Abstract
Small interfering RNAs (siRNAs) and microRNAs (miRNAs) are processed by the ribonuclease Dicer from distinct precursors, double-stranded RNA (dsRNA) and hairpin RNAs, respectively, although either may guide RNA silencing via a similar complex. The siRNA pathway is antiviral, whereas an emerging role for miRNAs is in the control of development. Here, we describe a virulence factor encoded by turnip yellow mosaic virus, p69, which suppresses the siRNA pathway but promotes the miRNA pathway in Arabidopsis thaliana. p69 suppression of the siRNA pathway is upstream of dsRNA and is as effective as genetic mutations in A. thaliana genes involved in dsRNA production. Possibly as a consequence of p69 suppression, p69-expressing plants contained elevated levels of a Dicer mRNA and of miRNAs as well as a correspondingly enhanced miRNA-guided cleavage of two host mRNAs. Because p69-expressing plants exhibited disease-like symptoms in the absence of viral infection, our findings suggest a novel mechanism for viral virulence by promoting the miRNA-guided inhibition of host gene expression.
INTRODUCTION
RNA silencing is a generic term describing related gene silencing processes that include posttranscriptional gene silencing (PTGS) in plants, double-stranded RNA (dsRNA)–mediated RNA interference in animals, and quelling in fungi (Ding, 2000; Matzke et al., 2001; Vance and Vaucheret, 2001). A unifying feature of RNA silencing is the production of small interfering RNAs (siRNAs) that are 21 to 26 nucleotides long and of both sense and antisense polarities (Hamilton and Baulcombe, 1999; Hamilton et al., 2002; Plasterk, 2002). siRNAs are processed from dsRNA precursors by the endoribonuclease Dicer, become incorporated into the RNA-induced silencing complex that contains at least one Argonaute (AGO) protein, and guide specific cleavage of complementary mRNAs to a central position of an siRNA/mRNA duplex (Hammond et al., 2000, 2001; Bernstein et al., 2001; Elbashir et al., 2001). MicroRNAs (miRNAs) are a new class of small RNAs that may control development in animals and plants (Ambros et al., 2003). miRNAs are present in one polarity and are derived from RNA transcripts that have potential to form hairpin structures. miRNAs are also a product of Dicer and found in a complex similar if not identical to the RNA-induced silencing complex, although miRNAs may guide either cleavage or translational arrest of target mRNAs (Ambros et al., 2003).
In plants, PTGS can be induced by transgenes that encode either a highly transcribed sense RNA or an inverted repeat RNA (IR-RNA) that is folded into a long dsRNA. Several genes essential for sense RNA–induced PTGS have been cloned from Arabidopsis thaliana. These include a cellular RNA-dependent RNA polymerase (RdRP) homolog (SGS2/SDE1), AGO1, an RNA helicase gene (SDE3), and genes that encode a coiled-coil protein (SGS3) and a novel protein (HEN1) (Fagard et al., 2000; Mourrain et al., 2000; Boutet et al., 2003). Current models place these genes upstream of dsRNA because none of these genes are required for IR-RNA–induced PTGS (Dalmay et al., 2001; Beclin et al., 2002). In A. thaliana, both Dicer-like 1 (DCL1) and HEN1 are involved in the production of miRNAs (Park et al., 2002; Reinhart et al., 2002), and there is evidence that miRNAs function as siRNAs to specify cleavage of their mRNA targets (Llave et al., 2002; Rhoades et al., 2002; Kasschau et al., 2003).
A biological function for RNA silencing, first established in plants, is as an adaptive defense against RNA and DNA viruses (Covey et al., 1997; Ratcliff et al., 1997; Al-Kaff et al., 1998; Vance and Vaucheret, 2001). Recent work has demonstrated a similar antiviral role for RNA silencing in the animal kingdom (Li et al., 2002, 2004). The antiviral response may be triggered by virus-specific dsRNA intermediates produced during genome replication, by transcription from converging promoters, or through recognition of viral RNAs by host RdRP, as proposed in plants (Voinnet et al., 2000; Szittya et al., 2002). As a counterdefense, viruses encode proteins such as helper component-proteinase (HC-Pro) and Cucumber mosaic virus 2b that suppress RNA silencing at various steps in the pathway (Anandalakshmi et al., 1998; Brigneti et al., 1998; Kasschau and Carrington, 1998; Mlotshwa et al., 2002; Silhavy et al., 2002). The identification of a silencing suppression function typically provides a molecular basis for previously observed defects in viral accumulation and spread of virus mutants that lack a functional suppressor (Li and Ding, 2001; Mlotshwa et al., 2002).
Here, we report the identification of a viral suppressor of RNA silencing that also plays a unique role in the induction of viral disease. The suppressor, p69, is encoded by turnip yellow mosaic virus (TYMV), a plus-strand RNA virus prevalent both in cultivated and wild species of the Brassicaceae family of dicotyledonous plants (Morch et al., 1988; Skotnicki et al., 1992). Previous work has shown that p69 is essential for virus spread and influences viral yields and symptom severity in infected plants but is dispensable for viral replication in single cells (Bozarth et al., 1992; Tsai and Dreher, 1993). Using distinct PTGS systems with defined silencing triggers, we found that p69 represents a unique viral suppressor of RNA silencing by targeting a step upstream of dsRNA production in the cellular RdRP-dependent branch of RNA silencing. However, transgenic A. thaliana plants expressing p69 alone displayed disease-like symptoms, indicating a role of p69 in disease induction that is independent of its suppression of the host PTGS mechanism targeting viral RNAs. Further analyses revealed a significantly upregulated miRNA pathway in p69-expressing plants, including elevated levels of miRNAs and enhanced miRNA-guided cleavages of two host mRNAs that encode putative transcriptional factors. Thus, p69 is distinct to the viral PTGS suppressor HC-Pro that was recently shown to inhibit miRNA silencing (Kasschau et al., 2003). We propose that enhanced miRNA silencing is a consequence of a negative feedback regulation of the RNA silencing pathway triggered by p69 suppression and that miRNAs can play a pathogenic role in the induction of viral diseases.
RESULTS
p69 Inhibits PTGS and DNA Methylation Induced by Sense RNA Transgenes
The first indication that TYMV might encode a suppressor of RNA silencing came from TYMV infection of A. thaliana plants that carry a silencing 35S-β-glucuronidase (GUS) sense RNA transgene at the L1 locus (Elmayan et al., 1998). We found that TYMV infection of L1 plants resulted in a restored GUS expression in the newly emerged leaves as shown by histochemical staining (Figure 1B).
Figure 1.
p69 Is a PTGS Suppressor in A. thaliana.
(A) TYMV genome organization.
(B) to (D) Suppression of the GUS RNA silencing in line L1 by TYMV 2 weeks after infection (B), by a 35S-P69 transgene (C), or a TYMV amplicon transgene (D). Photos of the same plants before GUS staining are also shown in the bottom panels in (C) and (D).
TYMV contains three genes, two of which are encoded in out-of-frame overlapping open reading frames (Figure 1A). The viral RdRP and p69 are translated from the genomic RNA with their initiation codons seven nucleotides apart, whereas the coat protein (CP) is produced from a subgenomic RNA that is 3′ coterminal with the genomic RNA (Morch et al., 1988; Skotnicki et al., 1992). We predicted that the suppressor activity of TYMV was encoded by P69 based on its role in viral spread and virulence that is similar to the known viral suppressors (Bozarth et al., 1992; Tsai and Dreher, 1993; Li and Ding, 2001).
