Abstract
BACKGROUND
The importance of human epidermal growth factor receptor 2 (HER2) as a prognostic and predictive marker in invasive breast cancer is well established. Accurate assessment of HER2 status is essential to determine optimal treatment options.
METHODS
Breast cancer tumor tissue samples from the VIRGO observational cohort tissue substudy that were locally HER2-negative were retested centrally with both US Food and Drug Administration (FDA)-approved immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assays, using FDA-approved assay cutoffs; results were compared.
RESULTS
Of the 552 unique patient samples centrally retested with local HER2-negative results recorded, tumor samples from 22 (4.0%) patients were determined to be HER2-positive (95% confidence interval [CI] = 2.5%-5.7%). Of these, 18 had been tested locally by only one testing methodology; 15 of 18 were HER2-positive after the central retesting, based on the testing methodology not performed locally. Compared with the 530 patients with centrally confirmed HER2-negative tumors, the 22 patients with centrally determined HER2-positive tumors were younger (median age 56.5 versus 60.0 years) and more likely to have ER/PR-negative tumors (27.3% versus 22.3%). These patients also had shorter median progression-free survival (6.4 months [95% CI = 3.8-15.9 months] versus 9.1 months [95% CI = 8.3-10.3 months]) and overall survival (25.9 months [95% CI = 13.8-not estimable] versus 27.9 months [95% CI = 25.0-32.9 months]).
CONCLUSIONS
This study highlights the limitations of employing just one HER2 testing methodology in current clinical practice. It identifies a cohort of patients who did not receive potentially efficacious therapy because their tumor HER2-positivity was not determined by the test initially used. Because of inherent limitations in testing methodologies, it is inadvisable to rely on a single test to rule out potential benefit from HER2-targeted therapy. Cancer 2014;120:2657–2664.
Keywords: breast cancer, human epidermal growth factor receptor 2, immunohistochemistry, fluorescence in situ hybridization, local and central HER2 testing, discordance rate
INTRODUCTION
Overexpression or amplification of human epidermal growth factor receptor 2 (HER2) is reported in 15% to 20% of breast cancers,1,2 and its importance as a prognostic/predictive marker in invasive breast cancer is well established.1,3,4 Prior to the introduction of trastuzumab in the metastatic setting, HER2-positive breast cancer was associated with more aggressive disease with a poorer prognosis than HER2-negative breast cancer.1–3,5,6 In women with early-stage HER2-positive breast cancer, trastuzumab, in addition to standard adjuvant chemotherapy, significantly improves disease-free survival and overall survival (OS).7–10 Use of trastuzumab in the adjuvant setting has been projected to prevent more than 55,000 recurrences over 25 years in the United States.11 In addition, data from randomized clinical trials have consistently shown that treatment of HER2-positive metastatic breast cancer (MBC) with HER2-targeted agents (trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine [T-DM1]) has improved clinical outcomes.6,12–16 Thus, accurate determination of tumor HER2 status is critical in identifying patients most likely to benefit from HER2-directed therapy, and in avoiding use of unwarranted or potentially detrimental therapy.17–19
HER2 testing is typically performed using either immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). IHC evaluates receptor protein expression on the cellular surface and FISH assesses HER2 gene copy number in the nucleus. IHC is scored semiquantitatively as 0, 1+, 2+, or 3+, based on staining intensity, pattern, and percentage of tumor cells stained.20 FISH directly examines HER2 gene amplification by use of fluorescent-labeled nucleic acid probes to enumerate the average number of HER2 gene copies within the nuclei of tumor cells, and is usually scored as a ratio of HER2 signal to the copy number of chromosome 17 (CEP17).20 According to the American Society of Clinical Oncology (ASCO)–College of American Pathologists (CAP) HER2 testing guidelines, either test can be used in the determination of HER2 status.20,21 Disagreement as to the most appropriate HER2 test or test algorithm exists, and the results of both tests can be affected by technical issues. Several reports have noted discordance between HER2 test results from local versus large reference laboratories in patients with locally determined HER2-positive breast cancer evaluated for trastuzumab-based clinical studies.22–25
Most prior studies reporting testing results for patients with HER2-positive breast cancer have discussed false-positive results observed, based on central confirmatory testing. In contrast, there are few published data on the false-negative rate obtained after examining the concordance of central and local testing results on locally determined HER2-negative breast cancer. A better understanding of this aspect of HER2 testing accuracy is needed, because patients whose tumors are incorrectly diagnosed as HER2-negative may forgo the opportunity to potentially benefit from treatment with HER2-directed therapies.
