Abstract
The use of tumour-associated antigens for cancer immunotherapy studies is exacerbated by tolerance to these self-antigens. Tolerance may be broken by using ex vivo monocyte-derived dendritic cells (DCs) pulsed with self-antigens. Targeting tumour-associated antigens directly to DCs in vivo is an alternative and simpler strategy. The identification of cell surface receptors on DCs, and targeting antigens to DC receptors, has become a popular approach for inducing effective immune responses against cancer antigens. Many years ago, we demonstrated that targeting the mannose receptor on macrophages using the carbohydrate mannan to DCs led to appropriate immune responses and tumour protection in animal models. We conducted Phase I, I/II and II, clinical trials demonstrating the effectiveness of oxidised mannan-MUC1 in patients with adenocarcinomas. Here we summarise DC targeting approaches and their efficacy in human clinical trials.
In the last two decades, dendritic cells (DCs) have surfaced as powerful cells, to target antigens to initiate T-cell immunity by efficient uptake, processing and presentation of endocytosed antigens.1 Many of the DC cell surface receptors have been identified, including mannose receptor (MR), DC-SIGN, L-SIGN, LSECtin, CIRE, Langerin, MGL, Dectin-1, Dectin-2, DNGR-1, MICL, CLEC2, CLEC12B, LOX-1, DCIR, BDCA-2, DEC205, scavenger receptor, DC-ASGPR, FIRE, DC-STAMP and Toll-like receptors (TLRs).2 Targeting of these receptors is becoming an efficient strategy to improve the immunogenicity of antigens in DC-based anticancer immunotherapy, especially in pre-clinical animal models and in in vitro DC antigen presentation and T-cell stimulation assays. A major challenge for vaccine design is targeting antigens to DCs in vivo in humans, facilitating cross-presentation, and conditioning the microenvironment for Th1- and Th2-type effective T-cell immune responses. Here we present DC immunotherapeutic approaches in preclinical stages with emphasis to those in human clinical trials.
Immune responses by targeting the MR
The MR is a carbohydrate (mannose, fucose, glucose, maltose, GlcNAc) binding C-type membrane lectin expressed by DCs and macrophages (Table 1). We had demonstrated over 20 years ago that antigen uptake via the MR results in processing and presentation of peptide epitopes via the MHC class I and class II pathways.3, 4, 5, 6, 7 These studies were the first to indicate the MR to be a viable DC cell surface receptor for antigen delivery for vaccine development. In addition, peptides and proteins conjugated to mannose have been shown to stimulate MHC class II-specific T cells with 200–10 000-fold higher efficiency as compared to non-mannose conjugated antigens.8 The MUC1 antigen conjugated to oxidised mannan (poly-mannose, comprising aldehydes) leads to rapid and 1000 times more efficient MHC class I presentation to CD8+ T cells with a preferential T1 response, compared to MUC1 antigen conjugated to reduced mannan (no aldehydes).5 Both oxidised and reduced mannan stimulate bone marrow-derived DCs and enhance OTI/OTII T cells in vitro, and in vivo they induce a mature phenotype of lymph node and splenic DCs.9 Oxidised and reduced mannan stimulate interleukin (IL)-1beta and tumour necrosis factor-alpha, with oxidised mannan stimulating interferon (IFN)-gamma and IL-12p40 cytokines, and reduced mannan stimulating IL-4, IL-10 and IL-13.9 The stimulation of DCs was demonstrated to be TLR-4 dependent.9, 10, 11 Further, ex vivo targeting of macrophages or DCs with oxidised