Abstract
The directed differentiation toward erythroid (E) or megakaryocytic (MK) lineages by the MK-E progenitor (MEP) could enhance the ex vivo generation of red blood cells and platelets for therapeutic transfusions. The lineage choice at the MEP bifurcation is controlled in large part by activity within the intracellular signal transduction network, the output of which determines the activity of transcription factors (TFs) and ultimately gene expression. Although many TFs have been implicated, E or MK differentiation is a complex process requiring multiple days, and the dynamics of TF activities during commitment and terminal maturation are relatively unexplored. Herein, we applied a living cell array for the large-scale, dynamic quantification of TF activities during MEP bifurcation. A panel of hematopoietic TFs (GATA-1, GATA-2, SCL/TAL1, FLI-1, NF-E2, PU.1, c-Myb) was characterized during E and MK differentiation of bipotent K562 cells. Dynamic TF activity profiles associated with differentiation towards each lineage were identified, and validated with previous reports. From these activity profiles, we show that GATA-1 is an important hub during early hemin- and PMA-induced differentiation, and reveal several characteristic TF interactions for E and MK differentiation that confirm regulatory mechanisms documented in the literature. Additionally, we highlight several novel TF interactions at various stages of E and MK differentiation. Furthermore, we investigated the mechanism by which nicotinamide (NIC) promoted terminal MK maturation using an MK-committed cell line, CHRF-288-11 (CHRF). Concomitant with its enhancement of ploidy, NIC strongly enhanced the activity of three TFs with known involvement in terminal MK maturation: FLI-1, NF-E2, and p53. Dynamic profiling of TF activity represents a novel tool to complement traditional assays focused on mRNA and protein expression levels to understand progenitor cell differentiation.
Keywords: transcription factor activity, megakaryocytic, erythroid, differentiation, dynamic networks, inference
Introduction
Hematopoietic stem and progenitor cells (HSPCs) give rise to all of the various blood cell lineages. In particular, erythroid (E) and megakaryocytic (MK) cells are closely related, and derive from a common progenitor cell, the MK-E progenitor (MEP). More efficient E commitment and differentiation of MEPs derived from HSPCs or induced pluripotent stem cells would improve prospects for ex vivo generation of red blood cells for transfusions (Griffiths et al., 2012). Similarly, strategies to culture MEPs that increase the yield of MK cells could substantially enhance platelet production. The supply of platelets from volunteer donors is currently limited by the inability to store platelets for more than 5 days (Stroncek and Rebulla, 2007). MK cells are unique in that they dramatically increase their DNA content and volume by undergoing multiple rounds of endomitosis (DNA replication without cell division) to become polyploid (>4N) cells. Treatment of MK progenitor cells with nicotinamide (NIC) dramatically increases MK polyploidization in ex vivo cultures (Giammona et al., 2006, 2009; Leysi-Derilou et al., 2012), which is due, in part, to inhibition of sirtuin deacetylases (SIRTs). Nevertheless, the mechanism of NIC action remains unclear (Giammona et al., 2009).
Commitment of MEPs to a lineage and subsequent maturation is directed by the cumulative effects of signaling pathways, which orchestrate a complex network of transcription factor (TF) activities (Doré and Crispino, 2011). TFs link extracellular and intracellular signals to mRNA, and ultimately protein output. The relevance of a particular TF during differentiation has traditionally been assayed through mRNA and protein levels, and more recently through chromatin immunoprecipitation (ChIP) to confirm the presence of a TF at a relevant genetic locus. Recently, we developed and validated a living cell array for the large-scale quantification of dynamic TF activities. This assay directly quantifies the activity of TFs, rather than abundance of mRNA or protein, and can be applied repeatedly to quantify TF activity through lineage commitment and differentiation (Bellis et al., 2011; Weiss et al., 2010).
In this study, we applied the TF activity assay to investigate E versus MK commitment and differentiation using the model cell line K562, which resembles MEPs in that it is bipotent for the E and MK lineages (Sutherland et al., 1986). K562 cells have been widely used for investigating E and MK differentiation programs (Georgantas et al., 2007; Leary et al., 1987), and this extensive characterization aided in the assay validation. Many of the factors shown to influence E and/or MK differentiation of K562 cells have been validated in primary hematopoietic cells (Eisbacher et al., 2003; Elagib et al., 2003; Ishiko et al., 2005; Loughran et al., 2008; Randrianarison-Huetz et al., 2010). We selected a panel of seven TFs known to be involved in E/MK differentiation and monitored their dynamic activities throughout the differentiation process. First, we examined the divergence in TF activities associated with the bifurcation between the E and MK lineages. We then utilized an ensemble tree-based inference algorithm (GENIE3) to infer the TF regulatory network for both lineages and performed a topological analysis of the inferred network (Huynh-Thu et al., 2010). The impact of knocking out the GATA-1 TF on the subsequent response of the TF network was also determined. Finally, we investigated the dynamic TF activity associated with NIC promotion of MK maturation using the CHRF-288-11 (CHRF) cell line, which resembles MK progenitors (Fuhrken et al., 2007). The previously established NIC-mediated inhibition of SIRTs, as well as changes in metabolism due to increased NAD+ concentration (Giammona et al., 2009), were expected to influence TF activities. TF activity arrays can provide unique perspectives on cell differentiation, which may ultimately be translated into strategies to more effectively promote production of cells in specific lineages.
Materials and Methods
Reagents
Phorbol 12-myristate 13-acetate (PMA), hemin, benzidine dihydrochloride, and nicotinamide were obtained from Sigma–Aldrich (St. Louis, MO). CD41a and GlyA antibodies were from BD Biosciences (San Jose, CA). Annexin V antibody was from ebiosciences (San Diego, CA).
Virus Production
Lentivirus was produced by co-transfecting HEK-293T cells with previously described lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G) (Dull et al., 1998) and lentiviral vectors such as pLenti-TRE-dsGFP-ffluc and GATA-1-directed TRC lentiviral short hairpin RNA vectors (Open Biosystems, Huntsville, AL) using Lipofectamine 2000 (Life Technologies, Carlsbad, CA). After 48 h, supernatants were collected and cell debris was spun down and removed. Viruses were concentrated using PEG-it (Systems Biosciences, Mountain View, CA) and re-suspended in phosphate buffered saline (PBS). Lentivirus titers were determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, NY).
Cell Culture, Differentiation, and Transduction
K562 cells were maintained in exponential growth in RPMI 1640 media, supplemented with 10% fetal bovine serum (FBS) from Hyclone (Logan, UT) and 1% penicillin/streptomycin (pen/strep). CHRF cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM) with 10% FBS and 1% pen/strep. The MK differentiation of either cell line was stimulated by the addition of 10 ng/mL PMA. For E differentiation, K562 cells were treated with 30 µM hemin.
Transduction of GATA-1-targetting lentiviral shRNA into K562 cells was performed by spinoculation. Two days after transduction, cells were cultured with media containing 2 µg/mL puromycin (Life Technologies) for 7 days. Cells were then cultured in regular media for 3 days before being used for experiments. GATA-1 down-regulation was confirmed by real-time PCR.
Flow Cytometry
To analyze E and MK differentiation, cells were washed 2 × with PBS supplemented with 2 mM ethylenediaminetetra-acetic acid (EDTA) and 0.5% bovine serum albumin (BSA) (PEB) and then incubated with the relevant antibodies at 4°C for 30 min. Following antibody staining, cells were again washed 2× with PEB and then stained with DAPI for 10– 15 min at room temperature (RT). Alternatively, in the case of performing Annexin V staining, cells were washed with Annexin V binding buffer, incubated with Annexin V antibody for 15 min at RT, re-washed 1×, and then stained with DAPI. All samples were acquired on a LSRII flow cytometer (BD Biosciences).
Benzidine Staining to Assess Hemoglobin Content
Benzidine staining was performed similar to that previously described (Lam et al., 2000). Briefly, 100,000 cells were washed 2x with cold PBS, and resuspended in 27 µL of PBS. Next, 3 µL of benzidine solution (0.2% w/v in 0.5M acetic acid) and 1 µL of H2O2 were added. After approximately 10 min, total cells and stained (blue) cells were counted on a hemacytometer. At least 200 total cells were counted for each condition at each time point.
Quantitative Reverse Transcription-PCR (qRT-PCR)
RNA was prepared from 5 × 105 K562 cells by use of the Absolutely RNA Microprep Kit (Stratagene, Santa Clara, CA). Before reverse transcription, 1 µg of RNA was treated with RNase-free DNase I. Purified RNA (0.5 µg) was reverse transcribed with random hexamers using the Superscript III first-strand synthesis system (Life Technologies). Beta actin was used as an internal control. Primers were as follows: hGATA-1 forward primer: 5′-gggatcacactgagcttgc-3′; reverse: 5′-acccctgattctggtgtgg-3′; h-β actin forward primers: 5′-gcacagagcctcgccttt-3′; reverse: 5′-ggaatccttctgacccatgc-3′. Real-time PCR was performed in a real-time PCR detection system (Bio-Rad, Hercules, CA). The cycling conditions were as follows: pre-treatment at 95°C for 15 s, then 40 cycles of denaturation at 95°C for 5 s, and extension at 60°C for 30 s. Relative gene expression was quantified using the comparative ΔCT (CT: cycle threshold) method.