VIRGO is a large, disease-based, observational cohort study that enrolled 1267 women with primarily HER2-negative MBC and included an optional tissue-based substudy. Here, we assessed discordance between local and central HER2 testing in women with HER2-negative breast cancer in the tissue substudy.
MATERIALS AND METHODS
VIRGO Study Design
VIRGO is a multicenter, prospective observational cohort study of 1267 women with HER2-negative MBC enrolled between June 2008 and January 2011. Two cohorts were included: patients with HER2-negative MBC who were treated with first-line chemotherapy and patients with hormone receptor–positive MBC (HER2-negative or -positive) treated with first-line endocrine therapy. The primary objective of VIRGO was to characterize clinical outcomes for various subgroups of patients with advanced breast cancer, as defined by patient clinical and tumor characteristics and treatment patterns. VIRGO also included an optional exploratory tissue-based substudy, for which separate consent for participation was obtained. Central HER2 testing of tissue samples to examine discordance was not a prespecified analysis.
Enrolled patients received treatment and evaluations for their advanced breast cancer as determined by their treating physicians, according to the standard of care and clinical practice at each study site. The VIRGO study design and protocol were approved by a central institutional review board and, when necessary, by the institutional review board at each site. All patients signed an informed consent and authorization to disclose their health information.
Data Collection
At baseline, demographic data, breast cancer–specific history, and prior cancer treatment history (chemotherapy, surgery, radiation), including metastatic sites and history and medical history, including cardiovascular risk factors, concomitant medication use, Eastern Cooperative Oncology Group performance status, selected laboratory tests, ejection fraction, or shortening fraction (if performed) were collected.
After enrollment, data elements were collected at quarterly follow-up visits and included study discontinuation status (including death), treatment status, disease response status, concomitant medication use, cardiovascular arterial disease status, interval surgical history, interval radiotherapy history, protocol-specified (selected) adverse events, and health economic data (for those who consented).
Definitions
Local HER2 testing refers to the initial testing performed on the tumor tissue samples that classified their HER2 status. Central HER2 testing refers to the testing performed in this study. HER2-positivity in central testing was defined according to FDA-approved assay cutoffs (IHC score of 3+: uniform, intense circumferential membrane staining in > 10% of invasive tumor cells; FISH+: HER2/CEP17 ratio ≥ 2.0). Notably, these testing criteria were the same as those used in the pivotal clinical trials of trastuzumab in the adjuvant HER2-positive breast cancer setting7,8,10 and are consistent with the updated 2013 ASCO/CAP HER2 testing guidelines.21
HER2 Assays
Available tissue samples were retested centrally with both FDA-approved HER2 IHC and FISH assays at a single reference laboratory (Clarient, Aliso Viejo, Calif). The IHC HercepTest kit was used to determine HER2 protein expression according to manufacturer's instructions (Dako, Carpenteria, Calif). The FISH PathVysion HER2 DNA probe kit/HER2/CEP17 probe mixture (Abbott Molecular, Des Plaines, Ill) was used to determine HER2 and CEP17 gene copy numbers. Because results obtained by this central HER2 testing could have had significant clinical implications affecting treatment decisions, after ethical consultation, study investigators whose patients were found to have tumors that tested HER2-positive centrally were informed of the results by certified mail.
Statistical Analysis
Results obtained by central retesting of tumor samples were compared with local testing results. The discordance rate and its 95% confidence interval (CI) were calculated. Tumors with unknown/missing local HER2 status were excluded from primary analyses. A sensitivity analysis was performed assessing the impact of including the patients with centrally HER2-negative tumors with unknown/missing local HER2 status, assuming their tumors to have tested HER2-negative locally, on the discordance rate.
