Abstract
Background
A phase II trial of conformal radiotherapy (CRT) for pediatric high-grade glioma (HGG) was performed to evaluate disease control and late effects.
Methods
Between July 1997 and January 2003, 34 pediatric patients (median age, 13.2 ± 6.7 years) with HGG were enrolled on an International Commission on Radiation Units and Measurements Report 50-compliant prospective trial using CRT with a 2 cm clinical target volume margin. Baseline and serial evaluations were performed to assess functional outcomes.
Results
Median follow-up for the entire group was 18 months (range, 2–134 months). Twenty (58.8%) patients developed local progression, and 6 (17.6%) patients developed distant progression. Progression-free and overall survival at 10 years were 18.8% ± 6.9% and 16.8% ± 6.5%, respectively. At baseline, 40% of patients evaluated for intelligence quotient (IQ) scored below 85. Measures of cognitive function obtained during the first 12 months fit a mixed model with a quadratic function. The relationship between IQ and time was -1.1883 points/month for the linear term and 0.07728 points/month for the quadratic term (P = .0454). IQ decreased between baseline and 6 months and then increased slightly through 12 months. The opposite was found for (all P values of the quadratic term) activities of daily living (P = .0155), socialization (P = .0049), and the composite score (P = .0257) of adaptive behavior.
Conclusion
CRT using a 2 cm clinical target volume margin in pediatric HGG demonstrated tumor control comparable to conventional radiation therapy. Disrupted cognitive and adaptive behavioral functioning were present at baseline and throughout the course of disease.
Keywords: high-grade glioma, radiation therapy, pediatrics
Central nervous system (CNS) tumors are the second most common malignancy and the most common form of solid tumor among children.1–3 Although they are frequently diagnosed in the adult population, high-grade glioma (HGG) comprises only 8%–12% of pediatric CNS tumors and commonly includes anaplastic astrocytoma (AA) and glioblastoma multiforme (GBM).4 After initial resection, radiotherapy (RT) is often used for pediatric patients with HGG. Exceptions include younger children, who may receive chemotherapy to delay or avoid RT because of concern about side effects.5,6 Despite aggressive therapy, survival remains poor. Five-year survival rates range from 10%–20%.1–3,7–9
There is increasing concern about the impact of treatment on quality of life and long-term effects in those diagnosed with high-grade tumors, especially those who might survive. Late effects from surgery and irradiation may be irreversible and progressive, affecting endocrine function, cognition, and other parameters. There is a heightened concern for younger children.5,10 In a recent report including patients with low-grade glioma, those aged 5 years or younger experienced the greatest decline in cognition with expected subnormal IQ (<85 points) evident 5 years following RT.6
To optimize treatment, spare normal tissue from irradiation, and minimize the risk of complications, conformal radiotherapy (CRT) was introduced for children with HGG. In 1997, we activated the first International Commission on Radiation Units and Measurements Report 50 compliant prospective trial for children with localized brain tumors to estimate the rate of local control and patterns of failure in patients treated with CRT. The study included children with HGG treated using a 2 cm clinical target volume margin. The trial aimed to evaluate the late sequelae of CRT in pediatric patients with HGG using ICRU targeting guidelines and estimating the incidence, time to onset, and severity of these effects. Despite shortened life expectancy, our goals were to document baseline morbidity and identify clinical factors that might predict complications. Although more recent trials have included similar CRT guidelines, none has serially evaluated functional outcomes for these patients. With the expectation of poor survivorship, neurophysiological effects may be overlooked or their evaluation delayed, with the potential to impact quality of life or the ability of a patient to survive intense therapy. This report includes disease control and functional outcomes for children with HGG prospectively treated using CRT.
Materials and Methods
Patient Population
A total of 34 pediatric patients diagnosed with HGG were enrolled on a phase II prospective study of CRT at St. Jude Children's Research Hospital between July 1997 and January 2003. Pathology was reviewed on all participants. Participants were diagnosed with HGG based on institutional pathology review that included criteria for WHO grade III/IV glioma and included the pathological subtypes noted in this report. The mean age at the time of diagnosis was 12.2 years (range, 2–24.4 years). Eight were >18 years of age at the time of irradiation. Median time between diagnosis and CRT was 2 months (range, 1–69 months). Participants were characterized by age, sex, tumor histology, tumor location, previous chemotherapy, extent of surgical resection, number of surgical procedures, and other parameters. Twelve participants presented with hydrocephalus at the time of diagnosis, and a total of 14 participants required CSF shunt placement. Participant characteristics prior to CRT are described in Tables 1 and 2. This study was approved by the St. Jude Children's Research Hospital Institutional Review Board.
Table 1.