Transcription Factor Activity Arrays
TF reporters consist of a specific TF response element (TRE) cloned upstream of a minimal cytomegalovirus (CMV) promoter (TA) driving the gene for firefly luciferase (FLUC) and destabilized GFP packaged in self-inactivating lentiviral vectors (pGreenFire, System Biosciences). Increased binding on TRE by TFs results in increased luciferase production and a proportional increase in luminescence when an excess of substrate is added during imaging, thus providing a quantitative measure of relative transactivation. TF reporter specificity and sensitivity studies are referenced on the TRANSFAC (Matys et al., 2006) and Panomics database (Affymetrix, Redwood, CA). TF reporters were prepared by cloning specific binding elements into the pGreenFire lentiviral backbone. Each lentiviral reporter consists of three repeats of a TF-specific binding element driving expression of FLUC, and a puromycin resistance cassette. K562 or CHRF cells were mixed with lentiviral vectors bearing TF reporter constructs at a multiplicitiy of infection (MOI) of approximately 10 virions per cell and centrifuged at 800g for 45 min at 32° C. After removing the supernatant, cell pellets were resuspended and treated with medium containing 1– 2 µg/mL puromycin to select transduced cells. K562 cells bearing reporter vectors were plated at 2 × 104/well in black 96-well plates (Greiner Bio-One, Monroe, NC) and treated with hemin (30 µM) or PMA (10 ng/mL) to induce E or MK differentiation, respectively. CHRF cells bearing reporter vectors were plated at 1 × 104/well and treated with PMA (10 ng/mL) or PMA + NIC (12.5 mM) to induce MK differentiation. To measure TF-activity-dependent luciferase production, d-luciferin (Molecular Imaging Products, Bend, OR) was added to wells to a final concentration of 1 mM, which had been previously determined to be well in excess of a limiting concentration. Following a 20-min equilibration period, luminescence in each well was measured using an IVIS Lumina LTE camera system (Caliper Life Sciences, Hopkinton, MA). Untransduced cells in arrays served as controls for non-enzymatic d-luciferin breakdown. Cells transduced with a minimal CMV-FLUC (denoted TA-FLUC) reporter construct without additional TF response elements served as controls for any differences in basal promoter activity between conditions. The media and the inducing agent (hemin or PMA) were exchanged for fresh media containing the inducing agent every other day.
TF Activity Data Processing
Luminescence values for each well on each day were divided by the average of three luminescence readings from corresponding TA-FLUC control wells to control for differences in basal TA promoter activity. Luminescence read-outs for a reporter TF(J)-r in cells treated with treatment Tx on day Dx after adjustment for basal transactivation from control reporter TA-r is therefore represented by the formula
Normalized luminescence values for each treatment on each day were then divided by the average of the normalized values for the respective untreated control (Veh) to correct for TF activity changes due to continued cell growth in arrays that cannot be attributed to differentiation. Normalized values can therefore be represented as:
Each TF activity was subsequently log-transformed to normalize the variance of TF(J)-r. Each array had three replicates per TF reporter and complete array experiments were repeated two times on different days. Plate position of cells expressing each TF reporter was varied between experiments beginning on different days.
Random Forest Inference
The GENIE3 algorithm was used to generate a network model for each set of time-points (Huynh-Thu et al., 2010). For the random forest (RF) algorithm, parameters were set using the following criteria: K (the number of features selected at random to generate each regression tree) was set to the number of TFs minus one. Ntrees (the total number of trees generated for the ensemble) was set to 1,000. The sign of the interaction (activating or inhibiting relationship) was determined by the sign of the correlation coefficient between the putative regulator and target TFs.
Assigning Confidence to Each Edge
GENIE3 receives a TF activity matrix as an input, and outputs a ranked list of edges and importance scores associated with each edge. For the confidence estimation procedure, each TF importance score was compared to a randomized score from a null model obtained by using internal sampling (randomly shuffling initial activity values by 10,000 iterations). By randomly shuffling the data, any association between TFs as predicted by the algorithm is removed. If a predicted interaction is observed in 4.99% of the null model predictions, then a P-value of 0.0499 is implied. The importance score is plotted against the number of false positives, and an importance score cut-off of 0.13 corresponding to a P-value of 0.1 was set for screening purposes.
Network Verification Methods
In the absence of a reference hematopoietic TF network, we derived a network of experimentally validated regulatory interactions from GeneGO (Metacore from Thomas Reuters). Direct interactions were downloaded from the GeneGO database, yielding a network of 34 interactions among seven TFs. The resulting networks from both GeneGO and RF inference were visualized by Cytoscape (Shannon et al., 2003).
Statistical Analysis
Results of experiments are presented as the mean ± standard deviation, unless otherwise indicated. Analysis of Living Cell Arrays (ALCA), an R package we have previously developed specifically for TF activity arrays, was used to visualize and analyze the data (Weiss et al., 2010). Unless noted otherwise differences in means were evaluated by a paired moderated t-test using false discovery rate correction (Benjamini and Hochberg, 1995; Smyth et al., 2005). A P <0.05 was considered to be statistically significant. We performed leave-one-out (LOO) cross validation on our network models to evaluate network sensitivity with respect to the presence/absence of transcription factors (Supplementary Methods).
Results
Reporter Lentiviruses Do Not Alter E or MK Differentiation of K562 Cells
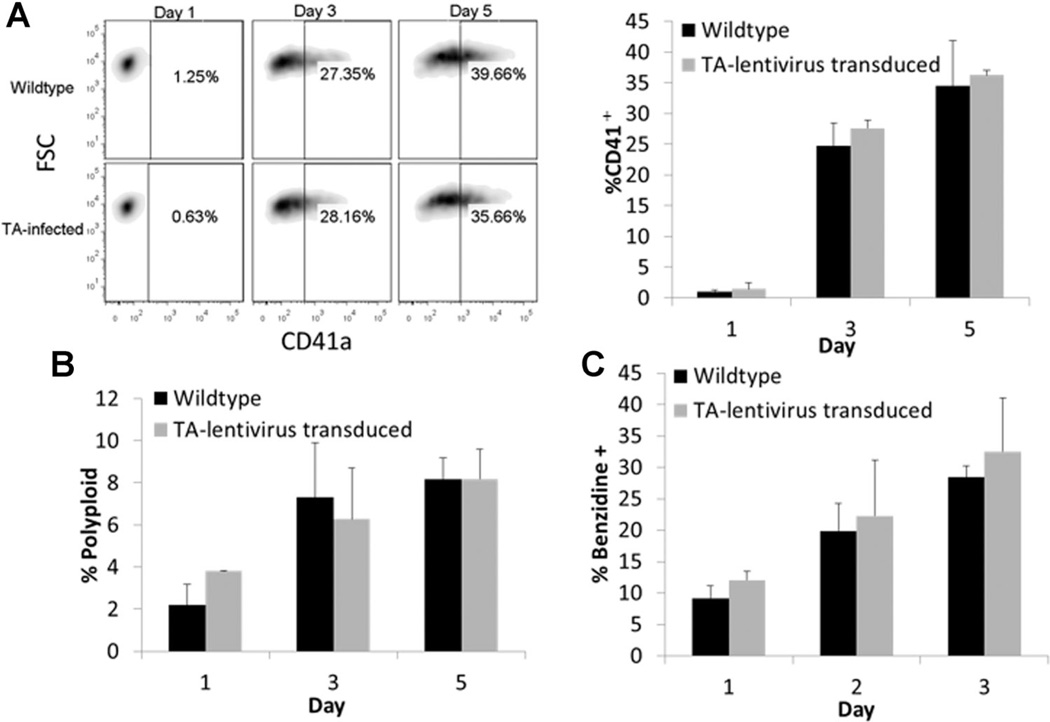
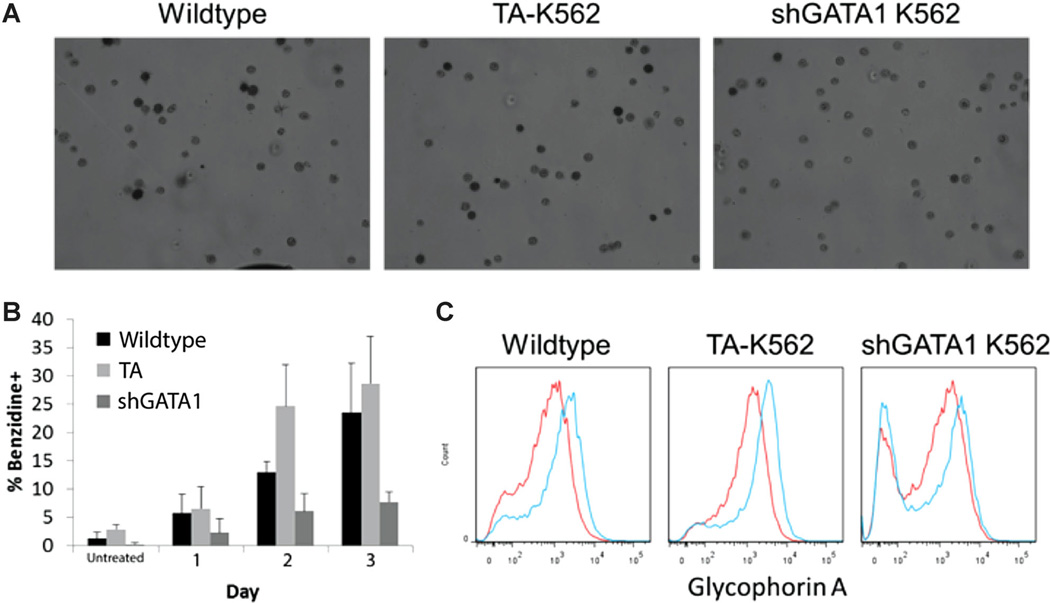
We initially sought to confirm that introducing the TF reporter lentiviruses would not substantially alter PMA-mediated MK or hemin-mediated E differentiation of K562 cells. Stably-transduced cells responded to both treatments similar to wild-type controls. Specifically, PMA-treated reporter cells acquired CD41 and underwent polyploidization similar to wild-type cells (Fig. 1A and B). Hemin-treated reporter cells became hemoglobinized (Fig. 1C) and upregulated expression of the erythroid antigen Glycophorin A (GlyA) to a similar extent as wild-type cells (data not shown). Thus, we subsequently characterized TF activity during E and MK differentiation using the generated reporter cell lines.
