Abstract
Gene delivery from biomaterials can create an environment that promotes and guides tissue formation. However, the immune response induced upon biomaterial implantation can be detrimental to tissue regeneration. Macrophages play a central role in mediating early phases of this response, and functional “polarization” of macrophages towards M1 (inflammatory) or M2 (anti-inflammatory) phenotypes may bias the local immune state at the implant site. Since gene delivery from biomaterial scaffolds can confer transgene expression in macrophages in vivo, we investigated whether transduction of macrophages with an IL-10 encoding lentivirus can (1) induce macrophage polarization toward an M2 phenotype even in an pro-inflammatory environment, and (2) prevent a shift in polarization from M2 to M1 following exposure to pro-inflammatory stimuli. IL-10 lentivirus delivery to pre-polarized M1 macrophages reduced TNF-α production 1.5-fold when compared to cells treated with either a control virus or a bolus delivery of recombinant IL-10 protein. IL-10 lentivirus delivery to naïve macrophages reduced the amount of TNF-α produced following an inflammatory challenge by 2.5-fold compared to cells treated with both the control virus and recombinant IL-10. At a mechanistic level, IL-10 lentivirus delivery mediated sustained reduction in NF-κB activation and, accordingly, reduced transcription of TNF-α. In sum, lentiviral delivery of IL-10 to macrophages represents a promising strategy for directing and sustaining macrophage polarization towards an M2 phenotype in order to promote local immune responses that facilitate tissue engineering.
Keywords: macrophage, macrophage phenotype, gene delivery, lentivirus, IL-10, transduction efficiency
Introduction
Regenerative medicine employs biomaterial scaffolds to support and promote tissue formation, and the innate immune response to the implanted biomaterials plays a crucial role in that post-injury microenvironment. The implantation and presence of foreign materials and damage-associated molecular patterns (DAMPs) trigger an immediate host immune response, primarily resulting from infiltration of neutrophils and monocytes. Infiltrating monocytes differentiate into macrophages as they enter the tissue and produce inflammatory cytokines (e.g., TNF-α, IFN-γ) and reactive oxygen species (ROS), leading to secondary tissue damage and scarring (Anderson et al., 2008; Boehler et al., 2011; Jones, 2008). The resulting tissue damage can be detrimental to regeneration or engraftment of implanted cells. Cell death causes extracellular release of high mobility group box 1 protein (HMGB1), which activates macrophages through Toll-like receptor 4 (TLR4), a receptor that also responds to microbial lipopolysaccharide (LPS) and induces robust immune activation during sepsis (Bell et al., 2006; Kim et al., 2013). However, macrophages also promote wound healing and tissue regeneration by secreting growth factors (Duffield, 2003) and phagocytosing cell debris (Xu et al., 2006), and preventing macrophage infiltration can lead to more extensive tissue damage and decreased ability to regenerate (Shechter et al., 2009; Tidball and Wehling-Henricks, 2007). An emerging concept in biomaterial-based applications involves targeting macrophages, through surface properties or gene/drug delivery, as a means to promote a regenerative response by macrophages (Boehler et al., 2011; Brown et al., 2009; Mokarram et al., 2012). We have recently reported on the localized expression of IL-10 as a means to influence immune cell infiltration in vivo (Gower et al., in press).
Harnessing the beneficial facets of the innate immune response while inhibiting the potential for injury is an attractive therapeutic strategy. Macrophage commitment to various phenotypes, which is termed functional “polarization,” has been described using a continuum ranging from classically activated, inflammatory (M1) states to alternatively activated, predominantly anti-inflammatory (M2) states (Murray and Wynn, 2011). The natural resolution of local immune responses involves a transition between these phenotypes (Martinez et al., 2008). This transition in macrophage phenotype can be induced in response to the presence of M2 cytokines such as IL-10 (Martinez et al., 2008), phagocytosis of cellular debris (Xu et al., 2006), or desensitization mediated by negative regulatory mechanisms induced following repeated exposure to inflammatory stimuli (Lawrence and Natoli, 2011; Murray and Wynn, 2011; Porta et al., 2009). M2 macrophages produce functional mediators including IL-10 and arginase-1 (Herbert et al., 2010; Murray and Wynn, 2011; Pesce et al., 2009), which inhibit production of inflammatory cytokines (Fiorentino et al., 1991) and ROS (Dokka et al., 2001). Thus, this distinct M2 phenotype enables macrophages to perform regenerative functions such as phagocytosis without promoting further secondary tissue damage.
Macrophages can adopt various phenotypes across the M1-M2 spectrum (Mosser and Edwards, 2008), and these phenotypes may change over time following changing cues in the extracellular environment. Experimentally, this “plasticity” has been investigated by sequentially exposing macrophages to pro-M1 and pro-M2 stimuli. Macrophages tend to bias their phenotype towards the first cytokine delivered and at least partially ignore subsequent stimuli, arguably due to cross-inhibition by opposing cytokine signal transduction pathways and downstream regulators (Erwig et al., 1998; Fiorentino et al., 1991). The ability of IL-10 and IFN-γ to inhibit signaling by the alternative pathway contributes to this bias towards the initial cytokines (Fiorentino et al., 1991; Herrero et al., 2003). Since reversing the initial phenotype may be challenging, in vitro studies in which the initial stimulus was removed and replaced with a second stimulus caused macrophages to transition between M1- and M2-like phenotypes (Porcheray et al., 2005; Stout et al., 2005). This plasticity may underlie the natural resolution of the immune response, and phenotype switching has been hypothesized (although not explicitly demonstrated) to occur in vivo (Arnold et al., 2007; Lee et al., 2011). Interestingly, chronic inflammation promotes sustained polarization towards an M1 phenotype, which does not resolve towards M2 over time (Kigerl et al., 2009; Pruss et al., 2011). Given these combined observations, we hypothesize that creating and sustaining an anti-inflammatory immune microenvironment at a biomaterial scaffold may be necessary to induce and retain an M2 macrophage-dominated host response that promotes regeneration in vivo. This hypothesis motivated this investigation into the efficacy of sustaining an anti-inflammatory environment to modulate macrophage phenotypes.