Clinical characteristics
| No. Patients Enrolled |
||
|---|---|---|
| # | % | |
| Sex | ||
| Male | 16 | 47 |
| Female | 18 | 53 |
| Race | ||
| White | 22 | 65 |
| Black | 11 | 32 |
| Other | 1 | 3 |
| Age at RT (years) | ||
| 2–4 | 6 | 18 |
| 5–9 | 9 | 26 |
| 10–14 | 8 | 24 |
| ≥15 | 11 | 32 |
| Histology | ||
| Anaplastic astrocytoma | 14 | 41 |
| Glioblastoma | 12 | 35 |
| Malignant glioneuronal tumor | 3 | 9 |
| Anaplastic oligodendroglioma | 2 | 6 |
| Astroblastoma | 1 | 3 |
| Anaplastic pleomorphic xanthroastrocytoma | 1 | 3 |
| Malignant neurocytoma | 1 | 3 |
| Region | ||
| Supratentorial | 30 | 88 |
| Infratentorial | 4 | 12 |
| Location | ||
| Infratentorial | ||
| Fourth ventricle | 1 | 3 |
| Cerebellum | 2 | 6 |
| Midbrain | 1 | 3 |
| Supratentorial | ||
| Thalamus | 12 | 35 |
| Frontal | 5 | 15 |
| Temporal | 5 | 15 |
| Parietal | 3 | 9 |
| Frontoparietal | 2 | 6 |
| Frontotemporal | 1 | 3 |
| Occipital | 1 | 3 |
| Parietooccipital | 1 | 3 |
| Extent of resection prior to RT | ||
| Biopsy only | 10 | 29 |
| Subtotal resection | 11 | 32 |
| Gross total resection | 13 | 38 |
| Hydrocephalus at diagnosis | ||
| Yes | 12 | 35 |
| No | 22 | 65 |
| Ventriculoperitoneal shunt | ||
| Yes | 14 | 41 |
| No | 20 | 59 |
| Chemotherapy | ||
| Adjuvant only | 3 | 9 |
| Neoadjuvant/concurrent | 12 | 35 |
| Neoadjuvant/concurrent/adjuvant | 14 | 41 |
| None | 5 | 15 |
Abbreviations: RT, radiation therapy
Table 2.
Surgical outcomes of patients with high grade glioma
| Age (yr) | Histology | Tumor Location | Extent of Resection | No. Surgeries | Hydrocephalus | Shunt | No. Shunt Revisions | Initial Steroid (mo)* | Survival From Diagnosis (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | MGN | Temporal | STR | 2 | No | Yes | 0 | 5* | 5 |
| 17 | Astroblastoma | IV ventricle | GTR | 2 | Diagnosis | No | 0 | 3 | 8 |
| 13 | GBM | Thalamus | BX | 4 | Diagnosis | Yes | 2 | 1 | 9 |
| 15 | GBM | Thalamus | GTR | 1 | No | No | 0 | 0 | 12 |
| 17 | GBM | Thalamus | STR | 5 | Diagnosis | Yes | 2 | 12* | 12 |
| 13 | GBM | Thalamus | STR | 4 | Diagnosis | Yes | 1 | 12* | 12 |
| 21 | AA | Thalamus | BX | 1 | Diagnosis | Yes | 0 | 13* | 13 |
| 4 | AA | Thalamus | BX | 3 | Diagnosis | Yes | 1 | 16* | 16 |
| 9 | GBM | Thalamus | STR | 2 | Diagnosis | Yes | 0 | 10 | 16 |
| 19 | GBM | Frontal | STR | 2 | None | No | 0 | 4 | 16 |
| 3 | AA | Thalamus | BX | 2 | None | Yes | 0 | 5 | 16 |
| 24 | AA | Midbrain | BX | 3 | Diagnosis | Yes | 1 | 3 | 17 |
| 8 | GBM | Cerebellum | GTR | 1 | None | No | 0 | 0 | 18 |
| 13 | AA | Temporal | STR | 2 | Diagnosis | Yes | 0 | 18* | 18 |
| 21 | GBM | Thalamus | BX | 3 | Diagnosis | Yes | 1 | 5* | 18 |
| 10 | AA | Thalamus | BX | 3 | Diagnosis | Yes | 2 | 19* | 19 |
| 5 | AA | Thalamus | BX | 1 | None | No | 0 | 1 | 19 |
| 6 | AA | FP | BX | 1 | None | No | 0 | 13 | 21 |
| 7 | AA | Occipital | STR | 2 | None | No | 0 | 0 | 21 |
| 6 | MGN | Temporal | STR | 4 | None | No | 0 | 3 | 24 |
| 9 | AA | FP | STR | 3 | None | No | 0 | 2 | 28 |
| 2 | AA | Cerebellum | GTR | 6 | Diagnosis | Yes | 0 | 1 | 31 |
| 14 | AA | Frontal | BX | 1 | None | No | 0 | 0 | 38 |
| 21 | GBM | Frontal | GTR | 3 | None | No | 0 | 6 | 41 |
| 23 | GBM | Parietal | GTR | 2 | None | No | 0 | 1 | 52 |
| 19 | AA | Temporal | GTR | 3 | None | No | 0 | 1 | 71 |
| 14 | GBM | Parietal | STR | 2 | None | No | 0 | 11 | 86 |
| 7 | GBM | Temporal | GTR | 3 | None | No | 0 | 1 | 88 |
| 13 | APXA | Parietal | GTR | 2 | None | No | 0 | 0 | Alive |
| 14 | MGN | FT | STR | 2 | None | No | 0 | 0 | Alive |
| 4 | AO | PO | GTR | 4 | None | No | 0 | 0 | Alive |
| 6 | AA | Frontal | GTR | 1 | None | No | 0 | 0 | Alive |
| 2 | AO | Frontal | GTR | 1 | None | No | 0 | 0 | Alive |
| 20 | MNC | Thalamus | GTR | 2 | None | Yes | 0 | 0 | Alive |
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodenroglioma; APXA, anaplastic pleomorphic xanthroastrocytoma; BX, biopsy only; FP, frontoparietal; FT, frontotemporal; GBM, glioblastoma; GTR, gross total resection; MGN, malignant glionneuronal tumor; MNC, malignant neurocytoma; PO, parietooccipital; STR, subtotal resection.