Figure 1.
Characterization of E and MK differentiation markers for K562 cells transduced with TF-reporter lentivirus. Percentage of CD41 acquisition (FSC is forward scatter) (A) and polyploidization (B) over time in TA-K562 cells compared to wild-type for PMA-induced differentiation. Percentage of hemoglobinized cells stained with benzidine (C) for hemin-induced differentiation. Results are from two independent time-course experiments. Error bars indicate standard deviation.
Analysis of TF Activity During E Versus MK Differentiation of K562 Cells
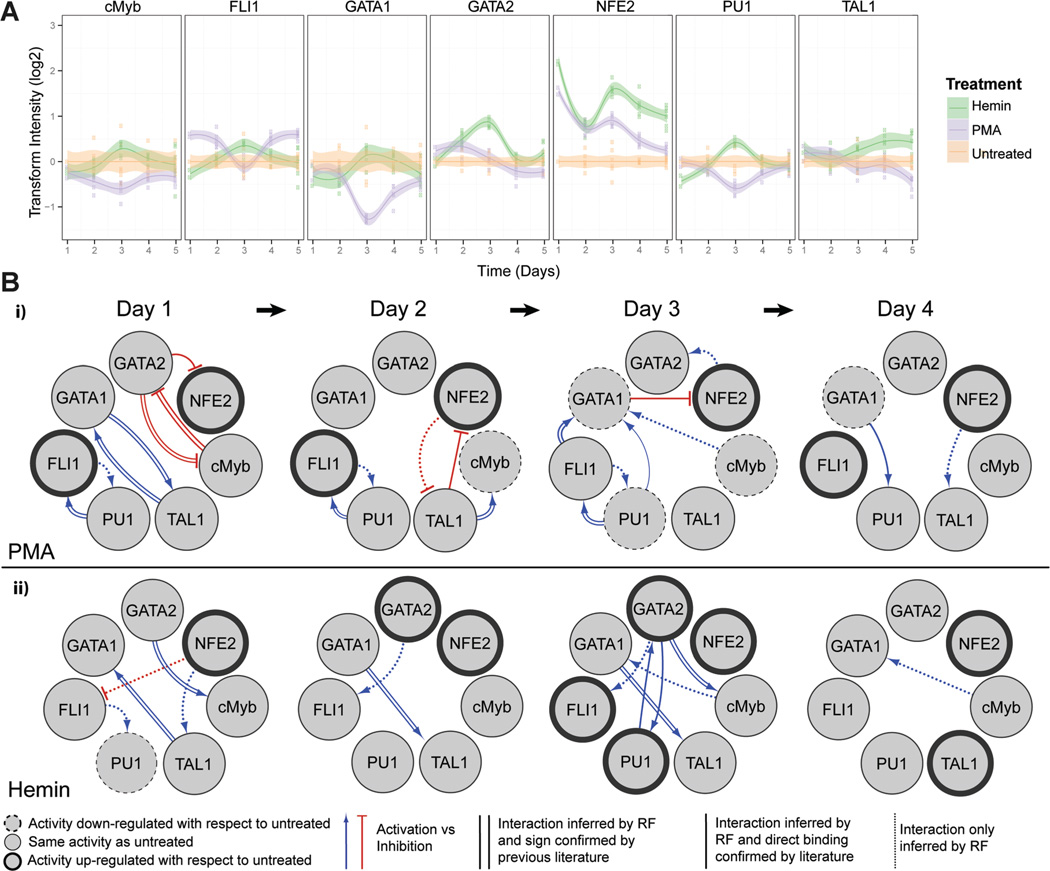
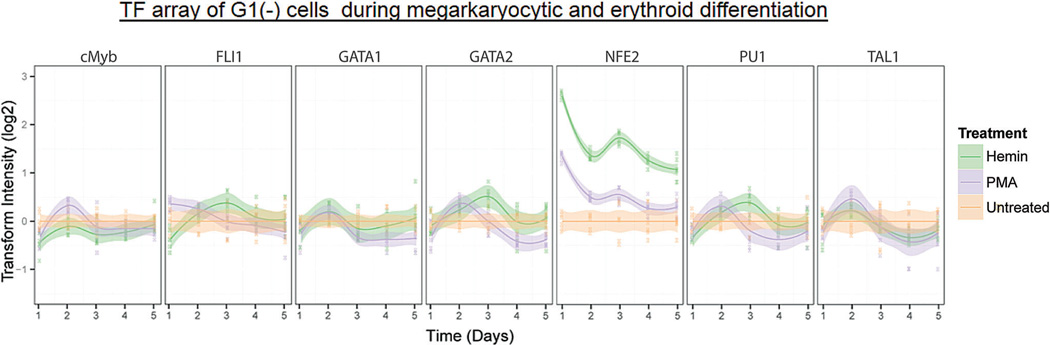
The activity profiles of 7 key hematopoietic TFs (Doré and Crispino, 2011) were quantified over the 5-day culture, during which cells differentiate to either E or MK phenotypes (Fig. 2A). Throughout MK differentiation, we noted a gradual reduction (Days 1–3) and recovery (Days 4–5) in the activities of GATA-1, c-Myb, and PU.1. TAL1, a key transactivating binding partner of GATA-1 (Xu et al., 2003), also had a slight decrease by Day 5. FLI-1, which promotes MK-lineage commitment and subsequent polyploidization (Raslova et al., 2003), was rapidly induced by PMA and remained significantly upregulated with respect to the untreated control (P <0.05) except for a transient decrease at Day 3. NF-E2, important for regulating platelet release from mature MKs (Shivdasani and Orkin, 1995), also showed an immediate and strong activation, but this activation gradually regressed to the level of untreated cells.
Figure 2.
Dynamic TF activity array and regulatory networks of K562 cells during MK or E differentiation. A: K562 cells transduced by each TF-activity-reporting lentivirus were treated with 10ng/mL of PMA or 30µM of hemin. TF activity was normalized by TA activity and represented by the ratio to vehicle-treated cells. Shaded regions denote 95% confidence intervals about the mean of six measurements (indicated by data points) from two independent transduction experiments. B: The dynamic TF regulatory networks for PMA- and hemin-treated K562 cells inferred from TF array data. The TF network shows target and regulator TFs (circles), as well as putative direct or indirect interactions between TFs (directed arrows). TF nodes (circles) indicate if TF activity is upregulated (bold outline), downregulated (dashed outline), or unchanged (thin outline) with respect to untreated control on the indicated day. Edge line styles depict whether the inferred interaction is a direct interaction confirmed by previous literature found in GENEGO (parallel lines), a direct interaction confirmed by previous literature but activation (blue lines) or inhibition (red lines) of the target is unknown (solid lines), or inferred interaction is novel (dotted lines).
