Abstract
The in vitro growth of ovarian follicles is an emerging technology for fertility preservation. Various strategies support the culture of secondary and multilayer follicles from various species including mice, non-human primate, and human; however, the culture of early stage (primary and primordial) follicles, which are more abundant in the ovary and survive cryopreservation, has been limited. Hydrogel-encapsulating follicle culture systems that employed feeder cells, such as mouse embryonic fibroblasts (MEFs), stimulated the growth of primary follicles (70–80 μm); yet, survival was low and smaller follicles (<70 μm) rapidly lost structure and degenerated. These morphologic changes were associated with a breakdown of the follicular basement membrane; hence, this study investigated ascorbic acid based on its role in extracellular matrix (ECM) deposition/remodeling for other applications. The selection of ascorbic acid was further supported by a microarray analysis that suggested a decrease in mRNA levels of enzymes within the ascorbate pathway between primordial, primary, and secondary follicles. The supplementation of ascorbic acid (50 μg/mL) significantly enhanced the survival of primary follicles (<80 μm) cultured in alginate hydrogels, which coincided with improved structural integrity. Follicles developed antral cavities and increased to diameters exceeding 250 μm. Consistent with improved structural integrity, the gene/protein expression of ECM and cell adhesion molecules was significantly changed. This research supports the notion that modifying the culture environment (medium components) can substantially enhance the survival and growth of early stage follicles. Biotechnol. Bioeng. 2014;111: 1417–1429.
Keywords: tissue engineering, regenerative medicine, ovarian follicle development, primary follicle, ascorbic acid, extracellular matrix, biomaterial, alginate hydrogel
Introduction
The in vitro growth and maturation of ovarian follicles is an emerging technology with the goal of expanding fertility preservation options for cancer patients (Woodruff, 2007a, b, 2010). Fertility preservation is necessary for girls and women, whose fertility is threatened by lifesaving cancer therapies, such as radiation and chemotherapy, which may diminish the follicle pool and trigger premature ovarian failure. Treatments to restore fertility include the transplantation of cryopreserved ovarian tissue, which may risk the reintroduction of cancer cells (Meirow et al., 2008; Shaw and Trounson, 1997). To avoid this risk, in vitro follicle culture, maturation, and fertilization techniques could potentially be used to produce mature eggs, and subsequently embryos, from cryopreserved ovarian tissue. In vitro culture systems have significantly advanced over the past 35 years (Eppig, 1977; Eppig and O’Brien, 1996; Lenie et al., 2004; Nayudu and Osborn, 1992; O’Brien et al., 2003; Pangas et al., 2003; Torrance et al., 1989); however, further advancements are necessary for the efficient growth of early stage (primordial and primary) follicles and human follicles capable of producing fertilizable eggs.
The three dimensional structure and cell–cell interactions of developing follicles have been maintained by biomaterials, such as alginate hydrogels (Augst et al., 2006; Kreeger et al., 2005, 2006; Pangas et al., 2003). Follicle culture on traditional flat substrates (tissue culture polystyrene) often results in the disruption of the normal follicular architecture (separation of the somatic cells from the oocyte). The maintenance of follicular structure that morphologically resembles in vivo grown follicles has been accomplished by encapsulation within hydrogels. Alginate-based hydrogel culture systems have supported the in vitro growth of secondary and multilayer mouse, non-human primate, and human follicles (Shikanov et al., 2009; West et al., 2007; Xu et al., 2006b, 2009a,b, 2011, 2013). Mouse follicles have produced eggs capable of fertilization and live births (Xu et al., 2006a). Furthermore, these systems provide a controlled and modifiable environment in which to study the mechanisms governing follicle development (Hornick et al., 2012; Kreeger et al., 2005, 2006; Parrish et al., 2011; Skory et al., 2013; West et al., 2007; Xu et al., 2006b).
As culture systems continue to advance, the focus is shifting to early stage (primary and primordial) follicles, which are more abundant in the ovary and survive cryopreservation, yet the biology is not well understood and successful culture has been limited (Abir et al., 2006; Itoh and Hoshi, 2000; Lenie et al., 2004; Muruvi et al., 2005, 2009; Saha et al., 2000). Early stage follicles have been grown via in situ organ culture or two-step culture systems (Eppig and O’Brien, 1996; Jin et al., 2010), in which ovarian tissue fragments containing early stage follicles are cultured in vitro. The tissue and stromal cells within the ovarian tissue provide physical support as well as signals that stimulate follicle growth. In alginate-based culture systems, the growth of isolated primary follicles has been achieved via co-culture with ovarian stromal cells (theca-interstitial cells; Tingen et al., 2011), mouse embryonic fibroblasts (MEFs; Tagler et al., 2012), and multiple follicles (Hornick et al., 2013). The hydrogel physically supports follicle structure (Hornick et al., 2012), while the feeder cells secrete paracrine factors that stimulate growth. These co-culture methods have been effective for early secondary follicles (90–100 μm), but primary follicles (70–80 μm) have low survival rates (Tagler et al., 2012). Therefore, these co-culture systems require additional factors to improve the survival of growing follicles and potentially culture smaller follicles.
In this report, we aimed to improve the survival and growth of primary follicles (60–70 μm) encapsulated in alginate hydrogels and co-cultured with MEFs (Tagler et al., 2013). The limited growth of early stage follicles results from a loss of follicle structure, which coincided with a breakdown of the follicular basement membrane that may result from damage during follicle isolation. Ascorbic acid, which has been associated with extracellular matrix (ECM) deposition/remodeling, has been previously identified to improve secondary and multilayer follicle (150–200 μm) survival (Murray et al., 2001; Rose et al., 1999; Thomas et al., 2001); and herein, we investigated ascorbic acid as a means to improve primary follicle culture in alginate hydrogels. In addition to investigating the influence of ascorbic acid on the survival and growth of primary follicles, we explored the underlying mechanisms, such as oxidative stress (antioxidant) and ECM gene/protein expression.
Materials and Methods
Animals and Materials
Follicles were isolated from the ovaries of CD1 female mice. Mice were maintained in accordance with the policies of the National Institutes of Health and Northwestern University’s Animal Care and Use Committee. A temperature, humidity, and light (12 hL/12 hD) controlled barrier facility within Northwestern University’s Center of Comparative Medicine (Chicago, IL) was used to house and breed the mice. Mice were provided with food and water ad libitum. The mice were fed Teklad Global (Madison, WI) irradiated chow (2,919 or 2,916) that does not contain soybean or alfalfa meal but does contain minimal phytoestrogens. Unless otherwise specified, all chemical were purchased from Sigma–Aldrich (St Louis, MO) and medium formulations were purchased from Life Technologies (Calsbad, CA). Sodium alginate (55–65% guluronic acid) was provided by FMC BioPolymers (Philadelphia, PA).