Herein, we investigated the capacity of lentiviral gene delivery to induce macrophage polarization to an M2 phenotype and limit or prevent phenotype switching to M1. Gene delivery was investigated based upon our previous observations that macrophages were transduced in vivo following gene delivery from biomaterial scaffolds (Boehler et al., 2013; Tuinstra et al., 2012), which provides an opportunity to directly influence macrophage phenotype. Gene delivery also enables sustained production of IL-10, which we hypothesized may promote an anti-inflammatory macrophage phenotype even in the presence of inflammatory stimuli. Initial studies investigated lentivirus gene delivery for its ability to transduce macrophages, with subsequent studies investigating the ability to overcome an inflammatory environment to induce and maintain an M2 phenotype. Creating and sustaining a local anti-inflammatory microenvironment to promote an M2 phenotype has numerous applications for enhancing regeneration or promoting the survival and function of transplanted cells in numerous regenerative medicine applications.
Materials and Methods
Cell Culture
RAW 264.7 cells (hereafter, RAW cells) and HEK-293T cells (Invitrogen, Carlsbad, CA) were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin, 1% sodium pyruvate, and 3.7 g/L sodium bicarbonate at 37°C and 5% CO2. RAW cells were passaged using 0.05 mM EDTA treatment followed by removal with a cell scraper.
Bone Marrow Isolation and Macrophage Differentiation
Bone marrow derived macrophages (BMDM) were harvested from the femurs of male 4–6 week-old C57BL/6 mice (Charles River) and cultured in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% FBS (Life Technologies), 1% penicillin/streptomycin (Life Technologies), and 20% L929 conditioned media in untreated cell culture plates. Media was replaced after 3 days. On day 7, cells were removed using 0.05 mM EDTA treatment followed by removal by rinsing or cell scraper (if necessary) and assayed for differentiation using flow cytometry for CD11b, as explained below.
Plasmids and Reagents
Human IL-10 was amplified from pUbC-hIL-10 (provided by Prof. David Dean, University of Rochester Medical Center) and ligated into the viral plasmid backbone, HIV-CSCG (provided by Prof. David Schaffer, University of California, Berkeley) using NheI and XhoI (New England Biosciences, Ipswich, MA). Lentivirus reporter constructs containing enhancer elements bound by NF-κB and encoding firefly luciferase were employed to assay transcription factor (TF) activity along with a negative control construct containing only a TATA box promoter (Panomics, Freemont, CA) by live imaging (Weiss et al., 2012). Ligated plasmids were transformed into DH5α cells (Invitrogen). Plasmids were purified using Qiagen reagents and stored in Tris–EDTA buffer (Qiagen, Venlo, Limburg, Netherlands) at −20°C.
Virus Production
Lentivirus was produced by co-transfecting HEK-293T cells with lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G) and vectors encoding the gene of interest (pLenti-CMV-hIL-10, pLenti-CMV-Luciferase, or pLenti-CMV-GFP) or a reporter construct (pLenti-TA-Luc or pLenti-NF-κB-Luc) using Lipofectamine 2000 (Roche Biosciences, Basel, Switzerland). After 48 h, supernatants were collected, cell debris was pelleted by low speed centrifugation, and clarified supernatant was removed. Viruses were concentrated using PEG-it (Systems Biosciences, Mountain View, CA) and re-suspended in PBS. Physical virus particle titers as determined by detection of viral genomes, (hereafter, “VP”), was quantified using a Lentivirus qPCR Titer Kit (Applied Biological Materials, Richmond, BC, Canada).
Live Imaging of Gene Expression
RAW or BMDM cells were initially seeded with 280 or 2,800 physical virus particles (VP)/cell of luciferase lentivirus (vLuc) and substrate D-luciferin (1 mM; Caliper, Hopkinton, MA) was added 4 h prior to imaging. For BMDM, luciferase expression was assayed at 3 and 4 days post-transduction. Cells were assayed daily for transgene expression of luciferase by adding the substrate and incubating for 4 h before imaging on the IVIS Spectrum (Caliper).
