delayed decrease in sCD30 levels within the first week have a higher incidence of rejection.58 These findings have been externally validated by other investigators mainly in the kidney transplant setting,59, 60, 61, 62, 63 with somewhat discordant results for other types of transplants.64, 65, 66 Nonetheless, a recent meta-analysis concluded that the pre-transplant levels of sCD30 exhibit only modest sensitivity and specificity values (0.70 and 0.48, respectively) for the subsequent occurrence of acute graft rejection,67 suggesting that the serial monitoring of this biomarker throughout the post-transplant follow-up may be more reliable for this purpose.
The above evidence raises the question whether the measurement of sCD30 levels may have a role in predicting infection in SOT recipients. Unfortunately, only a few single-center studies have explored this approach.61, 62, 64, 68 Nikaein et al.64 reported that heart transplant recipients with pre-transplant sCD30 serum levels <90 IU ml−1 had a higher 1-year cumulative incidence of infection compared with those above this cutoff value, although this finding was not tested by multivariate analysis. Surprisingly, the same group found that high pre-transplant levels were associated with the occurrence of infection after kidney transplantation, failing to provide a plausible explanation for these opposite results.68 In a large cohort of kidney transplant recipients, Wang et al.61 reported that pre-transplant sCD30 serum levels <120 U ml−1 were predictive for the development of post-transplant pneumonia after adjusting by other clinical variables, and suggested as underlying pathogenic mechanism that the low expression of CD30 by T cells could result in decreased production of interleukin-13, which in turn has a role in recruiting inflammatory cells into the lung. In another study, the same authors measured sCD30 concentrations at regular intervals until month 60, and found that recipients with pneumonia had significantly lower levels during the first 3 months than those without.62 From a technical point of view, monitoring of sCD30 has various potential advantages, including its molecular resistance to repeated thawing cycles, the availability of a commercial enzyme-linked immunosorbent assay with good intra- and inter-assay reproducibility, and the low volume of serum required (25 μl).69 Nevertheless, the real accuracy of this approach, as well as the optimal cutoff values, have still to be validated in separate cohorts and different types of infection before being implemented in clinical practice.
Intracellular concentration of ATP in stimulated CD4+ T cells
To date, the in vitro measurement of intracellular ATP (iATP) levels in peripheral blood CD4+ T cells following nonspecific stimulation with phytohemagglutinin is one of the few well-established strategies for functional immune monitoring in SOT recipients.70 The existence of a commercial assay (ImmuKnow; Cylex Inc., Columbia, MD, USA) approved by the Food and Drug Administration (FDA) in 2002 has contributed to this circumstance,71 as well as the amount of literature devoted since then to determine its real value for predicting post-transplant complications.72, 73, 74
Exposure of T cells to a mitogenic stimulus, such as phytohemagglutinin, leads to their metabolic activation and polyclonal expansion, a process in which the ATP synthesis and release precedes surface receptor expression, cytokine production and other subsequent events.7 Thus, the increases in iATP levels offer a proxy for the degree of functionality of the cell-mediated immune response.75 The protocol of the ImmuKnow assay is relatively simple. Heparinized whole blood is incubated with or without phytohemagglutinin (negative control) at 37 °C and 5% CO2 for 15–18 h. Paramagnetic particles coated with a monoclonal antibody to the human CD4 epitope are used to select CD4+ T cells from both the stimulated and non-stimulated wells. A lysing reagent is then added to release the iATP, which is measured by a luciferin/luciferase chemiluminescence method and expressed in ng ml−1.76 A population-based study comparing the assay results in healthy controls and SOT recipients established three categories to define patient's cell-mediated immune response: strong (⩾525 ng ml−1), moderate (226–524 ng ml−1) and low (⩽225 ng ml−1).76 Interestingly, iATP levels show a poor correlation with calcineurin inhibitor trough levels, total lymphocyte count or Th1/Th2 ratio.76, 77, 78, 79 Numerous authors have analyzed the predictive value of iATP for acute rejection, as recently summarized in a meta-analysis that found a relatively high specificity (0.75) but a low sensitivity (0.43), with significant heterogeneity across studies.73 The occurrence of post-transplant infection was also included as a primary outcome in most of these studies, encompassing kidney,80, 81, 82 liver,79, 83 heart77, 84 and lung78, 85 transplant populations. The frequency of monitoring and duration of follow-up differed, as well as the cutoff values used for iATP levels. Of note, the majority of studies analyzed post-transplant infection as an overall outcome, regardless of the nature of the complication or its pathogen. In the aforementioned meta-analysis, the pooled estimates for the predictive accuracy of iATP levels were poor (sensitivity of 0.58 and specificity of 0.69).73 A second meta-analysis focused only on liver transplant recipients reported better performance values (0.83 and 0.75, respectively).74 The utility of this approach has been also assessed for predicting some specific entities—including CMV and EBV infection,86 polyomavirus BK-associated nephropathy81 or invasive fungal infection79—usually in single-center studies with small sample sizes. A further application of the ImmuKnow assay could be predicting the progression from fungal colonization to invasive infection.85 A limitation shared by numerous studies lies in the indication for testing, triggered by the clinical suspicion of complication (that is, fever or deterioration of graft function) instead of being performed within a scheduled strategy for monitoring. The attribution of causality can be biased in the former setting, as the pathogen itself may be responsible for lowering iATP levels through mechanisms of T-cell exhaustion.87 On the other hand, the relative contribution of T-cell responsiveness (as measured by iATP concentrations) in controlling the infectious process differs according to the nature of the pathogen and it is likely that, by analyzing post-transplant infection as a single overall outcome, the actual predictive value of the assay may be underestimated. Accordingly, Husain et al.85 found that the levels of iATP were lower in lung transplant recipients with CMV disease and other viral infection as compared with those with bacterial or fungal infections, primarily controlled by the innate immunity. In addition, some studies have failed to show an association between single time point iATP values and the development of infection in the subsequent 90 days, raising the question whether the detection of changes over time by serial monitoring could be a more reliable approach.82 Finally, the blood sample storage time between collection and testing has been recently demonstrated to act as an unexpected source of variability in the assay results.88
In conclusion, although a functional monitoring approach based on the determination of iATP in stimulated CD4+ T cells seems appealing for a number of reasons, the optimal application of this assay in clinical practice still remains to be determined.
Summary
Although non-pathogen-specific assays show promise as an objective measure of immune function in SOT recipients, it remains to be seen how they would be utilized in clinical practice. Larger interventional trials that show their utility for adjustment of immunosuppression or modification of prophylaxis are needed. In addition, the timing and frequency of performance of these assays needs to be established. Further, a combination of nonspecific assays may have improved predictive value for infection or rejection compared with a single assay. Another non-pathogen-specific immune assay that combines the assessment of both innate and adaptive immune responses for transplant recipients is currently under development (personal communication, Cellestis Ltd, Melbourne, Australia).