Demographics were compared between patients with centrally HER2-positive tumors and patients with centrally HER2-negative tumors. Disease-free interval (DFI) was defined as the interval from the date of the first diagnosis of breast cancer to metastatic/recurrent diagnosis. Median progression-free survival (PFS) and OS and their 95% CIs were calculated for the 2 groups, through use of the Kaplan-Meier method.
RESULTS
Tissue Samples
Of the 1267 patients enrolled in VIRGO, 776 submitted samples to the tissue substudy. Of those, 637 unique patient samples were adequate for central retesting using both the IHC and FISH assays. Eighty-five samples were excluded from the analyses described here for reasons outlined in Fig. 1.
Figure 1.
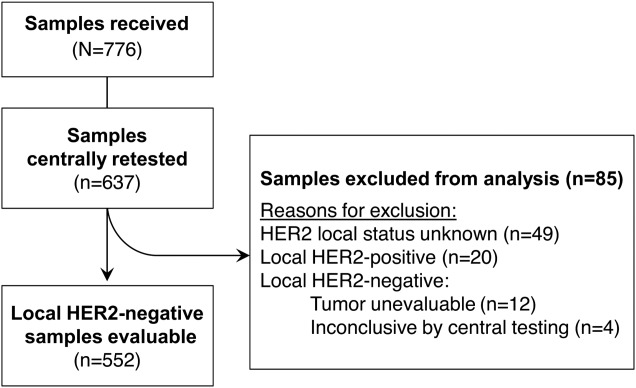
Flowchart of patient samples that were tested. Abbreviation: HER2, human epidermal growth factor receptor 2.
Clinical Results
Of the 552 locally HER2-negative unique patient samples that were tested centrally and included in the analysis, 530 were confirmed to be HER2-negative. The remaining 22 samples (4.0%; 95% CI = 2.5%-5.7%) were found to be HER2-positive by central testing. Local versus central IHC and FISH HER2 testing results for the discordant cases are shown in Table 1 and Table 2. Only one local laboratory had more than one discordant case (n = 2).
Table 1.
Summary of Discordant Cases
| Local HER2-Negative | Central HER2-Positive | |||
|---|---|---|---|---|
| IHC 3+ only | FISH+ onlya | IHC 3+ and FISH+a | Total | |
| IHC alone | 0 | 4 | 4 | 8 |
| FISH alone | 6 | 3 | 1 | 10 |
| IHC and FISH | 2 | 2 | 0 | 4 |
| Total | 8 | 9 | 5 | 22 |
Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
FISH+ defined as HER2/CEP17 ≥ 2.0.
Table 2.
Discordant Cases: HER2-Negative Locally; HER2-Positive Centrally
| Patient | Local Testing | Central Testing | ||
|---|---|---|---|---|
| HER2 IHC | HER2 FISH | HER2 IHC | HER2 FISH | |
| 1 | 1+ | N/A | 3+ | 3.7 |
| 2 | N/A | 1.1 | 3+ | 1.1 |
| 3 | N/A | 1.8 | 2+ | 2.4 |
| 4 | 2+ | 1.0 | 3+ | UE |
| 5 | 0 | 1.2 | 1+ | 4.5 |
| 6 | 1+ | N/A | 2+ | 2.1 |
| 7 | N/A | 1.0 | 3+ | 1.1 |
| 8 | 1+ | N/A | 3+ | 9.2 |
| 9 | 2+ | N/A | 3+ | 6.8 |
| 10 | N/A | 1.2 | 2+ | 3.1 |
| 11 | N/A | 1.4 | 3+ | 1.0 |
| 12 | N/A | 1.8 | 2+ | 2.1 |
| 13 | N/A | 1.0 | 3+ | 0.9 |
| 14 | 2+ | 1.5 | 3+ | 1.2 |
| 15 | 1+ | N/A | 0 | 8.7 |
| 16 | 2+ | 1.9 | 2+ | 2.1 |
| 17 | 1+ | N/A | 1+ | 2.1 |
| 18 | N/A | 1.6 | 3+ | 1.0 |
| 19 | N/A | 1.0 | 3+ | 1.0 |
| 20 | N/A | 0.3 | 3+ | 3.5 |
| 21 | 0 | N/A | 3+ | 2.3 |
| 22 | 2+ | N/A | 0 | 8.0 |
Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; N/A, not available; UE, unevaluable.