Transduction and Cytokine Treatment
Macrophages were seeded and cultured for 3 days, media was changed, and cells were exposed to one of four treatment options: (i) addition of cytokine, virus, or control treatment for 48 h followed by exposure to LPS (100 ng/mL; Sigma–Aldrich, Saint Louis, MO), (ii) cells were treated with a simultaneous delivery of anti-inflammatory treatment/ control treatment and an pro-inflammatory (pro-M1) stimulus consisting of IFN-γ (10 ng/mL, R&D Systems, Minneapolis, MN) and LPS (100 ng/mL), (iii) cells were treated with a staggered delivery with pro-M1 stimuli followed by an anti-inflammatory treatment/control treatment 24 h later, and (iv) cells were first treated with an anti-inflammatory treatment/control treatment for 48 h followed by media removal and replacement with media containing pro-M1 stimuli. Media was only changed after treatment in the fourth treatment condition. Anti-inflammatory/control treatment variables were no treatment, human recombinant IL-10 protein (10 ng/mL; R&D Systems), 280 VP/cell of GFP lentivirus (vCtrl), and 280 VP/cell of human IL-10 lentivirus (vhIL-10). A dose–response experiment was performed using a range of IL-10 concentrations, which demonstrated that concentrations >10 ng/mL did not affect TNF-α production (Supplementary Fig. S1). Recombinant human IL-10 was delivered in order to separately measure murine IL-10 produced by the macrophages. Concentrations of murine TNF-α and IL-10 in the supernatant were subsequently assayed by ELISA (R&D systems) according to manufacturer instructions. Note that the ELISA kit used for IL-10 quantification allows for distinguishing human IL-10 from murine IL-10, as the antibodies are not cross reactive as reported by the manufacturer and confirmed in our validation studies (data not shown). After 3 days of treatment (a total of 6 days post-cell seeding), supernatants were collected, and cell debris was spun down at 20,000g for 10 min at 4°C.
Quantitative PCR
RNA was isolated using an RNA Easy Minikit and reverse transcribed by an Omniscript RT kit (Qiagen). Transcripts were quantified using Taqman Gene expression assays for TNF-α and GAPDH (endogenous control) and Taqman Universal PCR mix (Applied Biosystems, Carlsbad, CA) on a Bio-Rad iCycler. Data were analyzed using the ΔΔCt method. Negative fold difference values were calculated by taking the negative inverse of the fold difference if the initial fold difference was <1.
Live Imaging of Transcription Factor (TF) Activity Cell Arrays
For TF reporter cell arrays, BMDM with 280 VP/cell of NF-κB TF reporter or TA control lentivirus and cultured for 2.5 days before replacing the differentiation media and adding D-luciferin (1 mM; Caliper). After 72 h post-seeding, cells were exposed to one of four treatment options that are discussed above and imaged at 0 (pre-treatment), 2, 4, 8, 12, and 24 h each day for the 3 days of treatment. D-Luciferin was added to the wells daily. TF array data were analyzed by first normalizing to pre-treatment time point and then to the corresponding TA condition.
Flow Cytometry
RAW cells were seeded with 280 or 2,800 VP/cell of GFP lentivirus (vGFP). The 280 VP/cell condition was also tested in the presence of M1 cytokines. For BM cells, only 280 VP/cell condition was tested and transduction was also assayed at 3 and 4 days post-seeding (+/− M1 cytokines). Cells were suspended by scraping in EDTA and blocked with a solution containing 1% normal mouse and rat serum (Sigma–Aldrich) and anti-mouse CD16/32 (eBioscience, San Diego, CA) to block Fc receptors. Cells were then stained for viability using DAPI (Invitrogen) and stained for differentiation with APC-conjugated M1/70 against CD11b (BD Biosciences, San Jose, CA). Data were acquired on a BD LSR II cytometer and analyzed using FlowJo software (Treestar, Ashland, OR).
Statistical Analysis
For multiple comparisons, statistical significance between groups was determined by ANOVAwith post-hoc testing. For single comparisons, the statistical significance between pairs was determined by unpaired two-tailed t-test. All statistics test significance using a P-value of 0.05 unless noted. Error bars represent SE in all figures and n refers to biological replicates. Prism (GraphPad) software was used for all data analysis.
Results
Transgene Expression From RAW Macrophage Cell Line
Lentiviral gene delivery to RAW macrophages, which exhibit phenotypes that span the spectrum from M1 to M2 and thus comprise a suitable model system for this investigation, was initially investigated to determine the appropriate viral dose for potentially modulating phenotype. Preliminary results using lentivirus encoding for GFP determined that the ratio of physical virus particles (quantified by qPCR) to infectious units (quantified by flow cytometry of transduced HEK 293T cells) was approximately 280:1 (data not shown). Therefore, RAW cells were transduced with 280 and 2,800 physical virus particles per cell (VP/cell), which corresponds to a multiplicity of infection (MOI) of 1 and 10, respectively, in HEK 293T cells. The lower virus dose resulted in 5% GFP+ RAW cells, with the higher dose generated 35% GFP+ cells (Fig. 1A). Live imaging of cells transduced with virus encoding luciferase demonstrated that the lower virus dose generated detectable luciferase output by day 3, and the higher dose generated detectable luciferase output by day 2 (Fig. 1B). Furthermore, the higher dose also produced significantly greater luciferase activity (≈2-fold) at the later time points relative to the low dose (Fig. 1B). Finally, RAW cells transduced with 280 VP/cell of GFP-encoding virus in the presence of M1 cytokines (IFN-γ + LPS) significantly increased transduction efficiency, suggesting that M1 polarization does not preclude lentiviral transduction and supporting our overall strategy (Fig. 1C).
Figure 1.

Lentivirus dose and transgene expression from RAW macrophage cell line. (A) Transduction efficiency was assessed by flow cytometry detection of GFP at day 3 (n = 3–6). Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test (P < 0.001). *Significant difference compared to 0 VP/cell. **Significant difference compared to both 0 and 280 VP/cell. (B) Transgene expression was observed using live imaging of luciferase expression (n = 3–4). Statistical analysis completed using a two-way ANOVA with Bonferronis post-hoc test. *Significant difference compared to background (P < 0.01). **Significant difference compared to both background and 280 VP/cell (P < 0.05). (C) Transduction efficiency was tested in the presence of inflammatory cytokines (n = 4–6). Statistical analysis completed using an unpaired t-test. **Significant difference compared to 280 VP/cell (P < 0.0001).