Gene Expression During Early Stage Follicle Development (mRNA Microarray)
Previously established procedures (Xu et al., 2006b) for ovary/follicle isolation were followed with slight modifications. Primordial follicles were isolated from the ovaries of mice at post-natal day 3 and day 4 (Skory et al., 2013). Primary follicles (70–90 μm in diameter) were mechanically isolated from day 10 ovaries. Two-layered secondary follicles (100–130 μm in diameter) were mechanically isolated from day 12 ovaries. Follicles were then aspirated. Each sample was collected in triplicate. RNA isolation from the ovary (primordial follicles) or from the entire follicles (primary and two-layered secondary) was performed as previously described (Skory et al., 2013). RNA was hybridized in MouseRef-8 v2 Expression BeadChips from Illumina and time series transcriptomics were employed to determine the genes that were differentially expressed over time. Normalization, transformation, and determination of adequate and present probes were performed as previously described (Skory et al., 2013). Briefly, data were transformed and normalized with the variance stabilization transformation method (Lin et al., 2008) and normalized by robust spline normalization (Du et al., 2008). Differentially expressed genes over time were assessed using limma (Smyth, 2004). Differentially expressed genes with a fold change per day of greater than to 1.4 and FDR (false discovery rate) corrected P-value less than 0.01 were considered significant.
Follicle Isolation and Encapsulation
Previously established procedures (Tagler et al., 2012) for follicle isolation and alginate encapsulation were followed with slight modifications. Briefly, individual secondary (diameter: 90–100 μm) and primary follicles (diameter: 60–80 μm) were mechanically isolated from 7–10-day-old CD1 mice using insulin gauge needles in dissection medium (Leibovitz’s L-15 medium supplemented with 1% fetal bovine serum and 0.5% penicillin–streptomycin). Follicles were separated into size classes based on average initial diameter (60, 70, 80, 90, and 100 μm). After isolation, the follicles were washed and stored in maintenance medium (Minimum Essential Medium Eagle Alpha Modification [aMEM] supplemented with 1% fetal bovine serum and 0.5% penicillin–streptomycin). Follicles were then transferred into 5–6 μL solutions of 0.25% alginate and immersed into a 50 mM CaCl2 and 140 mM NaCl solution for 1–2 min to crosslink. Encapsulated follicles were then co-cultured with mouse embryonic fibroblasts (MEFs).
Mouse Embryonic Fibroblast (MEF) Co-Culture
Follicles were co-cultured with MEFs according to previously established procedures (Tagler et al., 2012). Briefly, Inactivated MEFs (Life Technologies #S1520-100) were thawed, resuspended, and seeded according to the manufacturer’s procedures. A hemocytometer was used to determine cell concentration, and viability was determined to be approximately 90% via trypan blue staining. MEFs were seeded at 2.0 × 104 viable cells/well in a 96-well flat–bottom culture plate (Corning Costar #3596). After 20 h of culture (overnight), the cells were washed once in 100 μL of PBS for 5 min and 100 μL of fresh follicle growth medium was added to each well. Following follicle isolation and encapsulation, individual alginate beads were placed into each well. The co-culture was conducted at 37°C in 5% CO2 for 6–14 days. Every 2 days, half the medium (50 μL) was replaced with fresh growth medium.
Ascorbic Acid (AA) and Control Growth Medium Formulations
The ascorbic acid (AA+) growth medium consisted of αMEM medium supplemented with 3 mg/mL bovine serum albumin (BSA; MP Biomedicals, Solon, OH), 1 mg/mL bovine fetuin, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium (ITS), 10 mIU/mL recombinant human follicle-stimulating hormone (FSH) (A.F. Parlow, NHPP, NIDDK), and 50 μg/mL sodium l-ascorbic acid (Sigma #A4034; Thomas et al., 2001). Fresh AA growth medium was made every 2 days. The control growth þ medium without ascorbic acid (−AA) consisted of αMEM supplemented with BSA, fetuin, ITS, and FSH.
Follicle Survival and Growth Measurements
Images of follicles were collected every other day using an inverted Leica DM light microscope (Leica, Wetzlar, Germany). Follicle diameters were measured using ImageJ software (National Institutes of Health, Bethesda, MD). An average of two perpendicular diameter measurements was used for each follicle at each time point. Survival was determined via follicle/oocyte morphology. Non-growing follicles or follicles with fragmented, shrunken, degenerated, or detached oocytes were considered dead.
Confocal Imaging
Follicles were fixed in 4% paraformaldehyde for 45 min at room temperature and then washed in a blocking solution containing 0.2% sodium azide, 2% goat serum, 1% bovine serum albumin, and 0.1% Triton X-100. Next, follicles were stained with primary antibodies for laminin (1:100, rabbit anti-mouse, Sigma #L9393), rhodamine phalloidin (f-actin; 1:50, Life Technologies #R415), and DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) (1 μg/mL, Life Technologies #D1306). Follicles were then washed three times for 10 min in wash buffer and then incubated at room temperature for 1 h with the secondary antibody (Alexa Fluor® 568 goat anti-rabbit IgG, 1:50, Life Technologies #A-11011). Finally, follicles were mounted on Teflon printed microscope slides (Electron Microscopy Sciences, PA). Samples were imaged on Zeiss LSM 510 Meta Confocal Microscope using 40× oil objectives.
Oxidative Stress
The OxiSelect™ In Vitro ROS/RNS Assay Kit (Cell Biolabs #STA-347) was used to test conditioned medium for reaction oxygen and nitrogen species (ROS/RNS). Half the growth medium (50 μL) was collected on days 2, 4, and 6 and stored in −80°C. The protocol provided by the manufacturer was followed.
Extracellular Matrix (ECM) and Cell Adhesion Molecules Gene Expression (qRT-PCR)
The mouse extracellular matrix (ECM) and cell adhesion molecules qRT-PCR (Quantitative Real-Time PCR) array (Qiagen, Hilden, Germany) was utilized to determine differences in the gene expression (mRNA levels) between follicles (<75 μm) cultured in vitro in the presence and absence of ascorbic acid. RNA extraction, cDNA synthesis, and qRT-PCR array were performed according to the manufacturer’s instructions. Follicles in each group were removed from alginate beads on day 6 of culture by incubating in 10 mIU/mL alginate lyase for 10 min. The follicles were then flash frozen in liquid nitrogen in extraction buffer and stored at −80°C. RNAwas extracted using RNeasy Micro Kit (Qiagen) and cDNA was generated using pathway specific primers to enrich for mouse ECM and cell adhesion molecules genes. Genomic DNA was also eliminated in this step. Amplified cDNA was added to RT2 SYBR Green qPCR master mix (Qiagen) and 10 mLwas added to a 384-well plate, with each well containing pre-dispensed gene-specific primer sets. Included on the plate was 1 genomic DNA control, 3 reverse transcription controls (RTC), and 3 positive PCR controls (PPC). The plate was sealed and loaded onto an ABI 7900 HT thermal cycler. ABI 7900 HT SDS software version 2.3 (Applied Biosystems, Foster City, CA) was used to determine the threshold cycle (Ct) values for all genes on the qPCR array. Using the RT2 Profiler PCR Array Data Analysis software version 3.5 (Qiagen), the Ct values were normalized against the average Ct of housekeeping genes (Actb, B2m, and Gusb) to generate delta Ct (ΔCt). The 2−ΔΔCt method (Livak and Schmittgen, 2001) was used to determine fold change. Fold-change (2−ΔΔCt) was calculated as the normalized gene expression (2−ΔCt) of follicles cultured in the presence of ascorbic acid (AA+) divided by the normalized gene expression (2−ΔCt) of follicles cultured in the absence of ascorbic acid (AA−).
