Abstract
One of the most challenging areas of pharmaceutical research is ocular drug delivery. The unique anatomy and physiology of the eye impedes drug permeation to deeper ocular tissues. Nanosized carrier systems such as nanoparticles, liposomes, suspensions, dendrimers, and nanomicelles are being explored for ocular drug delivery. In this review, we have focused on application of emerging nanomicellar carrier systems in ocular drug delivery. Nanomicelles are nanosized vesicular carriers formed from amphiphilic monomer units. Surfactant and polymeric micellar nanocarriers provide an amenable means to improve drug solubilization, develop clear aqueous formulations and deliver drugs to anterior and posterior ocular tissues. Nanomicelles due to their amphiphilic nature encapsulate hydrophobic drugs and aid in drug delivery. Various methods are employed to develop nanosized micellar formulations depending upon the physicochemical properties of the drug. Nanomicellar carriers appear to be promising vehicles with potential applications in ocular drug delivery. In this review, we attempted to discuss about the progress in ocular drug delivery research using nanomicelles as carriers from the published literature and issued patents. Also, with regards to ocular static and dynamic barriers which prevent drug permeation, a brief discussion about nanomicelles, types of nanomicelles, their methods of preparation and micellar strategy to overcome ocular barriers, delivering therapeutic levels of drugs to anterior and posterior ocular tissues are discussed.
Keywords: Barriers, conjunctival/scleral pathway, drug delivery, nanotechnology, nanomicelles, patents, posterior segment, retina-choroid
INTRODUCTION
Several diseases are known to affect anterior and posterior segment of the human eye. Diseases affecting the anterior ocular tissues are easily and effectively treated with topical dosing. But diseases affecting the posterior ocular tissues are difficult to treat and if left untreated may lead to vision loss. Therapeutic agents administered by oral and intravenous routes may not reach targeted ocular tissues due to sub-optimal physicochemical properties of the drug [1, 2] and various ocular anatomical barriers. Therefore, currently employed drug delivery strategies for posterior ocular tissues are local and invasive i.e., by intravitreal, periocular, subconjunctival and suprachoroidal injections. These modes are employed (i) to minimize adverse effects associated with large doses that were previously observed with traditional methods (oral and intravenous administrations) and (ii) to attain therapeutic drug levels at the target tissue with no/minimal side effects. However, local drug administration methods have their own limitations such as retinal detachment, endophthalmitis, pseudoendophthalmitis and patient non-compliance [3–5]. Moreover, repeated administrations are required to maintain therapeutic drug concentrations at diseased site. Effective disease treatment depends on efficient drug delivery approach. To minimize frequent drug administrations and to attain therapeutic drug concentrations, sustained/controlled drug release is desired which may be achieved with a delivery system. The major requirement for any efficient delivery/carrier system is to act as a vehicle for the entrapped therapeutic agent and to deliver the drugs to the targeted site precisely and effectively. Design of an ocular drug delivery system depends on three major key aspects (i) provide a patient compliant route of drug administration (ii) control drug release kinetics from the carrier and (iii) deliver the drug to the targeted site in therapeutic concentrations. The ultimate goal of any drug delivery system is to improve drug efficacy and reduce toxicity. To achieve therapeutic drug concentrations, current research is focused on the development of nanoscaled carrier systems such as liposomes, nanoparticles, dendrimers, and nanomicelles based sustained drug delivery. These carrier systems have the ability to sequester therapeutic agents in their core and modulate drug release kinetics. Therefore, sustained drug release from the carrier systems may help reduce frequent drug administrations. Drug entrapped/loaded nanocarriers are being employed to deliver drugs to anterior and posterior ocular tissues following different routes of administrations. Though effective, these administration routes are invasive and often associated with ocular complications [3–5] thus reducing patient compliance. In this review we describe various ocular barriers which impede drug entry into deeper ocular tissues and mainly focus on nanomicellar ocular drug carriers that can efficiently overcome these biological barriers and deliver therapeutic drug levels to anterior and posterior ocular tissues.
AN OVERVIEW OF OCULAR ANATOMY
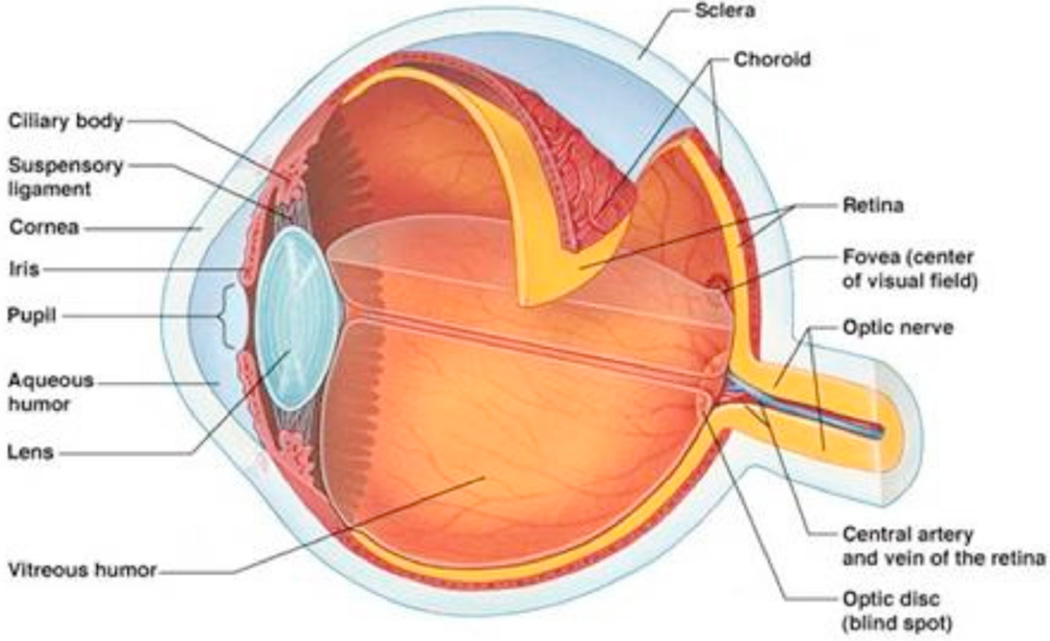
Human eye can be divided into two segments (a) anterior segment and (b) posterior segment (Fig. 1). Anterior segment occupies front one-third and posterior segment occupies the remaining two-third of the globe. Anterior segment of the eye primarily includes cornea, iris, ciliary body, aqueous humor, crystalline lens and conjunctiva. Posterior segment consists of sclera, choroid, retina, macula, vitreous humor and the optic nerve.
Fig. (1).
Structure of human eye.
BARRIERS IN OCULAR DRUG DELIVERY
Drug delivery to anterior segment ocular tissues is impeded due to the presence of ocular barriers which makes drug delivery more difficult and challenging. Another most challenging task in ocular drug delivery is back of the eye (retina-choroid) delivery. To modulate and improve ocular drug delivery, it is important to overcome these ocular barriers, which can be classified into two different types as (i) anterior segment barriers and (ii) posterior segment barriers.