IL-10 Lentivirus Delivery to Macrophages Promotes an M2 Phenotype
To establish a simple system for evaluating macrophage polarization, the M1 phenotype was defined as high expression of TNF-α and low expression of IL-10, and M2 was defined as the opposite profile. IFN-γ pre-treatment of RAW cells for 48 h followed by LPS activation induced a cytokine secretion profile characteristic of an M1 phenotype (Fig. 2). Conversely, 48 h pre-treatment with IL-10 followed by LPS activation induced a M2 cytokine secretion profile. For our purposes, TNF-α secretion levels were selected as a representative and functionally relevant metric of the M1/M2 phenotype, particularly as it pertains to inflammation post-implantation of biomaterials. Note that cells were treated with human recombinant IL-10 (hIL-10), and IL-10 production was determined by measurement of murine IL-10. Viral delivery of IL-10 (vhIL-10) at 280 VP/cell significantly reduced TNF-α production and increased IL-10 production compared to the luciferase virus control (vCtrl). However, the 2,800 VP/cell dose did not significantly reduce TNF-α production compared to the 280 VP/cell condition. Based upon these observations and our desire to avoid vector-mediated macrophage activation or cell death, a dose of 280 VP/cell was used for all subsequent experiments. Notably, the cytokine profiles obtained using low dose IL-10 virus were similar to those obtained using recombinant IL-10 protein, indicating that IL-10 lentivirus delivery mediates robust polarization towards an M2 phenotype.
Figure 2.

IL-10 lentivirus delivery induces an M2 phenotype. Production of (A) TNF-α and (B) murine IL-10 were assayed to characterize the macrophage phenotype. Pre-treatments were given on day 3 followed by addition of LPS (100 ng/mL) on day 5 (except for the no treatment control). The pre-treatments included IFN-γ (M1 ctrl; 10 ng/mL), human IL-10 (M2 ctrl; 10 ng/mL), control virus (vCtrl; 280, 28000 VP/cell), and human IL-10 virus (vhIL-10; 280, 28000 VP/cell). Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test. *Significant difference compared to IFN-γ (P < 0.01). **Significant difference compared to vCtrl (P < 0.001).
IL-10 Lentivirus Delivery Confers Resistance to Inflammatory Stimuli
Although macrophage responses to various individual stimuli have been characterized, the manner in which macrophages integrate multiple stimuli to arrive at a polarization outcome is relatively less explored (Chuang and Leonard, in preparation). Thus, we next investigated whether lentiviral IL-10 promotes an M2 phenotype in macrophages that (a) receive both pro-M1 and pro-M2 stimuli simultaneously or (b) are pre-polarized to an M1 phenotype prior to exposure to pro-M2 stimuli. The canonical pro-M1 stimulus is a combination of IFN-γ + LPS (Martinez et al., 2008; Stout et al., 2005). When this stimulus was provided simultaneously with the IL-10 lentivirus, TNF-α secretion was reduced 25-fold compared to virus control (Fig. 3A). Simultaneous treatment with recombinant IL-10 protein and this pro-M1 stimulus induced a similar reduction in TNF-α secretion when compared to the pro-M1 control condition, which is consistent with previous reports (Fiorentino et al., 1991). Although the recombinant IL-10 protein and IL-10 lentivirus both reduce TNF-α protein levels in supernatant, qPCR analysis indicated that recombinant IL-10 protein treatment decreased the number of TNF-α transcripts by 2.5-fold more than the lentivirus at 8 h post-M1 treatment. However, at 56 h post-M1 treatment, the ratio reversed to yield 1.3-fold greater reduction in TNF-α transcripts following lentivirus delivery versus recombinant protein treatment (Fig. 3B). This trend may result from a lag in IL-10 production following virus delivery as well as sustained virus-mediated IL-10 production.
Figure 3.

IL-10 lentivirus delivery overcomes simultaneous delivery of inflammatory factors to induce an M2 phenotype. (A) Cells were seeded on day 0, indicated factors were added at day 3, and media was collected at day 6 for analysis of TNF-α by ELISA (n = 5). The treatments included an M1 treatment (IFN-γ (10 ng/mL) + LPS (100 ng/mL)), M1 + human IL-10 (hIL-10; 10 ng/mL), M1 + control virus (vCtrl; 280 VP/cell), and M1 + human IL-10 virus (vhIL-10; 280 VP/cell). Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test (P < 0.001). *Significant difference compared to M1. **Significant difference compared to M1 + vCtrl. (B) Fold change in TNF-α mRNA transcripts was determined at 8 and 56 h post-M1 treatment. Data are representative data from three experimental replicates.
