Abstract
Background
Patients with central nervous system (CNS) malignancies represent an “at-risk” population for contracting influenza, particularly if they are receiving ongoing chemotherapy, radiation, and/or glucocorticoid treatment. The Centers for Disease Control endorses vaccination for these patients, although data are not available to indicate whether they mount an immunologic response adequate to achieve clinical protection.
Methods
A pilot prospective cohort study was designed to evaluate the immunogenicity of the standard-dose trivalent inactivated influenza vaccine in patients with malignant CNS tumors. Baseline data collection included diagnosis, chemotherapy, timing of chemotherapy or radiation relative to vaccination, and glucocorticoid dose. Serum samples were collected at baseline, day 14, day 28, and month 3 following vaccination. Samples were tested using hemagglutinin inhibition to determine seroconversion (4-fold rise in titer) and seroprotection (titer >1:40).
Results
A total of 38 patients were enrolled (mean age, 54 years ±13.5 years, 60.5% male, 94.7% Caucasian, and 5.3% African American). CNS tumor diagnoses included glioblastoma multiforme (55.2%), other high-grade glioma (13.2%), low-grade glioma (15.8%), and primary CNS lymphoma (15.8%). At enrollment, 20 patients (52.6%) were taking glucocorticoids, 25 (65.8%) were on active chemotherapy, and 3 (7.9%) were undergoing radiation. Seroconversion rates at day 28 for the A/H1N1, A/H3N2, and B strains were 37%, 23% and 23%, respectively. Seroprotection was 80%, 69%, and 74%, respectively. All rates were significantly lower than published rates in healthy adults (P < .001).
Conclusion
Influenza vaccine immunogenicity is significantly reduced in patients with CNS malignancies. Future studies are needed to determine the causative etiologies and appropriate vaccination strategies.
Keywords: central nervous system malignancy, chemotherapy, influenza vaccine
Influenza is a common RNA virus that causes yearly epidemics with an illness typically presenting with fever, myalgias, constitutional symptoms, and upper and/or lower respiratory tract symptoms. Patients with malignancies receiving chemotherapy are at increased risk for influenza-related illness1,2 and have been shown to have higher complication and mortality rates.3–5 The Centers for Disease Control (CDC) recommends annual influenza vaccination for all persons older than 6 months.6 The CDC endorses the practice of influenza vaccination for patients with malignancies, although little concrete evidence exists to suggest that patients with malignancies and/or those undergoing active chemotherapy mount a sufficient immunologic response to achieve clinical protection.
In the general population, influenza vaccination results in prevention of infection in ∼70%–90% of healthy young adults.7,8 Serologic response is high, with seroconversion rates of 75%–80% and seroprotection rates of around 95% based on hemagglutinin inhibition (HI) titer assessment.9,10 Studies have demonstrated reduced immunogenicity in elderly populations, with seroconversion rates of 23%–51% and seroprotection rates of 68%–97%,11 as well as other groups including patients with end-stage renal disease,12 renal transplants,13,14 liver transplants,15 and lung transplants,16 HIV populations,17 and others.
Investigation of influenza vaccine efficacy in patients with malignancies has been limited. Cancer patients are vaccinated at rates well below 50%. Lack of awareness of the current recommendations, fear of side effects, and concerns about vaccine efficacy have been cited as the primary reasons for not offering vaccination.18 Studies have demonstrated seroconversion and seroprotection rates similar to healthy adults following vaccination of patients with lung cancer19 as well as those with a variety of solid tumors.20 In patients with hematologic malignancies, seroconversion rates have been reported to be lower (21%).21 Some studies have evaluated the efficacy of single vaccination, while others have suggested additional efficacy using a multidose regimen.22
The efficacy of influenza vaccination has not been evaluated in patients with central nervous system (CNS) malignancies. Nevertheless, this is a potentially robust population for study given the immunosuppressive effects of gliomas and its associated treatment,23 variable degrees of therapy-induced lymphopenia and neutropenia, and the inclusion of glucocorticoids into pharmocotherapeutic regimens. The current study pilots an investigation into influenza vaccine immunogenicity in a group of patients with CNS malignancy.