To test this possibility, the P69 gene was introduced into A. thaliana ecotype C24 under the control of the 35S promoter of Cauliflower mosaic virus. It is striking that 7 of the 11 35S-P69 transformants obtained showed developmental defects (Figure 2, left; see also Supplemental Figure 1 online). The expression of P69 was hardly detectable in the remaining four lines that were morphologically indistinguishable from either wild-type plants or any of the eight 35S-ΔP69 transformants (data not shown). The pleiotropic defects of the P69 plants cosegregated with the transgene in backcrosses with wild-type plants and included severe dwarf, pale-colored leaves with reduced cell sizes, late flowering, short siliques, and decreased fertility. For example, line 3 homozygous for a single 35S-P69 transgene (P69c) began flowering 48.5 d after sowing with 13.4 rosette leaves on average (n = 65) in contrast with wild-type plants that flowered 42.4 d with 11.8 rosette leaves (n = 61) under our growth conditions. The developmental defects observed in the P69 plants mimicked, but were more severe than, the disease symptoms found in wild-type plants systemically infected with TYMV (Figure 2, right). Thus, p69 alone is able to confer pathogenicity independent of TYMV infection, further extending the finding by Tsai and Dreher (1993).
Figure 2.
p69-Conferred Virulence in A. thaliana.
Transgenic line P69c (left) expressing P69 causes pleiotropic developmental defects that resemble the phenotype displayed by a plant infected systemically with TYMV (right).
Further analyses confirmed that p69 is a potent suppressor of sense RNA–induced RNA silencing. High levels of GUS activity (Figure 1C) and GUS mRNA (Figure 3A, top panel) were detected in the F1 progeny plants of crosses between line P69c and line L1. The distinct GUS staining pattern in the L1xP69c plants (Figure 1C) may be caused by the constitutive expression of P69 possibly before or during the GUS transgene was silenced, as compared with that in TYMV-infected L1 plants (Figure 1B) in which GUS silencing was established before virus inoculation. By contrast, 35S-GUS remained silenced in the F1 progeny of a similar cross between the wild type and L1 (Figures 1C and 3A). Furthermore, whereas the GUS-specific siRNAs accumulated to high levels in L1xC24 plants, they were undetectable in L1xP69c plants (Figure 3B). Notably, these RNA analyses showed that suppression of GUS RNA silencing in P69c plants was as effective as in the sgs2 mutant that contains a defective cellular RdRP (Figures 3A and 3B) (Mourrain et al., 2000). Interestingly, silencing suppression as measured by the accumulation of both GUS mRNA and siRNAs, was less efficient in L1xP69d plants that expressed P69 mRNA at a lower level (Figure 3A, cf. lanes 3 and 4). Using 35S constructs directing translation of either the full-length or the first 45 codons of p69 and gene delivery by Agrobacterium tumefaciens coinfiltration, we further showed that p69 actively suppressed RNA silencing targeted against a 35S-controlled green fluorescent protein (GFP) sense transgene in Nicotiana benthamiana (see Supplemental Figure 2 online).
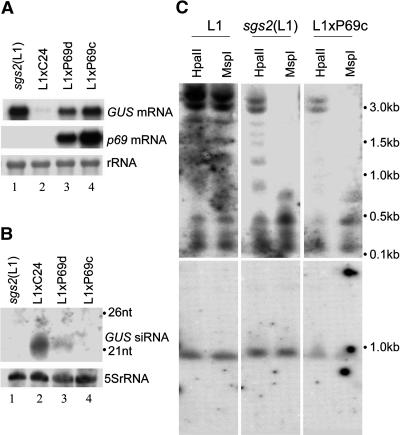
Figure 3.
Molecular Characterization of P69 Suppression of PTGS Induced by a Sense RNA Transgene in A. thaliana.
The detection of the GUS mRNA (A), GUS siRNAs (B), and GUS DNA methylation (C) in sgs2(L1), L1xC24, L1xP69c, and L1xP69d seedlings. In contrast with HpaII, MspI cleavage is not blocked to methylation at sites with overlapping CG. Equal loading and completion of DNA digestion was demonstrated by rehybridization of the filter for SPL3 (bottom panel of [C]), whereas equal loading of small RNAs was shown by reprobing for 5S rRNA (B). The positions of 21- and 26-nucleotide RNA markers are indicated (B).
The autonomous RNA silencing in L1 plants is associated with cytosine methylation within the coding sequence of the 35S-GUS transgene (Mourrain et al., 2000). As expected, the GUS DNA in the L1 plants was partially resistant to digestion by the methylation-sensitive restriction enzymes HpaII and MspI (Figure 3C, left panel). By contrast, the GUS DNA from the L1xP69c plants, in which the GUS siRNAs were undetectable, was digested completely by MspI, although the digestion by HpaII, sensitive to methylation at both overlapping and nonoverlapping CG sites, was less efficient (Figure 3C, right panel). This pattern of demethylation was similar to that found previously in the sgs2 mutant in the L1 background (Figure 3C, middle panel) (Mourrain et al., 2000). Thus, p69 expression also prevented the methylation of GUS DNA as completely as in the sgs2 mutant. These results establish p69 as a unique suppressor of RNA silencing because none of the known viral suppressors have been shown to eliminate both siRNA accumulation and transgene methylation associated with a silencing sense RNA transgene (Mallory et al., 2001; Guo and Ding, 2002).
P69 Inhibits PTGS Induced by a Virus-Derived Amplicon Transgene
Viral RNAs are silenced in plants via both the cellular and viral RdRP pathways (Dalmay et al., 2000b; Voinnet et al., 2000). We analyzed p69 suppression of PTGS that targets an amplicon transgene that encodes a replication-competent recombinant potato virus X (PVX) carrying GFP (PVX:GFP) in A. thaliana lines A and GxA (Dalmay et al., 2000a). The autonomous silencing of the amplicon requires active replication of PVX:GFP RNA but is SDE1 independent as shown in line GxA. Line G contains a highly expressed 35S-GFP transgene that is silenced in line GxA by the amplicon. In contrast with amplicon silencing, silencing of the 35S-GFP transgene in line GxA requires SDE1 and is associated with methylation of the GFP and PVX:GFP transgenes (Dalmay et al., 2000a).
RNA gel blot hybridizations detected significant accumulation of PVX:GFP genomic and subgenomic RNAs in both A and GxA plants either after systemic infection with TYMV (Figure 4A, lanes 4 and 5) or after the 35S-P69 transgene was introduced from line P69b, P69c, or P69d through genetic crosses (Figure 4B, lane 3 and lanes 6 to 8). Accumulation of GFP mRNA was also detected in (GxA)xP69 plants (Figure 4B, lanes 6 to 8) and in GxA plants infected with TYMV (Figure 4A, lane 4). Suppression of the amplicon and GFP RNA silencing was weaker in lines (GxA)xP69b and (GxA)xP69d than in (GxA)xP69c and undetectable in (GxA)xP69h and (GxA)xP69i as in lines (GxA)xΔP69a and (GxA)xΔP69b. These results indicate that the silencing suppression activity was determined by the expression levels of the P69 transgene and by whether or not the P69 transgene was translatable in these plants (Figure 4B). As expected from previous work (Dalmay et al., 2000b), the GFP mRNA and the genomic and subgenomic RNAs from PVX:GFP were detected in the sde1(GxA) mutant (Figure 4B, lane 13). Interestingly, the accumulation level was lower for the GFP mRNA and higher for the viral RNAs in (GxA)xP69 plants (Figure 4B, lanes 6 to 8) than in the sde1(GxA) mutant (Figure 4B, lane 13). Thus, p69 suppression of sense RNA silencing is incomplete, suggesting a differential suppression by p69 and the genetic mutation in SDE1of RNA silencing directed against the viral amplicon and sense-transgene transcripts.