Next, we investigated whether established M1 macrophages could be converted to an M2 phenotype by transduction with IL-10 lentivirus. RAW cells treated with IFN-γ + LPS for 24 h prior to IL-10 lentivirus delivery, secreted 1.5-fold less TNF-α than did cells treated with IFN-γ + LPS followed by IL-10 protein or virus control (Fig. 4A). Staggered delivery of pro-M1 stimuli followed by IL-10 protein delivery after 24 h had a modest decrease in TNF-α production when compared to the M1 control condition. Neither virus nor protein delivery was able to induce the transition towards the M2 phenotype that was observed for simultaneous delivery of these factors, possibly due to the potential IFN-γ to inhibit IL-10 signaling (Herrero et al., 2003) or that much of the TNF-α was secreted before addition of IL-10. The ability of IL-10 protein delivery to prevent transcription of TNF-α 8 h after delivery was abrogated by M1 pre-conditioning stimuli (Fig. 4B), which likely contributed to the inability of IL-10 protein delivery to induce an M2 phenotype. The enhanced ability of IL-10 lentivirus delivery to reduce secretion of TNF-α may result from its twofold greater reduction of TNF-α transcripts at 32 h after delivery (56 h post-M1) (Fig. 4B). Together, these data suggest that sustained IL-10 production suppresses TNF-α production by M1 macrophages more robustly than does transient exposure to recombinant IL-10.
Figure 4.

Lentivirus delivery of IL-10 overcomes an established M1 phenotype. (A) Cells were seeded on day 0, M1 stimuli were added at day 3, indicated factors were added at day 4, and media was collected at day 6 for analysis of TNF-α by ELISA (n = 4). The treatments included an M1 treatment (IFN-γ (10 ng/mL) + LPS (100 ng/mL)), M1, human IL-10 (hIL-10; 10 ng/mL), M1, control virus (vCtrl; 280 VP/cell), and M1, human IL-10 virus (vhIL-10; 280 VP/cell). Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test. *Significant difference compared to M1 (P < 0.05). **Significant difference compared to both M1, IL-10 and M1, vCtrl (P < 0.001). (B) Fold change in TNF-α mRNA transcripts was determined at 32 and 56 h post-M1 treatment. Data are representative data from three experimental replicates.
IL-10 Lentivirus Delivery Maintains an M2 Phenotype Upon Challenge With pro-M1 Stimuli
Maintenance of an M2 phenotype with lentivirus delivery was investigated by pre-conditioning macrophages with IL-10 protein or lentivirus and subsequently challenging them with pro-M1 stimuli. Macrophages pre-conditioned with IL-10 lentivirus prior to addition of pro-M1 stimuli exhibited a 2.5-fold decrease in TNF-α production and a 2.5-fold increase in IL-10 production when compared to cells pretreated with either recombinant IL-10 protein or control virus control prior to pro-M1 stimuli addition (Fig. 5). Moreover, when macrophages were pre-conditioned with recombinant IL-10 protein and then the IL-10-containing media was replaced with media containing pro-M1 stimuli, TNF-α production approached that of cells that had not been pre-treated with IL-10. This transient effect of recombinant IL-10 is consistent with prior reports that macrophages plastically shift their phenotypes following sequential exposure to different stimuli (Porcheray et al., 2005; Stout et al., 2005). Notably, these results also demonstrate that the level of IL-10 expressed following transduction with IL-10 lentivirus was sufficient to maintain an M2-biased phenotype and prevent the plastic transition toward an M1 phenotype.
Figure 5.
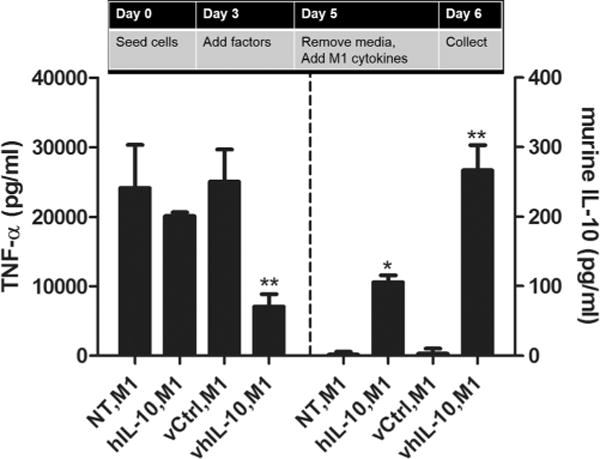
Lentivirus delivery of IL-10 to sustain an M2 phenotype following an inflammatory challenge. Cells were seeded on day 0, indicated factors were added at day 3, media was replaced by media containing M1 stimuli at day 5, and media was collected at day 6 for analysis of TNF-α and IL-10 by ELISA (n = 4–6). The treatments included an M1 treatment (IFN-γ (10 ng/mL) + LPS (100 ng/mL)), human IL-10 (hIL-10; 10 ng/mL), M1, control virus (vCtrl; 280 VP/cell), M1, and human IL-10 virus (vhIL-10; 280 VP/cell), M1. Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test. *Significant difference compared to NT, M1 (P < 0.001). **Significant difference compared to both IL-10, M1 and vCtrl, M1 (P < 0.01).
Transduction of Differentiating Bone-Marrow Derived Macrophages (BMDM) With IL-10 Lentivirus for Induction or Retention of an M2 Phenotype
The ability of the IL-10 lentivirus to modulate macrophage phenotype was next investigated using primary bone-marrow derived macrophages (BMDM). To determine whether lentiviral transduction efficiency changes during BMDM differentiation, BMDM were transduced with 280 VP/cell at varying times during the differentiation/phenotype induction process. Transduction of isolated bone marrow cells immediately after isolation (day 0) did not result in transgene expression above background until day 4, at which point the expression levels were relatively low (Fig. 6A). However, transduction of differentiating BMDM at day 3 or day 4 of the differentiation process induced transgene expression within 24 h of transduction at levels that were significantly greater than was observed 24 h after transduction at day 0 (Fig. 6A).