Materials and Methods
This pilot prospective study was designed at a single institution for patients with a clinical or histopathological diagnosis of primary CNS malignancy. The study was approved by the local institutional review board, and each patient provided informed consent. All patients presenting in the year of study who had not received the standard yearly influenza vaccine were eligible and were offered enrollment. Exclusion criteria included standard contraindications to influenza vaccination. Baseline data were collected on age, sex, diagnosis, chemotherapeutic regimen, glucocorticoid use, timing of treatment, and other clinical indicators. Participants received the standard-dose trivalent inactivated vaccine by intramuscular injection as per national recommendations.24 Sera were obtained at baseline, as well as at day 14 (±3 days), day 28 (±3 days), and month 3 (±2 weeks) after immunization.
Postvaccination titers to each strain contained within the seasonal 2011–12 influenza vaccine (A/California/7/2009 [H1N1]), A/Perth/16/2009 [H3N2]), and B/Brisbane/60/2008) were measured by hemagglutination inhibition (HI) assay, as previously described.25 Sera pretreated overnight with receptor-destroying enzyme underwent serial 2-fold dilutions on V-bottom 96-well microtiter plates, starting at 1:10 dilution. An equal volume of inactivated influenza vaccine antigen was added to the wells and subsequently incubated with chicken red blood cells (0.5% suspension in phosphate-buffered saline) at room temperature for 1 hour. The highest serum dilution at which hemagglutination was still completely inhibited was reported as the HI antibody titer for each strain. Seroconversion was defined as a 4-fold increase in HI titer between baseline and 28-day sample. Seroprotection was defined as a 28-day titer of at least 1:40. HI was also measured at 3 months to determine the longevity of the serologic response. Additional laboratory investigations on baseline samples included immunologic assessment for CD4 count, CD8 count, CD4/CD8 ratio, CD28 expression on CD8 positive T cells (CD28+CD8+ TC)—a measure of immune senescence—and quantitative immunoglobulin levels (IgM, IgA, and IgG).
Data on seroconversion and seroprotection rates were compared with published values for healthy adults9,10 and groups known to respond poorly to the vaccine.11 Data analysis was performed using SAS Version 9.3. Descriptive statistics were calculated for the study population. Geometric mean titers were calculated at each time interval. Univariate analyses were performed to assess differences in seroconversion and seroprotection. CD4, CD8, CD4/8 ratio, CD28+CD8+ TCs, and quantitative Ig levels were categorized into tertiles to assess immunologic associations. Frequencies and percentages were calculated using categorical variables, and statistical significance was assessed using Fisher' exact tests. For continuous variables, means and standard deviations were calculated, and statistical significance was assessed using Wilcoxon rank sum tests. Predetermined statistical significance for all tests was .05.
Results
A total of 38 participants were enrolled. Descriptive statistics are provided in Table 1 and show mean age of 54 years (±13.5 years), 60.5% male, 94.7% Caucasian, and 5.3% African American. Diagnoses included glioblastoma multiforme (55.2%), other high grade glioma (13.2%), low grade glioma (15.8%), and primary CNS lymphoma (15.8%). Mean duration of diagnosis was 2 years (±2.9 years). At enrollment, 20 participants (52.6%) were taking glucocorticoids, 17 were taking dexamethasone, and 3 were on prednisone. A total of 25 participants (65.8%) were on active chemotherapy including temozolomide, bevacizumab, cilengitide, methotrexate, rituximab, and IMC-3G3. Three participants (7.9%) were receiving active radiation therapy. Prior treatments varied (Supplemental Table 1). Both relatively treatment-naive and heavily treated patients were represented, with 76% of participants having been treated with 1 prior chemotherapy regimen and 24% having >1 prior regimen. Most participants were previously radiated (79%), but the timing of such radiation varied (median, 6 months; range, 1–135 months) including 18 who had received radiation within 1 year. Three individuals were excluded from further analysis due to death or disease progression.
Table 1.