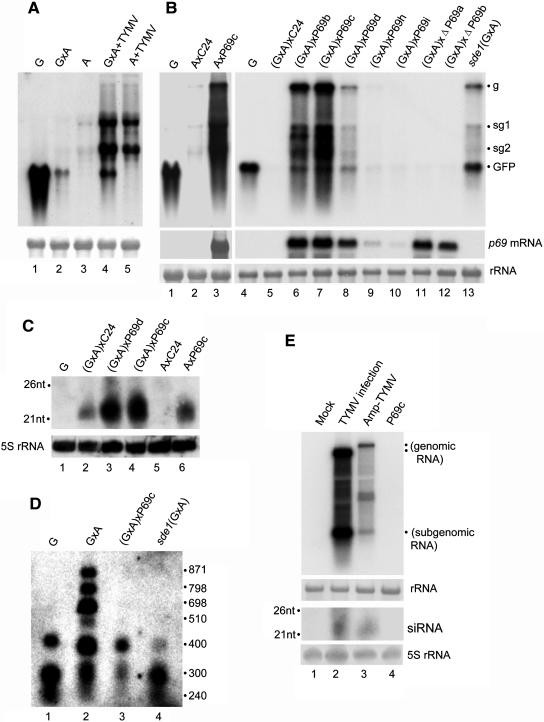
Figure 4.
Molecular Characterization of p69 Suppression of PTGS Induced by a Virus-Derived Amplicon Transgene.
(A) Silencing suppression in amplicon lines infected with TYMV (lanes 4 to 5). Samples from mock-inoculated lines were included as controls (lanes 1 to 3). PVX:GFP genomic (g) and subgenomic (sg) RNAs as well as GFP mRNA are indicated.
(B) Silencing suppression by the P69 transgene introduced into lines A and GxA by genetic crosses with the maternal parent listed first (this is used throughout the text). Except for G and sde1(GxA), all plants analyzed were hemizygous F1 plants. Total RNA loaded was 5 μg in lanes 1 to 3 and 3 μg in lanes 4 to 13 and equal loading was visualized by methylene blue staining of rRNA.
(C) Detection of siRNAs by a GFP-specific probe in hemizygous F1 amplicon plants crossed with P69c, P69d, or C24. A similar pattern of siRNA accumulation was detected using a PVX-specific probe.
(D) Detection of the GFP DNA methylation. Note that HaeIII cleavage is not blocked to methylation at sites with overlapping CG. The positions of DNA standards (in base pairs) are shown at the right.
(E) Detection of TYMV high and low molecular weight RNAs in either the TYMV-based amplicon plants or wild-type plants infected TYMV. The filters were probed with labeled DNA and RNA sequences corresponding to nucleotides 5591 to 6260, respectively, of the TYMV genome. The amplicon transgene contained an insertion of nonviral sequence (1.6 kb) upstream of the viral CP gene and thus gave rise to a recombinant viral genomic RNA longer than the wild-type TYMV genomic RNA, although the size of the CP subgenomic RNA remained unaltered.
We further examined the methylation status of the 35S-GFP transgene in (GxA)xP69c plants using the methylation-sensitive restriction enzyme HaeIII (Figure 4D). The GFP DNA extracted from (GxA)xP69c plants (lane 3) was completely digested by HaeIII as found for the GFP DNA extracted from either line G (lane 1) or the sde1(GxA) mutant (lane 4). This was in contrast with the GFP DNA extracted from GxA plants that was partially resistant to the digestion by HaeIII (Figure 4D, lane 2), as was found previously (Dalmay et al., 2000a). These results indicate that p69 also suppresses PTGS mediated by the viral amplicon either alone or in combination with a homologous transgene and that p69 suppression is associated with elimination of transgene methylation.
Hybridizations using a probe complementary to the GFP sequence detected the accumulation of siRNAs both in AxP69c and (GxA)xP69c plants (Figure 4C). In fact, the siRNAs became more abundant in these amplicon lines after P69 was introduced (Figure 4C, cf. lanes 3 and 4 with lane 2 or lane 6 with lane 5). Because Ax P69c plants did not carry the 35S-GFP transgene, all of the siRNAs detected in these plants must have come from the PVX:GFP amplicon transgene. Thus, p69 suppression of amplicon silencing led to enhanced accumulation of both viral genomic/subgenomic RNAs and siRNA, as found in the sde1(GxA) mutant (Dalmay et al., 2000b), rather than siRNA elimination observed in L1xP69c plants. This suggests that p69 expression did not prevent Dicer cleavage of virus-derived dsRNAs into siRNAs.
Unlike the PVX-based amplicon that induces consistent silencing in transgenic tobacco and A. thaliana plants (Angell and Baulcombe, 1997; Dalmay et al., 2000a), transgenic A. thaliana plants carrying a TYMV-based amplicon transgene displayed the characteristic TYMV symptoms (Figure 1D, bottom left) and suppression of the GUS transgene silencing as shown by GUS staining after the amplicon was introduced into line L1 by genetic crosses (Figure 1D, top left), suggesting that p69 also suppresses silencing induced by the TYMV amplicon. Compared with the 35S-P69 transgene (Figure 1C), however, suppression of GUS silencing by the TYMV amplicon was less effective (Figure 1D); this was probably caused by silencing of the amplicon transgene as indicated by detection of TYMV-specific siRNAs in the amplicon plants (Figure 4E, bottom panel, lane 3) and much lower levels of the genomic and subgenomic viral RNAs in those plants than in TYMV-infected plants (Figure 4E, top panel, cf. lanes 3 and 4). Consistent with the findings from the PVX amplicon plants, p69 expression from TYMV also did not prevent accumulation of viral siRNAs in TYMV-infected plants (Figure 4E, bottom panel, lane 2).
p69 Does Not Inhibit PTGS Induced by IR-RNA Transgenes
RNA silencing induced by IR-RNA transgenes is distinct to sense and virus RNA silencing because neither the cellular nor viral RdRP is involved in the formation of the dsRNA inducer from a nuclear self-complementary IR-RNA transcript (Beclin et al., 2002). Thus, we next determined if P69 interfered with silencing of the phytoene desaturase (PDS) gene mediated by an IR-RNA transgene (IRPDS), which caused a dominant photobleaching phenotype. An IRPDS/− heterozygote (in Columbia [Col-0] background) that contained a single copy of the transgene was crossed with P69c (in C24 background) and C24, respectively, and the F1 progenies were allowed to grow in the MS medium and MS plus hygromycin (for selection of the IRPDS and/or 35S-P69 positive individuals). The progeny of C24xIRPDS/− segregated 1:1 for hygromycin sensitive (HYGS) to hygromycin resistant (HYGR), but all of the 12 HYGR individuals showed photobleaching (Figure 5B). All of the 19 progenies from P69cxIRPDS/− carried the 35S-P69 transgene and were HYGR; however, only 10 of these F1 plants showed photobleaching (Figure 5C). When grown on MS medium (Figure 5A), both F1 populations from crosses C24xIRPDS/− (n = 29) and P69cxIRPDS/− (n = 52) contained ∼50% individuals displaying the photobleaching phenotype. These results indicate that the presence of 35S-P69 did not suppress the PDS silencing phenotype of IRPDS.