During E differentiation, we observed early, strong activation of both NF-E2 (Day 1) and GATA-2 (Day 2). NF-E2 activity subsequently fluctuated, but remained >2-fold higher than in untreated cells, while GATA-2 activity peaked at Day 3. TAL1, important for terminal E differentiation (Kassouf et al., 2008; Xu et al., 2003), was significantly upregulated with respect to the untreated control by Day 3 and had increased activity throughout the differentiation period. Although an initial slight repression was observed relative to untreated cells, GATA-1 and PU.1 activities trended toward an increase with respect to control until peaking at Day 3 and declining thereafter. The activities of c-Myb and the MK-specific TF FLI-1 remained similar to that of untreated cells beyond Day 3.
The observed trends in our activity data were consistent with literature reports of expression data, and with functional studies describing each TF’s specific role in regulating E/MK differentiation (see below). However, we note that changes in expression level do not always correspond to changes in TF activity level due to posttranslational regulatory mechanisms, and emphasize that TF activity measurements should not be considered as dynamic measurements on the protein level.
TF Regulatory Network Describing E Versus MK Differentiation
Next, we utilized the TF activity data to construct a regulatory network that identifies putative positive and negative interactions between TFs. The network was inferred by applying tree ensemble-based models to predict a set of possible regulators for each TF (Fig. 2B). We sought to evaluate the number of inferred edges that are supported by evidence of direct binding from literature. Of the 35 edges predicted for E/MK differentiation over 5 days, 20 edges had supporting evidence of direct binding. Although these relationships have been identified by other authors as direct binding, we note that the relationship inferred by the network could be direct or the result of indirect interactions. For several of the edges with evidence of direct binding, sources could not confirm whether the regulatory TF activated or inhibited the target TF. The probability of an edge within the network shown in Figure 2B was investigated using a leave-one-out (LOO) analysis. All interactions in the resulting network were present in those culminating from LOO analysis, though not all interactions were present in every LOO network in which a TF had been removed. The frequency of a sustained linkage within networks lacking a single TF reflects confidence of that interaction in the resulting model. The ranked list of interactions for the LOO analysis at each time point can be found in Supplementary Table SIII.
In the reconstructed networks, active regulatory interactions, as well as strong regulatory hubs (i.e., nodes with a large number of interactions), have been identified in both PMA (Fig. 2Bi) and hemin (Fig. 2Bii) networks. Judged by the number of connections, GATA-1 appears to be an influential TF with respect to both hemin- and PMA-mediated differentiation and is known to recruit many proteins to mediate chromatin remodeling (Escamilla-Del-Arenal and Recillas-Targa, 2008; Grass et al., 2003; Im et al., 2005). According to the model, GATA-1 is a target of most TFs that were screened. In both PMA and hemin networks, GATA-1 was found to participate in a mutually activating relationship with TAL1. Microarray and genome-wide experiments indicate that TAL1 forms a complex with GATA-1, and several other proteins, to synergistically promote the expression of genes involved in cellular development and cell growth/proliferation (Kassouf et al., 2008). The activation of these links early in both hemin- and PMA-induced differentiation reflects that these processes are essential to commitment for both E and MK fate. GATA-1 was also inferred to be activated by c-Myb during Day 3 and 4 of hemin-induced differentiation, and Day 3 for PMA-induced differentiation. Although they are not direct binding partners, c-Myb and GATA-1 have been reported to act in concert in MK progenitor cells to promote proliferation and commitment to differentiation (García et al., 2011).
In addition, the inferred networks identified characteristic interactions for hemin- and PMA-induced differentiation of K562 cells. For PMA-induced differentiation, FLI-1 and PU.1 participated in a mutually activating relationship for a majority of the timeline that was observed. PU.1 has been identified to activate FLI-1 transcription and subsequently stimulate the expression of MK-specific genes (Athanasiou et al., 1996; Starck et al., 1999). During hemin-induced differentiation, GATA-2 and PU.1 were predicted to act cooperatively in Day 3, consistent with reports in which their combination specifies mast cell fate in hematopoietic progenitors (Walsh et al., 2002). Additionally, GATA-2 and c-Myb were inferred to participate in a mutually inhibiting relationship in the PMA network, while during hemin-induced differentiation, GATA-2 was predicted to activate c-Myb. Evidence of reciprocal target binding sites has been found in GATA-2 and c-Myb promoters, but it is not known whether this relationship is activating or inhibiting (Lorenzo et al., 2011; Wilson et al., 2010). Our results suggest that both cooperative and antagonistic interplay exist between c-Myb and GATA-2, and that the nature of the relationship may contribute to the specification of either E or MK commitment.
Generation and Characterization of GATA-1-Depleted K562 Cells
The well-established importance of GATA-1 (confirmed by the preceding network analysis) in regulating normal E and MK differentiation motivated studies with the silencing of GATA-1 during K562 cell differentiation, and the measurement of TF activity profiles. Inhibition of GATA-1 is predicted to disrupt differentiation for both E and MK lineages.
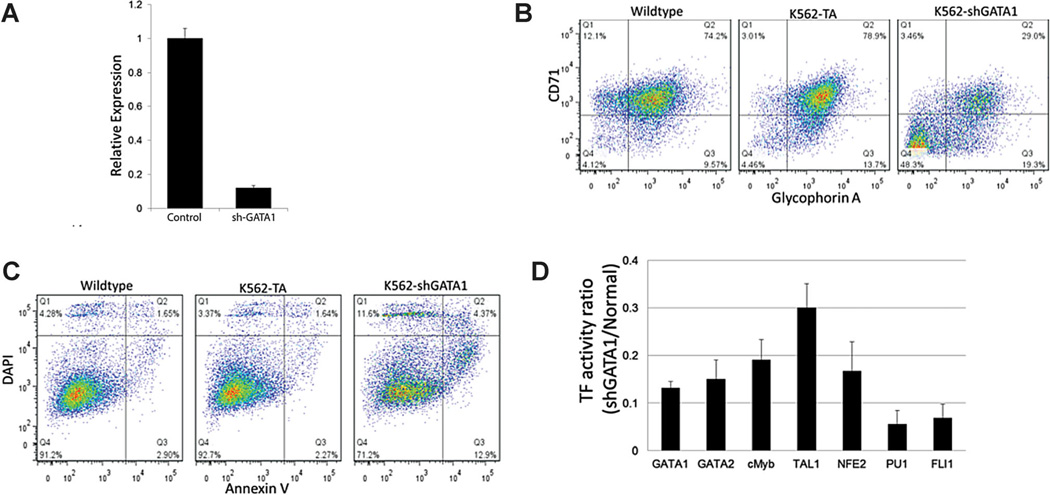
Lentivirus encoding shRNA against GATA-1 was delivered, with 90% knockdown of GATA-1 mRNA confirmed by qRT-PCR (Fig. 3A). This potent silencing of GATA-1 greatly reduced K562 cell expression of the constitutively expressed E antigens GlyA and CD71 (Fig. 3B). In particular, a substantial subpopulation (∼50%) of GlyA−CD71− cells emerged, suggesting a definitive regression from the MEP-like phenotype. In addition, GATA-1 silencing increased the fraction of apoptotic (AnV+DAPI−) and non-viable cells (DAPI+) and significantly decreased the proliferation rate (Fig. 3C and data not shown), consistent with reports on the protective effects exerted by GATA-1 (Ohneda and Yamamoto, 2002). Further, the basal level of all TF activities was significantly reduced (to 5–30% of wild-type level) by GATA-1 knockdown (Fig. 3D). These profound reductions further indicate the importance of GATA-1 for the maintenance of the MEP-like phenotype. We considered the possibility that these cells may have undergone lineage switching, but in an initial test we did not detect substantial expression of the myeloid antigens CD11b or CD33 (data not shown).
Figure 3.
Characterization of GATA-1-silenced K562 cells in control media. A: qRT-PCR of GATA-1 mRNA levels in shGATA-1 cells. B: Expression of the erythroid antigens CD71 and Glycophorin A. C: DAPI and Annexin V staining of shGATA-1 K562 cells. Data is representative of two independent transduction experiments. D: TF activity normalized by TA activity and represented by the ratio to normal K562 cells. All tested TF activities were significantly reduced (P < 0.05) by GATA-1 knockdown. Error bars in (A) and (D) represent the standard deviation.