Figure 6.

Gene expression changes in differentiating bone marrow-derived macrophages (BMDM). Differentiating BMDM were transduced with 280 VP/cell of lentivirus. (A) Transgene expression for cells transduced at either day 0, 3, or 4 of the differentiation process (n = 4). Statistical analysis completed using a two-way ANOVA with Bonferronis post-hoc test. *Significant difference compared to background (P < 0.05). **Significant difference compared to cells transduced at day 0 (P < 0.001). ***Significant difference compared to cells transduced at day 3 (P < 0.0001). (B) The treatments included no treatment (NT), GFP virus at day 3 (NT + vGFP), GFP virus and M1 treatment (IFN-γ (10 ng/mL) + LPS (100 ng/mL)) at day 3 (M1 + vGFP), vGFP at day 4 (NT, vGFP), M1 treatment at day 3 and vGFP at day 4 (M1, vGFP), vGFP at day 3 (vGFP, NT), vGFP at day 3 and M1 treatment at day 6 (with media change) (vGFP, M1). Transduction efficiency was assessed by flow cytometry detection of GFP for cells transduced at day 6 (n = 3). Statistical analysis completed using an unpaired t-test (P < 0.01). *Significant difference compared to NT, vGFP. **Significant difference compared to vGFP, NT.
To investigate whether lentiviral transduction efficiency and transgene expression vary with macrophage phenotype, GFP lentivirus (280 VP/cell) was delivered to BMDM either simultaneously with pro-M1 stimuli or following pre-conditioning cells for 24 h with pro-M1 stimuli. Transduction efficiency was fourfold greater for BMDM than for RAW cells (Figs. 1B and 6B). Interestingly, delivering pro-M1 stimuli prior to virus delivery results in no transduced cells; however, simultaneous addition of pro-M1 stimuli and virus resulted in a modest 1.5-fold increase in transgene expression and virus addition followed by replacement with media containing pro-M1 stimuli resulted in a threefold increase in transgene expression (Fig. 6B).
To determine whether differentiating primary BMDM exhibit the same responses to sequential and simultaneous treatments as were observed with RAW cells (Figs. 3–5), BMDM were exposed to similar scenarios (Fig. 7A–C). As observed with RAW cells, IL-10 protein and IL-10 lentivirus delivery both shifted cells toward an M2 phenotype (Fig. 7A) and reduced TNF-α production when delivered simultaneously with pro-M1 stimuli (Fig. 7B). However, neither IL-10 protein nor IL-10 lentivirus delivery significantly reduced TNF-α production when delivered 24 h after pro-M1 stimuli (Fig. 7B). This discrepancy between results obtained with RAW cells and BMDM for the staggered delivery condition is likely due to low levels of transduction for BMDM following presentation of M1 cytokines (Figs. 1C and 6B), which would result in low levels of IL-10 produced. Pre-treatment of BMDM with lentiviral IL-10 sustained a fivefold reduction in TNF-α production following subsequent change to media containing pro-M1 stimuli (Fig. 7C). Interestingly, the virus control reduced TNF-α levels below that of the untreated condition, which we hypothesize my result from moderation activation of macrophages by viral transduction that induces a negative feedback mechanism that subsequently reduced LPS-mediated induction of TNF-α expression. In particular, lentiviral RNA may directly activate Toll Like Receptor 3 (TLR3) (Breckpot et al., 2010), and viral preparations may contain traces of HMGB1, a ligand for TLR4 (Kim et al., 2013). Each of these pathways may induce regulatory mechanisms that interfere with subsequent LPS-induced signaling through TLR4 (Bode et al., 2012; Kinjyo et al., 2002). Importantly, while these mechanisms could modestly impact TNF-α levels, lentivirus-mediated expression of IL-10 is required to convey the robust suppression of TNF- α expression (Fig 7C).
Figure 7.

Lentivirus delivery of IL-10 to differentiating BMDM overcomes inflammatory stimuli and sustains an M2 phenotype. IL-10 lentivirus delivery to differentiating BMDM was investigated using the three previously described in vivo scenarios. TNF-α production was assayed by ELISA to determine the phenotype of differentiating macrophages when confronted with (A) LPS activation, (B) overcoming inflammatory stimuli, and (C) an overwhelming inflammatory challenge (n = 8). Statistical analysis completed using a one-way ANOVA with Tukeys post-hoc test. *Significant difference compared to (A) IFN-γ (10 ng/mL) (P < 0.001) or (B) M1 (IFN-γ (10 ng/mL) + LPS (100 ng/mL)) (P < 0.001). **Significant difference compared to (A) both human IL-10 (hIL-10; 10 ng/mL) and luciferase virus (vCtrl; 280 VP/cell) (P < 0.05), (B) M1 + vCtrl (P < 0.001), or (C) both hIL-10, M1 and vCtrl, M1 (P < 0.001). (D) NF-κB transcription factor activity was assayed using live imaging of cells for the situation of retaining an M2 phenotype in the presence of an inflammatory challenge (n = 3). Statistical analysis completed using a two-way ANOVA with Bonferronis post-hoc test. *Significant difference compared to hIL-10, M1 (P < 0.001).