Pathogen-specific monitoring
CMV-specific immune response
Human CMV is one of the major causes of infection-related morbidity in SOT recipients and entails a non-negligible mortality in the absence of specific prevention. In addition, CMV exerts an indirect detrimental impact on both patient and graft outcome through its immunomodulatory effects. Those recipients with no pre-existing CMV-specific immunity at the time of primary infection (that is, the seronegative recipient of an organ from a seropositive donor (D+/R−)) have the highest incidence of CMV disease.89 As other herpesviruses, following primary infection CMV enters into a state of lifelong latency within numerous cellular types, including macrophages, neutrophils, fibroblasts, endothelial and epithelial cells.90 The virus uses a large repertoire of immune-evasion mechanisms, such as the inhibition of human leukocyte antigen (HLA)-restricted antigen presentation.91 Those recipients that are seropositive for CMV at transplantation (R+) face the risk of either viral reactivation or superinfection (reinfection), which in turn may involve a wide range of clinical presentations, from asymptomatic viremia to life-threatening tissue-invasive infection.89 This risk is modulated by different variables (type of transplant, use of T-cell-depleting antibodies as induction therapy, maintenance immunosuppression regimen, co-infection with other herpesviruses or presence of certain polymorphisms in genes regulating innate immunity, among others).92
Owing to its extraordinary immunogenicity, CMV is able to trigger robust responses from virtually every arm of the immune system.28 However, the T-cell-mediated adaptive immune response is by far predominant in the protection against CMV. As recently reviewed,93 IFN-γ-producing CMV-specific CD8+ T cells have a crucial role in limiting CMV viremia during the initial acute phase of primary infection, whereas the CD4+ T-cell subset seems to be more relevant in establishing long-term immune control. Although cytotoxic CD8+ T cells may target a great variety of viral proteins (approximately 70% of the viral proteome), the responses against tegument phosphoprotein pp65 and immediate early-1 (IE-1) antigens are largely essential.94 Of note, CMV infection induces the accumulation of late-stage differentiated CD4+ and CD8+ T cells, which exhibit an immunophenotype of replicative senescence with loss of CD27 and CD28 expression.95, 96 Natural killer and regulatory T cells also contribute to these processes.97, 98 The enumeration and ex vivo assessment of the functionality of CMV-specific T cells is being increasingly advocated to categorize the actual risk of developing CMV disease in a given patient.99 In fact, the recent International Consensus on CMV suggests that these assays may be applicable in the clinical setting.100 Such an approach should allow to individualize the prophylaxis strategy against CMV: patients at low risk—with an adequate CMV-specific cell-mediated immunity—would no longer benefit from viral load surveillance and/or antiviral prophylaxis, whereas these efforts could be devoted to those patients unable to mount (in case of primary infection) or reconstitute (if reactivation) a proper response.93 Hence, the currently available assays for the monitoring of CMV-specific T-cell-mediated immunity are reviewed (Table 2), as well as the clinical evidence supporting their application.
Table 2. Currently available methods for monitoring of CMV-specific T-cell-mediated immune response in SOT recipients (modified from Egli et al. 93).
| Characteristic | QuantiFERON-CMV | ELISpot | Intracellular cytokine staining | MHC-tetramer staining |
|---|---|---|---|---|
| Required sample (volume) | Whole blood (3–5 ml) | PBMCs (10 ml) | PBMCs or whole blood (1–2 ml) | PBMCs (0.5–1 ml) |
| Turnaround time | 24 h | 24–48 h | 8–10 h | 1–2 h |
| Antigen | Pool of 22 different peptides mapped within pp65, pp50, IE-1, IE-2 and gB | Individual peptide/peptide library/whole virus lysate/CMV (VR-1814)-infected immature dendritic cells | Individual peptide/peptide library/whole virus lysate/CMV (VR-1814)-infected immature dendritic cells | Individual peptide (pp65, IE-1, pp50) |
| Functional analysis | Yes | Yes | Yes | No (unless associated to intracellular cytokine staining) |
| Phenotypic characterization | No | No | Yes | Yes |
| Differentiation between CD8+ and CD4+ T cells | No (detects mostly CD8+ T cells) | No | Yes | Yes |
| Required knowledge on epitope | No | No | No | Yes |
| Required knowledge on individual HLA-type | No | No | No | Yes |
| Advantages | Simple to perform and highly standardized. CE-approved commercial assay with increasing clinical experience | CE-approved assay recently commercialized. Potential for freeze PBMCs and ship to reference laboratory for testing | Gold standard. Most data available with this technique. Potential for freeze PBMCs and ship to reference laboratory for testing | CE-approved assay recently commercialized. High specificity. Short turnaround time |
| Limitations | Not differentiation between CD8+ and CD4+ T cells. Sensitive to lymphopenia (high rate of indeterminate results in patients treated with ATG). Limited to widespread HLA types | Lack of technical standardization. No defined cutoff values. Need for purified PBMCs and access to an ELISpot reader. No differentiation between CD8+ and CD4+ T cells. Not FDA approved | Labor intensive. Lack of technical standardization. Need for access to a flow cytometer | Labor intensive. Lack of technical standardization. Need for purified PBMCs and access to a flow cytometer. Not FDA approved |
Abbreviations: ATG, antithymocyte globulin; CE, Conformité Européenne; CMV, cytomegalovirus; ELISpot, enzyme-linked immunosorbent spot assay; FDA, Food and Drug Administration; gB, glycoprotein B; HLA, human leukocyte antigen; MHC, major histocompatibility complex; PBMCs, peripheral blood mononuclear cells.
QuantiFERON-CMV assay
The QuantiFERON-CMV assay (Cellestis Ltd) is a commercially available enzyme-linked immunosorbent-based assay that measures the release of IFN-γ mostly by CMV-specific CD8+ T cells after in vitro stimulation of whole blood with a pool of 22 immunogenic viral peptides. Most of them are mapped within pp65 and IE-1 proteins, and their presentation appears restricted by some widespread HLA class I haplotypes that encompass the most common HLA types present in the general population.101, 102 The QuantiFERON-CMV assay is technically simple to perform. A heparinized whole blood sample is drawn into three different tubes: one coated with the viral epitopes (CMV tube), one containing no antigen used as negative control (nil tube), and one containing the phytohemagglutinin or positive control (mitogen tube). After vigorous shaking, the tubes are incubated overnight at 37 °C. The supernatants are subsequently harvested and the levels of IFN-γ measured by enzyme-linked immunosorbent assay. According to the manufacturer, the results should be interpreted as follow: nonreactive at <0.2 IU ml−1 (CMV minus nil) and ⩾0.5 IU ml−1 (mitogen minus nil); reactive at ⩾0.2 IU ml−1 (CMV minus nil) and any value in the mitogen tube; and indeterminate at <0.2 IU ml−1 (CMV minus nil) and <0.5 IU ml−1 (mitogen minus nil).101 In some studies, non-reactive and indeterminate results have been analyzed together for statistical purposes. An alternative cutoff value at 0.1 IU ml−1 has been shown to perform better in some studies.99, 103 Although not FDA-approved, the QuantiFERON-CMV assay is Conformité Européenne marked for commercial use in Europe.
Most of the experience recently gained in CMV-specific immune monitoring in SOT recipients derives from the QuantiFERON-CMV assay (Table 3). Its prognostic value has been assessed for predicting CMV-related outcomes in different clinical scenarios:
Pre-transplant risk-stratification in seropositive patients. The baseline assessment of the risk of CMV infection conventionally relies on the pre-transplant IgG serostatus, under the assumption that CMV-seropositive patients (R+) have pre-existing immunity. Nevertheless, Cantisán et al.104 have recently found that about one-third of R+ transplant candidates actually lack a proper CMV-specific cell-mediated response as assessed by QuantiFERON-CMV assay. Interestingly, these patients were more likely to develop post-transplant CMV replication than those with a reactive assay before transplantation. The authors concluded that this strategy may eventually contribute to reclassify the current risk-stratification, as R+ patients with a pre-transplant non-reactive assay should be managed as high-risk patients. A planned intervention study will test this hypothesis.