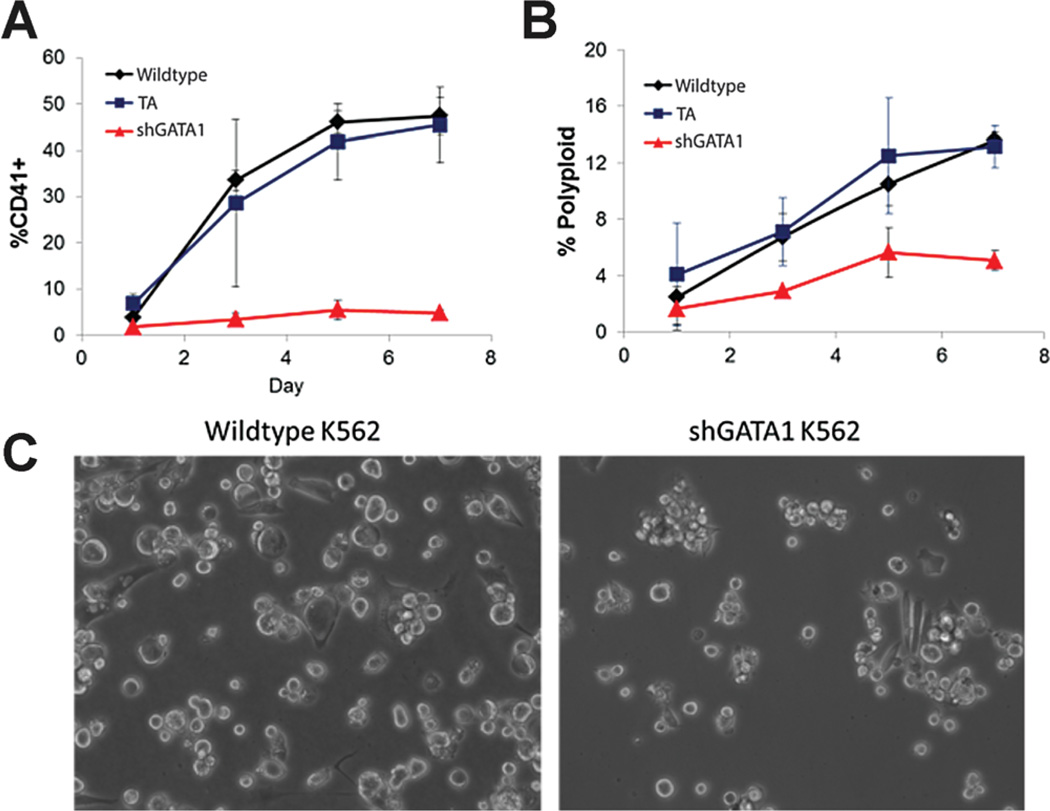
Next, we examined the E and MK differentiation of GATA-1-silenced K562 cells. GATA-1-silenced K562 cells were unresponsive to PMA treatment, failing to acquire CD41 (Fig. 4A) or undergo polyploidization (Fig. 4B) and typically retaining a small, round, undifferentiated morphology (Fig. 4C). Additionally, GATA-1-silenced cells did not respond to hemin, as they failed to produce hemoglobin (Fig. 5A and B) or upregulate GlyA expression (Fig. 5C). These results were consistent with the preceding network analysis, where GATA-1 was predicted to be essential for both E and MK differentiation.
Figure 4.
Characterization of PMA-induced MK differentiation of GATA-1-silenced K562 cells. Ploidy (A) and CD41 expression (B) were monitored for 1 week after PMA induction to assess MK differentiation. Data is from two independent time-course experiments. Error bars represent the standard deviation. C: GATA-1-silenced cells retained a small, rounded morphology 5 days post-PMA treatment.
Figure 5.
Characterization of hemin-induced E differentiation of GATA-1-silenced K562 cells. TA-transduced, sh-GATA1 and wild-type K562 cells were induced with hemin for 3 days in comparison to wild-type and TA transduced cells. Erythroid differentiation was assessed by hemoglobinization using benzidine staining (A: representative light micrographs on Day 3; B: quantification) and Glycophorin A expression (C). Representative flow cytometry histograms on day 3 for uninduced (red lines) and hemin-induced (blue lines) cells. Data is from two independent time-course experiments. Error bars in (B) represent the standard deviation.
Analysis of TF Activity Profiles and Regulatory Network in GATA-1-Depleted Cells
Next, we considered the TF activities of GATA-1-depleted cells during differentiation using the dynamic TF activity array (Fig. 6). During MK differentiation, we found that PU.1 and c-Myb activities were no longer repressed by PMA treatment in GATA-1-silenced cells. In addition, although FLI-1 activity was initially elevated, the increase was not sustained. Surprisingly, NF-E2 activation remained robust, indicating that this event is not far downstream from the initial activation of protein kinase C by PMA. Analysis of the dynamics of TF activity at short time points (e.g., hours post-PMA addition) confirmed this to be true, as NF-E2 exhibited a rapid increase in activity, reaching its apex prior to 24 h of PMA treatment (data not shown).
Figure 6.
Dynamic TF activity array and GATA1-silenced-K562 cells during E or MK differentiation. GATA-1-down-regulated K562 cells were transduced by each TF-activity-reporting lentivirus and treated with PMA or hemin. TF activity was normalized by TA activity and represented by the ratio to vehicle-treated cells. Shaded regions denote the 95% confidence interval about the mean for eight measurements (indicated by data points) from two independent transduction experiments.
For E differentiation, the increase in GATA-2 activity was much less than for wild-type cells and the increase in TAL1 activity observed for wild-type cells did not occur in GATA-1-silenced cells. However, as with PMA treatment, hemin rapidly induced NF-E2 activity despite GATA-1 depletion. Taken together, these results demonstrate that, aside from NF-E2, the TF activity trends shown in Figure 2A are GATA-1-dependent and are specifically associated with E and MK differentiation.
Finally, we also created an interaction network for GATA-1-silenced cells, similar to that shown in Figure 2B. As expected, this network substantially differed from that found during the differentiation of wild-type cells (Supplemental ). Of note, a majority of the characteristic interactions identified for PMA- and hemin-induced differentiation in wild-type cells were no longer present. In particular, as may be expected, the mutually activating relationship between GATA-1 and TAL1 was no longer present in early PMA- and hemin-induced differentiation, and GATA-1 no longer appears to be a target of FLI-1, PU.1, and c-Myb in PMA-induced differentiation. Depletion of GATA-1 also resulted in mutually activating relationships between TAL1 and c-Myb (hemin and PMA) and between PU.1 and GATA-2 (PMA only) that were not observed for wild-type cells.
TF Activity and Regulatory Network for Nicotinamide Influencing MK Polyploidization
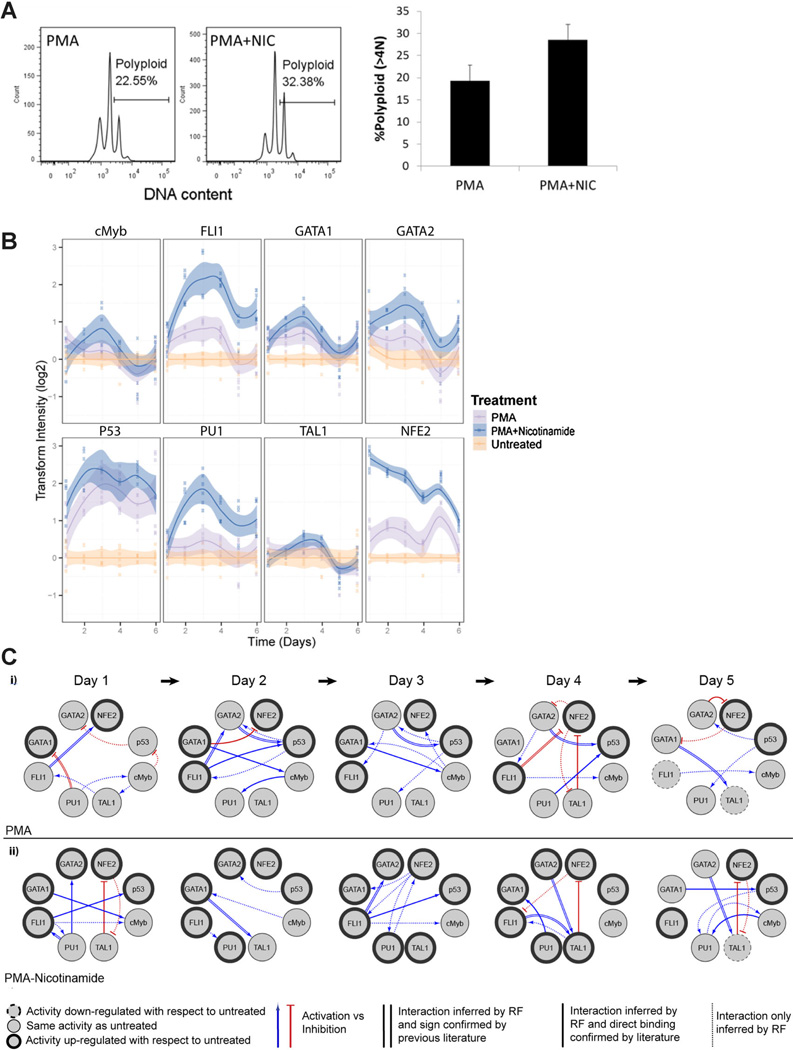
We previously established the potent beneficial effects of NIC treatment on MK maturation (Giammona et al., 2006; Giammona et al., 2009). We investigated the mechanism of NIC action by profiling TF activities and constructing a putative regulatory network using the MK cell line CHRF, which we have extensively validated as a model of MK differentiation (Fuhrken et al., 2007). CHRF cells are committed to the MK lineage, yet are terminally differentiated by PMA addition, becoming polyploid and forming proplatelet-like extensions. Importantly, as with primary MK cells, CHRF cells substantially increase their ploidy in response to NIC (Giammona et al., 2006).