Western Blot (Immunoblot) Analysis and Protein Quantification
Follicles (<75 μm) were cultured in growth medium with or without ascorbic acid for 7 days. Surviving follicles were removed from alginate beads by incubating with 50 μL of αMEM containing 10 IU/mL alginate lyase for 10 min. The follicles were then lysed in lysate buffer (20 mM Tris–HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA) and flash frozen in liquid nitrogen by treatment group. Samples were stored in −80°C until further analysis. After thawing, the lysates were electrophoresed on 4–12% SDS–PAGE gels and then transferred to nitrocellulose for immunoblot analysis. The blots were probed by polyclonal anti-fibronectin antibody (abcam, Cambridge, MA) overnight in 4°C followed by anti-rabbit secondary antibody conjugated to horseradish peroxidase (Zymed, San Francisco, CA). Proteins were detected by ECL primer (GE HealthCare Life Sciences, Pittsburgh, PA) and exposed to X-ray film (Kodak, Rochester, NY). The same blot was stripped using stripping buffer (Thermo Scientific) and re-probed with monoclonal anti-α-tubulin (Sigma, St. Louis, MO) followed by an anti-mouse secondary antibody conjugated to horseradish peroxidase. The blot was detected by the same method described above. Protein quantification was done using densitometric analysis on ImageJ Imaging Software.
Statistical Analysis
Secondary and primary follicle survival and growth (in the presence and absence of ascorbic acid) data were collected via ten independent cultures of approximately 40–80 follicles each. The extracellular matrix (ECM) and cell adhesion protein gene expression (mRNA) (qRT-PCR) data were collected via four biological replicates consisting of approximately 30 follicles per condition. Western blot data were collected via three independent cultures of approximately 20–50 follicles each. Oxidative stress measurements were collected via 10–12 independent conditioned medium samples. Statistical analysis was performed using Microsoft Excel and GraphPad Prism. Follicle growth and oxidative stress data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey for single time point comparisons. Survival data were analyzed using the Ka-plan–Meier log-rank test. For gene expression, P-values were calculated based on the Student’s t-test of the replicate 2−ΔCt values for each gene in the control and ascorbic acid treated groups. Western blot data were analyzed using a one-tailed unpaired Student’s t-test. A P-value less than 0.05 was considered statistically significant.
RESULTS
Primary Follicle Survival With Mouse Embryonic Fibroblast (MEF) Co-Culture
Primary follicles (average initial diameter of 60–70 μm) co-cultured with MEFs had low survival rates and exhibited the loss of follicle structure (Fig. 1). The survival rates were 23% for 70 μm (day 14), and 0% for 60 μm follicles (day 18). In non-surviving follicles, the oocyte typically separated from the somatic cells and degenerated. Analysis of the degenerating follicles by confocal imaging for laminin demonstrated the disruption of the basement membrane, despite the presence of the encapsulating alginate hydrogel. This phenotype suggested that the extracellular matrix (ECM) and/or basement membrane were insufficient to support follicle development.
Figure 1.
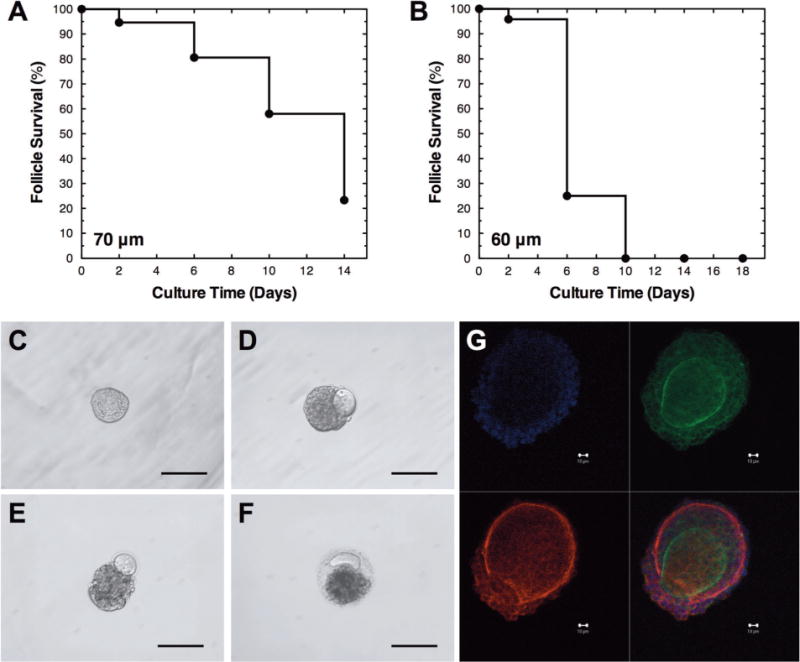
Primary follicle survival with mouse embryonic fibroblast (MEF) co-culture. The survival of (A) 70 μm and (B) 60 μm primary follicles cultured with MEFs. Representative images of primary follicles on (C) day 0, (D) day 6, (E) day 10, and (F) day 16. (G) Confocal images of representative non-growing follicle cultured on day 6 (red, laminin; green, f-actin; and blue, nucleus). Follicles have low survival, which is typically characterized by the loss of follicle structure, extruded oocyte, ruptured basement membrane, and degeneration. The scale bars represent (C–F) 100 μm and (G) 10 μm. Sample sizes are presented in Table I.
Literature searches identified ascorbic acid as a factor that influences ECM deposition/remodeling (Murray et al., 2001; Thomas et al., 2001), and microarray data was utilized to determine if the expression of genes within the ascorbate pathway changed during the primordial-to-primary and primary-to-secondary follicle transitions (Supplemental Table I). Follicles at the primordial and primary stage had a significant decline in mRNA levels for genes that encode enzymes within with the ascorbate/aldarate pathway, and a similar decline was observed in the primary-to-secondary follicle transition. This decline in expression of enzymes suggests that endogenous production of ascorbic acid may decline in vivo, as ascorbic acid may not be required given the presence of stromal cells and ECM. However, a decline in ascorbic acid production may limit the ability of the follicle to stimulate ECM deposition in response to damage that occurred due to isolation. The combination of basement membrane breakdown and altered expression of enzymes within the ascorbate pathway led to the hypothesis that ascorbic acid could limit basement membrane breakdown during early stage follicle culture. Hence, this hypothesis was investigated via ascorbic acid supplementation as a means to improve the structural integrity of developing primary follicles.