*These participants were unable to be weaned off steroids from the time of their diagnosis until their death.
Radiotherapy Treatment Planning
Radiation was delivered using megavoltage linear accelerators with photon energies of 6MV or 15MV. CT and MR images were used to define the target volumes and normal structures. Target volume definitions were consistent with the ICRU Report 50. The gross tumor volume (GTV) included the tumor bed, residual tumor, or both, using enhanced and nonenhanced tumor based on the T1-weighted, T2-weighted, and fluid-attenuated inversion recovery MR imaging. The clinical target volume (CTV) was defined by the gross tumor volume, which was surrounded by a 2 cm anatomically constrained margin. An additional expansion of 0.5 cm in 3 dimensions defined the planning target volume. A variety of methods were used for patient localization and verification. Participants received a dose of 59.4 Gy delivered in fractions of 1.8 Gy prescribed to the planning target volume. Radiation was delivered daily over a 6½ week period (range 54–59.4 Gy). One participant discontinued CRT after 3 fractions secondary to progressive neurological symptoms and died 2 months later after shunt failure.
Radiologic Follow-up and Assessment of Treatment Response
Participants were evaluated with neuroimaging 4–6 weeks after completion of treatment, followed by serial MR imaging every 3 months after completion for the first 2 years and every 6 months thereafter for an additional 5 years. Tumor progression was based upon neuroimaging. Local failure was defined as either a pathologically proven recurrence within the treatment field or a combination of clinical characteristics and image. Distant failure was defined as evidence of recurrence within the cerebrospinal fluid (CSF) or craniospinal axis, separate from the initial radiation treatment volume.
Evaluation of Endocrinopathy
Prior to CRT, baseline endocrine abnormalities were assessed using screening studies and provocative testing of hypothalamic-pituitary function. Participants receiving corticosteroids at the time of initial evaluation did not undergo testing. Serum was obtained for TSH, T4, T3, free T4, cortisol, prolactin, IGF-1, IGF-BP, LH, FSH, testosterone, and estradiol (when applicable). The following tests were administered: L-dopa test for growth hormone secretion, TSH surge and TRH stimulation test for evaluation of the hypothalamic-thyroid axis, 1 μg ACTH test or the metyrapone test for assessment of ACTH reserve, and GnRH stimulation test to determine gonadotropin response. These evaluations were performed at baseline and at 6, 12, 36, and 60 months after treatment, and screening studies were completed when appropriate.
Assessment of Hearing Effects
Participants underwent intermittent testing to assess the integrity of the conductive mechanism with a pure tone audiogram. Air conductions of 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz were evaluated in dB HL. Permanent hearing loss (HL) was defined as 2 consecutive hearing threshold measurements, 6 months apart, that were >25 dB HL. Participants were evaluated annually.
Assessment of Neurocognitive Effects
Prior to CRT, participants were referred for neuropsychological testing. This study included age-appropriate assessment of the following areas: global intellectual ability, academic achievement, social-emotional adjustment, and adaptive functioning.11–18 The measure of global intellectual ability used in this study was estimated based on the Information, Similarities, and Block Design subtests from the age-appropriate Wechsler Scale14–17 using a formula presented by Sattler.19 This method for estimating IQ correlates highly with IQ scores derived from the full assessment (r = 0.93). Neuropsychological testing was performed prior to CRT, 6 months post CRT, and annually thereafter if the participant was alive and cognitively intact to complete the evaluations.
Statistical Analysis
The Kaplan-Meier method was used to estimate the distribution of progression-free survival (PFS) and overall survival (OS).20,21 PFS was measured from initiation of CRT to any type of progression. OS was measured from the initiation of CRT to the time of death. The log-rank test was used to compare differences between survival curves of subgroups. The Fisher' exact test was used to test dependence of 2 categorical variables in Table 1. Mixed-effect modeling was used to estimate change in neuropsychological measures over time. All analyses were done using SAS version 9.2. The significance level for statistical tests was P < .05.
Results
Disease Control and Survival Outcomes
Median follow-up including all participants was 18 months (range, 2–134 months). At the time of last follow-up, disease progression was identified in 26 (76%) participants, with median time to progression of 9 months (range, 3–48 months). Local failure occurred in 20 (59%) participants and distant failure in 6 (18%). Three participants experienced recurrence at a new site within the brain: 2 experienced neuraxis dissemination, and one participant had an isolated spinal metastasis. The median OS was 16 months (range, 3–88 months). At last follow-up, 28 (82%) participants had died: 26 died from disease progression, one died after shunt failure, and one died after a seizure. Among the 6 survivors, the following tumor subtypes were noted: anaplastic pleomorphic xanthroastrocytoma, anaplastic oligodendroglioma (n = 2), malignant neurocytoma, malignant glioneuronal tumor, and anaplastic astrocytoma.
Local failure-free survival at 1 year was estimated at 59.6% ± 8.6%. PFS and OS estimates at 10 years were 18.8% ± 6.9% and 16.8% ± 6.5%, respectively. (Supplementary data 1) Factors significant in the analysis of PFS included histological subtype (P = .0016), hydrocephalus (P = .0003), CSF shunt requirement (P = .0096), and extent of resection (P = .0031). Those who had classical HGG subtypes of glioblastoma multiforme and anaplastic astrocytoma had inferior outcomes compared with those diagnosed with other high-grade subtypes. (Supplementary data 2).