After establishing CHRF TF-reporter cell lines, we quantified the dynamic TF activities in response to treatment with either PMA or PMA plus NIC over 6 days of differentiation. Cells harvested from the array at the final day (Day 6) confirmed that NIC treatment consistently increased polyploidization under the culture conditions (Fig. 7A). TF activity analysis (Fig. 7B) revealed that, consistent with K562 cells, PMA tended to increase the activities of NF-E2 and FLI-1 versus untreated cells (typically 1.5- to 2-fold induction). Also, similar to K562 cells, GATA-2 and TAL1 activities remained essentially constant after PMA induction. However, the decreases in GATA-1, PU.1, and c-Myb activities characteristic of K562 cells were not observed for CHRF cells. These differences likely result from the greater maturity of untreated CHRF cells (MK progenitor phenotype) relative to K562 cells (MEP phenotype), so that the basal levels of the aforementioned TFs may have already been down-regulated prior to PMA treatment. CHRF cells express wild-type p53, which we have shown to negatively regulate MK polyploidization (Fuhrken et al., 2008). p53-activity increased throughout MK differentiation, consistent with our previous observation using electrophoretic mobility shift assay (EMSA) that p53-DNA-binding increases during CHRF cell MK differentiation (Fuhrken et al., 2008). Adding NIC strongly enhanced the activities of FLI-1, PU.1, and NF-E2, while moderately enhancing p53 and GATA-2 activities. We attempted to confirm the effects of NIC on some of the aforementioned TFs during MK differentiation of primary CD34þ HSPCs, but were limited by low bioluminescence signal for most reporters, probably due to low transduction efficiency. However, we did confirm that NIC significantly enhanced NF-E2 activity (2- to 3-fold) during primary MK differentiation (data not shown), and have previously shown that NIC increases p53-binding activity (Giammona et al., 2009).
Figure 7.
Phenotypic characterization and dynamic TF activity array and regulatory networks of CHRF cells during MK maturation. A: CHRF cells were harvested at Day 6 to confirm that NIC enhanced MK ploidy during culture within the array (P< 0.05). Ploidy histogram shown is representative of three independent experiments. Error bars represent the standard deviation. B: Dynamic TF activity profiles. TF activity was normalized by TA activity and represented by the ratio to untreated cells. Shaded regions denote the 95% confidence interval of the mean of six measurements (indicated by data points) from two independent transduction experiments. Error bars indicate standard deviation. C: TF regulatory network was inferred for CHRF cells during MK maturation with PMA, and PMA plus NIC treatments. p53 was added as an additional node to identify potential TFs that regulate its activity. TF networks show target and regulator TFs (circles), as well as putative director indirect interactions between TFs (directed arrows). TF nodes indicate if TF activity is upregulated (bold outline), downregulated (dashed outline), or unchanged (thin outline) with respect to untreated control on indicated day. Edge weights depict whether the inferred interaction is a direct interaction confirmed by previous literature found in GENEGO (parallel lines), a direct interaction confirmed by previous literature but activation (blue lines) or inhibition (red lines) of the target is unknown (solid lines), or inferred interaction is novel (dotted lines).
To identify possible mechanisms for the response of CHRF cells to NIC treatment, functional TF networks were created and characteristic relationships were identified. Out of 68 edges inferred, 32 edges were found to have evidence in the literature for direct binding. After NIC stimulation, NF-E2 appears to be a potent cooperator with GATA-1, PU.1, and FLI-1 (Day 3), which are known to be regulators of MK gene expression (Labbaye et al., 1995; Shivdasani and Orkin, 1995). During PMA treatment without NIC, p53 expression appears to be primarily regulated by GATA-2. With NIC treatment, p53 activation does not strongly correspond to GATA-2 regulation and appears to cooperate with FLI-1 and GATA-1. We observed fewer edges to p53 with NIC treatment. In contrast, there were more edges to PU.1 with NIC. TAL1 and NF-E2 exhibited mutual inhibition, especially in cultures with NIC. Overall, little similarity was observed between the functional TF networks inferred for PMA treatment in the absence or presence of NIC.
Discussion
The distinct TF activity profiles we observed during E versus MK differentiation of K562 cells provide a unique perspective for understanding how these cells develop toward two disparate phenotypes. In particular, measuring TF activities enhances our understanding of previously known expression patterns. For instance, in K562 cells GATA-1 is generally accepted to be down-regulated at the protein level during treatment with either PMA or hemin (Cheng et al., 1994; Ueki et al., 2004). Yet, we only observed decreased activity for PMA-treated cells (Fig. 2A). This suggests that, during hemin treatment, there may be a mechanism by which declining levels of GATA-1 are offset to maintain a relatively steady level of activity. Previously, hemin was reported to increase GATA-1 DNA-binding affinity (Schnekenburger et al., 2004), while PMA decreases GATA-1 activity (as determined by EMSA) (Li et al., 2008; Partington and Patient, 1999). This discrepancy in GATA-1 function combined with TF activity results herein suggests that in K562 cells terminal E differentiation corresponds to less GATA-1, but in a transcriptionally active form, while MK differentiation may correspond to GATA-1 depletion without activation. Our inferred networks suggest that TAL1, PU.1, and c-Myb are key cooperators with GATA-1 (Fig. 2B), but the precise mechanism by which differentiating K562 cells activate and/or degrade GATA-1 remains to be determined. A report by De Thonel et al. (2010) suggests that acetylation may play a key role by promoting GATA-1 transactivation, while also enhancing its degradation by the proteasome. Under PMA treatment, we also observed a steady decrease in the activity of TAL1, which is known to form transcriptional activating complexes with GATA-1 (Kassouf et al., 2008; Lahlil et al., 2004; Tripic et al., 2009). The fact that independent reporters for GATA-1 and TAL1 both exhibited reduced activity after PMA addition suggests that the activity of GATA-1 + TAL1-containing complexes may likewise be reduced. Conversely, we observed a late upregulation of TAL1 activity during hemin-mediated differentiation of K562 cells, which is consistent with its reported requirement in terminal erythroid differentiation (Kassouf et al., 2008).
Unlike GATA-1, GATA-2 activity transiently increased during the first three days of hemin treatment and recovered to basal level, while there was no significant change under PMA treatment (Fig. 2A). Our analysis reveals interesting insights for GATA-2 function in the differentiation of K562 cells and offers an explanation to inconsistencies observed in the literature. Harigae et al. (2006) reported that GATA-2 overexpression resulted in greater expression of globin genes, the transferrin receptor, and heme content. However, an earlier study found that GATA-2 overexpression biased K562 cells towards the MK lineage (Ikonomi et al., 2000). Our results that GATA-2 activity initially increases and then declines after 4–5 days of hemin treatment, support the idea that ectopic GATA-2 promotes E progenitor cell proliferation, but interferes with their terminal differentiation, and that GATA-2 levels decline during the later stages of erythroid differentiation (Briegel et al., 1993; Labbaye et al., 1995).
We anticipated a decrease in FLI-1 activity in response to hemin, since FLI-1 antagonizes commitment of MEPs to the erythroid lineage in favor of MK lineage (Athanasiou et al., 2000; Kawada et al., 2001; Starck et al., 2003). However, hemin did not alter basal FLI-1 activity. FLI-1 is known to be required for normal polyploidization (Hart et al., 2000) and is important for coordinating the expression of numerous early and late MK proteins, including CD41, CD42, mpl, and Pf4 (Deveaux et al., 1996; Doré and Crispino, 2011). Since K562 cells already appear to exhibit a bias toward the erythroid lineage (e.g., express the erythroid-specific antigens CD71 and GlyA), this suggests that K562 cells may have already down-regulated FLI-1 expression and/or activity prior to treatment. In support of this, expression of luciferase from a FLI-1 promoter in untreated K562 cells was found to depend on provision of exogeneous FLI-1 (Frontelo et al., 2007).
Our analysis also provides interesting insights into the activity of NF-E2. In particular, we found that NF-E2 is strongly and rapidly activated by the addition of hemin or PMA. Furthermore, this activation does not depend on the presence of GATA-1 (Fig. 6) or the ability to differentiate to the E and MK lineages as normally occurs in K562 cells (Figs. 4 and 5). This indicates that NF-E2 activation is not far downstream in the intracellular signaling that occurs upon K562 cell stimulation with either hemin or PMA.