Of the 22 centrally determined HER2-positive tumors, 18 tumors were tested locally by only one testing methodology (8 by IHC alone and 10 by FISH alone). Four tumors were tested locally by both IHC and FISH. Of the 18 tumors tested by one methodology locally, 15 yielded positive results on the testing methodology not performed locally. In 10 of 15 cases, the central testing methodology not performed locally was the only HER2-positive result. Eleven of 22 centrally determined HER2-positive tumors showed discordant IHC and FISH results, with 7 IHC 3+/FISH-negative cases and 4 FISH-positive/IHC 0 or 1+ cases. All 7 IHC 3+/FISH-negative cases showed heterogeneous IHC staining with a mixture of strong, moderate, and weakly stained cells, with the strong staining component at the 10% cutoff. An example of a tumor in which there were discordant testing methodology results, potentially due to tumor heterogeneity, is shown in Fig. 2.
Figure 2.
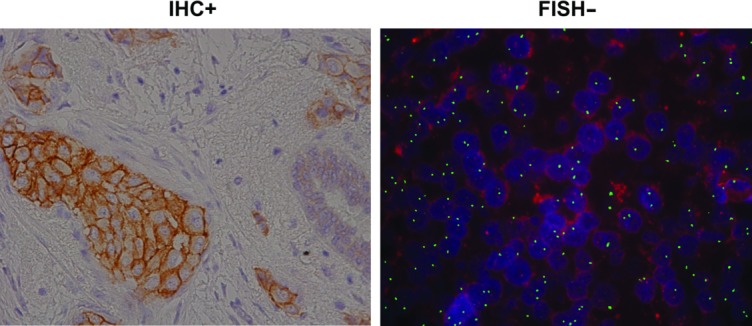
Example of discordant immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) testing results (courtesy of Clarient, Inc.).
Compared with the 530 patients with centrally HER2-negative tumors, the 22 patients found to have centrally HER2-positive tumors were younger (median age, 56.5 versus 60.0 years, respectively) and more likely to have estrogen receptor/progesterone receptor (ER/PR)–negative tumors (27.3% versus 22.3%; Table 3). Patients with centrally HER2-positive tumors were also more likely to have advanced histologic grade compared with patients with centrally HER2-negative tumors. For patients initially diagnosed with early-stage breast cancer that recurred, 18 patients with centrally determined HER2-positive tumors (15 patients with stage I through III breast cancer at time of initial diagnosis and 3 patients with unknown stage) had a median DFI of 30.4 months versus 43.1 months for the 399 recurrent patients with centrally confirmed HER2-negative tumors.
Table 3.
Demographic and Clinical Characteristics of Patient Testing Groups
| Characteristic | Local HER2-Negative, Central HER2- Positive (n = 22) | Local HER2-Negative, Central HER2-Negative (n = 530) | Local HER2- Negative (n = 1078)a |
|---|---|---|---|
| Age (median, years) | 56.5 | 60.0 | 60.0 |
| Clinical stage at first diagnosis, n (%) | |||
| I | 1 (4.5) | 80 (15.1) | 162 (15.0) |
| IIA | 4 (18.2) | 100 (18.9) | 209 (19.4) |
| IIB | 3 (13.6) | 77 (14.5) | 152 (14.1) |
| IIIA | 2 (9.1) | 56 (10.6) | 116 (10.8) |
| IIIB | 2 (9.1) | 29 (5.5) | 49 (4.5) |
| IIIC | 3 (13.6) | 33 (6.2) | 54 (5.0) |
| IV | 4 (18.2) | 118 (22.3) | 249 (23.1) |
| Unknown | 3 (13.6) | 37 (7.0) | 87 (8.1) |
| Histological grade at first diagnosis, n (%) | |||
| I | 0 | 43 (8.1) | 78 (7.2) |
| II | 6 (27.3) | 183 (34.5) | 333 (30.9) |
| III | 10 (45.5) | 221 (41.7) | 427 (39.6) |
| Unknown | 6 (27.3) | 83 (15.7) | 240 (22.3) |
| ER/PR status | |||
| ER– and PR– | 6 (27.3) | 118 (22.3) | 220 (20.4) |
| ER+ and/or PR+ | 15 (68.2) | 406 (76.6) | 833 (77.3) |
| ER unknown and PR unknown | 0 (0.0) | 6 (1.1) | 21 (1.9) |
| ER– and PR unknown | 1 (4.5) | 0 | 4 (0.4) |
| Disease-free intervalb | (n = 18) | (n = 399) | (n = 805) |
| (median, months) | 30.4 | 43.1 | 45.4 |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Includes patients not enrolled in VIRGO tissue substudy
Defined as the interval from the date of the first diagnosis of breast cancer to metastatic/recurrent diagnosis.