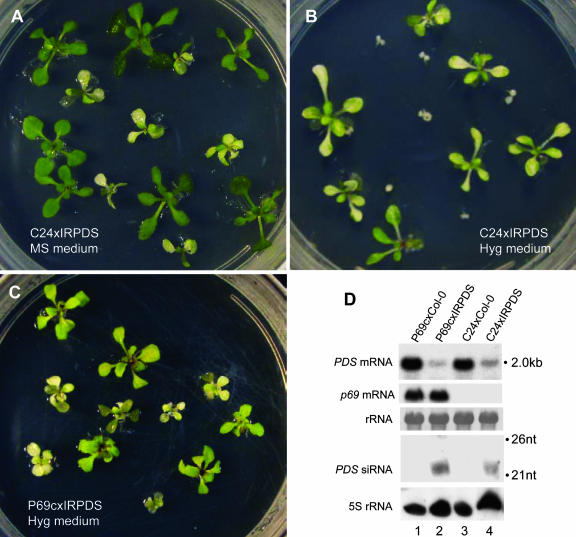
Figure 5.
p69 Does Not Suppress the IR-RNA–Induced PTGS Targeting of PDS.
(A) to (C) Genetic segregation of the photobleaching RNA interference phenotype of the IRPDS transgene after crossing an IRPDS heterozygote (IRPDS/−, in Col-0 background) with either C-24 or a P69c homozygote (in the C-24 background). A representative plate is shown for each except for P69cxIRPDS, which did not show difference with or without hygromycin selection. P69c plants appear yellow in contrast with the photobleached plants (C).
(D) Detection of P69 mRNA and PDS mRNA/siRNAs. For comparison to the level of PDS mRNA in the C24xCol-0 genetic background with and without P69, RNA samples from the progeny of C24xCol-0 (lane 3) and P69cxCol-0 (lane 1) were included as controls. Equal loading was monitored for mRNA by rRNA staining and for small RNAs by reprobing for 5S rRNA.
RNA analysis showed that expression of the IRPDS transgene in C24xIRPDS plants resulted in the accumulation of PDS-specific siRNAs (Figure 5D, lane 4) and an ∼75% knockdown of the PDS mRNA (Figure 5D, top panel, cf. lanes 3 and 4). Consistent with lack of P69 suppression indicated by the phenotypic analysis, P69 expression in the IRPDS-expressing plants was not associated with an increased accumulation of PDS mRNA (Figure 5D, cf. lanes 2 and 4). Furthermore, we found that TYMV infection of A. thaliana plants that carry the 35S-GFP transgene targeted by an IR-RNA transgene did not suppress GFP silencing as shown by RNA analyses (see Supplemental Figure 4 online). Thus, potent p69 suppression of RNA silencing triggered by sense RNA transgenes but not by the IR-RNA transgene suggests that p69 disrupts a cellular function leading to dsRNA synthesis.
p69 Enhances miRNA-Mediated Cleavage of Two Target mRNAs
Similar to IR-RNA, the hairpin precursor of miRNAs enters the RNA silencing pathway without new dsRNA synthesis. Thus, we predicted that p69 would not inhibit mRNA cleavage by endogenous miRNAs. We first examined the cleavage of the SCL6-IV mRNA by miR171, which occurs at positions centered in the middle of the miRNA–mRNA duplex so that both the full-length mRNA (SCL6-IVf) and a shorter 3′ fragment of ∼1.3 kb (SCL6-IV3′) are detected by a 3′-proximal probe in wild-type A. thaliana (Llave et al., 2002). We found that this cleavage indeed was not blocked in the presence of p69 because both of the RNA species were detected in P69c plants (Figure 6A). Notably, a decrease in the accumulation of the full-length mRNA and a corresponding increased accumulation of the 3′ fragment was detected in P69c plants as compared with wild-type plants (Figure 6A). This suggests that p69 may enhance RNA silencing mediated by the miRNA pathway.
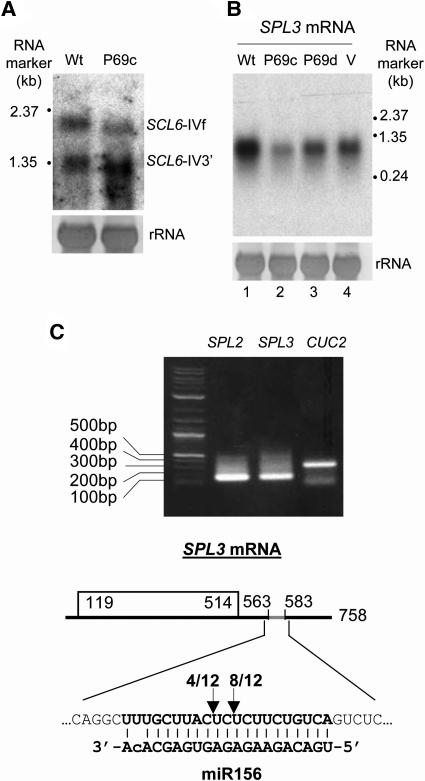
Figure 6.
Reduced Accumulation of Two miRNA-Targeted mRNAs in p69-Expressing Plants.
(A) Detection of full-length SCL6-IV mRNA and its 3′ cleavage product by miR171 in wild-type C-24 and P69c plants.
(B) Accumulation of SPL3 mRNA in P69c and P69d plants as well as in C-24 plants before (Wt) and after systemic TYMV infection (V).
(C) Determination of the miR156 cleavage sites within SPL3 mRNA. The 5′-terminal portion of the predicted 3′ cleavage product for SPL3 mRNA as well as SPL2 and CUC2 mRNAs (Kasschau et al., 2003; used here as controls) was amplified by 5′RACE (top panel), and the dominant products were recovered, cloned, and sequenced. The arrows indicate the positions of two cleavage sites inferred (bottom panel). The number of the 5′RACE clones that correspond to each site is indicated.
We further examined the steady state mRNA levels of SQUAMOSA-promoter binding protein-like gene 3 (SPL3), a predicted target of miR156 (Rhoades et al., 2002). SPL3 transcription is developmentally regulated, and constitutive overexpression of SPL3 resulted in early flowering (Cardon et al., 1997). RNA gel blotting analysis revealed significant decreases (0.7- to 2-fold) in the accumulation of the SPL3 mRNA in two independent p69 lines as compared with wild-type plants (Figure 6B). The reduction was more pronounced in line P69c than in line P69d (cf. lanes 2 and 3), which was correlated with a higher expression level of the P69 transgene in P69c plants as detected by RNA gel blot hybridization (Figure 4B, lanes 7 and 8). A modest decrease in the accumulation of SPL3 mRNA was also observed in wild-type plants infected with TYMV (Figure 6B, lane 4).
miR156 has mismatches to SPL3 mRNA in contrast with both miR171 and siRNAs that are perfectly complementary to their target mRNAs (Llave et al., 2002; Plasterk, 2002). Thus, we determined if there was cleavage of SPL3 mRNA within the predicted miR156/mRNA duplex, which contains one mismatch at the second nucleotide from the 3′ terminus of miR156 in addition to a G:U wobble (Rhoades et al., 2002) (Figure 6C). For this purpose, cDNA corresponding to the predicted 3′ fragment of SPL3 mRNA was amplified using the RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) essentially as described (Llave et al., 2002). Total mRNAs isolated from P69c plants were ligated to a 45-nucleotide RACE adapter RNA and reverse transcribed using a downstream SPL3-specific primer. Two rounds of nested PCR using the cDNAs obtained as templates and two sets of RACE and gene-specific primers yielded a discrete band that migrated within the predicted size range (Figure 6C, top panel). Sequencing and alignment of the cloned PCR products derived from the SPL3 mRNA identified two cleavage sites in the center of the predicted miR156/mRNA duplex (Figure 6C, bottom panel). Therefore, cleavage at the predicted duplex of miR156 and SPL3 mRNA indeed occurred in the P69c plants.