Follicle Survival in the Presence (±AA) of Ascorbic Acid
Secondary (average initial diameter of 90–100 μm) and primary follicles (average initial diameter of 60–80 μm) were cultured in the presence of 50 μg/mL ascorbic acid for 14–18 days. Ascorbic acid significantly enhanced the survival of 60–70 μm primary follicles (Fig. 2, and Table I) encapsulated in alginate hydrogels and co-cultured with MEFs, and these follicles cultured demonstrated similar morphologies to in vivo grown follicles (Fig. 3). In the presence of ascorbic acid, the survival rates at the end of follicle culture were 48% for 70 μm (day 14), and 17% for 60 μm follicles (day 18), a significant increase relative to the absence of ascorbic acid. The survival of 80, 90, and 100 μm follicles was not significantly impacted by the presence of ascorbic acid (Supplemental Fig. 1). Moreover, for all size classes, no significant differences were observed with respect to follicle growth between conditions.
Figure 2.
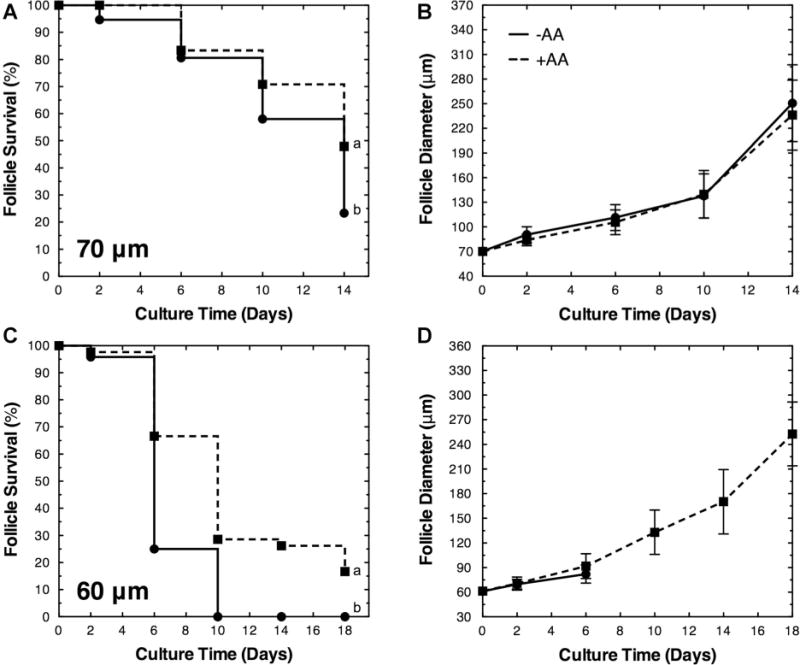
Ascorbic acid enhances the survival of primary follicles (60–70 μm). The survival and growth (diameter mean ± standard deviation) of (A and B) 70 μm and (C and D) 60 μm primary follicles cultured in the absence (−AA) and presence (+AA) of ascorbic acid. Significant differences between survival curves were indicated by different lower case letters (a and b, P < 0.05). No significant differences in growth were observed (P > 0.05). Sample sizes are presented in Table I.
Table I.
Survival and growth of primary follicles (60–70 μm).
| Size | Condition | Day 0 (n) | Day 2 (n, %) | Day 6 (n, %) | Day 10 (n, %) | Day 14 (n, %) | Day 18 (n, %) |
|---|---|---|---|---|---|---|---|
| 70μm | +AA | 48 | 48 (100%) | 40 (83%) | 34 (71%) | 23 (48%)* | n.d. |
| −AA | 150 | 142 (95%) | 121 (81%) | 87 (58%) | 35 (23%) | n.d. | |
| 60μm | +AA | 42 | 41 (98%) | 28 (67%) | 12 (29%) | 11 (26%) | 7 (17%)* |
| −AA | 24 | 23 (96%) | 6 (25%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Size | Condition | Day 0 (μm) | Day 2 (μm) | Day 6 (μm) | Day 10 (μm) | Day 14 (μm) | Day 18 (μm) |
|---|---|---|---|---|---|---|---|
| 70μm | +AA | 70 ±3 | 84 ±7 | 106 ± 15 | 140 ± 29 | 236 ± 43 | n.d. |
| −AA | 71 ±3 | 91 ±10 | 111 ± 16 | 138 ±27 | 251 ±47 | n.d. | |
| 60μm | +AA | 61 ±3 | 71 ±8 | 92 ±15 | 133 ±27 | 170 ± 39 | 253 ± 39 |
| −AA | 61 ±3 | 70 ±6 | 82±11 | n.s. | n.s. | n.s. |
−AA (70 μm) data referenced from Tagler et al. (2012).
−AA is control medium or αMEM supplemented with BSA, fetuin, ITS, and FSH.
Significant differences between conditions were indicated by asterisks (P < 0.05).
Diameter presented as mean ± standard deviation.
n.d. indicates experiment not done.
n.s. indicates no surviving follicles.
Figure 3.
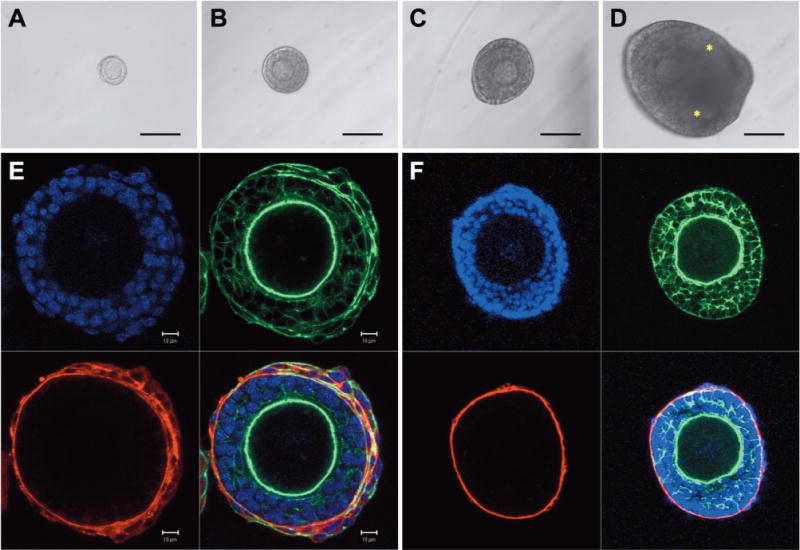
Primary follicle survival in the presence of ascorbic acid (+AA). Representative images of primary follicles (60–70 μm) cultured in the presence of ascorbic acid on (A) day 0, (B) day 6, (C) day 10, and (D) day 16. Confocal images of (E) growing follicle cultured with ascorbic acid on day 6 and (F) in vivo grown secondary follicle control (red, laminin; green, f-actin, and blue, nucleus). Ascorbic acid significantly increased the survival rate. In the presence of ascorbic acid, primary follicles grew to approximately 250 μm in diameter and developed antral cavities (asterisks). The scale bars represent (A–D) 100 μm and (E) 10 μm.