ANTERIOR SEGMENT BARRIERS
Upon topical application, drug molecules are prevented from reaching targeted ocular tissues due to anterior static barriers (corneal epithelium, blood aqueous barrier) and dynamic barriers (conjunctival blood and lymphatic clearance and tear drainage). A large fraction of a topically applied drug into the cul-de-sac is lost due to pre-corneal barrier - tear film. Upon topical drop application, the drug concentrations are reduced due to tear dilution, drug binding to tear proteins and accelerated clearance. Usually, the volume of dose for topical drop administration ranges from 20–50 µL. From the applied dose, precorneal pocket holds only 7–10 µL by replacing tears which is already present in the precorneal pocket (6). Such excess administered dose may be lost due to spill out from the precorneal pocket or drainage through the nasolacrimal duct [6–8]. Precorneal tear drainage washes the topically instilled drops/solutions within first 15–30 seconds causing a significant reduction in drug contact time and ultimately reducing ocular bioavailability (< 5%) [9–11].
Cornea represents a multilayered, avascular mechanical barrier which hinders transport of exogenous substances/ drug molecules from pre-corneal pocket. It can be subdivided into three different layers (a) corneal epithelium (highly lipophilic), (b) stroma (hydrophilic) and (c) mono cell layered endothelium (lipophilic). Mature corneal epithelial cells act as a barrier to drug absorption and restrict paracellular diffusion into deeper tissues due to tight junctions [12]. Corneal epithelia being relatively lipophilic allows ready absorption of hydrophobic drugs relative to hydrophilic drugs [13]. In contrary, hydrophilic stroma acts as a rate limiting barrier for the permeation of hydrophobic molecules. Corneal endothelium forms a cellular barrier between aqueous humor and the stroma. This barrier possesses leaky tight endothelial junctions which allow free movement of macromolecules between stroma and the aqueous humor [14]. Mostly, drug absorption into the aqueous humor occurs by transcorneal diffusion of drug. Efflux transporters expressed on the plasma membrane of corneal cells is another important significant barrier which restricts drug permeation from reaching targeted ocular tissues. Expression of drug efflux pumps such as P-glycoprotein (P-gp), multidrug resistant proteins (MRPs), breast cancer resistant protein (BCRP) and their active involvement in drug efflux at cell surface were identified and demonstrated on human and rabbit cornea [15–17].
Posterior to cornea: iris, ciliary body and choroid which represent the uvea of the eye are present. These tissues are vascularized and play significant role in impeding drug transport into intraocular tissues. Ciliary body secretes aqueous humor into the narrow cleft present between the back of iris and lens. Flow of aqueous humor is opposite to the direction of drug entry/flow and may limit drug availability to intraocular tissues by draining the drug through trabecular meshwork into canal of Schlemm. Aqueous humor turnover rate (2–3 µL/min) was found to eliminate large hydrophilic drugs [12]. Also, expression of drug efflux pump, P-gp, on the apical side of ciliary non-pigmented epithelium, iris and ciliary muscle cells was reported to cause drug efflux leading to sub-therapeutic levels [18, 19]. Active agents may be eliminated into systemic circulation from aqueous humor by easily penetrating into the anterior endothelial surface of the iris microvasculature. Hence, therapeutic molecules present in the aqueous humor may be absorbed by iris pigments and drained from the anterior segment into the iris blood circulation [20, 21]. Endothelial cells of iris/ciliary blood vessels and the non-pigmented ciliary epithelium forms another barrier known as blood-aqueous barrier (BAB). BAB regulates exchange of solutes between anterior and posterior segments [22, 23] by forming tight junctions at the cellular levels. Tight junctions at the cellular level impede nonspecific drug entry into deeper ocular tissues and act as a barrier. Small lipophilic drugs that cross BAB and enter uveal blood circulation were found to eliminate more rapidly than larger hydrophilic molecules.
The upper eyelid and the anterior surface of sclera are covered by a thin transparent membrane called conjunctiva which extends to the end of corneal limbus. Conjunctiva acts as a permeability barrier to topically applied drugs due to the presence of epithelial tight junctions with a trans-epithelial electrical resistance (TEER) over 1500 Ω.cm2 [24]. Also, conjunctiva is highly vascularized, richly supplied with lymphatics and involved in the production and maintenance of tear film. Therefore, topically instilled drugs into the culde-sac may be carried away by systemic and lymph circulation while traversing across the thin layer. At cellular levels, functional expression of efflux pumps such as P-gp and MRP1 may limit/restrict drug transport across conjunctiva [25]. Drug molecules which could overcome these barriers and traverse across the conjunctiva may reach posterior ocular tissues through trans-scleral pathway.
POSTERIOR SEGMENT BARRIERS
Drug permeation to posterior ocular tissues is impeded by the presence of static (sclera, retinal pigment epithelium and blood capillary endothelial cells) and dynamic barriers (choroidal blood and lymph circulation). In addition, expression of efflux pumps on the cell membrane acts as a significant permeation barrier.
Sclera is white part of the globe which is continuous with cornea and extends from limbus to the posterior segment of the eye. It is mostly composed of collagen fibers and mucopolysaccharides with poorly developed vasculature [26, 27]. Thickness of sclera differs from anterior to posterior region. It is thick near the limbus and gradually decreases at the equator. Towards the posterior end, at optic nerve, sclera is almost double in thickness relative to anterior thickness. It offers higher permeability than cornea but relatively lesser than conjunctiva. Scleral permeability is strongly dependent on molecular radius, physicochemical properties and surface charge of the permeating molecule. Drug permeability gradually recedes with increasing molecular radius and hydrophobicity, thus limiting permeability through aqueous scleral pores [28–30]. Also, the opposite surface charge of drug molecules may restrict drug permeability due to charge interactions with negatively charged proteoglycans matrix [31].
Choroid is sandwiched between sclera and Bruch’s membrane. Choroid is a highly vascularized and fenestrated tissue supplying blood to inner retina. Thickness of choroid in a new born is 200 µm which gradually reduces with age. Not only age, but other disease factors also affect the choroidal thickness such as age related macular degeneration (AMD), choroidal atrophy, and high myopia [32, 33]. At and above age 90, the choroidal thickness reduces below the half (80 µm) of the initial original thickness (200 µm) [34].
Bruch’s membrane lies below the choroid and above the retinal epithelial cell membrane. It is a thick basement membrane produced by collaboration of choriocapillaries and RPE. Change in thickness of Bruch’s membrane occurs in opposite direction to choroid with age i.e., thickness increases with age [35]. Variation in thickness causes calcification of elastic fiber: higher collagen fiber cross-linking and glycosaminoglycan turnover [36–38]. Changes in the choroid and Bruch’s membrane thickness may affect drug transport from sclera into inner ocular tissues. Moreover, highly lipophilic drugs may be drained into choroidal systemic circulation restricting drug molecules from reaching inner ocular tissues (retina).