Hydrocephalus, Shunt Placement, Surgical Procedures, and Neurological Deficits
Twelve participants (35%) were found to have hydrocephalus at the time of diagnosis, and 11 initially required CSF shunt placement. During the time interval of this study, 14 (41%) participants required CSF shunt placement, 7 (21%) required shunt revisions, and 3 required additional revisions. Participants with CSF shunts required a more protracted course of steroids at diagnosis. All 6 of the long-term survivors did not present with hydrocephalus, nor did they require CSF shunt placement. Macroscopic gross-total resection with no evidence of residual tumor on postoperative neuroimaging was performed in 38% of participants, near-total resection (minimal residual disease) in 3%, subtotal resection in 32%, and biopsy only in 29%. The mean number of surgeries was 2.4 (range, 1–6), and 14 (41%) participants had multiple surgical procedures, which were most commonly performed at the time of tumor progression. Fourteen (41%) participants presented with seizures at the time of diagnosis, and 5 (15%) developed new-onset seizures after initial surgical intervention. Twenty (59%) participants had some degree of hemiparesis, and 23 (68%) had documented cranial nerve deficits prior to CRT. After CRT, 3 (9%) participants underwent resection to remove necrotic tissue. One participant was diagnosed with a dysembryoplastic neuroepithelial tumor 9 years after CRT and was treated by topectomy for intractable seizures.
Endocrinopathy
Provocative endocrine testing was performed at baseline for 23 participants. This revealed that 35% had peak growth hormone (GH) values <10 ng/mL, and 13% had peak GH values <7 ng/mL. Five of the 6 remaining surviving participants (83%) developed GH deficiency; however, only one participant received GH replacement therapy. Among those who survived at least 2 years, the incidence of hormone replacement was determined: GH (0%), thyroid hormone replacement (36.4%), glucocorticoid replacement (45.5%), and gonadotropin-releasing hormone (0%). Table 3 depicts a summary of participants who developed hormonal deficiencies. Hypothalamic-pituitary-axis (HPA) dysfunction was noted in 5 (25%), 11 (55%), 12 (60%), and 13 (65%) participants at baseline and 1, 2, and 4 years, respectively, 12 of whom required hydrocortisone replacement. At the time of initial diagnosis, 28 (82%) participants had documented requirement for dexamethasone. Fifteen (44%) participants received dexamethasone therapy during radiation. On average, the length of steroid use was 6 months (range, 0–19 months). A total of 8 (24%) participants could not be weaned from dexamethasone prior to death and were treated for a median of 12 months (range, 5–19 months). A total of 8 of 18 (44%) participants had abnormal metyrapone testing, 3 of whom are still living today. Four participants developed hypogonadism 3, 6, 37, and 76 months after enrollment. Two participants required sex hormone replacement within 2 years of radiotherapy.
Table 3.
Summary of hormonal deficiencies over time*
| Baseline n (%) | 1 year n (%) | 2 year n (%) | 4 year n (%) | Received HRT n | |
|---|---|---|---|---|---|
| Growth hormone (n = 26) | 4 (15%) | 9 (35%) | 11 (42%) | 13 (50%) | 1 |
| HPT Axis (n = 26) | 3 (12%) | 6 (23%) | 6 (23%) | 7 (27%) | 7 |
| HPA Axis (n = 20) | 5 (25%) | 11 (55%) | 12 (60%) | 13 (65%) | 12 |
| GNRH (n = 26) | 0 | 2 (8%) | 2 (8%) | 3 (12%) | 2 |
Abbreviations: HPT, hypothlamic-pituitary-thyroid axis; HPA, hypothalamic-pituitary-adrenal axis; GNRH, gonadotropin releasing hormone.
*No documentation of patients requiring DDAVP.
Evaluation of Hearing Loss
Hearing evaluation was performed on 31 (91%) participants. Median follow-up was 12 months (range, 0–108 months). A total of 6 participants had evidence of hearing deficiency at levels >25 dB. Four participants had a single documentation of hearing deficits levels >25 dB with no additional follow-up; they died 6, 10, 11, and 14 months after the last evaluation. Hearing loss was recorded at baseline for one participant and 12 months for the others. Two participants had high frequency (6000–8000 Hz) hearing loss at baseline and 6 months; one had undergone 2 previous surgical resections, and the other had undergone 3 previous surgical resections. Only 6 participants had follow-up >3 years. None of the 6 living participants had documented hearing deficiencies at any frequency.
Evaluation of Neurocognitive Effects
Thirty-two (94%) participants underwent formal baseline neurocognitive testing. Prior to CRT, 40% presented with estimated intellectual ability (ESIQ) that fell below a score of 85. Closer inspection of scores revealed that 2 (6%) participants had superior range (score ≥120) standard scores, 3 (9%) had high-average (110–119) standard scores, 12 (38%) had average-range (90–109) standard scores, 7 (22%) had low-average (80–89) standard scores, 7 (22%) had borderline-range (70–79) standard scores, and one (3%) had an extremely low-range (≤69) standard score.