Our live cell array also provided insight into the effects of NIC on terminal MK differentiation, demonstrating enhancement of FLI-1, PU.1, NF-E2, GATA-2, and p53 activities. Although essential for cytoplasmic maturation and proplatelet formation, NF-E2 does not affect MK ploidy, (Shivdasani and Orkin, 1995). Thus, we do not expect increased NF-E2 activity to be related to NIC’s enhancement of ploidy, but it likely affects other genes related to MK maturation. With regard to p53, NIC treatment increases p53 acetylation at K382 (reported to promote cell cycle arrest and apoptosis [Roy et al., 2005; Tang et al., 2008]) and enhances its DNA-binding activity during MK differentiation (Giammona et al., 2009), consistent with observations from the array. The relationship between NIC-mediated increases in ploidy and increases in p53 activity remains unclear. Further exploring this relationship, as well as a possible dependence of NIC-mediated ploidy enhancement on FLI-1, will require further study.
The dynamic nature of the TF activity profiling assay provides the ability to capture how TF networks functionally rewire during differentiation. Novel and known characteristic relationships were inferred for PMA- and hemin-mediated differentiation, which illustrates changes in TF regulation during MK and E differentiation. Directed edges represent causal influences, but such predicted influences may be indirect and not be mediated by a direct binding relationship. The causal influence (assume inferred activation) of TF B by TF A could be explained by TF A binding to the promoter sequence of the gene controlling the transcription of TF B. This interaction could also be explained by a more complicated process, such as transcription factor A binding to a gene that encodes a metabolic enzyme producing a metabolite which in turn regulates the transcription of B. These detailed biochemical events are hidden in the observed set of variables. Although likely biological mechanisms have been described for known TF interactions, further experimental verification is needed to uncover the biochemical mechanisms involved in novel relationships.
In conclusion, our analysis revealed distinct changes in TF activity profiles with the induction of E versus MK differentiation. Activities of some TFs were consistent with and expected from previous reports, which verifies our dynamic TF activity analysis. A tree-ensemble-based inference method was employed to create characteristic networks describing active regulatory interactions, and this highlighted several functional relationships previously identified in the literature. In addition, we analyzed the effects GATA-1 silencing and verified GATA-1 as an early, essential regulator for both E and MK differentiation in K562 cells, as indicated by the network analysis and previous reports. The modeling of our array data has identified a number of potential interactions, some of which are validated within existing databases (e.g., GeneGo), and others for which no precedent in the literature is available. Taken together, this K562 cell model captures some, but not all, aspects of primary human progenitor cell differentiation and ongoing studies are investigating these observations in primary cells. Ultimately, TF activity data generated during such experiments will complement traditional methods to increase understanding of the regulation of gene expression that is critical for stem cell differentiation. Models that integrate mRNA, protein, and TF activity data will ultimately provide a more complete picture of the critical regulatory events throughout the differentiation process.
Supplementary Material
Acknowledgments
This research was supported by U.S. National Science Foundation (NSF) grant CBET-0853603 (WMM) and National Institutes of Health (NIH) grants NCI 5U54CA143869-05 (WMM, LDS, NB), P50GM081892 (LDS), and R01GM097220 (LDS). MD was supported in part by NIH predoctoral Biotechnology Training Grant T32GM008449. JW was supported in part by NSF grant DGE-1007911. This work used facilities of the Northwestern University Flow Cytometry Core Facility and the Northwestern University Center for Advanced Molecular Imaging.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Contributor Information
Neda Bagheri, Email: n-bagheri@northwestern.edu.
William M. Miller, Email: wmmiller@northwestern.edu.
Lonnie D. Shea, Email: l-shea@northwestern.edu.
References
- Athanasiou M, Clausen PA, Mavrothalassitis GJ, Zhang XK, Watson DK, Blair DG. Increased expression of the ETS-related transcription factor FLI-1/ERGB correlates with and can induce the megakaryocytic phenotype. Cell Growth Differ. 1996;7:1525–1534. [PubMed] [Google Scholar]
- Athanasiou M, Mavrothalassitis G, Sun-Hoffman L, Blair DG. FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia. 2000;14:439–445. doi: 10.1038/sj.leu.2401689. [DOI] [PubMed] [Google Scholar]
- Bellis AD, Peňalver-Bernabé B, Weiss MS, Yarrington ME, Barbolina MV, Pannier AK, Jeruss JS, Broadbelt LJ, Shea LD. Cellular arrays for large-scale analysis of transcription factor activity. Biotechnol Bioeng. 2011;108:395–403. doi: 10.1002/bit.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- Briegel K, Lim KC, Plank C, Beug H, Engel JD, Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 1993;7:1097–1109. doi: 10.1101/gad.7.6.1097. [DOI] [PubMed] [Google Scholar]
- Cheng T, Wang Y, Dai W. Transcription factor egr-1 is involved in phorbol 12-myristate 13-acetate-induced megakaryocytic differentiation of K562 cells. J Biol Chem. 1994;269:30848–30853. [PubMed] [Google Scholar]
- De Thonel A, Vandekerckhove J, Lanneau D, Selvakumar S, Courtois G, Hazoume A, Brunet M, Maurel S, Hammann A, Ribeil JA, Zermati Y, Gabet AS, Boyes J, Solary E, Hermine O, Garrido C. Hsp27 controls Gata-1 protein level during erythroid cell differentiation. Blood. 2010;116:85–96. doi: 10.1182/blood-2009-09-241778. [DOI] [PubMed] [Google Scholar]
- Deveaux S, Filipe A, Lemarchandel V, Ghysdael J, Roméo PH, Mignotte V. Analysis of the thrombopoietin receptor (MPL) promoter implicates GATA and Ets proteins in the coregulation of megakaryocyte-specific genes. Blood. 1996;87:4678–4685. [PubMed] [Google Scholar]
- Doré LC, Crispino JD. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118:231–239. doi: 10.1182/blood-2011-04-285981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbacher M, Holmes ML, Newton A, Hogg PJ, Khachigian LM, Crossley M, Chong BH. Protein-protein interaction between fli-1 and gata-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative dna binding. Mol Cell Biol. 2003;23:3427–3441. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elagib KE, Racke FK, Mogass M, Khetawat R, Delehanty LL, Goldfarb AN. Runx1 and gata-1 coexpression and cooperation in megakaryo-cytic differentiation. Blood. 2003;101:4333–4341. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- Escamilla-Del-Arenal M, Recillas-Targa F. GATA-1 modulates the chromatin structure and activity of the chicken α-globin 3′ enhancer. Mol Cell Biol. 2008;28:575–586. doi: 10.1128/MCB.00943-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontelo P, Manwani D, Galdass M, Karsunky H, Lohmann F, Gallagher PG, Bieker JJ. Novel role for EKLF in megakaryocyte lineage commitment. Blood. 2007;110:3871–3880. doi: 10.1182/blood-2007-03-082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrken PG, Apostolidis PA, Lindsey S, Miller WM, Papoutsakis ET. Tumor suppressor protein p53 regulates megakaryocytic polyploidiza-tion and apoptosis. J Biol Chem. 2008;283:15589–15600. doi: 10.1074/jbc.M801923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrken PG, Chen C, Miller WM, Papoutsakis ET. Comparative, genome-scale transcriptional analysis of CHRF-288-11 and primary human megakaryocytic cell cultures provides novel insights into lineage-specific differentiation. Exp Hematol. 2007;35:476–489. doi: 10.1016/j.exphem.2006.10.017. [DOI] [PubMed] [Google Scholar]
- García P, Berlanga O, Vegiopoulos A, Vyas P, Frampton J. c-Myb and GATA-1 alternate dominant roles during megakaryocyte differentiation. J Thromb Haemost. 2011;9:1572–1581. doi: 10.1111/j.1538-7836.2011.04396.x. [DOI] [PubMed] [Google Scholar]
- Georgantas RW, Hildreth R, Morisot S, Alder J, Liu C, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34þ hematopoietic stem-progenitor cell microRNA expression and function: A circuit diagram of differentiation control. Proc Natl Acad Sci USA. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giammona LM, Fuhrken PG, Papoutsakis ET, Miller WM. Nicotinamide (vitamin B3) increases the polyploidisation and propla-telet formation of cultured primary human megakaryocytes. Br J Haematol. 2006;135:554–566. doi: 10.1111/j.1365-2141.2006.06341.x. [DOI] [PubMed] [Google Scholar]