Blood retinal barrier (BRB) is composed of inner and outer BRB. Outer BRB is sandwiched between choroid and retina and constitutes tight junctions of retinal pigment epithelial cells whereas inner BRB is composed of retinal capillary endothelial cells. Functions of these tight junctions are supported by astrocytes and Müller cells. Tight junctions regulate the exchange of substances between choroid and retina. Astrocytes are involved in maintaining the integrity and enhancing the barrier properties of retinal endothelial capillaries [39, 40]. BRB provides protection to retina from molecules circulating in retinal circulation. Retinal endothelial cells lack fenestrations and thus transport may be mediated by receptor, energy/adenosine triphosphate dependent fluid phase pinocytosis. Therefore, drug entry is highly restricted due to the presence of tight junctions. Also, expression and activity of P-gp, MRP efflux pumps on apical and basal sides of human retinal pigment epithelium have been reported [41, 42]. P-gp expression in rat retinal endothelial cells has also been reported [43]. In another study expression of ATP binding cassettes (P-gp, MRP4 and BCRP) in retinal vasculature of postnatal mouse has been reported. Presumably the expression of P-gp in retinal astrocytes was also observed [44]. Recently, different expression levels of MRP1, MRP4 and MRP5 efflux proteins in human retinal pigment epithelial cells were also reported [45].
Retina is the light sensitive part of the eye that lies just above the thick viscous fluid called vitreous humor. It is composed of neural and glial cells [46] and this covers the entire inner wall of the globe. It is separated from vitreous humor by presence of a thick cell layer composed of 10 distinct extracellular matrix proteins [47] called inner limiting membrane. Drugs injected into the vitreous humor are restricted from reaching retinal cells by this limiting membrane. Intravitreally administered drugs are eliminated due to aqueous humor turnover and uveal blood flow in the anterior chamber. On the other hand, in the posterior chamber, elimination takes place by drug transport across retina. Transcellular and paracellular routes are ways to transport drugs across RPE. Hydrostatic and osmotic forces are involved in transporting molecules from subretinal spaces to RPE from where elimination occurs [48].
Vitreous body is a clear, avascular, thick gelly like fluid that covers the space between the lens and the retina and aids in maintaining the structure of the globe. It is composed of 99.9% water, 0.01% hyaluronic acid, collagen and ions [49, 50]. Diffusion in vitreous regulates the drug movement, convective flow of vitreous fails to alter drug diffusion at any significant rate [51]. Currently, the major route of drug administration to the back of the eye is by intravitreal administration. Pathophysiological state of vitreous and molecular weight of active agents determine the diffusion kinetics.
Previously described ocular static and dynamic barriers prevent administered drug from reaching the targeted tissue. In order to reach the targeted ocular tissues, molecules need to possess sufficient hydrophilic and lipophilic balance (HLB) and also evade efflux pumps expressed on the cell membrane. Drug delivery systems such as microparticles, nanoparticles, oil in water emulsions, liposomes, suspensions and nanomicelles are constantly being developed to address the above issues. Nanomicellar formulation strategy appears to be a valuable approach which allows for improved drug solubility. It allows formulation of clear aqueous solution by encapsulating drugs in the nanomicellar core. Therefore, to better understand nanomicellar formulations we describe briefly nanomicelles, types of micelles, methods of preparation and their recent development and applications in ocular drug delivery to anterior as well as posterior ocular segments.
NANOMICELLES
A molecule possessing both hydrophilic (polar) and hydrophobic (non polar) groups is known as an amphiphilic molecule. These molecules when exposed to a suitable solvent orient to self-assemble and form normal or reverse nanomicelles depending on the type and degree of orientation. The hydrophilic portion orients towards the polar solvent whereas the hydrophobic section of the molecule orients away from the solvent. Due to this orientation, the hydrophobic parts are clustered in the core (flocks to the interior) while the hydrophilic portions are allied towards the outer surface to maximize contact with water [52, 53]. Such clustered aggregates are termed as normal nanomicelles. On the other hand, amphiphilic molecules undergo an opposite orientation mechanism to form reverse micelles. When exposed to a hydrophobic solvent system, the amphiphiles tend to form nanomicelles with hydrophobic region towards outside and the hydrophilic portion towards inside. Normal nanomicelles can be utilized to encapsulate, solubilize and deliver hydrophobic drugs. While reverse nanomicelles can be applied to encapsulate and act as better candidates for delivery of hydrophilic drugs [54]. Nanomicelles serve as excellent drug delivery systems owing to their ability to minimize drug degradation, reduce adverse side effects and improve drug bioavailability [55–57]. In ocular drug delivery, nanomicelles offer unique advantages due to their nanoscale size, aqueous clear/transparent drug formulation, encapsulate and solubilize hydrophobic drugs and enable high permeation through ocular epithelia with minimal or no irritation. Nanomicelles can be formed with either surfactants or polymeric systems.
SURFACTANT NANOMICELLES
Surfactants are amphiphilic molecules comprising a hydrophilic head and a hydrophobic tail. The head of surfactant molecules could be charged (anionic or cationic), dipolar (zwitterionic), or non-charged (nonionic). Anionic surfactants such as sodium dodecyl sulfate (SDS), cationic surfactant: dodecyltrimethylammonium bromide (DTAB), nonionic surfactants such as n-dodecyl tetra (ethylene oxide) (C12E4), Vitamin E TPGS, octoxynol-40 and zwitterionic surfactants including dioctanoyl phosphatidylcholine (C8-lecithin) are commonly used. The tail of surfactant is generally a long chain hydrocarbon and less frequently a halogenated or oxygenated hydrocarbon or siloxane chain [58, 59].
Surfactants at lower concentrations tend to adsorb onto surfaces or interfaces and alter the surface or interfacial free energy. Generally, surfactants are known to reduce the surface or interfacial free energy. Surfactants form micelles when dissolved in water at concentrations above the critical micelle concentration (CMC). A subtle balance of intermolecular forces such as hydrophobic, steric, electrostatic, hydrogen bonding and Van der Waals interactions are crucial in the micellization process. The major attractive force is an outcome of the hydrophobic effect associated with the lipophilic surfactant tails and the main opposing repulsive force results from steric and electrostatic interactions between the hydrophilic surfactant heads. Low free energy of the system due to removal of hydrophobic fragments from the aqueous environment and the re-establishment of hydrogen bond network in water results in nanomicelle formation. Another important parameter is the aggregation number which represents the average number of surfactant monomers in each micelle of a nanomicellar solution. Since nanomicelles are formed by the non-covalent aggregation of individual surfactant monomers, these structures could be spherical, cylindrical or planar (discs or bilayers). Moreover, the shape and size of nanomicelles can be modulated by altering the chemical structure of surfactant and by changing conditions such as temperature, total surfactant concentration, number of surfactants, ionic strength and pH. Spherical micelles can transform one-dimensionally into cylindrical micelles or two-dimensionally into bilayers or discoidal nanomicelles. In particular, this transformation is controlled by the surfactant heads since both one and two-dimensional development necessitates bringing the surfactant heads closer to each other to reduce the available interfacial area per surfactant molecule at the nanomicelle surface. This property determines the curvature or shape of the nanomicelle surface [53, 60].