Oxidative Stress
Ascorbic acid was initially proposed based on its effects on ECM deposition/remodeling; however, ascorbic acid is also an antioxidant and we subsequently investigated whether the antioxidant properties contributed to follicle survival (Luck et al., 1995; Meister, 1994). Oxidative stress was measured as the total reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the conditioned medium (Fig. 4). The control medium (−AA) had ROS and RNS levels that did not significantly change throughout culture. Follicles cultured in the presence of ascorbic acid +AA) had similar levels of oxidative stress at day 2, with modest increases at day 4 and 6. As a control, an alternative antioxidant, glutathione (GSH), was added to the culture medium. Supplementing glutathione (0.2, 1, and 2 mM) did not improve follicle survival or growth compared to the control (−AA and −GSH; Supplemental Table II). Measurement of ROS and RNS levels with 0.2 mM glutathione indicated that the oxidative stress was comparable to the ascorbic acid condition (Fig. 4). The greatest concentration of glutathione tested (2 mM) substantially increased the ROS and RNS levels at the initial time point, which declined with time in culture. The modest levels of ROS and RNS in the control culture media combined with the inability of glutathione to improve follicle survival suggested that the antioxidant activity of ascorbic acid was not the critical factor influencing primary follicle survival.
Figure 4.
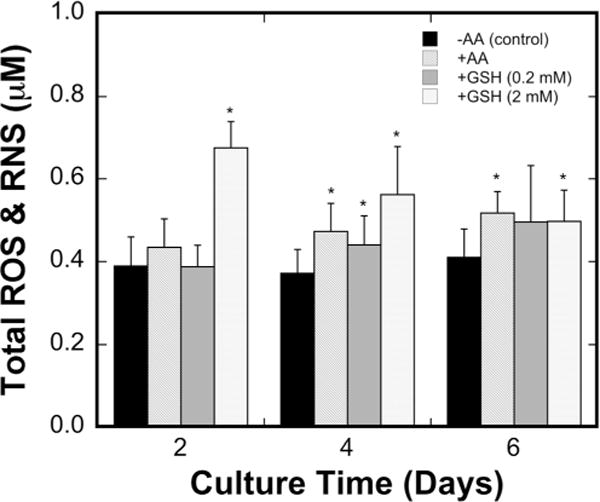
Oxidative stress in conditioned medium. Reactive oxygen species (ROS) and reactive nitrogen species (RNS; mean ± standard deviation) were measured in the control medium (−AA) and medium supplemented with ascorbic acid (+AA), 0.2 mM glutathione, and 2 mM glutathione on days 2, 4, and 6 of culture (n = 10 for control and n = 12 for AA/GSH conditions). Asterisks (*) indicate significant difference relative to control (P < 0.05).
Extracellular Matrix (ECM) and Cell Adhesion Molecules Gene (mRNA) Expression
The previously reported link between ascorbic acid and ECM deposition/remodeling (changes in protease and protease inhibitor expression; Murray et al., 2001; Thomas et al., 2001) was further investigated via the gene expression of 84 ECM and cell adhesion molecules. Primary follicles (<75 μm) were cultured for 6 days in the absence (−AA) and presence (+AA) of ascorbic acid. Significantly different gene expression levels between conditions were identified (Fig. 5, Table II [selected data], and Supplemental Table III [all data]). Of the 84 genes tested, 22 significantly changed between conditions (P < 0.05). Only seven of the significantly different genes had a fold change greater than two. Matrix metallopeptidase 10/12 (MMP-10/12), fibronectin 1 (Fn1), and collagen type IV alpha 2 (Col4a2) had a greater than twofold increase in gene expression. Cadherin 1 (Cdh1), ectonucleoside triphosphate diphosphohydrolase 1 (Entpd1), and integrin alpha 2 (Itga2) had a greater than twofold decrease in gene expression. Col4a2, Fn1, and Entpd1 are basement membrane components, and MMP-10/12 are proteases that can degrade collagen and fibronectin. Cdh1 and Itga2 are cell-adhesion molecules.
Figure 5.
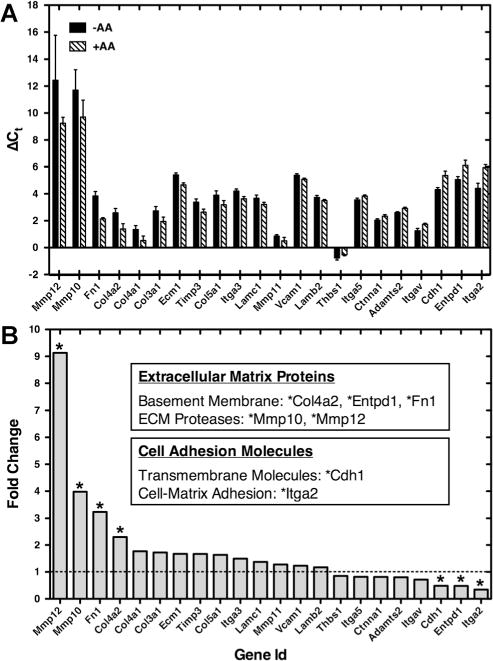
Ascorbic acid influences the gene expression of extracellular matrix (ECM) and cell adhesion molecules. (A) Significantly different (P < 0.05) ΔCt values (mean ± SD) of extracellular matrix (ECM) and cell adhesion molecules genes in the absence (−AA) and presence (+AA) of ascorbic acid. Ct is the number of cycles to exceed the analysis threshold (lower Ct values represent higher mRNA levels). ΔCt is the difference in gene expression between the gene of interest and a panel of housekeeping genes (Actb, B2m, and Gusb). (B) Fold change of genes in the absence (−AA) and presence (+AA) of ascorbic acid. Genes up-regulated greater than twofold (or down-regulated less than 0.5) were indicated with asterisks (*). Fold change was calculated as the normalized gene expression (2−ΔCt) of the AA+ sample divided the normalized gene expression (2−ΔCt) of the AA− sample.
Table II.
Gene expression of extracellular matrix (ECM) and cell adhesion molecules.