To begin investigating the mechanisms underlying the differences in phenotype retention between protein and gene delivery, transcription factor activity was investigated using a factor-specific reporter construct. This analysis focused on the transcription factor NF-κB, since numerous reports have connected this transcription factor to initiating an inflammatory response and the production of inflammatory cytokines in macrophages (Ahn and Aggarwal, 2005; Shames et al., 1998). This analysis was conducted exclusively in BMDM because transduction in RAW cells was not sufficient to analyze using the luciferase reporter constructs (data not shown). NF-κB activity peaked at 2 h post-treatment with pro-M1 stimuli, and this peak was threefold greater in cells pre-treated with IL-10 protein compared to those treated with IL-10 virus (Fig. 7D). Thus, the modality of pre-treatment with viral vector impacted the magnitude of NF-κB-induced gene expression rather than the timing of such gene expression. While decreased NF-kB activation in cells treated with the control virus could contribute to the observed decrease in TNF-α production, the expression of IL-10 notably led to further decreases in TNF-α production relative to cells treated with control virus alone (Fig. 7C), suggesting a contribution of other signaling pathways that are associated with TNF-α production. Since TNF-α expression is regulated at the transcriptional level by NF-κB, this pattern is also consistent with the observed reduction in TNF-α production following pre-treatment with IL-10 virus (Fig. 7C).
Discussion
In this manuscript, we demonstrate that lentiviral transduction of macrophages to confer sustained production of IL-10 can overcome an inflammatory microenvironment and sustain an M2 macrophage phenotype. These studies were motivated in part by the observation that untargeted lentivirus delivered from biomaterial scaffolds resulted in the transduction of macrophages in vivo (Boehler et al., 2013; Tuinstra et al., 2012), which likely results as a consequence of the foreign body response to the material and implantation. Scaffold-mediated gene delivery generally provides an opportunity for sustained, localized production of factors that can condition the environment and influence cellular responses. Thus, the intrinsic ability to transduce macrophages via scaffolds provides a unique modality by which to modulate macrophage phenotype in order to more effectively promote regeneration as opposed to inducing inflammation. Interestingly, the studies herein indicated that transducing approximately 5% of the cells in a population was sufficient to alter the phenotype of the overall cell population, when expressing a factor that can act in a paracrine fashion. However, a challenge with gene delivery is the inherent time delay before any gene product is expressed and accumulates to levels that can alter cellular processes. To wit, in this study, delivery of IL-10 protein suppressed inflammatory gene expression more robustly than did delivery of IL-10 virus at early time points post-delivery, but sustained expression of IL-10 eventually overcome this initial deficit and ultimately shifted macrophage phenotype towards M2. If this time delay proves to be meaningful in practice, protein and gene delivery might be combined to provide both immediate and sustained modulation of host responses. Importantly, the translation of this result to in vivo applications involving biomaterials must also consider the macrophage response to the biomaterial. For the commonly used biomaterial poly(lactide-co-glycolide), we have recently reported that the cytokines were more potent at influencing macrophage polarization than was the biomaterial (Gower et al., in press).
IL-10 lentivirus delivered to pre-conditioned M1 macrophages reduced their TNF-α production and pushed them towards an M2 phenotype in vitro, and translation of this finding to an in vivo setting would have therapeutic relevance for a multitude of applications. In cases of chronic inflammation or auto-immune disease (Ankeny and Popovich, 2009; Jones et al., 2002; Laliberte and Fehlings, 2013; Popovich and Jones, 2003), pushing macrophages towards an M2 phenotype could assist in resolving the detrimental immune activation and allowing healing or regeneration to occur. In contrast to chronic inflammation, normal wound healing concludes with a transition of local macrophages towards an M2 phenotype (Martinez et al., 2008). However, in cases of therapeutic intervention with drug/gene delivery, cell transplantation, or biomaterial implantation, an immune response can be mounted against the therapeutic materials, and while this response sometimes resolves on its own, a more rapid transition to M2 could enhance the survival and function of the newly regenerating tissues. Following implantation of a biomaterial, resident macrophages are expected to rapidly adopt a M1 phenotype (Boehler et al., 2011). According to our in vitro results, transduction of resident, inflammatory-activated macrophages may be limited (Figs. 1 and 6), likely due to different processing of viral particles in inflammatory macrophages (Zhang et al., 2009). This low-level of transduction for inflammatory-activated macrophages may require the transduction of naïve macrophages or other cell types infiltrating the scaffolds in order to convert the inflammatory-activated macrophages toward an M2 phenotype. Gene delivery may be more effective at converting peripheral infiltrating macrophages to M2, which arrive 1–7 days post-injury corresponding to the time at which transgene expression reaches its maximum level (Beck et al., 2010; Donnelly and Popovich, 2008). Due to the short half-life of lentivirus in vitro (Higashikawa and Chang, 2001; Shin and Shea, 2010), peripheral infiltrating macrophages may not be direct targets of transduction; however, based upon the observations described here, paracrine IL-10 production from transduced cells at the implant site could effectively compete with inflammatory stimuli in this milieu to bias infiltrating macrophages towards in M2 phenotype.