POLYMERIC NANOMICELLES
Polymeric nanomicelles represent a distinct class of nanomicelles which are formed by amphiphilic block-copolymers encompassing hydrophilic and hydrophobic monomer units. These spheres are represented by a distinct core-shell structure in which an inner core (hydrophobic) is enclosed by a shell (hydrophilic) [55, 61]. These structures consist of polymer chains and are spontaneously self-assembled due to hydrophobic or ion pair interactions between polymer segments. Unlike surfactants, these polymers could be synthesized or tailor made to meet specific requirements. The hydrophobic segments are often either a polyester (polycaprolactone, poly (d,l-lactide)), a polyether (polypropylene oxide), or a poly aminoacid (poly(β-benzyl-l-aspartate)) [62]. Poly (ethylene glycol) is often employed as the hydrophilic block [63]. More recently, application of new polymers including the hydrophobic polycarbonates and hydrophilic poly (N-vinyl-2-pyrrolidone) has been attempted [64, 65].
The higher the length of the hydrophilic segment, copolymers tend to exist in aqueous solvent (water) as unimers. On the other hand, molecules comprising long hydrophobic segment tend to form non-nanomicellar structures known as rods and lamellae [66]. Methodical and efficient control of the core-forming block structures may offer thermodynamic and kinetic stability to micelles by incorporating various materials for drug loading, release, activation and effective therapy. Polymeric micelles in general are more stable than nanomicelles made from conventional surfactants. Such structures have been reported to retain drug molecules for extended periods of time even in a much diluted condition in systemic fluids probably due to lower CMC (as low as 10−6 M) of amphiphilic copolymers with distinct core-shell structure [67–69]. The core forming segment of polymeric micelle should therefore provide high drug loading capacity, control drug release profile and better compatibility between the core-forming segment and the drug. The outer nanomicellar corona determines the hydrophilicity, charge, length and surface density of hydrophilic segments. It also offers effective steric protection to nanomicelle and allows incorporation of targeting ligands onto the nanomicellar shell for active and specific targeting which makes them highly attractive [70]. Polymeric micelles have been often employed for achieving extended circulation time, sustained release, favorable biodistribution, reduced side effects and lower toxicity [55, 71, 72].
METHODS OF MICELLE PREPARATION
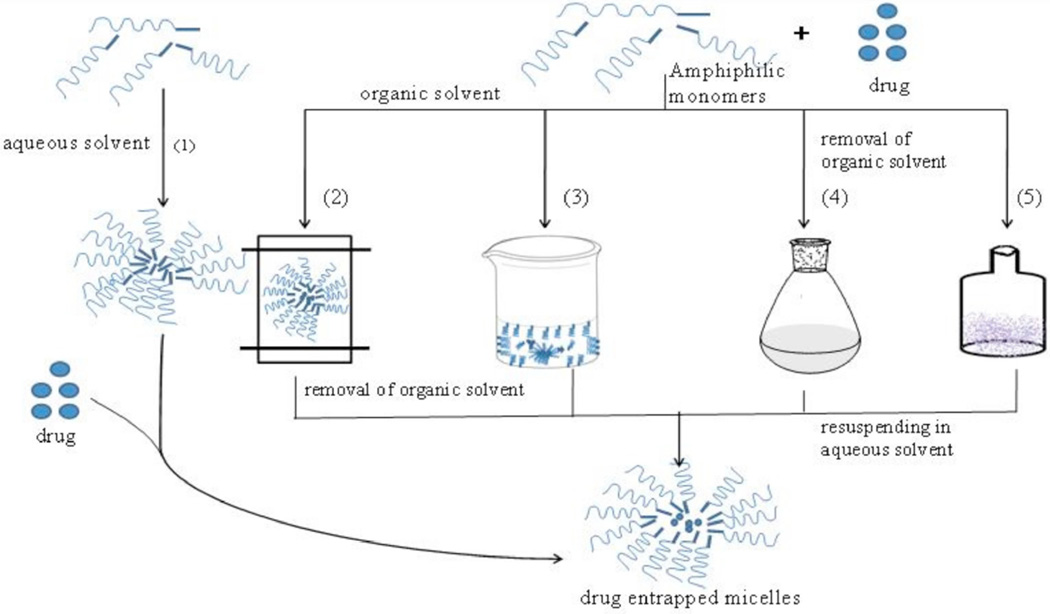
Generally, micelles are prepared by various methods which are divided into two major groups including, direct dissolution and dialysis methods (Fig. 2). These two classes of drug encapsulating procedures depend primarily on the physicochemical properties of the block copolymers [55, 73]. Particularly, the choice of the appropriate method is typically based on the extent of the solubility of a micelle-forming block co-polymer in an aqueous medium.
Fig. (2).
Micelle preparation methods: (1) simple dissolution (2) dialysis, (3) oil in water emulsion (4) solvent evaporation and (5) lyophilization or freeze drying.
Direct dissolution method is the frequently employed method for micelle preparation from copolymers with relatively high water solubility. This method involves dissolving the drug and the block copolymer directly in an aqueous media (distilled deionized water or buffer). Method of preparation may require stirring, heating and/or sonication in order to load drug into nanomicelles. Micelle formation is initiated through dehydration of the core forming blocks. This method has been generally employed for moderately hydrophobic polymers such as poloxamers and also to formulate polyion complex micelles (PICM) with a slight modification. For PICM, the copolymers and drug are dissolved individually in aqueous media where the micelles are formed when the two solutions are combined in suitable drug-polymer ratios [55, 74].
The dialysis method is frequently employed for micelle preparation from amphiphilic copolymers with low water solubility. Also this drug loading procedure is useful for copolymers which require a common organic solvent to solubilize. Organic solvents such as dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF), acetonitrile (ACN), tetrahydrofuran (THF), acetone or dimethylacetamide are commonly selected. For this method, the copolymer and the active agent are dissolved in a common organic solvent and micelle formation is stimulated by the addition of aqueous media (water) to the drug-copolymer mixture. The micelles are subsequently dialyzed against water for an extended time periods to eliminate organic solvent. Moreover, the selection of the solvent significantly influences the physical and drug encapsulation properties of the micelles. For example, presence of DMF resulted in an increase in drug loading efficiency relative to DMSO [75]. An optimal aqueous to organic solvent ratio may also influence micelle properties with respect to size, stability and drug loading capacity [76–79]. In case of water-miscible organic solvents, the copolymer mixture can be dialyzed against aqueous medium (water) to produce micelles due to slow removal rate of the organic solvent [55, 74].