Late-onset CMV disease after discontinuing antiviral prophylaxis. A single-center study recruited 108 patients at high risk for CMV disease not only on the basis of their D/R serostatus, but also because of the previous administration of antithymocyte globulin or the type of transplant. The QuantiFERON-CMV was tested at baseline and then monthly during the first 100 days (that is, the usual duration of antiviral prophylaxis with ganciclovir or valganciclovir). Late-onset CMV disease was less frequent in patients with a detectable IFN-γ response (⩾0.1 IU ml−1) at the end of prophylaxis as compared with those with a negative test (5.3% vs 22.9% P-value=0.038).103 These findings were recently validated in a larger, multicenter study only focused on D+/R− patients, in which the test was performed after discontinuation of antiviral prophylaxis (median duration of 98 days) and 1 and 2 months later. Patients with a positive result at any time had a cumulative incidence of subsequent CMV disease at month 12 significantly lower than those with negative or indeterminate results (6.4%, 22.2% and 58.3%, respectively; P-value <0.001). The positive predictive value for protection from CMV disease of having a positive QuantiFERON-CMV result was 0.90, although the negative predictive value was only moderate (0.27).105
Spontaneous clearance of asymptomatic CMV viremia. It is usually assumed that R+ patients are able to spontaneously control most episodes of asymptomatic, low-grade viremia resulting from CMV reactivation (or superinfection in D+/R+ patients) without the need for antiviral therapy. Lisboa et al.106 analyzed 37 recipients at intermediate risk for CMV disease (R+ patients not given T-cell-depleting antibodies or lung transplant recipients), and found that the occurrence of subsequent spontaneous viral clearance was higher in those with a reactive assay (⩾0.2 IU ml−1) at the onset of detectable viremia (92.3% vs 45.5% P-value=0.004). This result offers a preliminary evidence that the QuantiFERON-CMV assay may be useful in monitoring patients undergoing preemptive therapy and to guide the decision of initiating antiviral therapy according to the adequacy of their CMV-specific cell-mediated response.99
Table 3. Clinical scenarios in which monitoring of CMV-specific T-cell-mediated immune response has been evaluated, and suggestions for future studies.
| Clinical scenario | Predicted event | Previous studies | Monitoring method | Proposed intervention |
|---|---|---|---|---|
| High-risk patients (D+/R−, T-cell-depleting antibodies, lung transplantation) during antiviral prophylaxis | Late-onset diseasea | Yes103, 107, 108, 137 | QuantiFERON-CMV, ELISpot | Prolong antiviral prophylaxis or close monitoring for viremia if inadequate response |
| High-risk patients (D+/R−) after discontinuing antiviral prophylaxis | Late-onset diseasea | Yes105 | QuantiFERON-CMV | Prolong antiviral prophylaxis or close monitoring for viremia if inadequate response |
| High-risk patients (T-cell-depleting antibodies, lung or pancreas transplantation) after discontinuing antiviral prophylaxis | Late-onset diseasea | No | Prolong antiviral prophylaxis or close monitoring for viremia if inadequate response | |
| Pre-transplant assessment in intermediate-risk patients (R+ with no other factors) | Post-transplant viremia and/or disease | Yes104, 108 | QuantiFERON-CMV, ELISpot | Initiate antiviral prophylaxis in patients with inadequate response |
| Intermediate-risk patients (R+) on preemptive therapy with no concurrent viremia | Subsequent viremia and/or disease | Yes108, 109, 110, 112, 113, 138 | ICS, QuantiFERON-CMV, ELISpot, MHC-tetramer staining | Reduce the frequency and/or discontinue monitoring of viremia if adequate response |
| Intermediate-risk patients (R+) on preemptive therapy with asymptomatic viremia | Spontaneous clearance | Yes106 | QuantiFERON-CMV | Withhold antiviral therapy if adequate response |
| Active CMV infection or disease during antiviral treatment | Response to antiviral treatment | No | Decrease immunosuppression and/or modify antivirals if inadequate response | |
| Active CMV infection or disease after discontinuation of antiviral treatment | Post-treatment relapse | Yes115 | ICS | Initiate secondary prophylaxis if inadequate response |
| Acute graft rejection treated with steroid boluses and/or T-cell-depleting antibodies | Disease following anti-rejection therapy | No | Initiate prophylaxis if inadequate response |
Abbreviations: CMV, cytomegalovirus; ELISpot, enzyme-linked immunosorbent spot assay; ICS, Intracellular cytokine staining; MHC, major histocompatibility complex.
Refers to the occurrence of CMV disease after discontinuing antiviral prophylaxis with ganciclovir or valganciclovir (usually administered for 100–200 days).
ELISpot assay
The enzyme-linked immunosorbent spot (ELISpot) assay detects the release of IFN-γ by CD4+ and CD8+ T cells in CMV antigen-stimulated peripheral blood mononuclear cells (PBMCs). The stimulating antigen may be obtained from individual CMV peptides (that is, pp65 or IE-1), peptide libraries or whole virus lysates. Unlike the QuantiFERON-CMV, which determines the concentration of IFN-γ per ml of whole blood, the ELISpot assay quantifies the production of IFN-γ by assessing the number of spot-forming units in a given number of PBMCs. The IFN-γ secreted by activated cells is locally captured by a monoclonal antibody coated on a microtiter plate, and subsequently detected by using a second horseradish peroxidase-labeled antibody. Finally, individual spot-forming units are quantified with an image analysis software.28 Most of the previous studies with this method have relied on in-house assays,107, 108, 109 although a standardized assay (T-Track CMV; Lophius Biosciences GmbH, Regensburg, Germany) has been recently commercialized and is Conformité Européenne approved, and other commercial assays are also under development (Oxford Immunotec Ltd, Abingdon, UK). Further disadvantages are the time-consuming nature of the technique as compared with the QuantiFERON assay, and the lack of standardized cutoff values for classifying the adequacy of the T-cell response.99 The clinical experience with the ELISpot is relatively limited. By performing a monthly monitoring throughout the first year after kidney transplantation, Abate et al.107 found that the occurrence of low-grade viremia preceded an increase in CMV-specific T-cell responses, and that D+/R− patients on antiviral prophylaxis suffered from a delay in mounting CMV-specific immunity. Bestard et al.108 performed the ELISpot assay with different stimuli—pp65 and IE-1 peptides and whole virus lysate—in 137 kidney transplant recipients before transplantation. Patients who developed CMV infection had significantly lower pre-transplant anti-IE-1 T-cell responses compared with those who remained free of infection, with good sensitivity and negative predictive values (over 0.80 and 0.90, respectively) for cutoff values of 7–8 spot-forming unit × 103 PBMCs. Interestingly, no differences were found in the T-cell responses against pp65 or virus lysate, thus suggesting that IE-1 is crucial for triggering an effective CMV-specific cell-mediated immunity. A recent study comparing the performance of the two IFN-γ release assays (QuantiFERON-CMV and ELISpot) reported a similar ability for predicting CMV infection, although the areas under receiver operating curves were only modest (0.66 and 0.62, respectively).109
Flow cytometric intracellular cytokine staining
The intracellular cytokine stating (ICS) is based on the detection by flow cytometry of diverse Th1 effector cytokines (usually IFN-γ or tumor necrosis factor-α) in PBMCs or whole blood after stimulation with viral peptides, whole virus lysate or CMV-infected immature dendritic cells.99 As well as the enumeration of the CMV-specific T cells, the ICS also allows their phenotypic characterization through cell surface markers, and therefore this approach is regarded as the ‘gold standard' for assessing the CMV-specific cell-mediated immunity. The ICS method has been demonstrated to be useful in predicting the occurrence of CMV disease after kidney,110, 111, 112, 113 heart111 and lung111, 114, 115 transplantation. In one of the largest studies to date, Gerna et al.113 used immature dendritic cells infected with an endotheliotropic strain of CMV (VR-1814) to stimulate PBMCs and found that the presence of ⩾0.4 CMV-specific CD4+ and CD8+ T cells μl−1 was protective against CMV disease. Interestingly, in the absence of CMV-specific CD4+ T cells, the CD8+ subset was not able to consistently control alone viral replication. Among the disadvantages of the ICS method should be noted are its labor-intensive character and the lack of standardization in stimulating antigen preparation or protective cutoff values, as well as the requirement for a flow cytometer.