Baseline demographics
| Characteristic | n=38 |
|---|---|
| Age (years) | 54 ± 13.5 |
| Sex (male, %) | 23 (60.5%) |
| Race, n (%) | |
| Caucasian | 36 (94.7%) |
| African American | 2 (5.3%) |
| Diagnoses (n, %) | |
| Glioblastoma multiforme | 21 (55.3%) |
| Anaplastic astrocytoma | 2 (5.3%) |
| Low grade astrocytoma | 4 (10.5%) |
| Pontine glioma | 1 (2.6%) |
| High-grade oligodendroglioma | 1 (2.6%) |
| Low-grade oligodendroglioma | 1 (2.6%) |
| High-grade mixed glioma | 2 (5.3%) |
| Primary central nervous system lymphoma | 6 (15.8%) |
| Glucocorticoids (n, %) | 20 (52.6%) |
| Prednisone | 3 (15%) |
| Dexamethasone | 17 (85%) |
| Chemotherapy (n, %) | 25 (65.8%) |
| 5-day temozolomide | 13 (52%) |
| 21-day temozolomide | 2 (8.0%) |
| Bevacizumab | 3 (12.0%) |
| Cilengitide | 1 (4.0%) |
| IMC-3G3 | 1 (4.0%) |
| Temozolomide, bevacizumab | 1 (4.0%) |
| Temozolomide, cilengitide | 3 (12.0%) |
| Methotrexate, rituximab | 1 (4.0%) |
| Radiation (n, %) | 3 (7.9%) |
Baseline demographics for the population including patients actively receiving glucocorticoids, chemotherapeutic agents, or radiation at the time of or within 1 week of vaccine administration.
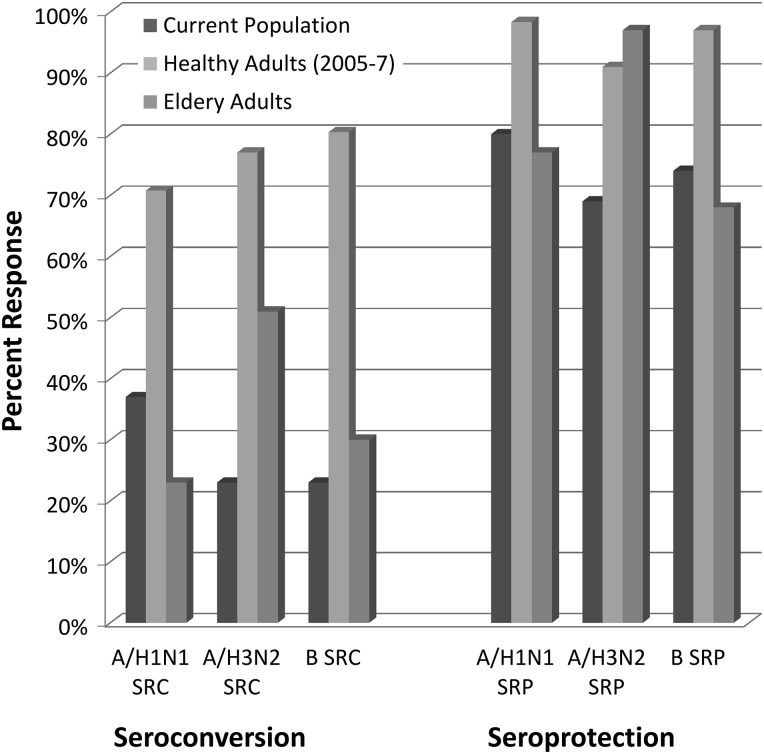
At 28 days, seroconversion rates for the A/H1N1, A/H3N2, and B strains were 37%, 23%, and 23%, respectively (Table 2). These rates were significantly lower than published seroconversion rates for healthy adults (P values <.001, Figure 1). Seroprotection rates at day 28 were 80%, 69%, and 74%, for each strain respectively. While these rates were greater than those for seroconversion, they were significantly lower than the published seroprotection rates for healthy adults (P values <.001). Baseline seroprotection in our population was 28% for all 3 strains and 46%, 49%, and 51% for the A/H1N1, A/H3N2, and B strains, respectively. There was no significant difference in seroconversion or seroprotection by age, sex, diagnosis, or grade of tumor (high vs low). There was no difference in serologic response based on glucocorticoid use, active chemotherapy, or the combination of glucocorticoids and chemotherapy. The size of each subgroup limited assessment of the effects of radiation, specific chemotherapeutic agent, or dose of glucocorticoid, but no significant differences in seroconversion or seroprotection were observed for any prior radiation, radiation within 1 year of vaccination, or number of prior chemotherapies.