Another method commonly employed is the dry-down or evaporation method wherein both the copolymer and active agent are dissolved in a common solvent or mixture of two miscible solvents. A drug-copolymer film is then formed upon stirring and drying the mixture. Micelles are spontaneously formed when the film is reconstituted with warm water or buffer [80–83]. The reconstituted samples may be sonicated or passed through a high-pressure extruder to prevent multimodal size distribution [64]. The extent of drug encapsulation through this method is largely determined by the common solvent initially employed for dissolving both the copolymer and active agent. Phase separation may likely be prevented during the evaporation process when a solvent, which can solubilize both the copolymer and drug, is selected.
Another method for formulating micelles involves dissolution of both the copolymer and the drug in a mixture of aqueous and organic solvent which is followed by lyophilization. The freeze dried mixture can be reconstituted to obtain drug-loaded micelles [79, 84]. Dimethylacetamide and tert-butanol have been generally employed as co-solvents because of their high vapor pressure which offers rapid sublimation followed by lyophilization [85]. The micelles produced by this method also demonstrate adequate shelf-life along with high water dispersibility [79].
In general, the method chosen for formulating drug encapsulated micelles would significantly influence both the physicochemical properties of the micelle and drug encapsulation efficiency. Nevertheless, the size and polydispersity indices of the formulations are impacted by the nature of the organic phase, the order of addition and the concentration of the copolymer in the organic solvent. Therefore, it would be beneficial to evaluate various preparation methods and optimize different parameters to develop and optimize a better formulation for an active pharmaceutical ingredient (API). Current research is focused on nanomicellar carriers for their application in anterior and posterior segment ocular drug delivery. The current literature showcasing the application of nanomicelles in ocular drug delivery is very limited. However, we made attempts to discuss the various nanomicellar approaches from recently published articles and patents in this area.
MICELLES IN ANTERIOR SEGMENT OCULAR DRUG DELIVERY
Researchers have utilized nanomicellar approach for topical delivery of different small molecules as well as several genes to the anterior segment of the eye. Polymeric and surfactant micelles have been reported for improving penetration of topically applied drugs through the cornea and hence enhancing ocular bioavailablity. In the following sections we describe about the research conducted in the development of nanomicelles for anterior segment drug delivery
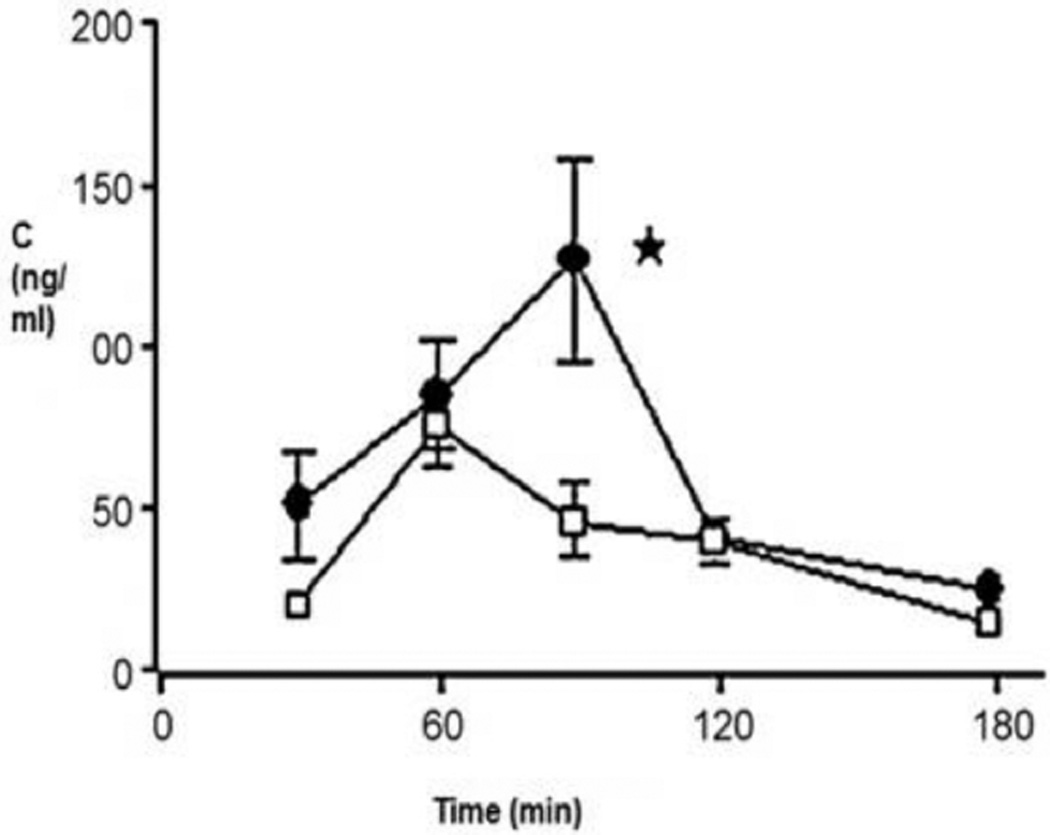
Polymeric micelles composed of copolymers of N-isopropylacrylamide (NIPAAM), vinyl pyrrolidone (VP) and acrylic acid (AA) cross-linked with N, N’-methylene bis-acrylamide (MBA) with encapsulated ketorolac have been reported for ocular delivery. A two fold increase in ocular bioavailability with no corneal damage was observed through excised corneal permeation studies on rabbit cornea relative to an aqueous suspension containing same amount of ketorolac as micelle nanoparticles [86]. Civiale and co-workers described copolymers of polyhydroxyethyl-aspartamide (PHEA), with side chains containing polyethylene glycol (PEG) and/or hexadecylamine (C16) (PHEA-PEG, PHEA-PEG-C(16) and PHEA-C(16) respectively), for ocular delivery [87]. Dexamethasone loaded micelles were prepared from PHEA-C(16) and PHEA-PEG-C(16). In vitro permeability studies conducted across primary cultured rabbit conjunctival and corneal epithelial cells indicated higher drug permeation for dexamethasone entrapped in either PHEA-C(16) or PHEA-PEG-C(16) micelles relative to drug solutions or suspensions. Additionally, in vivo ocular bioavailability studies were performed in rabbits with PHEA-PEG-C16 micelles. In vivo results were similar to in vitro data indicating higher bioavailability of dexamethasone entrapped in micelles relative to aqueous suspension of the same drug suggesting micelles as potential colloidal drug carriers for ocular drug delivery (Fig. 3) [88].
Fig. (3).
Dexamethasone concentrations in rabbit aqueous humour. PHEA-PEG-C16 micellar solutions (•); dexamethasone suspension (□), P < 0.05, Reproduced with permission from Ref [88].