In the first-line MBC setting, 22 patients with centrally determined HER2-positive tumors had shorter median PFS and OS compared with 530 patients with centrally confirmed HER2-negative tumors (median PFS, 6.4 months [95% CI = 3.8-15.9 months] versus 9.1 months [95% CI = 8.3-10.3 months]; median OS, 25.9 months [95% CI = 13.8-not estimable] versus 27.9 months [95% CI = 25.0-32.9 months]).
Sensitivity Analyses
Unknown Local HER2 Status
After querying the individual study sites, 49 of 85 tissue samples excluded from these analyses had unknown local HER2 status data, likely because of protocol deviations (Fig. 1). Of these, 48 were centrally determined to be HER2-negative, and one was unevaluable by central testing. Assuming these samples to be locally HER2-negative, the discordance rate would be 3.7% (22 of 600; Table 4).
Table 4.
Sensitivity Analysis Assessing the Impact of Unknown Local HER2 Status on the Discordance Rate
| Local Testing | Central Testing | Discordance | ||
|---|---|---|---|---|
| Negative (n) | Positive (n) | Total (n) | ||
| Negative | 530 | 22 | 552 | 4.0% |
| Unknowna | 48 | 0 | 48 | N/A |
| Total | 578 | 22 | 600 | 3.7% |
Abbreviations: HER2, human epidermal growth factor receptor; N/A, not available.
Tumor tissue from 1 of 49 patients with unknown local HER2 status was unevaluable by central testing.
Tumors That Were Locally IHC 2+, But Had No FISH Testing Results
Two patients with centrally HER2-positive tumors had local IHC 2+ results, but no FISH results were reported, indicating that the local laboratory did not follow the ASCO/CAP guideline of reflex testing of IHC 2+ cases by FISH. Patient records did not reflect treatment with HER2-directed therapy at any point. If these 2 patients are excluded, the discordant rate changes to 3.6% (20 of 550).
DISCUSSION
Accurate determination of HER2 status is critical to optimize clinical outcomes in patients with breast cancer. To date, most studies reporting discordance in HER2 testing results between local and central laboratories have focused on the false-positive results. Two prospective substudies from trastuzumab adjuvant trials (NSABP B-31 and NCCTG N9831)23,24 revealed discordance rates of 18% and 26%, respectively, when local HER2-positive samples were retested centrally by IHC and FISH. After central testing became mandatory in the NCCTG N9831 trial, an expanded analysis (N = 2535) found a lower discordance rate of 12%.25 Another analysis in a community-based setting by Reddy et al determined a false-positive rate of 14% to 16% when locally tested (by IHC) tumor samples were reexamined by IHC and FISH at a high-volume, centralized laboratory.19 Because patients whose tumors tested HER2-negative locally would generally not have been referred to trastuzumab-based studies and therefore would not have undergone central retesting, few data on false-negative rates exist. One exception is the analysis by Reddy et al, in which tumor samples that were locally determined to be HER2-negative were also assessed centrally, with a false-negative rate of 18% to 23% when local IHC results were compared with central IHC and FISH results, respectively.19 However, the high false-negative rate in this study was mainly driven by IHC 2+ cases, which represent 14% to 18% of the observed rate (and which contributed to the adoption of reflex FISH testing of IHC 2+ tumors).19
The tissue substudy of the VIRGO registry provides an important current resource to investigate the accuracy of testing for HER2-negative breast cancers in the community setting. In this study that evaluated a large number of samples, we found a discordance rate of 4.0%; 22 of 552 unique tumor samples initially found to be HER2-negative by local testing were determined to be HER2-positive upon central retesting. For these 22 samples, 18 were tested locally using only one testing methodology, and 15 of 18 yielded positive results using a testing methodology not performed locally.