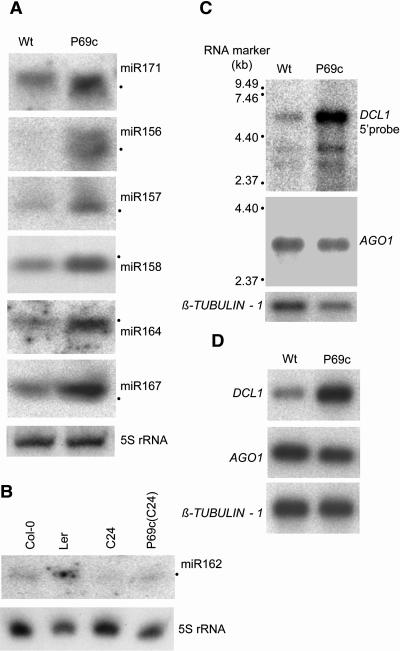
p69 Expression Increases the Accumulation Level of Seven miRNAs and DCL1 mRNA
The enhanced cleavage of miRNA targets observed in p69-expressing plants might be because of a higher accumulation level of miRNAs. To investigate this possibility, total RNA was extracted from newly bolted inflorescence and analyzed for the accumulation of seven A. thaliana miRNAs, miR156, miR157, miR158, miR162, miR164, miR167, and miR171. We found that each miRNA increased in abundance in the presence of the P69 transgene in P69c plants (Figures 7A and 7B). Similar increases were observed in the P69d plants but not in line G for miR156 and miR171, and we detected no difference in the accumulation of miR156, miR157, miR158, miR167, or miR171 between L1 and the sgs2(L1) mutant (see Supplemental Figure 3 online), suggesting that SDE1 may not be involved in miRNA biogenesis.
Figure 7.
Enhanced Accumulation of Seven miRNAs and mRNAs for DCL1 and SDE1/SGS2 in p69-Expressing Plants.
(A) and (B) Detection of miRNAs in P69c and wild-type C-24 plants as described in Methods. We noted that the level of miR162 was much lower in wild-type ecotype C-24 than in either Landsberg erecta (Ler) or Col-0.
(C) Detection of DCL1 and AGO1 mRNAs by RNA gel blot hybridizations. Two micrograms of poly(A)+ mRNAs from P69c and C-24 plants were loaded in each lane, and the filter was probed sequentially for the mRNA of AGO1, DCL1, and β-TUBULIN-1 (as a loading control). The full-length DCL1 and AGO1 mRNAs are 6.2 and 3.5 kb, respectively, as indicated.
(D) Real-time PCR analyses of the mRNA accumulation for AGO1, DCL1, and β-TUBULIN-1 in P69c and C-24 plants.
As the first step to investigate the mechanism of p69-mediated enhancement of miRNA silencing, we examined expression of two genes in the RNA silencing pathway using RNA gel blotting (Figure 7C) and real-time PCR (Figure 7D). No significant differences were detected in the accumulation of either AGO1 or β-TUBULIN-1 mRNA between P69c and wild-type plants. However, both analyses showed that mRNA of DCL1 was at least threefold higher in P69c plants than in wild-type plants.
DISCUSSION
In this work, we identified TYMV p69 as a unique viral virulence protein that suppresses the host RNA silencing antiviral defense but promotes RNA silencing of host genes mediated by miRNAs. Here, we discuss the mode of action of p69 and the potential role of p69 in viral pathogenesis by miRNAs.
p69 Suppresses RNA Silencing Upstream of dsRNA
Several lines of evidence indicate that among the viral suppressors characterized to date, p69 targets a unique step in the RNA silencing pathway. The fact that p69 suppressed PTGS induced by sense RNA transgenes but not by IR-RNA transgenes indicates that p69 inhibits a cellular function leading to dsRNA production. This conclusion is consistent with the observation that p69 had similar effects on RNA silencing to those of genetic mutations in cellular genes involved in dsRNA production such as SGS2/SDE1. For example, both P69 expression and the sgs2/sde1 mutation eliminated the siRNAs production (Figure 3B) and DNA methylation (Figures 3C) associated with RNA silencing induced by the sense RNA GUS transgene at the L1 locus (Mourrain et al., 2000). In the amplicon-based two-component silencing system (Figure 4), both P69 expression and the sgs2/sde1 mutation suppressed RNA silencing and DNA methylation of the 35S-GFP transgene, but both increased the accumulation of virus-specific siRNAs (Dalmay et al., 2000b). The increase in the production of viral siRNAs is likely from Dicer processing of the more abundant viral RdRP products after p69 suppression of silencing. However, these siRNAs may not be fully functional because of lack of both amplicon silencing and DNA methylation in the amplicon plants either expressing P69 or defective for SDE1 (Dalmay et al., 2000b; Mette et al., 2000).
PVX p25 also suppresses the host RdRP-dependent branch of RNA silencing (Voinnet et al., 2000). Interestingly, both p25 and p69 are essential for virus cell-to-cell movement as compared with a dispensable role for other viral suppressors in the cell-to-cell movement. Unlike p69, however, p25 is not active in the suppression of either established silencing targeted against sense RNA transgenes (Brigneti et al., 1998) or silencing targeted its own amplicon transgene (Angell and Baulcombe, 1997; Dalmay et al., 2000a). Furthermore, it appears that p25 expression also does not prevent DNA methylation of the 35S-GUS transgene in the tobacco host (Mallory et al., 2003). A recent work suggests that p25 acts downstream of dsRNA synthesis by a specific inhibition of the production of the longer siRNA species of 24 to 26 nucleotides (Hamilton et al., 2002). However, the recently described NSs protein of tomato spotted wilt virus (Takeda et al., 2002) may be mechanistically similar to p69.
p69 Suppression May Trigger a Negative Feedback Regulation
Our analysis revealed that P69 expression was associated with enhanced accumulation of all of the seven miRNAs examined and a correspondingly decreased accumulation of two mRNA targets of miRNAs. Increased accumulation was also observed for the mRNA of DCL1 (Figure 7C) and SDE1 (shown by real-time PCR analysis; our unpublished data) but not for the mRNA of AGO1 (Figure 7C). It is not known if p69 expression leads to increased expression of additional RNA silencing pathway genes, such as the homologs of DCL1 and SGS2/SDE1 (Finnegan et al., 2003; Yu et al., 2003) or if any of the p69-induced changes are mechanistically related. Given the known role of DCL1 in the production of miRNAs (Park et al., 2002; Reinhart et al., 2002), however, we propose that the RNA silencing pathway in A. thaliana is under a negative feedback regulation that can be triggered by p69 suppression and that DCL1 and SDE1 represent those genes in the pathway that are responsive to this feedback regulation at the level of transcription. It is possible that an upregulated DCL1 expression will lead to elevated levels of miRNAs by a more efficient processing of miRNA precursors, which may in turn cause enhanced cleavages of those host mRNAs targeted by miRNAs. In this regard, it will be interesting to determine if p69 expression also inhibits host gene expression by miRNA-guided translational repression (Aukerman and Sakai, 2003; Chen, 2003).