Polymeric micelle formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactides) (MPEG-hexPLA) as a novel excipient were evaluated for topical ocular delivery of a poorly water soluble drug, cyclosporin A (CsA). Excellent in vitro and in vivo ocular biocompatibility, transparency and stability of mPEG-hexPLA micelles were observed which suggested that CsA formulation may be employed as possible eye drop formulations [89]. Micelles composed of triblock copolymer Pluronic F127 (poly(oxyethylene)/ poly(oxypropylene)/poly (oxyethylene) were evaluated for ocular delivery of pilocarpine. Pharmacokinetic parameters (duration of miotic response and area under the miosis time curve) were improved with micellar formulation relative to standard pilocarpine solutions. Significant prolongation of miotic activity and increase in AUC of 64% were noted with micellar pilocarpine based solution [90].
Longer pre-corneal residence of topically applied drug solutions with mucoadhesive polymers in the formulation such as chitosan can improve ocular bioavailability. It is a cationic mucoadhesive polymer. In addition to mucoadhesive property, chitosan also possess permeation enhancing activity. This cationic polymer exerts its permeation effect by opening epithelial tight junctions. Recently, Pepic et al., have demonstrated application of chitosan micellar system as a novel eye drop formulation. Micellar formulations composed of polyoxyethylated nonionic surfactant Pluronic F127 (F127) and chitosan (CH) were prepared with dexamethasone (DEX) as a model drug. This study demonstrated that addition of CH significantly enhanced in vitro dexamethasone release thereby causing improved ocular bioavailability. The area under the curve (AUC) values showed 2.4-fold increase in bioavailability of dexamethasone when administered as F127/0.015 (w/v) % CH micellar system relative to a standard dexamethasone suspension (Table 1). These results provide evidence of improved intraocular dexamethasone absorption from the micellar systems, which was attributed to permeability enhancement by both F127 and mucoadhesive nature of CH [91].
Table 1.
Pharmacokinetic Parameters Following Ocular Administration of 0.1% DEX in Commercial Eye Drops or 0.025% DEX in Micelle Systems Based on F127 or F127 and CH [91].
| Ocular Formulations | Tmaxa(h) | Tminb(h) | AUC (% Increase in IOPt x h) | kel, appc (h−1) | AUCreld |
|---|---|---|---|---|---|
| F127/DEX in isotonic acetate buffer, pH 4.5 (F1) | 1.37±0.21* | 10.15±0.94* | 115.9±8.31* | 0.189±0.05* | 1.72 |
| F127/DEX/0.015 (w/v)% CH in isotonic acetate buffer, pH 4.5 (F4) | 1.25±0.23* | 11.23±0.56* | 162.8±11.23* | 0.148±0.05* | 2.41 |
| Commercial DEX (0.1, w/v %) eye drops | 2.26±0.14 | 8.39±0.38 | 67.6±9.42 | 0.319±0.06 | 1 |
Time needed to achieve peak IOP increase.
Duration of IOP increase response (the time interval needed for the IOP to return to its normal pretreatment value).
Approximate values calculated from the slope ln (% increase in IOP)/Δt for the terminal points of % increase in IOPt versus t curves.
Ratio of AUC to the value for the commercial DEX (0.1, w/v, %) eye drops.
Statistically significant difference as compared with commercial DEX (0.1, w/v, %) eye drops (p < 0.05).
Recently, a novel approach of photodynamic therapy (PDT) with dendrimer porphyrin (DP) loaded polyion complex (PIC) micelles were evaluated for selective accumulation in the pathologic corneal neovascularization area but not in normal ocular vessels. Nanocarriers consisting of PIC micelles demonstrated good stability, high drug-loading capacity, and excellent potential for controlled drug release. Selective accumulation of DP-micelles in the corneal neovascularization area was observed with no detectable level of DP-micelle in normal limbal vessels which appears to enhance permeability and retention (EPR) effect [92].
Topical gene delivery by means of eye drop holds promise for the treatment of a wide range of corneal diseases such as corneal neovascularization, dry eye syndrome, corneal scarring, corneal angiogenesis and inflammation. However, to improve the bioavailability of a topically delivered gene in ocular tissues, there is need to have optimally designed vehicles. Micellar formulations have opened up new approaches for topical ocular gene delivery. Liaw and co-workers have reported the application of PEO-PPO-PEO non-ionic co-polymeric micelles as a carrier for gene delivery. Stable and efficient transfer of plasmid DNA with lacZ gene in vivo with PEO-PPO-PEO non-ionic co-polymeric micelles was achieved, indicating potential use of block copolymers for DNA transfer [93]. In another study the same group reported non-invasive delivery of genes for two cornea specific promoters i.e. keratin 12 (K12) and keratocan utilizing PEO-PPO-PEO polymeric micelles (PM). β-Gal activity, considered as a measurement of transgene expression, was significantly elevated in both mouse and rabbit corneas following six doses of eye drop of pK12-Lac Z-PM three times a day. The probable mechanism of transfection was endocytosis of plasmid-PM as well as particle size dependent paracellular transport [94]. Following eye drop instillation of this gene encapsulated polymeric micelles in defective corneal epithelia mouse model, the mRNA level of bcl-x(L) was elevated by 2.2 fold with reduced corneal apoptosis [95]. This observation suggests the ability of micellar topical drops to non-invasively deliver the load at the targeted site.
MICELLES IN POSTERIOR SEGMENT OCULAR DRUG DELIVERY
Ocular pharmacologists and pharmaceutical scientists are currently utilizing nanomicellar carrier to deliver drugs non-invasively to posterior segment. Micelles not only aid in drug solubilization by entrapping lipophilic molecules in their hydrophobic core but also act as drug carrier/reservoir. AMD, uveitis, choroidal neovascularization (CNV), diabetic retinopathy (DR), posterior vitreoretinopathy (PVR) are posterior ocular diseases which can cause vision loss. Current delivery strategies via oral or intravenous routes may not be able to deliver drugs to posterior ocular tissues in therapeutic concentrations due to various ocular barriers discussed previously. On the other hand local ocular drug delivery strategies such as intravitreal, retrobulbar and suprachoroidal are associated with drug administration related side effects. In the following sections we provide an overview of different micellar formulations investigated to deliver drug to posterior ocular tissues (retina-choroid).