Patients with tumors found to be HER2-positive on central retesting had clinical characteristics (shorter DFI and PFS) consistent with those of the overall HER2-positive population. Using the discordant rate calculated in this analysis (4%) to extrapolate to the larger population of breast cancer cases, if one assumes there were 229,060 new cases of invasive breast cancer diagnosed in the United States in 2012,26 and 80% of those cases are HER2-negative based on local test results, the total number of patients potentially misdiagnosed would be 7330 (range, 4581-10,445) (Fig. 3). Based on World Health Organization global breast cancer incidence estimates,27 these results translate into 53,440 (range, 33,400–76,152) newly diagnosed patients annually worldwide who may forgo the opportunity to potentially benefit from HER2-directed therapies.
Figure 3.
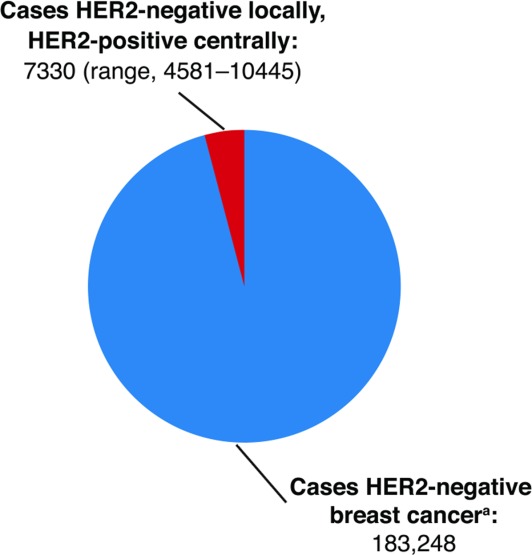
Estimated number of patients in the United States diagnosed annually with human epidermal growth factor receptor 2 (HER2)-negative tumors by local testing, but HER2-positive by central testing based on a discordance rate of 4.0%. aBased on American Cancer Society estimates of N = 229,060 new cases of breast cancer in 2012 and 80% HER2-negative.
There are several potential reasons for discordant HER2 testing results between laboratories. For example, a laboratory may not be using a properly validated assay(s) or the assay procedure may be altered from manufacturer's instructions, which can lead to inaccurate results. In addition, samples with tumor heterogeneity may lead to discrepant results.28,29 In this study, 50% of the 22 discrepant local versus central cases showed discordant centrally determined IHC and FISH results, possibly due to tumor heterogeneity. Interestingly, 6 of 7 IHC 3+/FISH-negative cases had a FISH ratio of approximately 1.0 but HER2 gene copy numbers > 2, which could be the result of polysomy or coamplification of both the HER2 gene and centromere region. Tumor heterogeneity has also been reported in other studies. For example, in a round-robin study evaluating HER2 testing of 389 tumor blocks from 3 large adjuvant trials (NCCTG N9831, BCIRG-006, and BCIRG-005) performed among 3 separate central laboratories, block-to-block heterogeneity was documented in 5% and 10% of the cases with FISH and IHC results, respectively, for cases with > 1 evaluable tumor block.30
Discordance may also arise from the inherent differences in the characteristics of IHC and FISH HER2 testing assays. IHC and FISH assays provide complementary results, each evaluating a different aspect of the biological events underlying HER2-positive cancer.31 Each assay has methodological and interpretive advantages and disadvantages that should be considered when analyzing HER2 test results. Erroneous results can stem from inadequate or overfixation, antigen retrieval, antibody sensitivity, or interpretation error.20 Preanalytic factors, including the time from tissue removal to tissue fixation, type and duration of fixation, or tissue processing, have the potential to negatively impact the consistency and reliability of HER2 testing.20,21 Rigorous quality control and standardization of the testing process are essential for achieving accurate and reproducible assay results. In the round-robin analysis described previously, the overall concordance between IHC and FISH results was 92% (343 of 373 blocks with both an adjudicated IHC and FISH result).30 In our study, of the 22 tumors yielding HER2-positive results by central retesting, there was discordance in results obtained on central IHC and FISH in 11 of the samples. At present, the clinical significance of discordance between IHC and FISH in terms of potential benefit from HER2-targeted therapy is unclear. The goal of pathologists has been to achieve a greater than 95% concordance between IHC-negative/FISH-negative and between IHC 3+/FISH-positive results. Yet, considering