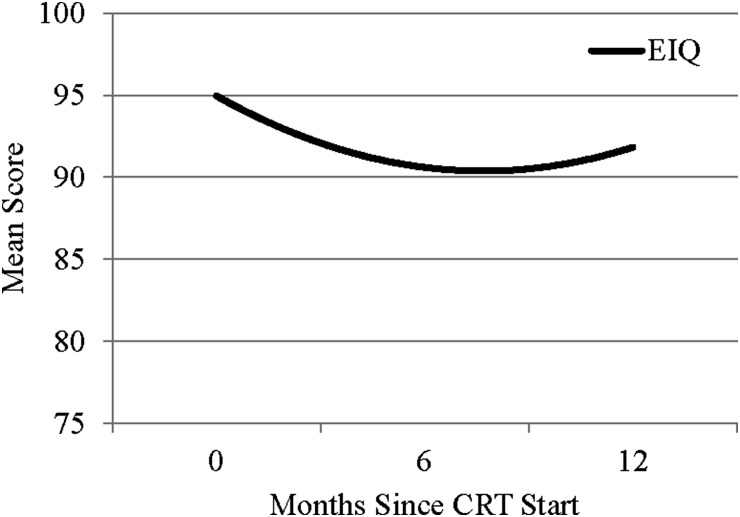
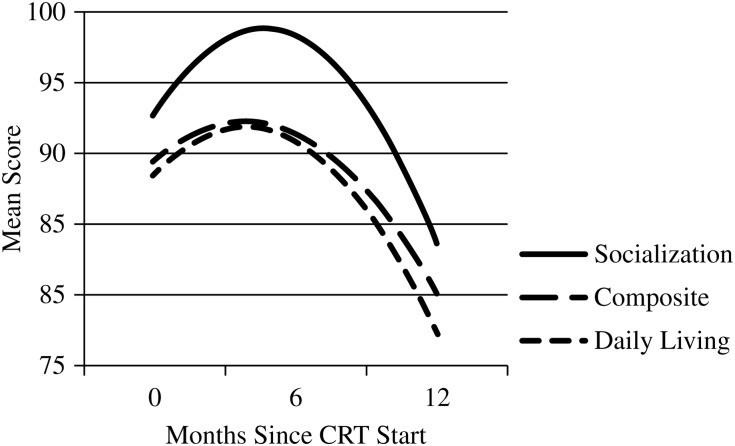
Twenty-three (68%) participants provided at least 2 measurements of ESIQ by the 12-month evaluation time. Mixed-effects modeling indicated a significant quadratic effect of evaluation time on ESIQ. The linear effect was a loss of 1.2 points per month (P = .0097), and the quadratic effect was an increase in 0.08 points per month (P = .0454), indicating that mean estimated ESIQ declined between baseline and the 6-month evaluation and then increased slightly at the 12-month evaluation (Fig. 1). Twenty participants provided at least 2 measurements of adaptive functioning. Modeling also indicated a significant quadratic effect of evaluation time on adaptive functioning including measures of daily living, socialization, and the composite score. The mean estimates increased between baseline and the 6-month evaluation and then decreased significantly by the 12-month evaluation (Fig. 2). Respectively, the linear and quadratic terms were 1.68 points/month and −0.22 points/month for daily living (P = .0155), 2.59 points/month and −0.28 points/month for socialization (P = .0049), and 1.39 points/month and −0.18 points per month for the composite score (P = .0257). There were no other significant changes on the other neuropsychological measures.
Fig. 1.
Mean estimated IQ (EIQ) during the first 12 months after conformal radiation therapy (CRT) for children with high-grade glioma.
Fig. 2.
Statistically significant nonlinear models of adaptive behavior during the first 12 months after conformal radiation therapy (CRT).
Only 5 (15%) participants were able to undergo long-term neurocognitive testing. The youngest participant completed RT at 2 years of age and is now a 12-year old special-education student with an ESIQ of 58 as assessed 5 years after enrollment. The second participant completed RT at 4 years of age with a baseline ESIQ of 104, and is currently a student with support from an Individualized Education Program (IEP). This participant has an ESIQ of 96 as assessed 10 years post RT. The third participant was treated with RT at the age of 6 with a baseline ESIQ of 92. This participant is now a student with IEP support who achieved an ESIQ of 76 10 years post RT. The fourth participant with a baseline ESIQ score of 116 underwent RT at the age of 13. Her ESIQ of 11.5 years after completion was 116, and she currently works as a nursing assistant. One participant who was treated at 20 years of age had a baseline ESIQ of 74 and is now unemployed with an ESIQ of 84 assessed 5 years after completion of RT. The fifth participant with a baseline ESIQ of 82 was treated at the age of 14 years, is currently unemployed, and achieved an ESIQ of 90 at 7 years post RT. He was the only survivor who underwent subtotal resection, while all others achieved gross total resection (GTR).
Discussion
Treatment of HGG continues to be a challenge for pediatric CNS tumors. Cognitive effects, endocrine abnormalities, growth, and developmental abnormalities associated with irradiation could not be definitively described for these participants since most did not survive. CRT has emerged as an attractive method to treat brain tumors because it maintains disease control and minimizes potential late toxicities. This study demonstrates that the use of CRT in the treatment of pediatric HGG results in the expected rate of disease control. Local progression continues to be a predominant cause for treatment failure, but the extent to which the treatment volume can be reduced is largely limited by pathological tumor characteristics. Our participants were treated on the basis of an institutional diagnosis, which appeared to be largely accurate for those diagnosed with AA and GBM; those diagnosed with other tumor types may have an outcome that is less concordant, leaving open the question about treating such diagnoses as high-grade tumors and using large CTV margins or concurrent or postirradiation chemotherapy.