Recent work indicates that the DCL1 mRNA is targeted for cleavage in A. thaliana plants by miR162 (Xie et al., 2003). miR162 also accumulated to a higher level in p69-expressing plants than in wild-type plants (Figure 7B), which intriguingly, did not lead to a correspondingly lower level of the DCL1 mRNA as expected, in contrast with miR156 and miR171. It is not clear if this is because of an overall lower accumulation level of miR162 in C24 than in Col-0 and Landsberg erecta (Figure 7B; Xie et al., 2003). Alternatively, miRNA cleavage of mRNAs may be a rate-limiting process so that it becomes ineffective to control mRNA accumulation of those genes that are under transcriptional induction. It is less likely, however, that the increased accumulation of DCL1 mRNA found in p69-expressing plants is a result of p69-mediated inhibition of miRNA cleavage because mRNA cleavages by miR156 and miR171 were not inhibited (Figure 6) and consistent HC-Pro inhibition of miRNA cleavages was observed for 10 target mRNAs (Kasschau et al., 2003; Xie et al., 2003). It should be pointed out that a putative role for DCL1 in elevated levels of miRNAs is based on an assumption that p69 does not inhibit miRNA-mediated translational arrest, which remains to be determined.
Viral Pathogenesis by miRNAs?
Recent work has established a regulatory role for miRNAs in the development of plants and animals by targeting mRNAs for either cleavage or translational repression (Carrington and Ambros, 2003; Bartel, 2004). Although p69 could play a role in viral pathogenesis by miRNA-independent mechanisms, an attractive hypothesis is that the disease symptoms/developmental defects associated with P69 expression in A. thaliana plants represent a consequence of enhanced miRNA-guided inhibition of host gene expression regardless of whether one or both mechanisms are used. For example, the late flowering phenotype of the P69 plants may be in part attributable to the p69-stimulated miR156 knockdown of SPL3 mRNA because it is known that constitutive overexpression of SPL3 results in early flowering (Cardon et al., 1997). Interestingly, the level of P69 expression is highest in the young leaves systemically infected with TYMV but decline as infected leaves expand and mature (Bozarth et al., 1992), possibly because of selective proteolysis by the proteasome (Drugeon and Jupin, 2002). Presumably, young tissues are most sensitive to developmental cues such as miRNAs, a suggestion consistent with the observation that systemic viral symptoms always appear first in young leaves. Thus, miRNAs may play a novel pathogenic role in the induction of viral diseases.
HC-Pro expression in transgenic plants is known to cause developmental defects (Anandalakshmi et al., 2000). Recent studies show that even though miRNAs accumulate to elevated levels in the presence of HC-Pro, miRNA cleavage of target mRNAs is inhibited in HC-Pro–expressing plants, as found previously for PTGS induced by either sense- or IR-RNA transgenes (Anandalakshmi et al., 1998; Kasschau and Carrington, 1998; Mallory et al., 2002, 2003; Kasschau et al., 2003; Xie et al., 2003). Accordingly, it has been proposed that the role of HC-Pro in disease development is to inhibit host developmental pathways that depend on negative regulation by miRNAs (Kasschau et al., 2003). Therefore, our work on p69 of TYMV not only establishes a distinct mechanism in viral suppression of the RNA silencing antiviral defense, but also suggests an alternative model for the induction of viral diseases by upregulating the role of miRNAs in the inhibition of host gene expression.
METHODS
Plant Materials and Growth Conditions
L1 carries a silenced GUS transgene in the Arabidopsis thaliana ecotype Col-0 background, and sgs2 is a loss-of-function mutant of L1 at the SGS2/SDE1 locus (Mourrain et al., 2000). Lines G, A, and GxA (in C-24 ecotype background) correspond to GFP142, Amp243, and GFP142xAmp243, respectively (Dalmay et al., 2000a). sde1-3 was derived from GxA (Dalmay et al., 2000b).
The P69 coding sequence of TYMV-Blue Lake (BL) (Skotnicki et al., 1992) was cloned into pCambia1301 by replacing the GUS sequence between the 35S promoter and nopaline synthase terminator to give 35S-P69. The start codon (ATG) of the viral RdRP, which is four nucleotides downstream of the start codon for p69, was mutated to ACG in 35S-P69. As a result, the RdRP open reading frame (ORF) is likely not to be translated from 35S-P69 because the next Met codon in the RdRP ORF is 95 codons downstream. A single G-to-T substitution converting the 46th codon of ORF P69 into a stop codon (TAA) was introduced to create 35S-ΔP69. 35S-P69 and 35S-ΔP69 were transferred into A. thaliana ecotype C24 as described (Clough and Bent, 1998). The IR-RNA transgene targeting PDS contained an inverted repeat corresponding to nucleotides 128 to 532 of the PDS mRNA. The IR-RNA targeting GFP corresponded to nucleotides 360 to 716 of the GFP mRNA. Inserted between the repeats of either PDS or GFP was the third intron of A. thaliana actin gene 11 (nucleotides 1957 to 2111; GenBank accession number ATU27981). The PDS and GFP IR-RNA cassettes were cloned into pCambia1300 between 35S promoter and nopaline synthase terminator and transformed into wild-type Col-0 and line G (Dalmay et al., 2000a), respectively. Transformants were selected by hygromycin at 20 mg/L. Lines with a single copy transgene were selected by a 3:1 segregation of HygR and HygS among selfed progeny. Homozygous lines for 35S-P69 or 35S-ΔP69 were used in crosses. For plant inoculation, purified viral particles derived from an infectious plasmid containing the full-length TYMV-BL cDNA (Skotnicki et al., 1992) under the 35S promoter (A. Mackenzie, unpublished data) in the pCass vector as described (Ding et al., 1995) were used. TYMV amplicon plants, which were kindly supplied by N.H. Chua, carried the minimal 35S promoter (−46 to +1)–controlled TYMV-BL cDNA with a 1.6-kb insertion of nonviral sequence between the viral RdRP and CP genes (Aoyama and Chua, 1997). These plants became symptomatic without dexamethasone induction, possibly because of a leaky transcription.
Seeds were either imbibed on wet filter paper at 4°C for 7 d before planting on the Florobella potting compost/sand mix (3:1) or surface sterilized for growth on MS medium with/without antibiotics, but both were maintained in a growth room (16 h light/8 h darkness, 20 to 23°C).
DNA and RNA Analyses
The analysis of DNA methylation of either the GFP coding sequence (Dalmay et al., 2000a) or the GUS coding sequence (Mourrain et al., 2000) was performed as described using 32P-labeled DNA probes corresponding to the full coding sequences. As a control, the GUS filter was rehybridized with a SPL3-specific probe because there is only one HpaII/MspI recognition sequence (CCGG) within the SPL3 genomic DNA.