the curative potential of trastuzumab treatment in the adjuvant setting, a false-negative rate of 5% should be deemed disproportionate. Laboratories should endeavor to lower this percentage of false-negative tests to as close to 0% as possible.20 Although some errors would inevitably remain due to human error, many inaccuracies could be eliminated by following manufacturer's instructions, participating in laboratory proficiency testing, or retesting equivocal cases with another test or on a second tumor specimen, as recommended in the ASCO/CAP guidelines20,21 as well as retesting HER2-negative results with another test if only one test is used, as suggested by results from VIRGO.
Although central retesting of patients' tumor samples is not feasible in all cases, in this study, approximately two-thirds of the tumor samples locally determined to be HER2-negative yielded positive results centrally by the testing methodology not performed locally. A better understanding of HER2 testing discrepancies is clinically relevant, because patients who have tumors that are incorrectly diagnosed as HER2-negative are denied the potential benefit derived from treatment with HER2-directed therapies. Indeed, the Herceptin (trastuzumab) package insert highlights the importance of accurate HER2 testing by stating that relying on a single testing method to rule out potential trastuzumab benefit is inadvisable.32
The 2007 ASCO/CAP recommendations for HER2 testing algorithms endorsed either IHC testing with reflex testing by FISH for IHC 2+ cases, or FISH testing alone.20 These algorithms were recently revised to recommend consideration of repeat testing if results seem discordant with other histopathologic findings and encouraged close communication between pathologists and oncologists.21 Although the new guidelines did not go as far as recommending reflex testing with the alternative assay for all HER2-negative cases,21 a recent modeling analysis projected that retesting samples that were IHC 0, IHC 1+, and FISH-negative on their first test from women with early-stage breast cancer was a cost-effective strategy.33 The VIRGO tissue substudy data presented here further support the idea of retesting all HER2-negative cases. HER2 status may be assessed by testing either the gene or protein in each tissue sample using FISH and IHC testing, respectively; however, in the case of an initial negative test by either testing methodology, one should consider testing by the other methodology, especially in the presence of high-risk clinical or pathologic features. This would ensure that HER2-positive cases are not missed by just performing one test and would in part address the inherent limitations of currently available HER2 testing methodologies.
This substudy has certain limitations. Because of the small number of patients with centrally determined HER2-positive tumors (n = 22), data regarding clinical characteristics and outcomes of these patients are limited and should be interpreted with caution. It is also unclear whether these patients ever had the opportunity to be treated with HER2-targeted therapy and, if so, how they might have responded to such treatment. In addition, this study was performed in a population of patients with MBC, which may have resulted in a higher false-negative rate compared with patients who had early-stage breast cancer, because this high-risk population (either relapsed following an earlier diagnosis or diagnosed with de novo stage IV disease) tends to have a higher HER2-positivity rate, in general. It is also not known how the assays were performed in the local laboratories or whether the tumor block initially tested was the same as that submitted for the VIRGO tissue-based substudy. Finally, inherent limitations with tissue testing, such as tumor heterogeneity, variations in HER2 assays, and preanalytical issues, including fixation, could have affected assay performance.