Modern neuroimaging to identify tumor extent has been previously evaluated.22,23 Hochberg and Pruitt reviewed autopsy records of patients with HGG and correlated them with CT imaging. Eighty-one percent of the patients failed within 2 cm of the primary site.24 A series from the University of Michigan evaluated 42 patients with recurrent grade III-IV gliomas after treatment with resection and RT (CT contrast + 3 cm to 45 Gy and CT contrast + 1.5 cm to 60 Gy). All patients experienced disease recurrence within 2 cm of the initial contrast enhancement. In 67% of patients, the entire recurrence fell within 2 cm margin.25 Similar confirmations have been made using CT and MR imaging sequences and correlating with stereotactic biopsy-proven tumor involvement.26 Three participants (9%) in our series developed leptomeningeal/spinal failure, which is similar to rates reported in the adult literature.27–29
The low rates of disease control in this study may be attributable to the fact that many participants had substantial residual disease after surgery. Twenty-nine percent of participants had biopsy only, 32% of participants underwent subtotal resection, and only 38% achieved a GTR. The extent of surgical resection is one of the most important clinical prognostic factors in children with supratentorial HGG and demonstrates improved survival in those achieving >90% surgical resection.7,30 The extent of surgical resection as a prognostic variable on outcome has previously been reported in CCG-943 and echoed the impact of improved survival in these participants.31 Participants who did not have a GTR had a 5-year PFS rate of 11% (±4%). Similar to our findings, significant differences were described in survival between those who were able to achieve a GTR when compared with those who could only undergo subtotal resection or diagnostic biopsy.
Prior to initiation of RT, children with HGG often suffered significant sequelae from tumor and surgery. In this series, 40% of the participants presented with baseline estimated IQ scores below the average range. Participants exhibited an initial decline in estimated IQ at the 6-month follow-up that appeared to rebound by the 12-month follow-up. Conversely, participants exhibited an initial increase in adaptive functioning at the 6-month follow-up and then a decline by the 12-month follow-up. One of the limitations of this study was the limited follow-up and ability to associate clinical factors with neurocognitive decline. Because of premature death or cognitive devastation from tumor and treatment-related morbidity, successful neurocognitive testing was unobtainable for most participants. Most HGGs originate within the supratentorium, with ∼30%–50% located within the cerebral hemispheres, while the remainder arise from the basal ganglia, third ventricle, thalamus, and hypothalamus.32 Hydrocephalus and shunt placement as a consequence of tumor obstruction have been well reported as risk factors for developing adverse cognitive effects.33,34 A previous study at St. Jude Children's Research Hospital documented serial ventricular measurements of 59 children treated for ependymoma and evaluated the effect of hydrocephalus and shunt placement on cognition.35 Participants with higher ventricular measurement scores were more likely to have decreased IQ at the initiation of RT. The greatest improvement in IQ scores after RT was seen in participants with more extensive ventricular size at presentation and correlated with changes in ventricular measurements. Participants who required shunt placement had significantly lower IQ scores compared with those who did not undergo shunt placement. Previous series have demonstrated the impact of surgical morbidity on late neurocognitive function. A series by Merchant et al.36 evaluating CRT in 28 children diagnosed with craniopharyngioma revealed the negative impact of numerous surgical procedures, presence of hydrocephalus, shunt placement, and the need for revisions on IQ results. Participants who underwent 2–3 surgical procedures experienced a statistically significant decline in IQ during RT. Unfortunately, the invasiveness of surgery and the effects of radiation impact long-term cognitive function in pediatric patients with HGG. However, better outcomes have been demonstrated with those able to achieve a greater maximal safe resection. Patients with minimal surgical morbidity who have a stronger cognitive function prior to the initiation of RT are more likely to be spared the detrimental decrease in IQ scores as outcome toxicity. Hoppe-Hirsch et al.37 estimated how the extent of surgical resection and the volume of irradiated brain influenced cognitive outcomes in children treated for malignant tumors of the posterior fossa. At 1–2 years after surgery, 70–80% of the children maintained an IQ >90 when no operative complications occurred compared with 20–40% for those who experienced surgical complications including elevated intracranial pressure, neurological deterioration from preoperative status, meningitis, etc. A similar pattern was seen in the surviving participants in our study; those with intact cognitive function at the start of RT seemed to preserve function over time. However, one participant treated at 2 years of age could not complete any neurocognitive evaluations until 4 years after enrollment. This emphasizes not only the impact of RT but also the potential detriment of initial tumor involvement, surgical intervention, and supratentorial tumor location.
The present study included assessments of global intellectual ability, academic achievement, social-emotional/behavioral functioning, and adaptive functioning. Our results indicated that only global intellectual ability and adaptive functioning changed significantly over time. No significant change occurred on measures of academic or social-emotional/behavioral functioning. Although other areas of neurocognitive function are important and certainly worthy of study. At the time this study was initiated (1997), the literature on the importance of examining more specific areas of neurocognitive functioning was not available, and the neurocognitive battery was designed to be short and manageable for patients who were recently diagnosed and treated. Future studies may include a more comprehensive neurocognitive battery. Nevertheless, the present study results are noteworthy in that they examine functioning in the HGG patient population at baseline (prior to CRT) and then follow up with the 23 survivors again at 6 and 12-months post CRT, providing information on early patterns of change over time. Unfortunately, further longitudinal analyses were not possible due to the small number of survivors.