Table 2.
Seroresponse data
| Baseline | Day 28 | 3 Month | |
|---|---|---|---|
| GMT | |||
| A/H1N1 | 29.1 | 65.6 | 62 |
| A/H3N2 | 26.9 | 46.9 | 42.9 |
| B | 30.9 | 48.8 | 49.2 |
| Seroconversion | |||
| A/H1N1 | 37.1% | ||
| A/H3N2 | 22.9% | ||
| B | 22.9% | ||
| Seroprotection | |||
| A/H1N1 | 45.7% | 80.0% | 73.3% |
| A/H3N2 | 26.9% | 68.6% | 56.7% |
| B | 51.4% | 74.3% | 66.7% |
Serologic response to trivalent inactivated influenza vaccination including geometric mean titer (GMT), seroconversion (at day 28) and seroprotection.
Fig. 1.
Influenza vaccine immunogenicity. Comparison of influenza vaccine seroconversion and seroprotection between the study population, healthy controls (mean values, averaged over the indicated years of study), and elderly adults (who are known to respond poorly to the standard trivalent inactivated vaccine).
When comparing these data with published seroconversion and seroprotection rates in an elderly population, which has been previously reported to have substandard serologic response rates, there was no significant difference in seroconversion or seroprotection rates for the A/H1N1 and B strains between groups.11 For the A/H3N2 strain, seroconversion and seroprotection rates in our population were significantly less than those in the previously reported elderly population (23% vs 50.7%; P = .002 and 69% vs 96.5%; P < .001, respectively).
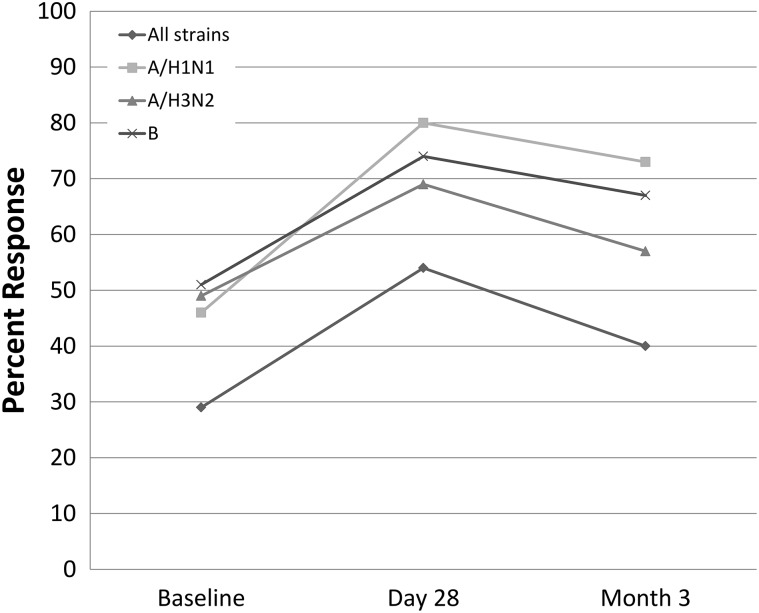
Overall, only 3 participants (8.6%) seroconverted to all 3 strains, and only 54.3% of participants were seroprotected to all 3 strains at day 28. Long-term seroprotection at 3 months was similar to 28-day seroprotection with rates of 73%, 57%, and 67% for the respective strains (Figure 2).
Fig. 2.
Long-term seroprotection. Graphical depiction of the seroprotection from baseline, day 28 to 3 months following vaccination for each of the 3 strains contained within the vaccine and a composite of all strains.