Conclusions
The data from the VIRGO study highlight a cohort of patients who were denied potentially efficacious therapy due to possibly inaccurate HER2 testing, which may impact a considerable number of patients with breast cancer. Given the magnitude of potential benefit provided by HER2-directed therapy, it is essential to accurately and reliably test for HER2 status so that patients may have the opportunity to receive the most efficacious treatment for their disease.
FUNDING SUPPORT
Support for third-party writing assistance for this manuscript was provided by Genentech, Inc. The VIRGO study was funded by Genentech, Inc.
CONFLICT OF INTEREST DISCLOSURES
Drs. Kaufman, Pegram, and Vogel have received consulting fees from Genentech. Drs. Rugo, Gralow, Vogel, Swain, and Kaufman have received research funding for their institutions from Genentech. Dr. Kaufman has received honoraria from Genentech. Drs. Brammer, Chau, and Yoo are (and Dr. Lalla was) full-time employees of Genentech and own Roche stock. All other authors made no disclosure.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neo oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Estevez LG, Seidman AD. HER2-positive breast cancer: incidence, prognosis, and treatment options. Am J Cancer. 2003;2:169–179. [Google Scholar]
- 4.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Piccart-Gebhart MJ, Procter M, et al. HERA Trial: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years median follow-up. Presented at the European Society for Medical Oncology (ESMO), October 2012, Vienna, Austria.
- 10.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese MD, Lalla D, Brammer M, et al. Estimating recurrences prevented from using trastuzumab in HER-2/neu-positive adjuvant breast cancer in the United States. Cancer. 2010;116:5575–5583. doi: 10.1002/cncr.25347. [DOI] [PubMed] [Google Scholar]
- 12.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 13.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 14.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. Erratum in N Engl J Med. 2013;368:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebeau A, Turzynski A, Braun S. Reliability of human epidermal growth factor receptor 2 immunohistochemistry in breast core needle biopsies. J Clin Oncol. 2010;28:3264–3270. doi: 10.1200/JCO.2009.25.9366. [DOI] [PubMed] [Google Scholar]
- 18.Mass RD, Press MF, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 19.Reddy JC, Reimann JD, Anderson SM, et al. Concordance between central and local laboratory HER2 testing from a community-based clinical study. Clin Breast Cancer. 2006;7:153–157. doi: 10.3816/CBC.2006.n.025. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond EH, Schwartz, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 22.Dybdal N, Leiberman G, Anderson S, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93:3–11. doi: 10.1007/s10549-004-6275-8. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;11:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 24.Roche PC, Suman VJ, Jenkins RB, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002;11:855–857. doi: 10.1093/jnci/94.11.855. [DOI] [PubMed] [Google Scholar]
- 25.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 26.R Siegel, D Naishadham, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 27.WHO International Agency for Research on Cancer. Globocan: Breast cancer Estimated Incidence, Mortality and Prevalence Worldwide. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed April 7, 2014.
- 28.Davila E, Amazon K. The clinical importance of the heterogeneity of HER2 neu. Case Rep Oncol. 2010;3:268–271. doi: 10.1159/000319020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao Y, Chou M-C, Fowler C, et al. Breast cancer heterogeneity: mechanisms, proofs, and implications. J Cancer. 2010;1:6–13. doi: 10.7150/jca.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez EA, Press MF, Dueck AC, et al. Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005) Breast Cancer Res Treat. 2013;138:99–108. doi: 10.1007/s10549-013-2444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks DG, Kulkarni S. HER2+ breast cancer: review of biologic relevance and optimal use of diagnostic tools. Am J Clin Pathol. 2008;129:263–273. doi: 10.1309/99AE032R9FM8WND1. [DOI] [PubMed] [Google Scholar]
- 32.Herceptin [package insert] South San Francisco, CA: Genentech; 2010. [Google Scholar]
- 33.Garrison LP, Jr, Lalla D, Brammer M, Babigumira JB, Wang B, Perez EA. Assessing the potential cost-effectiveness of retesting IHC0, IHC1+, or FISH-negative early stage breast cancer patients for HER2 status. Cancer. 2013;119:3113–3122. doi: 10.1002/cncr.28196. [DOI] [PubMed] [Google Scholar]