MHC-tetramer staining
In brief, main histocompatibility complex (MHC)-tetramers are complexes formed between HLA class I or class II molecules and antigenic peptides covalently linked to a fluorochrome, in order to allow the direct visualization of antigen-specific receptor-carrying T cells by using flow cytometry.116 Such tetramers are typically constructed by refolding MHC molecules in the presence of high concentrations of the antigenic peptide, followed by biotinylation of one chain of the MHC molecule. The resulting MHC-peptide complexes are bound in a 4:1 ratio to fluorophore-labeled streptavidin, thanks to the high affinity of the streptavidin–biotin interaction. The MHC-tetramer technology posses various advantages, including its high specificity, as well as the opportunity for surface and intracellular phenotyping or combination with functional assays.117 On the other hand, as the method is both epitope specific and HLA specific, it requires the knowledge of the individual HLA-type of the patient and large panels of tetramers should be available to be routinely implemented.28 Most of the clinical experience in the monitoring of the CMV-specific immunity by using this technology derived from hematopoietic stem-cell transplant recipients, with few studies in the SOT setting.116, 118, 119 The recent commercialization of a Conformité Européenne-marked assay (Dextramer CMV Kit; Immudex ApS, Copenhagen, Denmark), which uses different haplotypes covering about 95% of the European population, may contribute to the dissemination of this technique.
Other strategies for monitoring of CMV-specific immunity
It has been proposed that monitoring the expression of inhibitory costimulatory molecules on the surface of CMV-specific T cells, as a phenotypic marker of impaired immunity, may be useful in stratifying the risk for CMV infection. The upregulation of CD279, also known as programmed death-1 receptor, on total and CMV-specific CD8+ T cells was significantly associated with the development of CMV disease in a small group of high-risk (D+/R−) liver transplant recipients after discontinuation of antiviral prophylaxis.120 The serum levels of interleukin-10 showed a correlation with the overexpression of programmed death-1.121 In accordance with these findings, our group found that the frequencies of regulatory T cells (CD25+ FoxP3+)—which exert their function through the release of interleukin-10 and other inhibitory cytokines—were significantly higher at the onset of CMV viremia in patients failing to control the infection, and that having a ratio of CMV-3-specific CD4+ T cells to regulatory T cells above 0.4 predicted spontaneous clearance with good sensitivity and specificity values.98 Recently, Dirks et al.122 have proposed a rapid, stimulation-independent monitoring strategy based on assessing, by flow cytometry, the expression of programmed death-1 on CD28− CD27− CD4+ T cells. As CMV-specific T cells dominate among lymphocyte populations showing an immunophenotype of replicative senescence, the enumeration of CD27− CD28− CD4+ T cells could be used as a surrogate for CMV-specific immune response.
Other virus-specific immune responses
In parallel to the above attempts to characterize CMV-specific immunity, some efforts have been focused on other relevant viral agents for the SOT population, mainly EBV and BKPyV.123
EBV possesses the capacity to establish lifelong latency within the B-cell compartment following primary infection.124 The control of latent EBV infection in healthy individuals depends largely on the status of cell-mediated immune response.125 In SOT recipients, EBV-induced B-cell proliferation may be poorly controlled and lead to post-transplant lymphoproliferative disease.126, 127, 128 Therefore, the monitoring of EBV-DNA replication in peripheral blood is routinely used to identify those patients at risk of developing this complication, particularly in the EBV-seronegative pediatric population.129 Previous studies have demonstrated the feasibility of measuring the EBV-specific T-cell-mediated response by MHC-tetramer staining,130 ICS131 and ELISpot assay,9, 132 although with limited clinical application at this time. One potential role of these approaches would be to monitor the effect of newer therapeutic interventions in patients with uncontrolled EBV replication (that is, adoptive cellular immunotherapy with donor EBV-specific cytotoxic T cells after in vitro expansion).132
The human BKPyV is linked to the development of polyomavirus BK-associated nephropathy in 1–10% of kidney transplant recipients, an important cause of premature and irreversible graft loss.133 BKPyV is a small, non-enveloped, double-stranded DNA virus with a widespread distribution in the general population that establishes latency in urogenital epithelial cells.134 Failure to activate BKPyV-specific cell-mediated immunity in the setting of post-transplant immunosuppression leads to viral reactivation and, eventually, the occurrence of progressive cytopathic changes, interstitial fibrosis, and tubular atrophy in the renal graft.135 Some studies have analyzed, by means of IFN-γ ELISpot assay, the kinetics of the BKPyV-specific cellular immunity directed against various viral proteins (that is, nonstructural small and large T antigens and structural VP1-3 proteins).10, 136 Schachtner et al.136 found that the increase in the frequency of BKPyV-specific T cells was associated with the spontaneous clearance of viral reactivation and with the recovery of graft function after tapering of immunosuppression in kidney transplant recipients with biopsy-proven polyomavirus BK-associated nephropathy. Although still preliminary, this monitoring strategy could therefore be useful in guiding the optimal decrease of immunosuppression in presence of polyomavirus BK-associated nephropathy in an attempt to minimize the associated risk of graft rejection.
Future directions
Although notable advances in the development of novel strategies for post-transplant immune monitoring have been achieved, the precise role of these approaches in daily clinical practice is still far to be defined. Most of existing studies with nonspecific strategies are limited by small sample sizes, heterogeneity in baseline risk profiles of patients and lack of precise assessment of the different infectious syndromes and causative agents. Such caveats apply particularly to functional methods such as the ImmuKnow assay, so future studies should be performed in well-characterized subgroups of SOT recipients and be ultimately aimed at establishing pathogen- and graft-specific cutoff values (that is, prediction of fungal infection in lung transplant recipients), instead of using generic population-based categories of risk. Immune response is not a static process, nor is the risk of infection throughout the months following transplantation,2 so dynamic assessments by means of repeated testing at different points (early, intermediate and late post-transplant periods) should be encouraged. The potential additive value of predictive scores combining different assays is also worth exploring. Regarding the specific immune monitoring for CMV, Table 3 summarizes the different clinical scenarios in which this approach has been explored to date, as well as some unmet needs in the management of CMV infection in SOT recipients. Similarly, further studies should ideally be carried out on specific risk groups (that is, patients previously treated with T-cell-depleting antibodies) and achieve an adequate sample size, likely requiring multicenter collaborations. In that sense, the standardization of technical procedures and protective cutoff values is critical. Hopefully, definitive evidence on the utility of post-transplant immune monitoring will emerge in the years to come from intervention clinical trials.
Acknowledgments
Mario Fernández-Ruiz holds a research-training contract ‘Rio Hortega' (CM11/00187) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III).
DK and AH have a research grant from Roche. MF-R has no conflict of interest. DK has received consulting fees from Oxford Immunotec.