mannan-MUC1 and re-injection into mice induce T-cell responses and protect against MUC1 tumour challenge.3 Moreover, oxidised and reduced mannan complexed to DNA via poly-L-lysine induces cellular and humoral immune responses in mice,12, 13 and mannosylated dendrimers are endocytosed by bone marrow-derived and Flt3-L DCs, stimulate CD4 and CD8 T cells and protect against tumour challenge.10, 11 Studies by others have also demonstrated the efficacy of MR targeting by the use of mannan-coated cationic liposomes (nanoparticles) incorporating HIV-1 DNA,14, 15 or with mannosylated anionic liposomes having increased interaction to murine and human DCs.16 Mannosylated liposomes incorporating ErbB2 T-cell epitopes and TLR agonists induce strong immune responses,17 and bind to immature DCs.18 Mannan-coated poly(D, L-lactide-co-glycolic acid) nanoparticles have also been noted to induce strong T-cell responses.19 Mannosylation of chitosan microspheres (MCMs) incorporating Bordetella bronchiseptica antigen induces IgA antibody responses in mice.20 In addition, HER2 protein complexed to the cholesteryl group bearing mannan induces CD8+ T cells and rejects HER2+ tumours in mice.21 Likewise, human papillomavirus type-16, E7 peptide (744–762) conjugated to D-mannose effectively rejects tumours.22 Interestingly, keyhole limpet haemocyanin, widely used to aid in the generation of humoral and cellular immune responses, activates and matures DCs, and its interaction with DCs has been noted to be partially mediated by binding to the MR.23 Conjugation of anti-MR antibody to pmel17 melanoma antigen or to human chorionic gonadotropin beta protein results in strong T-cell responses.24, 25 Furthermore, ovalbumin expressed in yeast, resulting in naturally mannosylated ovalbumin, binds to MR and DC-SIGN and stimulates enhanced CD4+ T-cell responses in vitro,26 and enhances CD8+ T cells and IFN-gamma, IL-2, IL-4, and IL-5 in vivo.27 It is clear that in animal models mannosylated antigen is an effective approach to potentiate antigen immunogenicity, due to the enhanced antigen uptake and presentation by DCs and macrophages via uptake primarily by the MR. A number of animal studies have been reported on the use of the MR in inducing immunity, with potential to be of therapeutic benefit in human clinical trials.
Table 1. Cell surface receptors and their expression on cells.
| Receptor | Expression |
|---|---|
| Mannose receptor (CD206) | Macrophages, DCs |
| DC-SIGN (CD209, Clec4L) | Immature DCs, macrophages, endothelial vascular cells |
| L-SIGN or DC-SIGNR (CD299, Clec4M) | Liver sinusoidal cells, lymph nodes, endothelial vascular cells |
| LSECtin (Clec4G) | Lymph nodes, sinusoidal endothelial cells, DCs, Kupffer cells |
| Langerin (CD207, Clec4K) | Langerhans cells, CD103+ DCs, splenic CD8+DCs |
| MGL | Macrophages, immature DCs |
| Dectin-1 (DCAL-1, Cec7A) | Myeloid DCs, CD8−CD4− DCs, dermal DCs, monocytes, macrophages, neutrophils, T cells, B cells, mast cells, eosinophils, monocytes |
| DNGR-1 (Clec9A) | Murine CD8+ DCs not on CD4+ DCs, on CD11c+ DCs but not by CD11c− cells (B cells, T cells, NK cells, NKT cells, macrophages, granulocytes), plasmacytoid DCs, human blood DCsBDCA-3+ DCs) and monocytes (CD14+CD16−). Flt3 ligand bone marrow-derived CD8+ DCs |
| MICL (Clec12A) | Granulocytes, monocytes, macrophages, B cells, CD8+ T cells, peripheral blood and DCs |
| CLEC2 (Clec1B) | NK cells, monocytes, granylocytes, platelets, megakaryocytes, liver sinusoidal epithelial cells |
| CLEC12B | Macrophages, monocytes, DCs |