Attempts have been made to deliver agents by conventional route i.e., intravenous (IV) or topical drop administration, to the posterior segment ocular tissues after loading into nanomicellar constructs. Ryuichi et al., conducted initial studies to encapsulate FITC-P(Lys) in polyethylene glycol-block-poly-α,β-aspartic acid micelles [96, 97] to deliver the cargo to posterior ocular tissues (choroid). Micelles were formed spontaneously in aqueous environment due to electrostatic interactions between the carrier and drug molecules resulting in polyionic complex (PIC) micelles with narrow size distribution. Free FITC-P(Lys) served as a control. In vivo studies were conducted in rats after inducing CNV with a diode laser photocoagulator. Blood, retina-choroid levels and tissue distribution in highly vascularized organs was studied. Animals receiving free FITC-P(Lys) died within one hour of administration demonstrating drug toxicity. On the contrary, no death occurred in animals administered with FITC-P(Lys) encapsulated in PIC micelles. Drug encapsulated micellar formulation showed a Cmax at 4 hours following IV administration in retina-choroid and drug level was detected up to 7 days following single administration. Residual drug amounts in blood peaked to 7.8 % of initial dose and reached 0.5% within 24 h of single administration. Most importantly, drug induced toxicity was not observed with FITC-P(Lys) in PIC micelles which indicates that drug was encapsulated in nanomicellar core and was not freely available. Studies revealed that prolonged micellar circulation was achieved by controlling the polymer and drug charge ratios. From these results the authors speculated that longer circulation of micelles may aid in EPR effect at neovascularization site. Micellar constructs were observed to selectively accumulate at the pathologic neovascular site to a greater extent than in normal tissues [98, 99]. Effective accumulation of drug loaded nanomicellar constructs, due to EPR effect, at posterior ocular tissues indicated that PIC micelle formulation could deliver more of the dose to retina-choroid with no/minimal systemic adverse effects.
In another study, dendritic photosensitizer was encapsulated in poly(ethylene glycol)-block-poly(L-lysine) (PEG-b-P(Lys)) micellar construct for the treatment of exudative AMD [97, 100] with PDT. It involves a procedure in which a photosensitizer is injected into the subject. Photosensitizer selectively accumulates at the neovascular tissues. Mild laser light excitation is introduced to activate photosensitizer, generate and liberate cytotoxic oxygen free radicals (reactive oxygen species (ROS)) which selectively occludes the newly formed abnormal blood vessels [92]. In this study micelles were prepared by adjusting the molar ratio of carboxylic end group of dendritic photosensitizer (DP) and the lysine residue of polymer to one. In vitro cytotoxicity studies were performed under dark and light irradiation for DP and DP loaded PIC micelles. Cell viability was not affected under dark conditions with free DP and DP loaded PIC micelles where as irradiation caused DP loaded PIC micelles to produce higher cytotoxic effect relative to free DP. Higher cytotoxicity of the PIC micelles under light irradiation was utilized for the treatment of exudative AMD. To demonstrate the carrier properties of the micellar formulation, in vivo studies were conducted in rats by IV administration of DP encapsulated PIC micelles. Photocoagulation was induced in rat eye with diode laser photocoagulator. Following the induction of photocoagulation, DP loaded PIC micelles were administered and DP accumulation in choroidal neovascular site was observed. Mild laser light treatment was applied for the generation of ROS which destroyed/choked the abnormal vasculature and prevented further drug leakage. Histological studies revealed that PIC micelles selectively accumulated at the site of induced ocular lesions showing EPR effect. The micelles were observed to accumulate at ocular lesions as early as 0.25 h after IV administration and both free DP and DP loaded PIC micelles, reached Cmax at 4 hr. On the contrary, free DP disappeared within 24 hrs whereas PIC micelles were still detected after 24 h indicating micelle accumulation and at the lesion site with slow cellular uptake which may be due to negatively charged micellar corona. Laser treatment of DP loaded PIC micelle at 25 mins post-administration showed hypofluorescence indicating the choking of abnormal vasculature. Further, lower fluorescence was observed at 160 hrs of PIC micelle administration with laser treatment indicating improvement in leakage reduction (73.9% and 82.6% for day 1 and 7 respectively) at the lesion site due to the destruction of only choroidal neovascular endothelial cells. No destructive effect of PDT on normal endothelial cells was observed possibly due to low DP accumulation in normal endothelial cells [98, 99]. These studies also revealed that lower amount of laser light energy was required to treat the exudative AMD. These results indicate that small micellar size with negatively charged corona caused considerable EPR effect which may help in selective drug accumulation in the choroidal neovascular tissues with minimal/no drug induced adverse effects on normal cells.
CsA is indicated for the treatment of autoimmune diseases i.e, uveitis, Bechet’s disease, Sjogren’s syndrome, keratoconjunctivitis sicca and corneal transplantation. CsA is administered by traditional methods of drug delivery via oral or IV route to treat diseases affecting posterior ocular tissues. Ocular disease treatment with local topical drop instillation is preferred over traditional routes of administration to avoid drug induced side effects. CsA is a hydrophobic drug with poor aqueous solubility. Several attempts were made to develop ocular formulations using different vehicles such as cyclodextrins, vegetable oils, liposomes, micro and nanospheres [101–104]. None of the vehicles could deliver therapeutic concentrations of CsA to the targeted ocular tissues with no/minimal toxicity. Mitsuaki et al., [105] made attempts to compare the ocular pharmacokinetics and tissue drug distribution of CsA loaded in nonionic surfactant, oil and emulsion formulations. Nonionic surfactants such as tween 80, hydrogenated castor oil-60 (HCO-60®) and polyoxyl 40 stearate (MYS-40®) were selected. Among the three surfactants, higher drug solubility was obtained in MYS-40®. Drug solubility increased linearly with the weight percentage of MYS-40® at constant temperature. Further in vivo ocular tissue distribution and pharmacokinetic studies with 0.1% CsA formulation were conducted. Oil based formulations are generally undesirable for ocular administration because of their associated side effects such as ocular irritation and blurred vision. Single topical drop application of CsA containing MYS-40® aqueous formulation resulted in improved tissue drug accumulation. Higher drug concentrations were observed in all the ocular tissues relative to oil and emulsion based formulations. Single topical drop instillation was able to deliver the drug to posterior ocular tissues i.e, retina-choroid without any sign of irritation which is generally observed with oily formulations. With single drop aqueous formulation, pharmacokinetic study revealed improved AUC for all tissues including retina-choroid. Intraocular drug penetration with three different vehicles followed the following order: oil < emulsion < aqueous formulation. The difference in ocular tissue drug penetration was attributed to physicochemical properties of the vehicle/carrier. Release of hydrophobic agents from the carrier into the surrounding aqueous medium was regulated by the hydrophobicity of the carriers. Due to higher hydrophobicity of the carrier such as oils, drug partitioning into the aqueous environment was low. Therefore, low amount of drug was available for penetration into deeper ocular tissues. On the other hand nonionic surfactants have the ability to spontaneously form micelles in aqueous medium and dissolve hydrophobic drugs such as CsA. The generated micelles of size less than 200nm in diameter possess larger micellar surface area. Therefore higher drug release is expected from the micelles. Moreover, the associated side effects with topically applied oil and emulsion formulation may be avoided. Also, traditional methods of systemic drug administration to achieve therapeutic drug concentrations in posterior ocular tissues can be minimized. As a result drug induced systemic side effects can also be largely minimized. Topical drug instillation encapsulated in aqueous nonionic surfactant formulation may be used to deliver drugs to posterior ocular tissues non-invasively and treat posterior ocular diseases.