Several series have demonstrated that histology grade influences outcome, with WHO grade III (AA) tumor patients having superior outcomes to patients with WHO grade IV (GBM) tumors.7,30,38 In the current series, the majority of participants had a pathological diagnosis of AA or GBM. Finlay and colleagues published results of a phase III trial evaluating chemotherapy regimens in children with high-grade glioma; 5-year estimates were 33% and 36% for PFS and overall survival (OS), respectively.7 However, when evaluating outcomes in patients with AA and GBM, those with other eligible diagnoses experienced an estimated 5-year PFS of 64% and OS of 71%, which was significantly greater than the other 2 classifications (P < .02). In CCG 943, 5-year PFS for patients with AA was 28% (SE = 7%) and 16% (SE = 17%) for patients with GBM.29 In our current series, the 6 remaining participants had histologies of anaplastic pleomorphic xantroastrocytoma (n = 1), malignant neurocytoma (n = 1), malignant glioneuronal (n = 1), AA (n = 1), and anaplastic oligodendroglioma (n = 2). These participants may be eligible for smaller treatment volumes than the standard 2 cm CTV expansion, thus further diminishing radiation dose to normal structures without compromising tumor control.
Neuroendocrinological sequelae of radiation therapy have been studied extensively. Growth hormone deficiency (GHD) is one of the more common neuroendocrinological sequelae, developing after doses as low as 18 Gy and increasing the risk with doses 40–60 Gy.39,40 GHD has been implicated in growth failure, decreased bone density, and effect on cognition, social withdrawal, and memory. By 4 years from the time of enrollment, 13 of our 23 (56%) participants had documented GHD; however, only one participant was treated with hormone replacement therapy (HRT). This was mostly attributed to parental discretion and concern for tumorigenesis. In this study, there were a significant number of participants with hypothalamic-pituitary-adrenal (HPA) dysfunction, with 5 (25%) participants requiring replacement prior to CRT and 12 of 20 participants (60%) requiring replacement by 2 years, as determined by provocative testing. An additional 8 (24%) participants could not undergo provocative testing because of inability to be weaned from dexamethasone initiated at presentation, with a median use of 12.5 months (range 5–18 months). Our previous report evaluating late effects of CRT in 50 participants with low-grade glioma revealed similar patterns of endocrine dysfunction; however, the rates of HPA requiring HRT were much lower. Only 7 (14%) participants were deficient at baseline, and 11 (22%) required HRT at 2 years.6 This could be attributed to multiple factors including differences in tumor extent, radiation dose, longer treatment volumes, number of surgical interventions, and iatrogenic adrenal insufficiency from prolonged steroid use initiated to provide temporary relief from increased intracranial pressure and tumor-related edema. Withdrawal from steroids results in headache, lethargy, nausea, and other symptoms associated with adrenal insufficiency. Prolonged intake of glucocorticoids has been associated with adrenal gland hypotrophy, which may require recovery of several months following discontinuation of exogenous steroids.41 Adrenal suppression with its associated withdrawal symptoms has also been documented in 64 children with acute lymphoblastic leukemia who were treated with high-dose steroid, with adrenal suppression being present in 81% following last dose and achieving adrenal function within 10 weeks of discontinuation.42 Marked hypocortisolism requires hydrocortisone substitution in order to mimic circadian cortisol secretion.43 Close monitoring of these children is critical because they are at great risk for developing adrenal suppression, which would decrease their ability to mount an appropriate response in times of both mental and physical stress.
Conclusion
Conformal radiotherapy for childhood HGG represents an effective treatment modality with acceptable toxicity. Most failures occur within the high-dose region of the radiation volume, suggesting that improved brain-directed therapies are needed. Extent of resection, shunt status, and histopathological diagnosis are significant prognostic variables for survival. Although most succumb to their disease, these children are at risk early in their clinical course for developing adrenal insufficiency and other hormonal and neurocognitive effects, necessitating close monitoring and support.
Supplementary Material
Funding
Supported in part by the National Cancer Institute Cancer Center Support Grant No. CA21765, the American Cancer Society Research Project Grant No. RPG-99-252-01-CCE, and the American Lebanese Syrian and Associated Charities (ALSAC).
Supplementary Material
Acknowledgments
Conflict of interest statement. None of the authors of this manuscript has an actual or potential conflict of interest in the preparation or publication of this manuscript.