Herein, gene delivery was effective in maintaining an M2 phenotype due to sustained IL-10 expression preventing the plastic switching from an M2 to an M1 phenotype. In contrast, macrophages pre-treated with IL-10 protein quickly transitioned toward an M1 phenotype when environmental stimuli were experimentally changed from pro-M2 to pro-M1. The retention of an M2 phenotype when cells were subsequently exposed to IL-10 plus pro-M1 stimuli coincided with reduced NF-kB activity, potentially implicating this transcription factor as a key regulator of this phenotypic balance. (Fig. 7D). The need to maintain an M2 phenotype is exemplified by inflammation following spinal cord injury, in which macrophages that initially transition to an M2 phenotype revert to M1, resulting in a chronic immune response (Kigerl et al., 2009; Pruss et al., 2011) that could be prevented by sustained IL-10 production. Importantly, the M2 phenotype should be sustained only until the chronic inflammation is resolved, and should be not be permanent. Lentivirus delivery from biomaterials has sustained transgene expression for at least 28 days and subsequently returned to background levels (Boehler et al., 2013; Tuinstra et al., 2012), a duration that coincides with the peak of the chronic immune response in the spinal cord at 28 days (Kigerl et al., 2009). Lentivirus delivery was used herein due to the difficulty in transducing macrophages in vitro, and the translation of these results may also consider alternative strategies, such as non-viral vectors, non-integrating viruses, or sustained protein delivery, in order to create a prolonged yet transient anti-inflammatory environment. An additional consideration for the delivery vehicle will be its effect on the inflammatory response, and vector modifications, such as removing CpG motifs and development of “stealth” viruses, have reduced such responses (Croyle et al., 2001; Hyde et al., 2008).
In summary, we observed that delivery of IL-10 lentivirus promoted a transition away from an M1 macrophage phenotype and retained an M2 phenotype in the presence of inflammatory stimuli. IL-10 lentivirus transduced both RAW cells and BMDM to induce an M2 phenotype. IL-10 lentivirus delivery outperformed IL-10 protein in inducing a transition from an M1 to an M2 phenotype and retaining an M2 phenotype after switching cells from a pro-M2 to a pro-M1 environment. This comparative advantage likely resulted from sustained production of IL-10 via virus delivery. We propose that lentiviral delivery of IL-10 from biomaterial scaffolds can be employed in vivo to transduce infiltrating macrophages and induce them to an M2 phenotype to resolve inflammation following injury and implantation of a biomaterial for numerous applications in regenerative medicine.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institutes of Biomedical Imaging and Bioengineering, (NIBIB) at the National Institutes of Health (NIH)
Grant numbers: R01EB005678; R01EB009910; R01CA173745; R01GM097220
Footnotes
The authors declare no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158(3):1112–1121. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(Pt 2):433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CW, Jiang W, Reich CF, III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291(6):C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)–STAT3 axis. Cell Signal. 2012;24(6):1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239–240. doi: 10.2144/000113754. 242, 244 passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler RM, Shin S, Fast AG, Gower RM, Shea LD. A PLG/HAp composite scaffold for lentivirus delivery. Biomaterials. 2013;34(21):5431–5438. doi: 10.1016/j.biomaterials.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S, Keyaerts M, Collins M. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84(11):5627–5636. doi: 10.1128/JVI.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y, Leonard JN. Resolution of incoherent stimuli during macrophage polarization. in preparation. [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75(10):4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokka S, Shi X, Leonard S, Wang L, Castranova V, Rojanasakul Y. Interleukin-10-mediated inhibition of free radical generation in macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1196–L1202. doi: 10.1152/ajplung.2001.280.6.L1196. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: A story of Jekyll and Hyde. Clin Sci (Lond) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161(4):1983–1988. [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147(11):3815–3822. [PubMed] [Google Scholar]
- Gower RM, Boehler RM, Azarin SM, Ricci CF, Leonard JN, Shea LD. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials. 2014;35(6):2024–2031. doi: 10.1016/j.biomaterials.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O’Brien W, Cederbaum S, Christianson DW, Zimmermann N, Rothenberg ME, Finkelman FD. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184(11):6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, Ivashkiv LB. Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol. 2003;171(10):5034–5041. doi: 10.4049/jimmunol.171.10.5034. [DOI] [PubMed] [Google Scholar]
- Higashikawa F, Chang L. Kinetic analyses of stability of simple and complex retroviral vectors. Virology. 2001;280(1):124–131. doi: 10.1006/viro.2000.0743. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26(5):549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin Immunol. 2008;20(2):130–136. doi: 10.1016/j.smim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: Implications for autoimmune vaccine therapy. J Neurosci. 2002;22(7):2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17(5):583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- Laliberte AM, Fehlings MG. The immunological response to spinal cord injury: Helpful or harmful? Exp Neurol. 2013;247:282–285. doi: 10.1016/j.expneurol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: Back to basics. Trends Pharmacol Sci. 2003;24(1):13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: An asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106(35):14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, Schwab JM. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): Indices and resolution plateau. Brain Pathol. 2011;21(6):652–660. doi: 10.1111/j.1750-3639.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames BD, Selzman CH, Meldrum DR, Pulido EJ, Barton HA, Meng X, Harken AH, McIntyre RC., Jr Interleukin-10 stabilizes inhibitory kappaB-alpha in human monocytes. Shock. 1998;10(6):389–394. [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Shea LD. Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol Ther. 2010;18(4):700–706. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578(Pt 1):327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials. 2012;33(5):1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MS, Bernabe BP, Shikanov A, Bluver DA, Mui MD, Shin S, Broadbelt LJ, Shea LD. The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials. 2012;33(13):3548–3559. doi: 10.1016/j.biomaterials.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107(12):4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- Zhang X, Edwards JP, Mosser DM. The expression of exogenous genes in macrophages: Obstacles and opportunities. Methods Mol Biol. 2009;531:123–143. doi: 10.1007/978-1-59745-396-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