| LOX-1 (Clec8A) | Endothelial cells, smooth muscle cells, platelets, fibroblasts, macrophages |
| DCIR (Clec4A) | Plasmacytoid DCs, immature and mature monocyte-derived DCs, monocytes, macrophages, B cells |
| Dectin-2 (DCAL-2, Clec6A) | DCs, macrophages, neutrophils, monocytes |
| BDCA-2 (Clec4C) | Human blood DCs |
| DEC205 (CD205, Ly75) | DCs, thymic epithelial cells |
| Scavenger receptor | Macrophages |
| DC-ASGPR | Monocyte-derived DCs (CD14+CD34+), tonsilar interstitial-type DCs, granulocytes |
| F4/80 receptor | Macrophages |
| FIRE | Murine CD8−CD4+ and CD8−CD4− immature DCs, weakly on monocytes and macrophages |
| DC-STAMP | DCs, activated blood DCs |
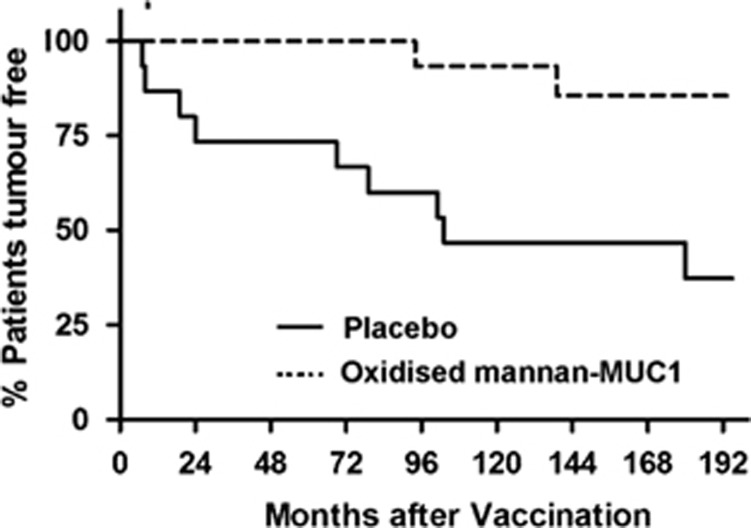
From 1994 to 1997, we injected humans with oxidised mannan-MUC1, targeting the MR on DCs. In 25 patients with advanced metastatic adenocarcinoma, high-IgG1 anti-MUC1 antibodies were generated in 13/25 patients, T-cell proliferation in 4/15 patients and cytotoxic T-cell responses in 2/10 patients.28 Upon further analysis, intracellular cytokines IFN-gamma and TNF-alpha were induced by CD4+CD69+ and CD8+CD69+ activated T cells from patients immunised with oxidised mannan-MUC1 as compared to T cells isolated from normal subjects.29 A further three phase I trials were performed in 41 patients with adenocarcinoma, of the (i) breast, (ii) colon and (iii) other organs, all of whom received oxidised mannan-MUC1 and cyclophosphamide. Sixty per cent of patients generated IgG1 antibody responses and 28% of patients generated cellular responses (proliferation, cytotoxic T cells or CD8 T cells secreting TNF-alpha and IFN-gamma).30 We previously reported the pilot phase III, double-blind placebo controlled trial, ISRCTN71711835, of 5-year immunological and clinical follow-up data,31 and 15-year clinical follow-up data,32 in early-stage breast cancer patients with no evidence of disease, injected with oxidised mannan-MUC1. We demonstrated induction of humoral and cellular responses and protection against recurrence in these breast cancer patients. The clinical follow-up of these patients 16.5 years later (as of 28 May 2014) demonstrate that there have been 2/16 late recurrences (mean 118 months post injection) in patients who received oxidised mannan-MUC1 and 9/15 recurrences (mean 65.8 months post injection) in patients in the placebo group (P=0.009) (Figure 1). In addition, no adverse events at any stage have been reported, up to 16.5 years later, including toxic events related to autoimmunity to self-antigen. Clearly, targeting the MR on DCs shows promise and oxidised mannan-MUC1 warrants inclusion as a harmless adjuvant therapy in the current management of patients with breast cancer. However, the number of patients in this study is small, and a large Phase III trial needs to be done in this cohort of patients. It is important to understand the status of disease of the patients at the time of immunotherapy treatment, as immunotherapy may not be clinically relevant in advanced cancer patients as opposed to early-stage patients. Interestingly, using oxidised mannan-MUC1 does not require further manipulation in order to activate and mature DCs. In fact, oxidised mannan not only binds to the MR, but also activates DCs via TLR-4; hence, mannan plays a dual role in this vaccination strategy. It is clear that targeting cell surface receptors on DCs, such as the MR, leads to immunity against otherwise poorly immunogenic self-antigens and has implications for enhancing the efficacy of cancer immunotherapy studies using self-antigens.