| Gene id | Description | Fold change | 95% CI | P-value |
|---|---|---|---|---|
| Significantly different (P < 0.05) and fold change greater than 2 or less than 0.5 | ||||
| * Mmp12 | Matrix metallopeptidase 12 | 9.13 | (0.00001, 29.91) | 0.0058 |
| * Mmp10 | Matrix metallopeptidase 10 | 3.98 | (0.00001, 9.30) | 0.0432 |
| * Fn1 | Fibronectin 1 | 3.24 | (2.48, 4.01) | 0.0000 |
| * Col4a2 | Collagen, type IV, alpha 2 | 2.31 | (1.52, 3.09) | 0.0073 |
| * Cdh1 | Cadherin 1 | 0.49 | (0.37, 0.60) | 0.0004 |
| * Entpd1 | Ectonucleoside triphosphate diphosphohydrolase 1 | 0.48 | (0.34, 0.63) | 0.0029 |
| * Itga2 | Integrin alpha 2 | 0.35 | (0.24, 0.45) | 0.0025 |
| Significantly different (P < 0.05) | ||||
| Col4a1 | Collagen, type IV, alpha 1 | 1.77 | (1.23, 2.30) | 0.0146 |
| Col3a1 | Collagen, type III, alpha 1 | 1.72 | (1.18, 2.26) | 0.0258 |
| Ecm1 | Extracellular matrix protein 1 | 1.67 | (1.43, 1.92) | 0.0007 |
| Timp3 | Tissue inhibitor of metalloproteinase 3 | 1.67 | (1.29, 2.04) | 0.0041 |
| Col5a1 | Collagen, type V, alpha 1 | 1.63 | (1.14, 2.13) | 0.0142 |
| Itga3 | Integrin alpha 3 | 1.49 | (1.26, 1.73) | 0.0023 |
| Lamc1 | Laminin, gamma 1 | 1.37 | (1.11, 1.64) | 0.0137 |
| Mmp11 | Matrix metallopeptidase 11 | 1.28 | (1.04, 1.51) | 0.0414 |
| Vcam1 | Vascular cell adhesion molecule 1 | 1.23 | (1.11, 1.35) | 0.0063 |
| Lamb2 | Laminin, beta 2 | 1.17 | (1.04, 1.31) | 0.0327 |
| Thbs1 | Thrombospondin 1 | 0.85 | (0.75, 0.96) | 0.0400 |
| Itga5 | Integrin alpha 5 (fibronectin receptor alpha) | 0.82 | (0.73, 0.91) | 0.0124 |
| Ctnna1 | Catenin (cadherin associated protein), alpha 1 | 0.81 | (0.73, 0.90) | 0.0095 |
| Adamts2 | A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 2 | 0.80 | (0.75, 0.86) | 0.0008 |
| Itgav | Integrin alpha V | 0.72 | (0.62, 0.81) | 0.0041 |
| Selected genes not significantly different (P > 0.05) | ||||
| Itgal | Integrin alpha L | 18.80 | (0.00001, 67.56) | 0.1597 |
| Itgam | Integrin alpha M | 14.84 | (0.00001, 42.17) | 0.0870 |
| Itga4 | Integrin alpha 4 | 6.97 | (0.00001, 25.77) | 0.1044 |
| Mmp9 | Matrix metallopeptidase 9 | 5.48 | (0.00001, 14.01) | 0.0807 |
| Syt1 | Synaptotagmin I | 4.96 | (0.00001, 15.09) | 0.1541 |
| Col2a1 | Collagen, type II, alpha 1 | 3.85 | (0.00001, 13.90) | 0.1954 |
| Thbs2 | Thrombospondin 2 | 3.28 | (0.06, 6.50) | 0.0513 |
| Col1a1 | Collagen, type I, alpha 1 | 3.18 | (0.76, 5.61) | 0.0529 |
| Timp1 | Tissue inhibitor of metalloproteinase 1 | 1.05 | (0.88, 1.22) | 0.4937 |
CI, confidence interval.
Indicates fold change greater than 2 (or less than 0.5) and statistically significant (P < 0.05).
Western Blots
The variations in gene expression were confirmed by performing Western blots for fibronectin, which exhibited significantly up-regulated gene expression in the presence of ascorbic acid. Primary follicles (<75 μm) were cultured for 7 days. Follicles cultured with ascorbic acid (+AA) produced a denser band for fibronectin than those cultured without ascorbic acid (−AA; Fig. 6). After determining the optical densities of the bands, normalized values were determined by calculating the ratio of the density of the band for fibronectin to that of α-tublin for each given sample. The +AA group was significantly greater than the −AA group.
Figure 6.
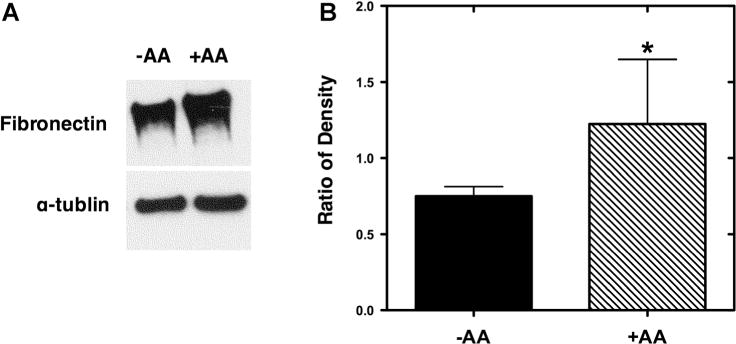
Western blots confirm fibronectin up-regulation. (A) Western blots were performed on follicles cultured in the absence (−AA) and presence (+AA) of ascorbic acid. To normalize differences in follicle numbers between experiments, α-tublin was used as a standard. (B) Densitometry ratios (mean ± standard deviation) of fibronectin compared to α-tublin (n = 3 for each condition). Significant differences between conditions were indicated via asterisks (*P < 0.05).
Discussion
The in vitro culture of primordial and primary follicles has been challenging (Abir et al., 1999; Itoh and Hoshi, 2000; Muruvi et al., 2005, 2009; Picton et al., 2008; Saha et al., 2000; Xu et al., 2013), in part due to the inability to maintain follicle structure. Follicle culture systems that utilize flat surfaces (Lenie et al., 2004) can support the growth of mouse primary follicles (80–90 μm), although physical manipulations are required to prevent somatic cell attachment and migration away from the oocyte. Some success has been achieved via in situ organ culture or two-step culture systems (Eppig and O’Brien, 1996; Jin et al., 2010). As with organ culture, hydrogels that encapsulate follicles can maintain the architecture and preserve cell–cell connections (Hornick et al., 2012). However, for the culture of early stage follicles in hydrogels, co-culture with supporting cells, such as stromal cells, is necessary to promote survival and growth (Hornick et al., 2013; Tagler et al., 2012; Tingen et al., 2011). More recently, mouse embryonic fibroblast (MEF) co-culture supported the growth of early secondary (90–100 μm) and late primary (70–80 μm) follicles; however, the survival of developing primary follicles was low and smaller follicles (<70 μm) rapidly degenerated (Tagler et al., 2012). To improve the survival of primary follicles, this study investigated ascorbic acid in order to enhance the extracellular matrix (ECM) and prevent separation of the oocyte from the somatic cells.
Ascorbic acid enhanced the survival of primary follicles (60–70 μm), complementing previous research that observed the improved survival of secondary and multilayer follicles (150–200 μm; Murray et al., 2001; Rose et al., 1999; Thomas et al., 2001). Ascorbic acid has been used as a component of various follicle/oocyte culture systems and studies (Dalvit et al., 2005; Durlinger et al., 2001; Eppig et al., 1998; Kim et al., 2004; Mao et al., 2002; McLaughlin and Telfer, 2010; Nation and Selwood, 2009; Nayudu et al., 2003; Nilsson et al., 2002; O’Brien et al., 2003; Rossetto et al., 2009; Tao et al., 2010; Telfer et al., 2008; Vitt et al., 1998; Wu et al., 2001). The study herein focused on primary follicles, and the presence of ascorbic acid improved follicle survival. Non-surviving follicles were observed to extrude the oocyte from the somatic cells, an obvious disruption in the basement membrane of the follicle. In the presence of ascorbic acid, follicle survival increased, and it was hypothesized that enhanced ECM would maintain the follicle architecture. The basement membrane, and more generally the ECM within the follicle, plays a critical role in various ovarian cell functions, such as adhesion, migration, survival, differentiation, and proliferation, via mechanical and chemical signals (Berkholtz et al., 2006b; Irving-Rodgers and Rodgers, 2006; Kreeger et al., 2006; Rodgers et al., 2003; Woodruff and Shea, 2007). The composition of the ECM in the murine follicles has been characterized, with collagen type IV and fibronectin present in the theca cell layer and basement membrane throughout follicle development (Berkholtz et al., 2006a; Rodgers et al., 1998).