References
- Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24:273–283. doi: 10.1016/j.idc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Fishman JA, AST Infectious Diseases Community of Practice Introduction: infection in solid organ transplant recipients. Am J Transplant. 2009;9 (Suppl 4:S3–S6. doi: 10.1111/j.1600-6143.2009.02887.x. [DOI] [PubMed] [Google Scholar]
- Kuypers DR. Immunosuppressive drug monitoring—what to use in clinical practice today to improve renal graft outcome. Transpl Int. 2005;18:140–150. doi: 10.1111/j.1432-2277.2004.00041.x. [DOI] [PubMed] [Google Scholar]
- Fleming JN, Weimert NA. Novel strategies for immune monitoring in kidney transplant recipients. Adv Chronic Kidney Dis. 2010;17:e63–e77. doi: 10.1053/j.ackd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5:341–358. doi: 10.2215/CJN.07111009. [DOI] [PubMed] [Google Scholar]
- Barraclough KA, Staatz CE, Isbel NM, Johnson DW. Therapeutic monitoring of mycophenolate in transplantation: is it justified. Curr Drug Metab. 2009;10:179–187. doi: 10.2174/138920009787522205. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Lichtman AH, Pillai S.Cellular and Molecular Immunology7th ed.Elsevier/Saunders: Philadelphia; 2012 [Google Scholar]
- Vitzthum F, Behrens F, Anderson NL, Shaw JH. Proteomics: from basic research to diagnostic application. A review of requirements & needs. J Proteome Res. 2005;4:1086–1097. doi: 10.1021/pr050080b. [DOI] [PubMed] [Google Scholar]
- Calarota SA, Chiesa A, Zelini P, Comolli G, Minoli L, Baldanti F. Detection of Epstein-Barr virus-specific memory CD4+ T cells using a peptide-based cultured enzyme-linked immunospot assay. Immunology. 2013;139:533–544. doi: 10.1111/imm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131–1139. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Florescu DF, Kalil AC, Qiu F, Schmidt CM, Sandkovsky U. What is the impact of hypogammaglobulinemia on the rate of infections and survival in solid organ transplantation? A meta-analysis. Am J Transplant. 2013;13:2601–2610. doi: 10.1111/ajt.12401. [DOI] [PubMed] [Google Scholar]
- Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transpl Infect Dis. 2003;5:181–186. doi: 10.1111/j.1399-3062.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Wieneke H, Otte B, Lang D, Heidenreich S. Predictive value of IgG subclass levels for infectious complications in renal transplant recipients. Clin Nephrol. 1996;45:22–28. [PubMed] [Google Scholar]
- Broeders EN, Wissing KM, Hazzan M, Ghisdal L, Hoang AD, Noel C, et al. Evolution of immunoglobulin and mannose binding protein levels after renal transplantation: association with infectious complications. Transpl Int. 2008;21:57–64. doi: 10.1111/j.1432-2277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz M, López-Medrano F, Varela-Peña P, Lora-Pablos D, García-Reyne A, González E, et al. Monitoring of immunoglobulin levels identifies kidney transplant recipients at high risk of infection. Am J Transplant. 2012;12:2763–2773. doi: 10.1111/j.1600-6143.2012.04192.x. [DOI] [PubMed] [Google Scholar]
- Doron S, Ruthazer R, Werner BG, Rabson A, Snydman DR. Hypogammaglobulinemia in liver transplant recipients: incidence, timing, risk factors, and outcomes. Transplantation. 2006;81:697–703. doi: 10.1097/01.tp.0000180531.66518.9e. [DOI] [PubMed] [Google Scholar]
- Goldfarb NS, Avery RK, Goormastic M, Mehta AC, Schilz R, Smedira N, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71:242–246. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]
- Kawut SM, Shah L, Wilt JS, Dwyer E, Maani PA, Daly TM, et al. Risk factors and outcomes of hypogammaglobulinemia after lung transplantation. Transplantation. 2005;79:1723–1726. doi: 10.1097/01.tp.0000159136.72693.35. [DOI] [PubMed] [Google Scholar]
- Yip NH, Lederer DJ, Kawut SM, Wilt JS, D'Ovidio F, Wang Y, et al. Immunoglobulin G levels before and after lung transplantation. Am J Respir Crit Care Med. 2006;173:917–921. doi: 10.1164/rccm.200510-1609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamani MH, Avery RK, Mawhorter SD, Young JB, Ratliff NB, Hobbs RE, et al. Hypogammaglobulinemia following cardiac transplantation: a link between rejection and infection. J Heart Lung Transplant. 2001;20:425–430. doi: 10.1016/s1053-2498(00)00331-4. [DOI] [PubMed] [Google Scholar]
- Farmer DG, Kattan OM, Wozniak LJ, Marcus E, Ponthieux S, Hwang V, et al. Incidence, timing, and significance of early hypogammaglobulinemia after intestinal transplantation. Transplantation. 2013;95:1154–1159. doi: 10.1097/TP.0b013e3182869d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhorter S, Yamani MH. Hypogammaglobulinemia and infection risk in solid organ transplant recipients. Curr Opin Organ Transplant. 2008;13:581–585. doi: 10.1097/MOT.0b013e3283186bbc. [DOI] [PubMed] [Google Scholar]
- Ganschow R, Lyons M, Kemper MJ, Burdelski M. B-cell dysfunction and depletion using mycophenolate mofetil in a pediatric combined liver and kidney graft recipient. Pediatr Transplant. 2001;5:60–63. doi: 10.1034/j.1399-3046.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109:581–591. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Giannella M, Alcala L, Sarmiento E, Fernández Yanez J, Palomo J, et al. Clostridium difficile-associated diarrhea in heart transplant recipients: is hypogammaglobulinemia the answer. J Heart Lung Transplant. 2007;26:907–914. doi: 10.1016/j.healun.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Sarmiento E, Rodríguez-Molina JJ, Fernández-Yanez J, Palomo J, Urrea R, Muñoz P, et al. IgG monitoring to identify the risk for development of infection in heart transplant recipients. Transpl Infect Dis. 2006;8:49–53. doi: 10.1111/j.1399-3062.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89:853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RK, Blumberg EA. Hypogammaglobulinemia: time to reevaluate. Am J Transplant. 2013;13:2517–2518. doi: 10.1111/ajt.12403. [DOI] [PubMed] [Google Scholar]
- Yamani MH, Avery R, Mawhorter SD, McNeill A, Cook D, Ratliff NB, et al. The impact of CytoGam on cardiac transplant recipients with moderate hypogammaglobulinemia: a randomized single-center study. J Heart Lung Transplant. 2005;24:1766–1769. doi: 10.1016/j.healun.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Carbone J, Sarmiento E, Del Pozo N, Rodríguez-Molina JJ, Navarro J, Fernández-Yanez J, et al. Restoration of humoral immunity after intravenous immunoglobulin replacement therapy in heart recipients with post-transplant antibody deficiency and severe infections. Clin Transplant. 2012;26:E277–E283. doi: 10.1111/j.1399-0012.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17:317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari E, Zhou W, Sacks S. Complement in organ transplantation. Curr Opin Organ Transplant. 2010;15:486–491. doi: 10.1097/MOT.0b013e32833b9cb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Imamura Y, Takada Y. Relationships between the haemolytic activities of the human complement system and complement components. Clin Exp Immunol. 1979;35:324–328. [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz M, López-Medrano F, Varela-Peña P, Morales JM, García-Reyne A, San Juan R, et al. Hypocomplementemia in kidney transplant recipients: impact on the risk of infectious complications. Am J Transplant. 2013;13:685–694. doi: 10.1111/ajt.12055. [DOI] [PubMed] [Google Scholar]