Figure 1.
Kaplan–Meier survival curve for placebo and oxidised mannan-MUC1 immunised patients using PRISM. % patients tumour free is shown after 195 months.
Ex vivo DC culture as vaccine approaches
Use of ex vivo-grown monocyte-derived DCs has recently been popular for cancer vaccine studies, with the large amount of DCs that could be generated with ease and not requiring large volume of cells. Ex vivo culture of monocyte-derived DCs, pulsed with p53 and re-injected into head and neck squamous cell carcinoma patients, demonstrated an increased T-cell frequency in 11/16 patients, IFN-gamma secretion by T cells in 4/16 patients and decrease in T regulatory cells in most patients.33 It was speculated from these studies that stronger DC maturation stimuli are required to improve vaccine efficacy. In addition, six uterine cancer patients injected with autologous ex vivo-grown DCs and electroporated with Wilms' tumour gene 1 showed HLA-A2-restricted T-cell responses.34 We have demonstrated that ex vivo culture of human monocyte-derived DCs and pulsing with oxidised mannan-MUC1 and re-injection into patients with adenocarcinoma resulted in strong cellular immune responses (IFN-gamma-secreting T cells), delayed type hypersensitivity responses and clinical responses, and was well tolerated in all 10 patients.35 DCs matured with lipopolysaccharide and IFN-gamma and pulsed with six HLA-A2+ HER-2/neu peptides injected into 27 in situ ductal carcinoma patients demonstrated T-cell immunity against the peptides that were present 1 year post immunisation.36 Likewise, immunisation with IFN-gamma and DCs pulsed with HLA-A2+ prostate cancer antigen peptides demonstrated stable disease in 4/12 patients, a significant slower rise in prostate serum antigen levels37 and stimulation of cytotoxic T cells.38
The use of autologous tumour lysates to pulse ex vivo-grown DCs represents a powerful approach, as numerous tumour epitopes could be presented simultaneously by the DCs. Indeed, maturation and pulsing of CD14+ DC precursors with autologous tumour lysates resulted in delayed-type hypersensitivity responses and Th1 cytokine secretion, and increased the number of NK cells and CD8+IFN-gamma+ cells in the blood of immunised double-negative breast cancer patients; however there was no difference in overall survival among the patients receiving or not receiving DC vaccine.39 A number of animal studies and human clinical trials have been completed using ex vivo-grown monocyte-derived DCs with promising results.40
Anti-DC cell surface receptor monoclonal antibody targeting
Recently, several studies have been reported that target antigens to DC receptors in clinical trials. Two phase I clinical trials were conducted in advanced epithelial carcinoma patients injected with CDX-1307 (recombinant human chorionic gonadotropin beta-chain (hCG-beta) fused to anti-MR monoclonal antibody, with or without the addition of GM-CSF and TLR-3 and TLR7/8 agonists). Superior humoral and T-cell responses and the longest duration of stable disease were noted when GM-CSF and TLR agonists were included, and anti-hCG-beta antibodies had tumour-suppressive functions in vitro.41 This vaccination regime is currently in Phase II clinical trials in newly diagnosed, resectable hCG-beta-expressing bladder cancers, where low tumour burden and early intervention may provide greater