When stratified into lowest, middle, or highest tertile by CD4 count, CD4/8 ratio, CD28+CD8+ TC values, or quantitative Ig levels, no statistical difference was observed in seroconversion or seroprotection. When stratified by CD8 count, those participants in the middle (CD8 282–534 cells/mm3) or highest (>534 cells/mm3) tertiles had a trend toward higher rates of seroprotection to the A/H3N2 and B strains (P = .068 and P = .073, respectively), but this was not observed for the A/H1N1 strain.
Discussion
In this pilot study, influenza vaccination immunogenicity in participants with primary CNS malignancies was significantly reduced from that seen in normal healthy adults. Seroconversion rates of 23%–37% and seroprotection rates of 69%–80% are comparable to those in populations known to respond poorly to the vaccine. Long-term immunogenicity also remained poor, with seroprotection rates as low at 57% at 3 months. Seroprotection rates are likely higher than seroconversion rates, owing to a significant degree of baseline seroprotection (46%–51%) in this population likely resulting from prior vaccination or influenza exposure. The reduced immunogenicity was not associated with age or grade of tumor.
Between 10% and 40% of adult oncology patients are infected with seasonal influenza annually.26 Influenza-related upper respiratory infections in cancer patients result in costly hospitalizations, delays in treatment of the underlying malignancy, and death.3 Concern also exists for the tendency of immune-suppressed patients to shed virus for prolonged periods during infection.27 Based on work using algorithmic mathematical modeling, it has been suggested that vaccination of cancer patients with life expectancy of >3 months within 5 years of a cancer diagnosis may reduce hospitalization and increase life expectancy.28
In noncancer patients known to respond poorly to the vaccine, a variety of alternative vaccination strategies have been considered. In both elderly and HIV-infected patients, high-dose (60 mcg/strain) trivalent influenza vaccination was found to induce a significantly greater seroprotection than standard-dose vaccination11,29,30 without affecting tolerability.31 In a case-control study comparing 1 and 2-dose regimens of ASO3-adjuvanted pandemic influenza vaccination to A/H1N1 in cancer patients, of which glioma patients accounted for 13% of the population, a statistically nonsignificant difference was observed in seroconversion (15.8% difference, 89.5% vs 73.7%) and seroprotection (21% difference, 94.7% vs 73.7%).32 To date, studies investigating high-dose or 2-dose regimens have not been performed specifically in glioma populations.
An underappreciated strategy for protecting vulnerable patients is to ensure immunization of the family members and caregivers who have frequent contact with at-risk patients. This practice has been termed “cocooning” and has proven particularly useful for prevention of pertussis and other infectious diseases in infants.33 Of course, immunization of health care workers is also recommended due to frequent contact with these patients and documented transmission of influenza to immunocompromised adults by health care personnel.34
Evaluation for causative factors associated with reduced immunogenicity is limited by the small sample size. No difference was observed by age or tumor grade. Subgroup analysis also failed to show significant associations with active glucocorticoid use, prior or active chemotherapy, or radiation. Recent studies suggest an immunosuppressing role of radiation therapy, although in this study, no differences in seroconversion or seroprotection were observed with any prior radiation, radiation within 1 year, or by CD4 count.35 It may be that the immunobiology underlying CNS malignancy, as opposed to treatment-specific factors, is driving impaired immunogenicity, but a much larger sample is necessary to explore this hypothesis. Since the 1970s, suppressed cellular and humoral immunologic responses have been repeatedly demonstrated in patients with CNS malignancies.36 While the extent of response has often correlated with the degree of anaplasia, significant immunosuppression has been demonstrated across tumor types from glioblastoma multiforme to meningioma.37 Numerous immunosuppressing factors have been implicated including tumor production of transforming growth factor-β2 (which suppresses lymphocyte function),38 production of prostaglandin E2 (which suppresses cellular immunity),39 downregulation of human leukocyte antigen class I antigens (which are important in intracellular antigen presentation),40 and others. Immunologic evaluation showed a trend toward higher seroprotection for 2 strains in patients with higher CD8 counts, but this was not statistically significant. Further large-scale, multicenter studies with a broader array of immunologic monitoring are required to confirm the causative factors associated with impaired immunogenicity.