- Carbone J, Micheloud D, Salcedo M, Rincon D, Banares R, Clemente G, et al. Humoral and cellular immune monitoring might be useful to identify liver transplant recipients at risk for development of infection. Transpl Infect Dis. 2008;10:396–402. doi: 10.1111/j.1399-3062.2008.00329.x. [DOI] [PubMed] [Google Scholar]
- Sarmiento E, del Pozo N, Gallego A, Fernández-Yanez J, Palomo J, Villa A, et al. Decreased levels of serum complement C3 and natural killer cells add to the predictive value of total immunoglobulin G for severe infection in heart transplant recipients. Transpl Infect Dis. 2012;14:526–539. doi: 10.1111/j.1399-3062.2012.00757.x. [DOI] [PubMed] [Google Scholar]
- Berger SP, Daha MR. Emerging role of the mannose-binding lectin-dependent pathway of complement activation in clinical organ transplantation. Curr Opin Organ Transplant. 2011;16:28–33. doi: 10.1097/MOT.0b013e3283425509. [DOI] [PubMed] [Google Scholar]
- Worthley DL, Johnson DF, Eisen DP, Dean MM, Heatley SL, Tung JP, et al. Donor mannose-binding lectin deficiency increases the likelihood of clinically significant infection after liver transplantation. Clin Infect Dis. 2009;48:410–417. doi: 10.1086/596313. [DOI] [PubMed] [Google Scholar]
- Verschuren JJ, Roos A, Schaapherder AF, Mallat MJ, Daha MR, de Fijter JW, et al. Infectious complications after simultaneous pancreas-kidney transplantation: a role for the lectin pathway of complement activation. Transplantation. 2008;85:75–80. doi: 10.1097/01.tp.0000297249.10654.f5. [DOI] [PubMed] [Google Scholar]
- Manuel O, Pascual M, Trendelenburg M, Meylan PR. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation. 2007;83:359–362. doi: 10.1097/01.tp.0000251721.90688.c2. [DOI] [PubMed] [Google Scholar]
- de Rooij BJ, van Hoek B, ten Hove WR, Roos A, Bouwman LH, Schaapherder AF, et al. Lectin complement pathway gene profile of donor and recipient determine the risk of bacterial infections after orthotopic liver transplantation. Hepatology. 2010;52:1100–1110. doi: 10.1002/hep.23782. [DOI] [PubMed] [Google Scholar]
- Cervera C, Balderramo D, Suárez B, Prieto J, Fuster F, Linares L, et al. Donor mannose-binding lectin gene polymorphisms influence the outcome of liver transplantation. Liver Transpl. 2009;15:1217–1224. doi: 10.1002/lt.21834. [DOI] [PubMed] [Google Scholar]
- Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- Calarota SA, Zelini P, De Silvestri A, Chiesa A, Comolli G, Sarchi E, et al. Kinetics of T-lymphocyte subsets and posttransplant opportunistic infections in heart and kidney transplant recipients. Transplantation. 2012;93:112–119. doi: 10.1097/TP.0b013e318239e90c. [DOI] [PubMed] [Google Scholar]
- Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated Cd4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6:753–760. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- De Castro N, Xu F, Porcher R, Pavie J, Molina JM, Peraldi MN. Pneumocystis jirovecii pneumonia in renal transplant recipients occurring after discontinuation of prophylaxis: a case-control study. Clin Microbiol Infect. 2010;16:1375–1377. doi: 10.1111/j.1469-0691.2009.03143.x. [DOI] [PubMed] [Google Scholar]
- Struijk GH, Gijsen AF, Yong SL, Zwinderman AH, Geerlings SE, Lettinga KD, et al. Risk of Pneumocystis jiroveci pneumonia in patients long after renal transplantation. Nephrol Dial Transplant. 2011;26:3391–3398. doi: 10.1093/ndt/gfr048. [DOI] [PubMed] [Google Scholar]
- Brunot V, Pernin V, Chartier C, Garrigue V, Vetromile F, Szwarc I, et al. An epidemic of Pneumocystis jiroveci pneumonia in a renal transplantation center: role of T-cell lymphopenia. Transplant Proc. 2012;44:2818–2820. doi: 10.1016/j.transproceed.2012.09.089. [DOI] [PubMed] [Google Scholar]
- Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, et al. CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation. 1998;65:1270–1272. doi: 10.1097/00007890-199805150-00022. [DOI] [PubMed] [Google Scholar]
- Ducloux D, Carron PL, Motte G, Ab A, Rebibou JM, Bresson-Vautrin C, et al. Lymphocyte subsets and assessment of cancer risk in renal transplant recipients. Transpl Int. 2002;15:393–396. doi: 10.1007/s00147-002-0410-4. [DOI] [PubMed] [Google Scholar]
- Thibaudin D, Alamartine E, Mariat C, Absi L, Berthoux F. Long-term kinetic of T-lymphocyte subsets in kidney-transplant recipients: influence of anti-T-cell antibodies and association with posttransplant malignancies. Transplantation. 2005;80:1514–1517. doi: 10.1097/01.tp.0000181193.98026.3f. [DOI] [PubMed] [Google Scholar]
- Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- Pellegrini P, Totaro R, Contasta I, Berghella AM, Carolei A, Adorno D. CD30 antigen and multiple sclerosis: CD30, an important costimulatory molecule and marker of a regulatory subpopulation of dendritic cells, is involved in the maintenance of the physiological balance between TH1/TH2 immune responses and tolerance. The role of IFNbeta-1a in the treatment of multiple sclerosis. Neuroimmunomodulation. 2005;12:220–234. doi: 10.1159/000085654. [DOI] [PubMed] [Google Scholar]
- Saini D, Ramachandran S, Nataraju A, Benshoff N, Liu W, Desai N, et al. Activated effector and memory T cells contribute to circulating sCD30: potential marker for islet allograft rejection. Am J Transplant. 2008;8:1798–1808. doi: 10.1111/j.1600-6143.2008.02329.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- Susal C, Pelzl S, Dohler B, Opelz G. Identification of highly responsive kidney transplant recipients using pretransplant soluble CD30. J Am Soc Nephrol. 2002;13:1650–1656. doi: 10.1097/01.asn.0000014256.75920.5b. [DOI] [PubMed] [Google Scholar]
- Wang D, Wu GJ, Wu WZ, Yang SL, Chen JH, Wang H, et al. Pre- and post-transplant monitoring of soluble CD30 levels as predictor of acute renal allograft rejection. Transpl Immunol. 2007;17:278–282. doi: 10.1016/j.trim.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Delgado JC, Pavlov IY, Shihab FS. Post-transplant increased levels of serum sCD30 is a marker for prediction of kidney allograft loss in a 5-year prospective study. Transpl Immunol. 2009;22:1–4. doi: 10.1016/j.trim.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Nafar M, Farrokhi F, Vaezi M, Entezari AE, Pour-Reza-Gholi F, Firoozan A, et al. Pre-transplant and post-transplant soluble CD30 for prediction and diagnosis of acute kidney allograft rejection. Int Urol Nephrol. 2009;41:687–693. doi: 10.1007/s11255-008-9505-x. [DOI] [PubMed] [Google Scholar]
- Wang D, Wu WZ, Chen JH, Yang SL, Wang QH, Zeng ZX, et al. Pre-transplant soluble CD30 level as a predictor of not only acute rejection and graft loss but pneumonia in renal transplant recipients. Transpl Immunol. 2010;22:115–120. doi: 10.1016/j.trim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wang D, Wu W, Yang S, Wang Q, Tan J. Post-transplant monitoring of soluble CD30 level as predictor of graft outcome: a single center experience from China. Transpl Immunol. 2012;27:146–150. doi: 10.1016/j.trim.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Billing H, Sander A, Susal C, Ovens J, Feneberg R, Hocker B, et al. Soluble CD30 and ELISA-detected human leukocyte antigen antibodies for the prediction of acute rejection in pediatric renal transplant recipients. Transpl Int. 2013;26:331–338. doi: 10.1111/tri.12049. [DOI] [PubMed] [Google Scholar]