potential for benefit, as demonstrated in our 16.5-year follow-up study. Similarly, NY-ESO-1, a cancer-testis antigen widely used in melanoma studies, fused with either anti-MR or anti-DEC205 antibodies,42 leads to stimulation of CD4+ and CD8+ T cells from peripheral blood mononuclear cells of cancer patients, as compared to only CD4+ T-cell stimulation when NY-ESO-1 is used without antibody conjugation. Thus, targeting either the MR or DEC205 on DCs is a promising vaccination strategy to induce strong cellular immune responses. Indeed, in a Phase I clinical trial using CDX-1401 (NY-ESO-1 fused to anti-DEC205 and mixed with TLR-3 and TLR-7/8 agonists) in 45 advanced cancer patients, 13 demonstrated stabilisation of disease with a median duration of 6.7 months and 2 patients showed tumour regression.43
Future directions
A strategy to improve the immunogenicity of antigens is by ‘antigen targeting'. DCs are a family of professional antigen-presenting cells that play a major role in the initiation of innate and adaptive immune responses against pathogens and tumours. Understanding the role of DC cell surface receptors aids in the understanding of the mechanism underlying their potent antigen-presenting capacity. An array of DC cell surface receptors have been discovered in the last 5–10 years, which function in inducing immune responses and individually shows promise as targets for vaccine delivery. Although all are in pre-clinical stages, we await data from clinical trials on the effectiveness of targeting different DC cell surface receptors. In addition, the discovery of TLRs and stimulation of DCs via TLR ligands has opened new avenues for designing DC-based cancer immunotherapeutics. It remains to be determined whether TLR-targeted approaches will result in enhanced immunogenicity in humans, as primarily seen in animal models. Moreover, chemokine receptors present on DCs such as CCR1, CCR2, CXCR4, CCR5, CCR6 and CXCR1 have been shown to generate enhanced immune responses in vitro and in animal models, as well as bacterial toxins, DC-binding peptides, internalisation peptides (Int) and Fc receptors. DC receptor targeting of antigens is a promising approach for cancer immunotherapy, and in particular targeting the MR on DCs results in protection of recurrence in breast cancer patients up to 16.5 years later. We await the next 5 years of new clinical data from immunotherapy studies targeting an array of DC cell surface receptors, including MR, DEC205, DC-SIGN, scavenger receptor, TLR, and so on, all of which show promise for stimulating strong immune responses and with potentially strong clinical outcomes. In light of the encouraging results regarding anti-CTLA-4 antibody (ipilimumab), now approved for metastatic melanoma, it will be interesting to see if the combination of antigen-specific vaccines with check-point inhibition will result in improved outcomes of cancer vaccines.
Acknowledgments
This work was supported by the New Idea Breast Cancer Funds, Hellenic Funds, Bosom Buddies Breast Cancer Foundation and Prolipsis Medical Centre Funds. At the time of the trial, VA was an NH and MRC R Douglas Wright Fellow 223316. All authors were also supported by the Austin Research Institute (VA, GAP, IFCM), Prolipsis Medical Centre (SV, AT, AT) and Victoria University (VA, LS).
The authors declare no conflict of interest.