The hypothesized connection between ascorbic acid and ECM deposition/remodeling in primary follicles was investigated through gene expression for numerous ECM proteins or enzymes. Collagen type IV, fibronectin, matrix metalloproteinase 10 (MMP-10), and matrix metalloproteinase 12 (MMP-12) were significantly different in the presence of ascorbic acid. Consistent with our results, previous studies investigating ascorbic acid identified significant changes in collagen (Hulmes, 1992; Luck et al., 1995; Ono et al., 1990; Pinnell, 1985). The enzymes MMP-10 and MMP-12 are reported to contribute to remodeling of collagen type IV, fibronectin, elastin, and plasminogen (Smith et al., 2002). Moreover, significant changes have been observed in matrix metalloproteinase (MMP-9; Thomas et al., 2001) and tissue inhibitor of metalloproteinase 1 (TIMP-1; Murray et al., 2001) due to ascorbic acid. Interestingly, changes in MMP-9 and TIMP-1 were not observed in our study. These differences may be attributable to follicle size class (primary vs. secondary/multilayer), animal species (mouse vs. bovine), or culture system (alginate hydrogel vs. tissue culture plates). The proposed hypothesis is that changes in gene expression function to strengthen the basement membrane of the developing primary follicles, preventing the loss of follicular structure (oocyte separating from the somatic cells). In contrast to secondary follicles, which have been cultured effectively without ascorbic acid, primary follicles may lack the ECM required to maintain follicular structure.
In addition to ECM proteins, significant changes in the gene expression of cell adhesion molecules were observed. Cadherin 1, which has been associated with oocyte/follicle development (Blaschuk and Farookhi, 1989; Carabatsos et al., 2000; Machell et al., 2000; Mackay et al., 1999; Wang and Roy, 2010), was significantly down-regulated by ascorbic acid. Integrin alpha 2, which was similarly downregulated with ascorbic acid, has been observed on granulosa cells boarding the basement membrane (Giebel and Rune, 1997; Giebel et al., 1996; Wehrenberg and Rune, 2000; Yamada et al., 1999). The down-regulation of both cadherin 1 and integrin alpha 2 is consistent with previous research that observed decreasing expression during primordial-to-primary-to-secondary follicle development (Giebel et al., 1996; Machell et al., 2000). These results demonstrate that ascorbic acid stimulates changes in cell adhesion molecules that are consistent with normal follicle development.
Ascorbic acid may also function as an antioxidant or free radical scavenger to improve follicle survival (Luck et al., 1995; Meister, 1994). Oxidative stress or the imbalance of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) is associated with cell damage/death and various reproductive diseases (Agarwal et al., 2012). However, in this study, ascorbic acid was not observed to lower the levels of ROS and RNS in conditioned medium from primary follicles co-cultured with MEFs. Previous studies have connected ascorbic acid to granulosa and theca cell death/proliferation (Duleba et al., 2004; Thomas et al., 2001; Tilly and Tilly, 1995). Other antioxidants, such as glutathione and vitamin E, also have been observed to improve embryo/follicle/ovary culture (Eppig et al., 2000; Nugent et al., 1998; Olson and Seidel, 2000; Tao et al., 2010; Wang et al., 2002). In this study, glutathione was tested but did not improve primary follicle survival, which suggested that antioxidant activity was not the critical factor. Stage-specific differences between secondary/multilayer follicles from previous studies and primary follicles reported herein may explain this discrepancy. Alternatively, glutathione may not be deficient or a limiting factor in our system.
In conclusion, we have demonstrated that ascorbic acid improves the survival of primary mouse ovarian follicles (60–70 μm). This culture system combined biomaterials (alginate hydrogels), co-culture with MEFs, and medium containing ascorbic acid to simultaneously support follicle structure/survival and stimulate growth. Without ascorbic acid, 60 μm primary follicles rapidly lost structure and degenerated within 10 days. In contrast, follicles cultured in the presence of ascorbic acid survived for 18 days and increased to diameters exceeding 250 μm. The mechanism of action involves gene/protein expression changes associated with ECM and cell adhesion molecules, though additional effects are possible. Future research may lead to novel strategies for in vitro follicle culture and fertility preservation.
Supplementary Material
Acknowledgments
The authors would like to thank Eugene Galdones, Ph.D. and Anaar Siletz, Ph.D. for technical assistance with the early stage follicle development microarray studies and Brendan Tran for technical assistance with the ascorbic acid supplementation studies. This research was supported by grants from the National Institutes of Health (NIH) (PL1EB008542), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPIR) (U54HD41857 and U54HD076188), National Health and Medical Research Council (NHMRC; Australia; C.J. Martin Fellowship to Y.M., GNT1016460), and Northwestern University (NIH Biotechnology Training Program and McCormick School of Engineering Fellowships to D.T.). The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NICHD, NHMRC, or Northwestern University. No competing financial interests exist.