Total plant RNA extraction and RNA gel blotting analysis were performed as described previously (Li et al., 1999). Hybridization probes were labeled with 32P using the Amersham Megaprime DNA labeling kit (Piscataway, NJ). SPL3, SCL6-IV, and PDS probes corresponded to nucleotides 293 to 532, 774 to 1485, and 128 to 532 of the SPL3, SCL6-IV, and PDS mRNAs, respectively. Total mRNAs were extracted from whole plants including leaves, stems, flowers, and siliques but not roots using the Qiagen Oligotex mRNA Midi kit (Valencia, CA). The probes for DCL1 (Jacobsen et al., 1999), AGO1 (Fagard et al., 2000), and β-TUBULIN-1 corresponded to nucleotides 475 to 2283, 484 to 748, and 500 to 992 of their respective mRNAs.
siRNA detection was as described (Guo and Ding, 2002). These siRNA filters were reprobed for the 5S rRNA to show equal loading. For miRNA detection, total RNA was isolated from newly bolted inflorescence, and 50 μg was loaded in each lane as described (Reinhart et al., 2002) and probed by end-labeled DNA oligonucleotides complementary to miRNA. All experiments on the DNA and RNA analyses were repeated at least twice.
Real-Time RT-PCR Analysis
Total cDNA was first synthesized using the oligo(dT)15 primer from total RNAs and used as templates for the real-time PCR with gene-specific primers. The amplified regions corresponded to nucleotides 721 to 949, 628 to 1057, 484 to 748, and 500 to 992 of the SDE1/SGS2 (RdRP), DCL1, AGO1, and β-TUBULIN-1 mRNAs, respectively. The LightCycler-FastStartDNA Master SYBR Green kit and LightCycler Instrument real-time PCR machine (Roche Applied Science, Penzberg, Germany) were used. The reactions were terminated when PCR was in the log-linear phase but before the later plateau phase, which was 27 cycles for RdRP and 29 for DCL1, AGO1, and β-TUBULIN-1. After confirming the identity by direct sequencing, PCR products were blotted and detected by hybridizations with gene-specific probes.
RLM-RACE
The FirstChoice RLM-RACE kit (Ambion, Austin, TX) was used for RLM-RACE essentially following the manufacturer's instructions. Briefly, poly(A)+ mRNA was obtained from flowering plants using Oligotex mRNA Midi kit (Qiagen) and directly ligated to RLM-RACE 5′RACE RNA oligo adaptor (45 nucleotides) before cDNA synthesis using oligo(dT)15 primer. The first round of nested PCR was performed using 5′RACE outer primer included in the kit and a gene-specific outer primer that was complementary to nucleotides 690 to 710 of SPL3 (At2g33810 complete cDNA), 1166 to 1185 of SPL2 (At5g43270), or 1075 to 1094 of CUC2 (At5g53950). For the second round, 5′RACE inner primer was used with the second set of gene-specific inner primers that were respectively complementary to nucleotides 665 to 685 of SPL3, 1134 to 1152 of SPL2, and 1057 to 1078 of CUC2. In each case, a unique gene-specific DNA fragment was amplified (Figure 6C). The PCR products were gel purified and cloned into pGEM-T Easy vector (Promega, Madison, WI) for DNA sequencing.
Supplementary Material
Acknowledgments
We thank F. Liu, J. Fei, and H. Guo for helpful discussion and technical support, H. Vaucheret for L1 lines, D. Baulcombe for amplicon lines, A. Mackenzie and A. Gibbs for the TYMV clone, N.H. Chua for the TYMV amplicon line, and Z. Wen, T. Dreher, and the anonymous reviewers for valuable comments on the manuscript. This work was supported first by A-STAR in the Institute of Molecular Agrobiology and then at the Institute of Molecular and Cell Biology. The work conducted at the University of California Riverside was supported by grants from the USDA and the National Institutes of Health.
Online version contains Web-only data.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Jin Rong Peng (pengjr@imcb.a-star.edu.sg) and Shou Wei Ding (shou-wei.ding@ucr.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.018986.
References
- Al-Kaff, N.S., Covey, S.N., Kreike, M.M., Page, A.M., Pinder, R., and Dale, P.J. (1998). Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science 279, 2113–2115. [DOI] [PubMed] [Google Scholar]
- Ambros, V., et al. (2003). A uniform system for microRNA annotation. RNA 9, 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandalakshmi, R., Marathe, R., Ge, X., Herr, J.M., Mau, C., Mallory, A., Pruss, G., Bowman, L., and Vance, V.B. (2000). A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290, 142–144. [DOI] [PubMed] [Google Scholar]
- Anandalakshmi, R., Pruss, G.J., Ge, X., Marathe, R., Mallory, A.C., Smith, T.H., and Vance, V.B. (1998). A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 95, 13079–13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell, S.M., and Baulcombe, D.C. (1997). Consistent gene silencing in transgenic plants expressing a replicating potato virus X RNA. EMBO J. 16, 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama, T., and Chua, N.H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11, 605–612. [DOI] [PubMed] [Google Scholar]
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, D.P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Beclin, C., Boutet, S., Waterhouse, P., and Vaucheret, H. (2002). A branched pathway for transgene-induced RNA silencing in plants. Curr. Biol. 12, 684–688. [DOI] [PubMed] [Google Scholar]
- Bernstein, E., Caudy, A.A., Hammond, S.M., and Hannon, G.J. (2001). Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366. [DOI] [PubMed] [Google Scholar]
- Boutet, S., Vazquez, F., Liu, J., Beclin, C., Fagard, M., Gratias, A., Morel, J.B., Crete, P., Chen, X., and Vaucheret, H. (2003). Arabidopsis HEN1: A genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. 13, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth, C.S., Weiland, J.J., and Dreher, T.W. (1992). Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology 187, 124–130. [DOI] [PubMed] [Google Scholar]
- Brigneti, G., Voinnet, O., Li, W.X., Ji, L.H., Ding, S.W., and Baulcombe, D.C. (1998). Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 17, 6739–6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cardon, G.H., Hohmann, S., Nettesheim, K., Saedler, H., and Huijser, P. (1997). Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: A novel gene involved in the floral transition. Plant J. 12, 367–377. [DOI] [PubMed] [Google Scholar]
- Carrington, J.C., and Ambros, V. (2003). Role of microRNAs in plant and animal development. Science 301, 336–338. [DOI] [PubMed] [Google Scholar]
- Chen, X. (2003). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303, 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Covey, S.N., Al-Kaff, N.S., Langara, A., and Turner, D.S. (1997). Plants combat infection by gene silencing. Nature 385, 781–782. [Google Scholar]
- Dalmay, T., Hamilton, A., Mueller, E., and Baulcombe, D.C. (2000. a). Potato virus X amplicons in Arabidopsis mediate genetic and epigenetic gene silencing. Plant Cell 12, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay, T., Hamilton, A., Rudd, S., Angell, S., and Baulcombe, D.C. (2000. b). An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101, 543–553. [DOI] [PubMed] [Google Scholar]
- Dalmay, T., Horsefield, R., Braunstein, T.H., and Baulcombe, D.C. (2001). SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 20, 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S.W. (2000). RNA silencing. Curr. Opin. Biotechnol. 11, 152–156. [DOI] [PubMed] [Google Scholar]