References
- 1.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Third Edition. World Health Organization; 2000. [Google Scholar]
- 2.National Cancer Institute. US population data. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1990-2007) DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2013. released November 2009. [Google Scholar]
- 3.CBTRUS Statistical Report. Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. 2011. [DOI] [PMC free article] [PubMed]
- 4.Louis DN, Ohgaki H, Wiesteler OD, et al. WHO Classification of Tumours of the Central Nervous System. Albany, NY: WHO Publication Center; 2007. [Google Scholar]
- 5.Duffner PK, Krischer JP, Burger PC, et al. Treatment of infants with malignant gliomas: the Pediatric Oncology Group experience. J Neurooncol. 1996;28(2–3):245–256. doi: 10.1007/BF00250203. [DOI] [PubMed] [Google Scholar]
- 6.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol. 1995;13(1):112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 8.Broniscer A, Chintagumpala M, Fouladi M, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol. 2006;76(3):313–319. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 9.Finlay JL, Zacharoulis S. The treatment of high grade gliomas and diffuse intrinsic pontine tumors of childhood and adolescence: a historical - and futuristic - perspective. J Neurooncol. 2005;75(3):253–266. doi: 10.1007/s11060-005-6747-7. [DOI] [PubMed] [Google Scholar]
- 10.Salcman M, Scholtz H, Kaplan RS, et al. Long-term survival in patients with malignant astrocytoma. Neurosurgery. 1994;34(2):213–219. doi: 10.1227/00006123-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 12.Albers CAGA. Test Review: Bayley Scales of Infant and Toddler Development. J Psychoeduc Assess. Third Edition. 2007;25(2):180–190. [Google Scholar]
- 13.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Services; 1984. [Google Scholar]
- 14.Wechsler D. The Wechsler Adult Intelligence Scale - Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 15.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence - Revised. San Antonio: The Psychological Corporation; 1989. [Google Scholar]
- 16.Wechsler D. The Wechsler Intelligence Scale for Children. Third Edition. New York: The Psychological Corporation; 1991. [Google Scholar]
- 17.Wechsler D. The Wechsler Individual Achievement Test. New York: The Psychological Corporation; 1992. [Google Scholar]
- 18.Woodcock RW, Johnson MB. Woodcock-Johnson tests of cognitive ability- Revised. New York: Riverside Publishing Co; 1989. [Google Scholar]
- 19.Sattler JH. Assessment of Children. Third Edition. San Diego: Jerome Sattler Publishing Inc; 1992. [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. An Stat. 1988;16(3):1141–1154. [Google Scholar]
- 21.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 22.Bashir R, Hochberg F, Oot R. Regrowth patterns of glioblastoma multiforme related to planning of interstitial brachytherapy radiation fields. Neurosurgery. 1988;23(1):27–30. doi: 10.1227/00006123-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Halperin EC, Bentel G, Heinz ER, et al. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989;17(6):1347–1350. doi: 10.1016/0360-3016(89)90548-8. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 25.Liang BC, Thornton AF, Jr., Sandler HM, et al. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75(4):559–563. doi: 10.3171/jns.1991.75.4.0559. [DOI] [PubMed] [Google Scholar]
- 26.Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 27.Arita N, Taneda M, Hayakawa T. Leptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcome. Acta Neurochir (Wien) 1994;126(2–4):84–92. doi: 10.1007/BF01476415. [DOI] [PubMed] [Google Scholar]
- 28.Jung T-Y, Jung S, Kim I-Y, et al. High grade gliomas: neuraxis dissemination pattern and prognosis. Neurosurg Q. 2007;17(3):151–155. [Google Scholar]
- 29.Lindsay A, Holthouse D, Robbins P, et al. Spinal leptomeningeal metastases following glioblastoma multiforme treated with radiotherapy. J Clin Neurosci. 2002;9(6):725–728. doi: 10.1054/jocn.2002.1079. [DOI] [PubMed] [Google Scholar]
- 30.Wolff JE, Gnekow AK, Kortmann RD, et al. Preradiation chemotherapy for pediatric patients with high-grade glioma. Cancer. 2002;94(1):264–271. doi: 10.1002/cncr.10114. [DOI] [PubMed] [Google Scholar]
- 31.Rutten EH, Kazem I, Slooff JL, et al. Post operative radiation therapy in the management of brain astrocytomata-retrospective study of 142 patients. Int J Radiat Oncol Biol Phys. 1981;7(2):191–195. doi: 10.1016/0360-3016(81)90436-3. [DOI] [PubMed] [Google Scholar]
- 32.Wolff JCP. Astrocytic Tumors, High Grade. London: Arnold: 2004. [Google Scholar]
- 33.Ralph K, Moylan P, Canady A, et al. The effects of multiple shunt revisions on neuropsychological functioning and memory. Neurol Res. 2000;22(1):131–136. doi: 10.1080/01616412.2000.11741049. [DOI] [PubMed] [Google Scholar]
- 34.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Med Pediatr Oncol. 2003;40(1):26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- 35.Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101(2 Suppl):159–168. doi: 10.3171/ped.2004.101.2.0159. [DOI] [PubMed] [Google Scholar]
- 36.Merchant TE, Kiehna EN, Kun LE, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(2 Suppl):94–102. doi: 10.3171/ped.2006.104.2.5. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst. 1995;11(6):340–345. doi: 10.1007/BF00301666. [DOI] [PubMed] [Google Scholar]
- 38.Sposto R, Ertel IJ, Jenkin RD, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7(2):165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 39.Merchant TE, Goloubeva O, Pritchard DL, et al. Radiation dose-volume effects on growth hormone secretion. Int J Radiat Oncol Biol Phys. 2002;52(5):1264–1270. doi: 10.1016/s0360-3016(01)02788-2. [DOI] [PubMed] [Google Scholar]
- 40.Sklar CA, Constine LS. Chronic neuroendocrinological sequelae of radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1113–1121. doi: 10.1016/0360-3016(94)00427-M. [DOI] [PubMed] [Google Scholar]
- 41.Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol. 2010;23(6):597–602. doi: 10.1097/WCO.0b013e32833e5a5d. [DOI] [PubMed] [Google Scholar]
- 42.Einaudi S, Bertorello N, Masera N, et al. Adrenal axis function after high-dose steroid therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50(3):537–541. doi: 10.1002/pbc.21339. [DOI] [PubMed] [Google Scholar]
- 43.Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23(2):167–179. doi: 10.1016/j.beem.2008.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.