This study does have limitations. This is a single-center pilot study that cannot be generalized to other populations. Prior vaccination history was not available, which limits correlation of these findings with baseline immunity. While prevaccination seroprotection ranged from 46%–51%, this is similar to the varying rates that have been reported previously and is not felt to significantly limit the determination of seroconversion.41 This study was not sufficiently powered to determine factors that may be associated with reduced immunogenicity in this population. As with many serologic studies of influenza vaccine, the clinical implications of these results in terms of the impact on influenza infection, morbidity and mortality related to influenza infection, and other clinical outcomes cannot be determined. In patients with CNS gliomas, the decreased life expectancy from the underlying pathology may prohibit such large-scale studies with clinical endpoints of influenza acquisition or secondary bacterial infection.
In conclusion, influenza vaccine immunogenicity is significantly reduced in patients with CNS malignancies and is similar to populations for which alternative strategies have been considered. Indications for annual vaccination remain at the discretion of the treating oncologist. Further investigation into alternative vaccination strategies is necessary to determine a regimen sufficient to induce adequate immunologic response in those with CNS tumors.
Supplementary Material
Funding
This study received no external funding. This research was supported by the Clinical Research Unit of the Wake Forest Translational Science Institute and the Comprehensive Cancer Center of Wake Forest University NCI CCSG P30CA012197 grant.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Kempe A, Hall CB, MacDonald NE, et al. Infuenza in children with cancer. J Pediatr. 1989;115(1):33–39. doi: 10.1016/s0022-3476(89)80325-7. [DOI] [PubMed] [Google Scholar]
- 2.Baerk WH, Mulloly JP. Pneumonia and influenza deaths during epidemics: Implications for prevention. Arch Intern Med. 1982;142(1):85–89. [PubMed] [Google Scholar]
- 3.Cooksley CD, Avritscher EB, Bekele BN, et al. Epidemiology and outcomes of serious influenza-related infections in cancer population. Cancer. 2005;104(3):618–628. doi: 10.1002/cncr.21203. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 5.Whimbley E, Elting LS, Cough RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13(4):437–440. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention: Prevention and control of influenza with vaccines: Recommendations for the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59:32–39. (No. RR-8) [Google Scholar]
- 7.Herrera GA, Iwane MK, Cortese M, et al. Influenza vaccine effectiveness among 50-64-year-old persons during a season of poor antigenic match between vaccine and circulating influenza virus strains: Colorado, United States, 2003-2004 . Vaccine. 2007;25(1):154–160. doi: 10.1016/j.vaccine.2006.05.129. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. MMWR Morb Mort Wkly Rep. 2008;57:1–60. [Google Scholar]
- 9.Beran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis. 2009;200(12):1861–1869. doi: 10.1086/648406. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, Gaglani MJ, Kevserling HL, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 2010;10:71. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falsey AR, Treanor JJ, Tornieporth N, et al. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 12.McGrath LJ, Kshirsagar AV, Cole SR, et al. Influenza vaccine effectiveness in patients on hemodialysis: an analysis of natural experiment. Arch Intern Med. 2012;172(7):548–554. doi: 10.1001/archinternmed.2011.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birdwell KA, Ikizler MR, Sannella ED, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54(1):112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito S, Meregalli E, Daleno C, et al. An open-label, randomized clinical trial assessing immunogenicity, safety and tolerability of pandemic influenza A/H1N1 MF59-adjuvanted vaccine administered sequentially or simultaneously with seasonal virosomal-adjuvanted influenza vaccine to paediatric kidney transplant recipients. Nephrol Dial Transplant. 2011;26(6):2018–2024. doi: 10.1093/ndt/gfq657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soesman NM, Rimmelzwann GF, Nieuwkoop NJ, et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61(1):85–93. [PubMed] [Google Scholar]
- 16.Mazzone PJ, Mossad SB, Mawhorter SD, et al. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir. 2001;18(6):971–976. doi: 10.1183/09031936.01.00215201. [DOI] [PubMed] [Google Scholar]