- Nikaein A, Spiridon C, Hunt J, Rosenthal J, Anderson A, Eichhorn E, et al. Pre-transplant level of soluble CD30 is associated with infection after heart transplantation. Clin Transplant. 2007;21:744–747. doi: 10.1111/j.1399-0012.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- Ypsilantis E, Key T, Bradley JA, Morgan CH, Tsui S, Parameshwar J, et al. Soluble CD30 levels in recipients undergoing heart transplantation do not predict post-transplant outcome. J Heart Lung Transplant. 2009;28:1206–1210. doi: 10.1016/j.healun.2009.05.041. [DOI] [PubMed] [Google Scholar]
- Matinlauri I, Hockerstedt K, Isoniemi H. High serum soluble CD30 does not predict acute rejection in liver transplant patients. Transplant Proc. 2006;38:3601–3604. doi: 10.1016/j.transproceed.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tai Q, Hong S, Kong Y, Shang Y, Liang W, et al. Pretransplantation soluble CD30 level as a predictor of acute rejection in kidney transplantation: a meta-analysis. Transplantation. 2012;94:911–918. doi: 10.1097/TP.0b013e31826784ad. [DOI] [PubMed] [Google Scholar]
- Spiridon C, Nikaein A, Lerman M, Hunt J, Dickerman R, Mack M. CD30, a marker to detect the high-risk kidney transplant recipients. Clin Transplant. 2008;22:765–769. doi: 10.1111/j.1399-0012.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- Susal C, Opelz G. Posttransplant sCD30 as a biomarker to predict kidney graft outcome. Clin Chim Acta. 2012;413:1350–1353. doi: 10.1016/j.cca.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Sottong PR, Rosebrock JA, Britz JA, Kramer TR. Measurement of T-lymphocyte responses in whole-blood cultures using newly synthesized DNA and ATP. Clin Diagn Lab Immunol. 2000;7:307–311. doi: 10.1128/cdli.7.2.307-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services USDoHaH Cylex Immune Cell Function Assay; K013169; 02 April2002
- Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82:663–668. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- Ling X, Xiong J, Liang W, Schroder PM, Wu L, Ju W, et al. Can immune cell function assay identify patients at risk of infection or rejection? A meta-analysis. Transplantation. 2012;93:737–743. doi: 10.1097/TP.0b013e3182466248. [DOI] [PubMed] [Google Scholar]
- Rodrigo E, López-Hoyos M, Corral M, Fabrega E, Fernández-Fresnedo G, San Segundo D, et al. ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012;18:1245–1253. doi: 10.1002/lt.23497. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today. 2000;21:192–199. doi: 10.1016/s0167-5699(00)01593-0. [DOI] [PubMed] [Google Scholar]
- Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17:77–88. doi: 10.1034/j.1399-0012.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Israeli M, Ben-Gal T, Yaari V, Valdman A, Matz I, Medalion B, et al. Individualized immune monitoring of cardiac transplant recipients by noninvasive longitudinal cellular immunity tests. Transplantation. 2010;89:968–976. doi: 10.1097/TP.0b013e3181cbabe6. [DOI] [PubMed] [Google Scholar]
- Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant. 2008;27:990–994. doi: 10.1016/j.healun.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Zhou T, Xue F, Han LZ, Xi ZF, Li QG, Xu N, et al. Invasive fungal infection after liver transplantation: risk factors and significance of immune cell function monitoring. J Dig Dis. 2011;12:467–475. doi: 10.1111/j.1751-2980.2011.00542.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Velasco P, Rodrigo E, Valero R, Ruiz JC, Fernández-Fresnedo G, López-Hoyos M, et al. Intracellular ATP concentrations of CD4 cells in kidney transplant patients with and without infection. Clin Transplant. 2008;22:55–60. doi: 10.1111/j.1399-0012.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Gralla J, Huskey J, Wiseman AC. Trends in immune function assay (ImmuKnow; Cylex) results in the first year post-transplant and relationship to BK virus infection. Nephrol Dial Transplant. 2012;27:2565–2570. doi: 10.1093/ndt/gfr675. [DOI] [PubMed] [Google Scholar]
- Huskey J, Gralla J, Wiseman AC. Single time point immune function assay (ImmuKnow) testing does not aid in the prediction of future opportunistic infections or acute rejection. Clin J Am Soc Nephrol. 2011;6:423–429. doi: 10.2215/CJN.04210510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T, et al. Immune cell functional assay in monitoring of adult liver transplantation recipients with infection. Transplantation. 2010;89:620–626. doi: 10.1097/TP.0b013e3181c690fa. [DOI] [PubMed] [Google Scholar]
- Kobashigawa JA, Kiyosaki KK, Patel JK, Kittleson MM, Kubak BM, Davis SN, et al. Benefit of immune monitoring in heart transplant patients using ATP production in activated lymphocytes. J Heart Lung Transplant. 2010;29:504–508. doi: 10.1016/j.healun.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, et al. Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation. 2009;87:1852–1857. doi: 10.1097/TP.0b013e3181a75ad2. [DOI] [PubMed] [Google Scholar]
- Gautam A, Fischer SA, Yango AF, Gohh RY, Morrissey PE, Monaco AP. Cell mediated immunity (CMI) and post transplant viral infections—role of a functional immune assay to titrate immunosuppression. Int Immunopharmacol. 2006;6:2023–2026. doi: 10.1016/j.intimp.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Hosking MP, Flynn CT, Botten J, Whitton JL, CD8+ Memory T. Cells appear exhausted within hours of acute virus Infection. J Immunol. 2013;191:4211–4222. doi: 10.4049/jimmunol.1300920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suviolahti E, Petrosyan A, Mirocha J, Ge S, Karasyov A, Thomas D, et al. Significant reduction of ATP production in PHA-activated CD4+ cells in 1-day-old blood from transplant patients. Transplantation. 2012;94:1243–1249. doi: 10.1097/TP.0b013e318270f322. [DOI] [PubMed] [Google Scholar]
- Razonable RR, Humar A, Practice ASTIDCo Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13 (Suppl 4:93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- Grefte A, van der Giessen M, van Son W. The TH circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993;167:270–277. doi: 10.1093/infdis/167.2.270. [DOI] [PubMed] [Google Scholar]
- Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- de la Torre-Cisneros J, Fariñas MC, Caston JJ, Aguado JM, Cantisán S, Carratalà J, et al. GESITRA-SEIMC/REIPI recommendations for the management of cytomegalovirus infection in solid-organ transplant patients. Enferm Infecc Microbiol Clin. 2011;29:735–758. doi: 10.1016/j.eimc.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55:1678–1689. doi: 10.1093/cid/cis818. [DOI] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V, Beswick M, et al. CMV and Immunosenescence: from basics to clinics. Immun Ageing. 2012;9:23. doi: 10.1186/1742-4933-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- Egli A, Silva M, Jr, O'Shea D, Wilson LE, Baluch A, Lisboa LF, et al. An analysis of regulatory T-cell and Th-17 cell dynamics during cytomegalovirus replication in solid organ transplant recipients. PLoS One. 2012;7:e43937. doi: 10.1371/journal.pone.0043937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel O.Clinical experience with immune monitoring for cytomegalovirus in solid-organ transplant recipients Curr Infect Dis Rep(e-pub ahead of print 29 September 2013). [DOI] [PubMed]
- Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007;9:165–170. doi: 10.1111/j.1399-3062.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Giulieri S, Manuel O. QuantiFERON(R)-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev Mol Diagn. 2011;11:17–25. doi: 10.1586/erm.10.109. [DOI] [PubMed] [Google Scholar]
- Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- Cantisán S, Lara R, Montejo M, Redel J, Rodríguez-Benot A, Gutiérrez-Aroca J, et al. Pretransplant interferon-gamma secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant. 2013;13:738–745. doi: 10.1111/ajt.12049. [DOI] [PubMed] [Google Scholar]
- Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56:817–824. doi: 10.1093/cid/cis993. [DOI] [PubMed] [Google Scholar]
- Lisboa LF, Kumar D, Wilson LE, Humar A. Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation. 2012;93:195–200. doi: 10.1097/TP.0b013e31823c1cd4. [DOI] [PubMed] [Google Scholar]
- Abate D, Saldan A, Fiscon M, Cofano S, Paciolla A, Furian L, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010;202:585–594. doi: 10.1086/654931. [DOI] [PubMed] [Google Scholar]
- Bestard O, Lucia M, Crespo E, Van Liempt B, Palacio D, Melilli E, et al. Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant. 2013;13:1793–1805. doi: 10.1111/ajt.12256. [DOI] [PubMed] [Google Scholar]
- Abate D, Saldan A, Mengoli C, Fiscon M, Silvestre C, Fallico L, et al. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol. 2013;51:2501–2507. doi: 10.1128/JCM.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sester M, Sester U, Gartner B, Heine G, Girndt M, Mueller-Lantzsch N, et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- Sester U, Gartner BC, Wilkens H, Schwaab B, Wossner R, Kindermann I, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant. 2005;5:1483–1489. doi: 10.1111/j.1600-6143.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Egli A, Binet I, Binggeli S, Jager C, Dumoulin A, Schaub S, et al. Cytomegalovirus-specific T-cell responses and viral replication in kidney transplant recipients. J Transl Med. 2008;6:29. doi: 10.1186/1479-5876-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G, Lilleri D, Chiesa A, Zelini P, Furione M, Comolli G, et al. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant. 2011;11:2463–2471. doi: 10.1111/j.1600-6143.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis. 2011;204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrand M, Tournay C, Peyrat MA, Eriksson BM, Wadstrom J, Wirgart BZ, et al. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation. 2000;69:2243–2250. doi: 10.1097/00007890-200006150-00005. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Cerundolo V, Dunbar PR. Tracking T cells with tetramers: new tales from new tools. Nat Rev Immunol. 2002;2:263–272. doi: 10.1038/nri777. [DOI] [PubMed] [Google Scholar]
- Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- Crough T, Fazou C, Weiss J, Campbell S, Davenport MP, Bell SC, et al. Symptomatic and asymptomatic viral recrudescence in solid-organ transplant recipients and its relationship with the antigen-specific CD8(+) T-cell response. J Virol. 2007;81:11538–11542. doi: 10.1128/JVI.00581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Zhou W, Lacey SF, Limaye AP, Diamond DJ, La Rosa C. Programmed death-1 receptor and interleukin-10 in liver transplant recipients at high risk for late cytomegalovirus disease. Transpl Infect Dis. 2010;12:363–370. doi: 10.1111/j.1399-3062.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks J, Tas H, Schmidt T, Kirsch S, Gartner BC, Sester U, et al. PD-1 analysis on CD28(−) CD27(−) CD4 T cells allows stimulation-independent assessment of CMV viremic episodes in transplant recipients. Am J Transplant. 2013;13:3132–3141. doi: 10.1111/ajt.12480. [DOI] [PubMed] [Google Scholar]
- Costa C, Saldan A, Cavallo R. Evaluation of virus-specific cellular immune response in transplant patients. World J Virol. 2012;1:150–153. doi: 10.5501/wjv.v1.i6.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. Epstein-Barr virus infection. New Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- San-Juan R, De Dios B, Navarro D, Garcia-Reyne A, Lumbreras C, Bravo D, et al. Epstein-Barr virus DNAemia is an early surrogate marker of the net state of immunosuppresion in solid organ transplant recipients. Transplantation. 2013;95:688–693. doi: 10.1097/TP.0b013e31827a4bd6. [DOI] [PubMed] [Google Scholar]
- Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, Saas P, et al. Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant. 2013;13:656–662. doi: 10.1111/ajt.12009. [DOI] [PubMed] [Google Scholar]
- Snow AL, Martinez OM. Epstein-Barr virus: evasive maneuvers in the development of PTLD. Am J Transplant. 2007;7:271–277. doi: 10.1111/j.1600-6143.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Rowe DT, Webber S, Schauer EM, Reyes J, Green M. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis. 2001;3:79–87. doi: 10.1034/j.1399-3062.2001.003002079.x. [DOI] [PubMed] [Google Scholar]
- Sugaya N, Kimura H, Hara S, Hoshino Y, Kojima S, Morishima T, et al. Quantitative analysis of Epstein-Barr virus (EBV)-specific CD8+ T cells in patients with chronic active EBV infection. J Infect Dis. 2004;190:985–988. doi: 10.1086/423285. [DOI] [PubMed] [Google Scholar]
- Guppy AE, Rawlings E, Madrigal JA, Amlot PL, Barber LD. A quantitative assay for Epstein-Barr Virus-specific immunity shows interferon-gamma producing CD8+ T cells increase during immunosuppression reduction to treat posttransplant lymphoproliferative disease. Transplantation. 2007;84:1534–1539. doi: 10.1097/01.tp.0000290232.65830.e7. [DOI] [PubMed] [Google Scholar]
- Yang J, Lemas VM, Flinn IW, Krone C, Ambinder RF. Application of the ELISPOT assay to the characterization of CD8(+) responses to Epstein-Barr virus antigens. Blood. 2000;95:241–248. [PubMed] [Google Scholar]
- Hirsch HH, Randhawa P, Practice ASTIDCo BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 (Suppl 4:179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- Nickeleit V, Hirsch HH, Binet IF, Gudat F, Prince O, Dalquen P, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999;10:1080–1089. doi: 10.1681/ASN.V1051080. [DOI] [PubMed] [Google Scholar]
- Schachtner T, Muller K, Stein M, Diezemann C, Sefrin A, Babel N, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11:2443–2452. doi: 10.1111/j.1600-6143.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- Weseslindtner L, Kerschner H, Steinacher D, Nachbagauer R, Kundi M, Jaksch P, et al. Prospective analysis of human cytomegalovirus DNAemia and specific CD8+ T cell responses in lung transplant recipients. Am J Transplant. 2012;12:2172–2180. doi: 10.1111/j.1600-6143.2012.04076.x. [DOI] [PubMed] [Google Scholar]
- Sund F, Lidehall AK, Claesson K, Foss A, Totterman TH, Korsgren O, et al. CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin Transplant. 2010;24:401–409. doi: 10.1111/j.1399-0012.2009.00976.x. [DOI] [PubMed] [Google Scholar]