References
- Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Delivery. 2013;2013:869718. doi: 10.1155/2013/869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulos V, Barnes N, Pietersz GA, McKenzie IF. Ex vivo targeting of the macrophage mannose receptor generates anti- tumor CTL responses. Vaccine. 2000;18:3174–3184. doi: 10.1016/s0264-410x(00)00090-6. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, McKenzie IF. Role of the mannose receptor in the immune response. Curr Mol Med. 2001;1:469–474. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, Pietersz GA, Gordon S, Martinez-Pomares L, McKenzie IF. Aldehyde-mannan antigen complexes target the MHC class I antigen- presentation pathway. Eur J Immunol. 2000;30:1714–1723. doi: 10.1002/1521-4141(200006)30:6<1714::AID-IMMU1714>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, Pietersz GA, Loveland BE, Sandrin MS, McKenzie IF. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc Natl Acad Sci USA. 1995;92:10128–10132. doi: 10.1073/pnas.92.22.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulos V, Pietersz GA, McKenzie IF. Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine. 1996;14:930–938. doi: 10.1016/0264-410x(95)00258-3. [DOI] [PubMed] [Google Scholar]
- Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- Sheng KC, Pouniotis DS, Wright MD, Tang CK, Lazoura E, Pietersz GA, et al. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118:372–383. doi: 10.1111/j.1365-2567.2006.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng KC, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, Apostolopoulos V, et al. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur J Immunol. 2008;38:424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- Sheng KC, Kalkanidis M, Pouniotis DS, Wright MD, Pietersz GA, Apostolopoulos V. The adjuvanticity of a mannosylated antigen reveals TLR4 functionality essential for subset specialization and functional maturation of mouse dendritic cells. J Immunol. 2008;181:2455–2464. doi: 10.4049/jimmunol.181.4.2455. [DOI] [PubMed] [Google Scholar]
- Tang CK, Sheng KC, Esparon SE, Proudfoot O, Apostolopoulos V, Pietersz GA. Molecular basis of improved immunogenicity in DNA vaccination mediated by a mannan based carrier. Biomaterials. 2009;30:1389–1400. doi: 10.1016/j.biomaterials.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Tang CK, Sheng KC, Pouniotis D, Esparon S, Son HY, Kim CW, et al. Oxidized and reduced mannan mediated MUC1 DNA immunization induce effective anti-tumor responses. Vaccine. 2008;26:3827–3834. doi: 10.1016/j.vaccine.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Cui Z, Hsu CH, Mumper RJ. Physical characterization and macrophage cell uptake of mannan-coated nanoparticles. Drug Dev Industrial Pharm. 2003;29:689–700. doi: 10.1081/ddc-120021318. [DOI] [PubMed] [Google Scholar]
- Toda S, Ishii N, Okada E, Kusakabe KI, Arai H, Hamajima K, et al. HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-coated liposomes and inhibited by anti-interferon-gamma antibody. Immunology. 1997;92:111–117. doi: 10.1046/j.1365-2567.1997.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G, Frokjaer S. Interaction of dendritic cells with antigen-containing liposomes: effect of bilayer composition. Vaccine. 2004;22:1903–1913. doi: 10.1016/j.vaccine.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Thomann JS, Heurtault B, Weidner S, Brayé M, Beyrath J, Fournel S, et al. Antitumor activity of liposomal ErbB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials. 2011;32:4574–4583. doi: 10.1016/j.biomaterials.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Espuelas S, Thumann C, Heurtault B, Schuber F, Frisch B. Influence of ligand valency on the targeting of immature human dendritic cells by mannosylated liposomes. Bioconj Chem. 2008;19:2385–2393. doi: 10.1021/bc8002524. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Haddadi A, Shayeganpour A, Samuel J, Lavasanifar A. Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles. Pharm Res. 2011;28:2288–2301. doi: 10.1007/s11095-011-0459-9. [DOI] [PubMed] [Google Scholar]
- Jiang HL, Kang ML, Quan JS, Kang SG, Akaike T, Yoo HS, et al. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29:1931–1939. doi: 10.1016/j.biomaterials.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Shiku H, Wang L, Ikuta Y, Okugawa T, Schmitt M, Gu X, et al. Development of a cancer vaccine: peptides, proteins, and DNA. Cancer Chemother Pharm. 2000;46 (Suppl:S77–S82. doi: 10.1007/s002800000179. [DOI] [PubMed] [Google Scholar]