- 17.Madhi SA, Dittmer S, Kuqanda L, et al. Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS. 2013;27(3):369–379. doi: 10.1097/QAD.0b013e32835ab5b2. [DOI] [PubMed] [Google Scholar]
- 18.Loulergue P, Mir O, Alexandra J, et al. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann Oncol. 2008;19(9):1658. doi: 10.1093/annonc/mdn531. [DOI] [PubMed] [Google Scholar]
- 19.Anderson H, Petrie K, Berrisford C, et al. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999;80(1/2):219–220. doi: 10.1038/sj.bjc.6690342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Methuku N, Coimbatore P, et al. Immunogenicity of an inactivated monovalent 2009 influenza A (H1N1) vaccine in patients who have cancer. Oncologist. 2012;17(1):125–134. doi: 10.1634/theoncologist.2011-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monkman K, Mahony J, Lazo-Langner A, et al. The pandemic H1N1 influenza vaccine results in low rates of seroconversion for patients with hematological malignancies. Leuk Lymphoma. 2011;52(9):1736–1741. doi: 10.3109/10428194.2011.584003. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau B, Loulergue P, Mir O, et al. Immunogenecity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23(2):450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 23.Tada M, de Tribolet N. Immunobiology of malignant gliomas. J Clin Neurosci. 1996;3(2):102–113. doi: 10.1016/s0967-5868(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control. Prevention and Control of Influenza – Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57(RR07):1–60. [PubMed] [Google Scholar]
- 25.Kendal A, Pereira M, Skehel J. Concepts and Procedures for Laboratory-Based Influenza Surveillance. Atlanta, GA: Centers for Disease Control; 1982. [Google Scholar]
- 26.Hicks KL, Chemaly RF, Kontoyiannis DP. Common community respiratory viruses in patients with cancer: more than just “common colds”. Cancer. 2003;97(10):2576–2587. doi: 10.1002/cncr.11353. [DOI] [PubMed] [Google Scholar]
- 27.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug resistant influenza A virus in an immunocompromised patient. N Engl JMed. 2003;348(9):867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 28.Avritscher EB, Cooksley CD, Geraci JM, et al. Cost-Effectiveness of Influenza Vaccination in Working-Age Cancer Patients. Cancer. 2007;109(11):2357–2364. doi: 10.1002/cncr.22670. [DOI] [PubMed] [Google Scholar]
- 29.Chen WH, Cross AS, Edelman R, et al. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine. 2011;29(16):2865–2873. doi: 10.1016/j.vaccine.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons. Ann Intern Med. 2013;158(1):19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166(10):1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 32.Hottinger AF, George AC, Bel M, et al. H1N1 Study Group. A prospective study of factors shaping antibody responses to the ASO3-adjuvanted influenza A/H1N1 vaccine in cancer outpatients. Oncologist. 2012;17(3):436–445. doi: 10.1634/theoncologist.2011-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grizas AP, Camenga D, Vazquez M. Cocooning: a concept to protect young children from infectious disease. Curr Opin Pediatr. 2012;24(1):92–97. doi: 10.1097/MOP.0b013e32834e8fe9. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Influenza Vaccination Information for Healthcare Workers. Available at: http://www.cdc.gov/flu/healthcareworkers.htm . Accessed: May 13, 2013.
- 35.Grossman SA, Ye X, Lesser G, et al. NABTT CNS Consortium. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nano R, Ceroni M. The immunobiology of malignant gliomas. Funct Neurol. 2005;20(1):39–42. [PubMed] [Google Scholar]
- 37.Mahaley MS, Jr, Brooks WH, Roszman TL, et al. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977;46(4):467–476. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- 38.Brooks WH, Netsky MG, Horwitz DA, et al. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterisation of a humoral immunosuppressive factor. J Exp Med. 1972;136(6):1631–1747. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawamura Y, Diserans A-C, de Tribolet N. In vitro prostaglandin E2 production by glioblastoma cells and its effect on interleukin-2 activation by oncolytic lymphocytes. J Neurooncol. 1990;9(2):125–130. doi: 10.1007/BF02427832. [DOI] [PubMed] [Google Scholar]
- 40.Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11(23):8304–8311. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 41.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197(4):490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.