- Giammona LM, Panuganti S, Kemper JM, Apostolidis PA, Lindsey S, Papoutsakis ET, Miller WM. Mechanistic studies on the effects of nicotinamide on megakaryocytic polyploidization and the roles of NADþ levels and SIRT inhibition. Exp Hematol. 2009;37:1340–1352. doi: 10.1016/j.exphem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci USA. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RE, Kupzig S, Cogan N, Mankelow TJ, Betin VMS, Trakarnsanga K, Massey EJ, Parsons SF, Anstee DJ, Lane JD. The ins and outs of human reticulocyte maturation. Autophagy. 2012;8:1150–1151. doi: 10.4161/auto.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigae H, Okitsu Y, Yokoyama H, Fujiwara T, Inomata M, Takahashi S, Minegishi N, Kaku M, Sasaki T. Induction of erythroid-specific genes by overexpression of GATA-2 in K562 cells. Int J Hematol. 2006;84:38–42. doi: 10.1532/IJH97.06020. [DOI] [PubMed] [Google Scholar]
- Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE. 2010;5:e12776. doi: 10.1371/journal.pone.0012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomi P, Rivera CE, Riordan M, Washington G, Schechter AN, Noguchi CT. Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp Hematol. 2000;28:1423–1431. doi: 10.1016/s0301-472x(00)00553-1. [DOI] [PubMed] [Google Scholar]
- Im H, Grass JA, Johnson KD, Kim S-I, Boyer ME, Imbalzano AN, Bieker JJ, Bresnick EH. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc Natl Acad Sci USA. 2005;102:17065–17070. doi: 10.1073/pnas.0506164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiko E, Matsumura I, Ezoe S, Gale K, Ishiko J, Satoh Y, Tanaka H, Shibayama H, Mizuki M, Era T, Enver T, Kanakura Y. Notch signals inhibit the development of erythroid/megakaryocytic cells by suppressing Gata-1 activity through the induction of hes1. J Biol Chem. 2005;280:4929–4939. doi: 10.1074/jbc.M406788200. [DOI] [PubMed] [Google Scholar]
- Kassouf MT, Chagraoui H, Vyas P, Porcher C. Differential use of SCL/TAL-1 DNA-binding domain in developmental hematopoiesis. Blood. 2008;112:1056–1067. doi: 10.1182/blood-2007-12-128900. [DOI] [PubMed] [Google Scholar]
- Kawada H, Ito T, Pharr PN, Spyropoulos DD, Watson DK, Ogawa M. Defective megakaryopoiesis and abnormal erythroid development in Fli-1 gene-targeted mice. Int J Hematol. 2001;73:463–468. doi: 10.1007/BF02994008. [DOI] [PubMed] [Google Scholar]
- Labbaye C, Valtieri M, Barberi T, Meccia E, Masella B, Pelosi E, Condorelli GL, Testa U, Peschle C. Differential expression and functional role of GATA-2, NF-E2, and GATA-1 in normal adult hematopoiesis. J Clin Invest. 1995;95:2346–2358. doi: 10.1172/JCI117927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlil R, Lécuyer E, Herblot S, Hoang T. SCL assembles a multifactorial complex that determines glycophorin A expression. Mol Cell Biol. 2004;24:1439–1452. doi: 10.1128/MCB.24.4.1439-1452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LT, Ronchini C, Norton J, Capobianco AJ, Bresnick EH. Suppression of erythroid but not megakaryocytic differentiation of human K562 erythroleukemic cells by notch-1. J Biol Chem. 2000;275:19676–19684. doi: 10.1074/jbc.M002866200. [DOI] [PubMed] [Google Scholar]
- Leary JF, Ohlsson-Wilhelm BM, Giuliano R, LaBella S, Farley B, Rowley PT. Multipotent human hematopoietic cell line K562: Lineage-specific constitutive and inducible antigens. Leuk Res. 1987;11:807–815. doi: 10.1016/0145-2126(87)90065-8. [DOI] [PubMed] [Google Scholar]
- Leysi-Derilou Y, Duchesne C, Garnier A, Pineault N. Single-cell level analysis of megakaryocyte growth and development. Differ Res Biol Divers. 2012;83:200–209. doi: 10.1016/j.diff.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Li C-Y, Fang F, Xu W-X, Xu C-W, Zhan Y-Q, Wang Z-D, Ding Y-L, Li Y-H, Sun H-B, Yang X-M. Suppression of EDAG gene expression by phorbol 12-myristate 13-acetate is mediated through down-regulation of GATA-1. Biochim Biophys Acta. 2008;1779:606–615. doi: 10.1016/j.bbagrm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Lorenzo PI, Brendeford EM, Gilfillan S, Gavrilov AA, Leedsak M, Razin SV, Eskeland R, Saether T, Gabrielsen OS. Identification of c-Myb target genes in K562 cells reveals a role for c-Myb as a master regulator. Genes Cancer. 2011;2:805–817. doi: 10.1177/1947601911428224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, Willson TA, Henley KJ, Ellis S, Voss AK, Metcalf D, Hilton DJ, Alexander WS, Kile BT. The transcription factor erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9:810–819. doi: 10.1038/ni.1617. [DOI] [PubMed] [Google Scholar]
- Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, Voss N, Stegmaier P, Lewicki-Potapov B, Saxel H, Kel AE, Wingender E. Transfac and its module transcompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–D110. doi: 10.1093/nar/gkj143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda K, Yamamoto M. Roles of hematopoietic transcription factors GATA-1 and GATA-2 in the development of red blood cell lineage. Acta Haematol. 2002;108:237–245. doi: 10.1159/000065660. [DOI] [PubMed] [Google Scholar]
- Partington GA, Patient RK. Phosphorylation of GATA-1 increases its DNA-binding affinity and is correlated with induction of human K562 erythroleukaemia cells. Nucleic Acids Res. 1999;27:1168–1175. doi: 10.1093/nar/27.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrianarison-Huetz V, Laurent B, Bardet V, Blobe GC, Huetz F, Duménil D. Gfi-1b controls human erythroid and megakaryocytic differentiation by regulating tgf-beta signaling at the bipotent erythro-megakaryocytic progenitor stage. Blood. 2010;115:2784–2795. doi: 10.1182/blood-2009-09-241752. [DOI] [PubMed] [Google Scholar]
- Raslova H, Roy L, Vourc’h C, Couedic JPL, Brison O, Metivier D, Feunteun J, Kroemer G, Debili N, Vainchenker W. Megakaryocyte polyploidization is associated with a functional gene amplification. Blood. 2003;101:541–544. doi: 10.1182/blood-2002-05-1553. [DOI] [PubMed] [Google Scholar]
- Roy S, Packman K, Jeffrey R, Tenniswood M. Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ. 2005;12:482–491. doi: 10.1038/sj.cdd.4401581. [DOI] [PubMed] [Google Scholar]
- Schnekenburger M, Morceau F, Duvoix A, Delhalle S, Trentesaux C, Dicato M, Diederich M. Increased glutathione S-transferase P 1-1 expression by mRNA stabilization in hemin-induced differentiation of K562 cells. Biochem Pharmacol. 2004;68:1269–1277. doi: 10.1016/j.bcp.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci USA. 1995;92:8690–8694. doi: 10.1073/pnas.92.19.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinfor-matics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- Starck J, Cohet N, Gonnet C, Sarrazin S, Doubeikovskaia Z, Doubeikovski A, Verger A, Duterque-Coquillaud M, Morle F. Functional cross-antagonism between transcription factors FLI-1 and EKLF. Mol Cell Biol. 2003;23:1390–1402. doi: 10.1128/MCB.23.4.1390-1402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck J, Doubeikovski A, Sarrazin S, Gonnet C, Rao G, Skoultchi A, Godet J, Dusanter-Fourt I, Morle F. Spi-1/PU.1 is a positive regulator of the Fli-1 gene involved in inhibition of erythroid differentiation in friend erythroleukemic cell lines. Mol Cell Biol. 1999;19:121–135. doi: 10.1128/mcb.19.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroncek DF, Rebulla P. Platelet transfusions. The Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- Sutherland JA, Turner AR, Mannoni P, McGann LE, Turc JM. Differentiation of K562 leukemia cells along erythroid, macrophage, and megakaryocyte lineages. J Biol Response Mod. 1986;5:250–262. [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripic T, Deng W, Cheng Y, Zhang Y, Vakoc CR, Gregory GD, Hardison RC, Blobel GA. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood. 2009;113:2191–2201. doi: 10.1182/blood-2008-07-169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki N, Zhang L, Hayman MJ. Ski negatively regulates erythroid differentiation through its interaction with GATA1. Mol Cell Biol. 2004;24:10118–10125. doi: 10.1128/MCB.24.23.10118-10125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, Friend DS, Stevens RL, Anastasi J, Singh H. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- Weiss MS, Peñalver Bernabé B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD. Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture. PLoS ONE. 2010;5:e14026. doi: 10.1371/journal.pone.0014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NK, Foster SD, Wang X, Knezevic K, Schütte J, Kaimakis P, Chilarska PM, Kinston S, Ouwehand WH, Dzierzak E, Pimanda JE, de Bruijn MFTR, Göttgens B. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Xu Z, Huang S, Chang L-S, Agulnick AD, Brandt SJ. Identification of a TAL1 target gene reveals a positive role for the lim domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol Cell Biol. 2003;23:7585–7599. doi: 10.1128/MCB.23.21.7585-7599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.