- Moyle PM, Olive C, Ho MF, Pandey M, Dyer J, Suhrbier A, et al. Toward the development of prophylactic and therapeutic human papillomavirus type-16 lipopeptide vaccines. J Med Chem. 2007;50:4721–4727. doi: 10.1021/jm070287b. [DOI] [PubMed] [Google Scholar]
- Presicce P, Taddeo A, Conti A, Villa ML, Della Bella S. Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Mol Immunol. 2008;45:1136–1145. doi: 10.1016/j.molimm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- He LZ, Ramakrishna V, Connolly JE, Wang XT, Smith PA, Jones CL, et al. A novel human cancer vaccine elicits cellular responses to the tumor-associated antigen, human chorionic gonadotropin beta. Clin Cancer Res. 2004;10:1920–1927. doi: 10.1158/1078-0432.ccr-03-0264. [DOI] [PubMed] [Google Scholar]
- Ramakrishna V, Treml JF, Vitale L, Connolly JE, O'Neill T, Smith PA, et al. Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules. J Immunol. 2004;172:2845–2852. doi: 10.4049/jimmunol.172.5.2845. [DOI] [PubMed] [Google Scholar]
- Lam JS, Huang H, Levitz SM. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS ONE. 2007;2:e1009. doi: 10.1371/journal.pone.0001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlen G, Strindelius L, Johansson T, Nilsson A, Chatzissavidou N, Sjöblom M, et al. Mannosylated mucin-type immunoglobulin fusion proteins enhance antigen-specific antibody and T lymphocyte responses. PLoS ONE. 2012;7:e46959. doi: 10.1371/journal.pone.0046959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas V, Lodding J, Maino VC, McKenzie IF. Flow cytometric measurement of intracellular cytokines detects immune responses in MUC1 immunotherapy. Clin Cancer Res. 2000;6:829–837. [PubMed] [Google Scholar]
- Karanikas V, Thynne G, Mitchell P, Ong CS, Gunawardana D, Blum R, et al. Mannan mucin-1 peptide immunization: influence of cyclophosphamide and the route of injection. J Immunother. 2001;24:172–183. [PubMed] [Google Scholar]
- Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Drakaki H, Loveland BE, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Br Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilaros S, Tsibanis A, Tsikkinis A, Pietersz GA, McKenzie IF, Apostolopoulos V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy. 2013;5:1177–1182. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- Schuler PJ, Harasymczuk M, Visus C, Deleo A, Trivedl S, Lei Y, et al. Dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res. 2014;20:2433–2444. doi: 10.1158/1078-0432.CCR-13-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman ZN, et al. Wilms' Tumor Gene 1 (WT1)—loaded dendritic cell mmunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer Res. 2013;33:5495–5500. [PubMed] [Google Scholar]
- Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- Koski GK, Koldovsky U, Xu S, Mick R, Sharma A, Fitzpatrick E, et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J Immunother. 2012;35:54–65. doi: 10.1097/CJI.0b013e318235f512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildenbrand B, Sauer B, Kalis O, Stoll C, Freudenberg MA, Niedermann G, et al. Immunotherapy of patients with hormone-refractory prostate carcinoma pre-treated with interferon-gamma and vaccinated with autologous PSA-peptide loaded dendritic cells—a pilot study. Prostate. 2007;67:500–508. doi: 10.1002/pros.20539. [DOI] [PubMed] [Google Scholar]
- Waeckerle-Men Y, Uetz-von Allmen E, Fopp M, von Moos R, Böhme C, Schmid HP, et al. Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma. Cancer Immunol Immunother. 2006;55:1524–1533. doi: 10.1007/s00262-006-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi CJ, Ning YL, Han YS, Min HY, Ye H, Zhu YL, et al. Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunol Immunother. 2012;61:1415–1424. doi: 10.1007/s00262-011-1192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantia-Smaldone GM, Chu CS. A review of dendritic cell therapy for cancer: progress and challenges. BioDrugs. 2013;27:453–468. doi: 10.1007/s40259-013-0030-9. [DOI] [PubMed] [Google Scholar]
- Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:232ra251. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]