Contract grant sponsor: National Institutes of Health (NIH)
Contract grant number: PL1EB008542 Contract grant sponsor: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
Grant numbers: U54HD41857; U54HD076188
Contract grant sponsor: National Health and Medical Research Council (NHMRC; Australia)
Contract grant number: GNT1016460
Contract grant sponsor: Northwestern University
Footnotes
Authors contributed equally to manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: Clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21(8):887–898. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14(5):1299–1301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006a;126(5):583–592. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006b;24(4):262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk OW, Farookhi R. Estradiol stimulates cadherin expression in rat granulosa cells. Dev Biol. 1989;136(2):564–567. doi: 10.1016/0012-1606(89)90283-2. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocytegranulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226(2):167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- Dalvit G, Llanes SP, Descalzo A, Insani M, Beconi M, Cetica P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod Domest Anim. 2005;40(2):93–97. doi: 10.1111/j.1439-0531.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- Du P, Kibbe W, Lin S. Lumi: A pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19(7):1519–1524. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Mouse oocyte development in vitro with various culture systems. Dev Biol. 1977;60(2):371–388. doi: 10.1016/0012-1606(77)90135-x. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163(1–2):109–116. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: Follicle-stimulating hormone and insulin. Biol Reprod. 1998;59(6):1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- Giebel J, de Souza P, Rune GM. Expression of integrins in marmoset (Callithrix jacchus) ovary during folliculogenesis. Tissue Cell. 1996;28(4):379–385. doi: 10.1016/s0040-8166(96)80024-3. [DOI] [PubMed] [Google Scholar]
- Giebel J, Rune GM. Relationship between expression of integrins and granulosa cell apoptosis in ovarian follicles of the marmoset (Callithrix jacchus) Tissue Cell. 1997;29(5):525–531. doi: 10.1016/s0040-8166(97)80053-5. [DOI] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27(6):1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145(1):19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJ. The collagen superfamily-diverse structures and assemblies. Essays Biochem. 1992;27:49–67. [PubMed] [Google Scholar]
- Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24(4):195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- Itoh T, Hoshi H. Efficient isolation and long-term viability of bovine small preantral follicles in vitro. In Vitro Cell Dev An. 2000;36(4):235–240. doi: 10.1290/1071-2690(2000)036<0235:EIALTV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93(8):2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, Gosden RG. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82(3):679–685. doi: 10.1016/j.fertnstert.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73(5):942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71(5):1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36(2):e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol Reprod. 1995;52(2):262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- Machell NH, Blaschuk OW, Farookhi R. Developmental expression and distribution of N- and E-cadherin in the rat ovary. Biol Reprod. 2000;63(3):797–804. doi: 10.1095/biolreprod63.3.797. [DOI] [PubMed] [Google Scholar]
- Mackay S, Nicholson CL, Lewis SP, Brittan M. E-cadherin in the developing mouse gonad. Anat Embryol (Berl) 1999;200(1):91–102. doi: 10.1007/s004290050263. [DOI] [PubMed] [Google Scholar]
- Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN. Effects of culture medium, serum type, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod. 2002;67(4):1197–1203. doi: 10.1095/biolreprod67.4.1197. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010;139(6):971–978. doi: 10.1530/REP-10-0025. [DOI] [PubMed] [Google Scholar]
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–1013. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269(13):9397–9400. [PubMed] [Google Scholar]
- Murray AA, Molinek MD, Baker SJ, Kojima FN, Smith MF, Hillier SG, Spears N. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction. 2001;121(1):89–96. doi: 10.1530/rep.0.1210089. [DOI] [PubMed] [Google Scholar]
- Muruvi W, Picton HM, Rodway RG, Joyce IM. In vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology. 2005;64(6):1357–1370. doi: 10.1016/j.theriogenology.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Muruvi W, Picton HM, Rodway RG, Joyce IM. In vitro growth and differentiation of primary follicles isolated from cryopreserved sheep ovarian tissue. Anim Reprod Sci. 2009;112(1–2):36–50. doi: 10.1016/j.anireprosci.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Nation A, Selwood L. The production of mature oocytes from adult ovaries following primary follicle culture in a marsupial. Reproduction. 2009;138(2):247–255. doi: 10.1530/REP-09-0028. [DOI] [PubMed] [Google Scholar]
- Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95(2):349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- Nayudu PL, Wu J, Michelmann HW. In vitro development of marmoset monkey oocytes by pre-antral follicle culture. Reprod Domest Anim. 2003;38(2):90–96. doi: 10.1046/j.1439-0531.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188(1–2):65–73. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114(2):341–346. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Olson SE, Seidel GE. Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol Reprod. 2000;62(2):248–252. doi: 10.1095/biolreprod62.2.248. [DOI] [PubMed] [Google Scholar]
- Ono M, Aratani Y, Kitagawa I, Kitagawa Y. Ascorbic acid phosphate stimulates type IV collagen synthesis and accelerates adipose conversion of 3T3-L1 cells. Exp Cell Res. 1990;187(2):309–314. doi: 10.1016/0014-4827(90)90096-s. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9(5):1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction. 2011;142(2):309–318. doi: 10.1530/REP-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136(6):703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- Pinnell SR. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J Biol Med. 1985;58(6):553–559. [PMC free article] [PubMed] [Google Scholar]
- Rodgers HF, Irvine CM, van Wezel IL, Lavranos TC, Luck MR, Sado Y, Ninomiya Y, Rodgers RJ. Distribution of the alpha1 to alpha6 chains of type IV collagen in bovine follicles. Biol Reprod. 1998;59(6):1334–1341. doi: 10.1095/biolreprod59.6.1334. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126(4):415–424. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- Rose UM, Hanssen RG, Kloosterboer HJ. Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol Reprod. 1999;61(2):503–511. doi: 10.1095/biolreprod61.2.503. [DOI] [PubMed] [Google Scholar]
- Rossetto R, Figueiredo JR, Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domest Anim Endocrinol. 2009;37(2):112–123. doi: 10.1016/j.domaniend.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Saha S, Shimizu M, Geshi M, Izaike Y. In vitro culture of bovine preantral follicles. Anim Reprod Sci. 2000;63(1–2):27–39. doi: 10.1016/s0378-4320(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod. 1997;12(3):403–405. [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30(29):5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory RM, Bernabé BP, Galdones E, Broadbelt LJ, Shea LD, Woodruff TK. Microarray analysis identifies COMP as the most differentially regulated transcript throughout in vitro follicle growth. Mol Reprod Dev. 2013;80(2):132–144. doi: 10.1002/mrd.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MF, Ricke WA, Bakke LJ, Dow MPD, Smith GW. Ovarian tissue remodeling: Role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191(1):45–56. doi: 10.1016/s0303-7207(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Smyth G. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(1):1544–6115. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD. Supplemented aMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2013;110(12):3258–3268. doi: 10.1002/bit.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, Shea LD. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng A. 2012;18(11–12):1229–1238. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, Liu Y, Fang FG, Ding JP, Zhang XR. Effects of L-ascorbic acid, alpha-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim. 2010;45(1):19–25. doi: 10.1111/j.1439-0531.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Leask R, Srsen V, Riley SC, Spears N, Telfer EE. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction. 2001;122(3):487–495. doi: 10.1530/rep.0.1220487. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136(1):242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, Woodruff TK. A macrophage and theca cell enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–820. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87(1):367–374. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Kloosterboer HJ, Rose UM, Mulders JW, Kiesel PS, Bete S, Nayudu PL. Isoforms of human recombinant follicle-stimulating hormone: Comparison of effects on murine follicle development in vitro. Biol Reprod. 1998;59(4):854–861. doi: 10.1095/biolreprod59.4.854. [DOI] [PubMed] [Google Scholar]
- Wang C, Roy SK. Expression of E-cadherin and N-cadherin in perinatal hamster ovary: Possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology. 2010;151(5):2319–2330. doi: 10.1210/en.2009-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–1277. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Rune GM. Spontaneous luteinization of antral marmoset follicles in vitro. Mol Hum Reprod. 2000;6(6):504–509. doi: 10.1093/molehr/6.6.504. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK. The emergence of a new interdiscipline: Oncofertility. Cancer Treat Res. 2007a;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. Oncofertility: Fertility preservation for cancer survivors. New York: Springer; 2007b. [Google Scholar]
- Woodruff TK. The Oncofertility Consortium–addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7(8):466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14(8 Suppl):6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod. 2001;64(1):375–381. doi: 10.1095/biolreprod64.1.375. [DOI] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28(8):2187–2200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: Effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26(5):1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009a;24(10):2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006a;12(10):2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006b;75(6):916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009b;81(3):587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Fujiwara H, Honda T, Higuchi T, Nakayama T, Inoue T, Maeda M, Fujii S. Human granulosa cells express integrin alpha2 and collagen type IV: Possible involvement of collagen type IV in granulosa cell luteinization. Mol Hum Reprod. 1999;5(7):607–617. doi: 10.1093/molehr/5.7.607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