- Ding, S.W., Li, W.X., and Symons, R.H. (1995). A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long-distance virus movement. EMBO J. 14, 5762–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugeon, G., and Jupin, I. (2002). Stability in vitro of the 69K movement protein of Turnip yellow mosaic virus is regulated by the ubiquitin-mediated proteasome pathway. J. Gen. Virol. 83, 3187–3197. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., Lendeckel, W., and Tuschl, T. (2001). RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmayan, T., Balzergue, S., Beon, F., Bourdon, V., Daubremet, J., Guenet, Y., Mourrain, P., Palauqui, J.C., Vernhettes, S., Vialle, T., Wostrikoff, K., and Vaucheret, H. (1998). Arabidopsis mutants impaired in cosuppression. Plant Cell 10, 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard, M., Boutet, S., Morel, J.B., Bellini, C., and Vaucheret, H. (2000). AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. USA 97, 11650–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan, E.J., Margis, R., and Waterhouse, P.M. (2003). Posttranscriptional gene silencing is not compromised in the Arabidopsis CARPEL FACTORY (DICER-LIKE1) mutant, a homolog of Dicer-1 from Drosophila. Curr. Biol. 13, 236–240. [DOI] [PubMed] [Google Scholar]
- Guo, H.S., and Ding, S.W. (2002). A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 21, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A., Voinnet, O., Chappell, L., and Baulcombe, D. (2002). Two classes of short interfering RNA in RNA silencing. EMBO J. 21, 4671–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A.J., and Baulcombe, D.C. (1999). A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286, 950–952. [DOI] [PubMed] [Google Scholar]
- Hammond, S.M., Bernstein, E., Beach, D., and Hannon, G.J. (2000). An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296. [DOI] [PubMed] [Google Scholar]
- Hammond, S.M., Boettcher, S., Caudy, A.A., Kobayashi, R., and Hannon, G.J. (2001). Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293, 1146–1150. [DOI] [PubMed] [Google Scholar]
- Jacobsen, S.E., Running, M.P., and Meyerowitz, E.M. (1999). Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126, 5231–5243. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D., and Carrington, J.C. (1998). A counterdefensive strategy of plant viruses: Suppression of posttranscriptional gene silencing. Cell 95, 461–470. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D., Xie, Z., Allen, E., Llave, C., Chapman, E.J., Krizan, K.A., and Carrington, J.C. (2003). P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell 4, 205–217. [DOI] [PubMed] [Google Scholar]
- Li, H.W., Li, W.X., and Ding, S.W. (2002). Induction and suppression of RNA silencing by an animal virus. Science 296, 1319–1321. [DOI] [PubMed] [Google Scholar]
- Li, H.W., Lucy, A.P., Guo, H.S., Li, W.X., Ji, L.H., Wong, S.M., and Ding, S.W. (1999). Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J. 18, 2683–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W.X., and Ding, S.W. (2001). Viral suppressors of RNA silencing. Curr. Opin. Biotechnol. 12, 150–154. [DOI] [PubMed] [Google Scholar]
- Li, W.X., Li, H., Lu, R., Li, F., Dus, M., Atkinson, P., Brydon, E.W., Johnson, K.L., Garcia-Sastre, A., Ball, L.A., Palese, P., and Ding, S.W. (2004). Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. USA 101, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave, C., Xie, Z.X., Kasschau, K.D., and Carrington, J.C. (2002). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297, 2053–2056. [DOI] [PubMed] [Google Scholar]
- Mallory, A.C., Ely, L., Smith, T.H., Marathe, R., Anandalakshmi, R., Fagard, M., Vaucheret, H., Pruss, G., Bowman, L., and Vance, V.B. (2001). HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal. Plant Cell 13, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory, A.C., Mlotshwa, S., Bowman, L.H., and Vance, V. (2003). The Capacity of transgenic tobacco to send a systemic RNA silencing signal depends on the nature of the inducing transgene locus. Plant J. 35, 82–92. [DOI] [PubMed] [Google Scholar]
- Mallory, A.C., Reinhart, B.J., Bartel, D., Vance, V.B., and Bowman, L.H. (2002). A viral suppressor of RNA silencing differentially regulates the accumulation of short interfering RNAs and micro-RNAs in tobacco. Proc. Natl. Acad. Sci. USA 99, 15228–15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke, M.A., Matzke, A.J.M., Pruss, G.J., and Vance, V.B. (2001). RNA-based silencing strategies in plants. Curr. Opin. Genet. Dev. 11, 221–227. [DOI] [PubMed] [Google Scholar]
- Mette, M.F., Aufsatz, W., van der Winden, J., Matzke, M.A., and Matzke, A.J.M. (2000). Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 19, 5194–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa, S., Voinnet, O., Mette, M.F., Matzke, M., Vaucheret, H., Ding, S.W., Pruss, G., and Vance, V.B. (2002). RNA silencing and the mobile silencing signal. Plant Cell 14, S289–S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch, M.D., Boyer, J.C., and Haenni, A.L. (1988). Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 16, 6157–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourrain, P., et al. (2000). Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101, 533–542. [DOI] [PubMed] [Google Scholar]
- Park, W., Li, J., Song, R., Messing, J., and Chen, X. (2002). CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk, R.H.A. (2002). RNA silencing: The genome's immune system. Science 296, 1263–1265. [DOI] [PubMed] [Google Scholar]
- Ratcliff, F., Harrison, B.D., and Baulcombe, D.C. (1997). A similarity between viral defense and gene silencing in plants. Science 276, 1558–1560. [DOI] [PubMed] [Google Scholar]
- Reinhart, B.J., Weinstein, E.G., Rhoades, M.W., Bartel, B., and Bartel, D.P. (2002). MicroRNAs in plants. Genes Dev. 16, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades, M.W., Reinhart, B.J., Lim, L.P., Burge, C.B., Bartel, B., and Bartel, D.P. (2002). Prediction of plant microRNA targets. Cell 110, 513–520. [DOI] [PubMed] [Google Scholar]
- Silhavy, D., Molnar, A., Lucioli, A., Szittya, G., Hornyik, C., Tavazza, M., and Burgyan, J. (2002). A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 21, 3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicki, M.L., Mackenzie, A.M., and Gibbs, A.J. (1992). Turnip yellow mosaic virus variants produced from DNA clones encoding their genomes. Arch. Virol. 127, 25–35. [DOI] [PubMed] [Google Scholar]
- Szittya, G., Molnar, A., Silhavy, D., Hornyik, C., and Burgyan, J. (2002). Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell 14, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, A., Sugiyama, K., Nagano, H., Mori, M., Kaido, M., Mise, K., Tsuda, S., and Okuno, T. (2002). Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 532, 75–79. [DOI] [PubMed] [Google Scholar]
- Tsai, C.H., and Dreher, T.W. (1993). Increased viral yield and symptom severity result from a single amino acid substitution in the turnip yellow mosaic virus movement protein. Mol. Plant Microbe Interact. 6, 268–273. [DOI] [PubMed] [Google Scholar]
- Vance, V., and Vaucheret, H. (2001). RNA silencing in plants–Defense and counterdefense. Science 292, 2277–2280. [DOI] [PubMed] [Google Scholar]
- Voinnet, O., Lederer, C., and Baulcombe, D.C. (2000). A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103, 157–167. [DOI] [PubMed] [Google Scholar]
- Xie, Z., Kasschau, K.D., and Carrington, J.C. (2003). Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 13, 784–789. [DOI] [PubMed] [Google Scholar]
- Yu, D., Fan, B., MacFarlane, S.A., and Chen, Z. (2003). Analysis of the involvement of an inducible Arabidopsis RNA-dependent RNA polymerase in antiviral defense. Mol. Plant Microbe Interact. 16, 206–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.