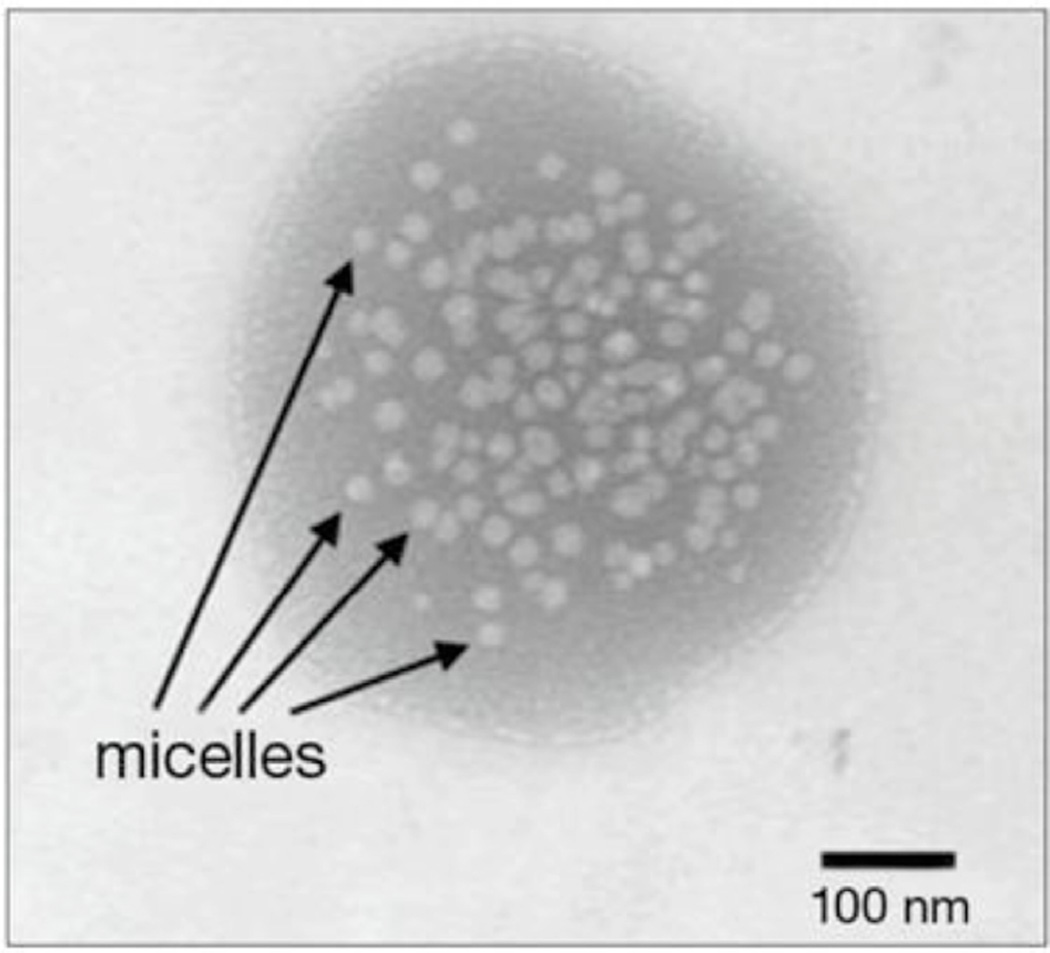
Recently Mitra et al., disclosed methods to deliver the therapeutics to ocular tissues with aqueous mixed nanomicellar topical eye drops in US patent application number US2009/0092665. Attempts have been made with biocompatible, biodegradable, amphiphilic polymeric surfactants to encapsulate drugs in the core of nanomicelles. Initially, studies were conducted to encapsulate a calcineurin inhibitor, voclosporin, in aqueous mixed nanomicellar formulation using two polymeric surfactants (Vitamin E TPGS and octoxynol-40) with different hydrophilic lipophilic balance (HLB) values. Of the polymers used, one was relatively hydrophilic than the other. Other drugs such as dexamethasone and rapamycin were also encapsulated in mixed nanomicellar formulation. The micelles were in the range of 10–25nm in size encapsulating voclosporin, (Fig. 4) [106, 107] dexamethasone and rapamycin with drug concentrations of 2, 1 and 2 mg/ml respectively [106, 108]. These developed formulations were clear/transparent and free flowing aqueous solutions.
Fig. (4).
Transmission electron micrograph (TEM) of 0.2% voclosporin loaded in mixed nanomicellar formulation.
Efficacy studies were conducted with 0.2% voclosporin mixed nanomicellar formulation using Optimmune® (CsA ophthalmic ointment) as control in canine keratoconjunctivitis sicca (KCS) model. Results were evaluated with the Schirmer tear test and corneal observation. Schirmer tear test is a measure of tear production in the eye. Test values were maintained well above the normal value (> 15 mm/min) while the control went below the threshold. Moreover, corneal adverse effects were not observed with twice daily application of voclosporin encapsulated mixed nanomicellar formulation indicating its safety. Formulation tolerability was evaluated with marketed formulation Restasis® as control in New Zealand White (NZW) rabbits. Hackett-MacDonald Scoring was applied to evaluate the formulation tolerability for two voclosporin (0.02 and 0.2%) and Restasis® formulations. A detailed 72 hour study was included for microscopic ocular examination. After a period of 1 hour, Restasis® caused the highest ocular irritation relative to two micellar voclosporin formulations. It was demonstrated that the novel mixed nanomicellar formulations were well tolerated and induced markedly low irritation in comparison to marketed oily emulsion based Restasis® formulation.
From the two formulations, 0.2% mixed nanomicellar formulation was used for toxicological evaluation in NZW rabbits. Toxicity was evaluated for 2 weeks and 13 weeks in NZW rabbits and for two weeks in Beagle dogs. Formulation safety and toxicity were evaluated with the help of macro and microscopic ophthalmic evaluations- electroretinography, ocular pathology, hematology, and tonometry. On the first and last day of the study voclosporin blood concentrations were determined. Dose dependent study was conducted with 0.14, 0.28 and 0.56 mg/eye/day in bilateral eyes. Results demonstrated that the formulation had no dose dependent adverse effect on specific function and histopathologic ocular indices in 2 and 13 week study. Minimal systemic exposure and accumulation with no toxicity was demonstrated with topical application of mixed nanomicellar formulation.
Drug levels were evaluated in anterior and posterior ocular tissues with single and once daily drop instillation in rabbit animal model. Pharmacokinetic studies with once-daily single drop application were studied in Albino New Zealand rabbits and pigmented Dutch Belted rabbits for 7 days. It appears that voclosporin lacks specific melanin binding and is not significantly metabolized in the ocular tissues upon single and repeated dosing. Cmax in both rabbit strains after single drop administration were 1.73 and 1.28 ng/ml respectively at a Tmax of 1 hr. On the other hand, multiple dosing in New Zealand rabbits resulted in Tmax of 0.5 hr. After 7 days, Cmax was 1.16 ng/ml. Interestingly with a single once daily dose application, higher drug concentrations were detected in the posterior ocular tissues with minimal and/or nondetectable drug levels in aqueous humor, lens and vitreous humor. Similar trend was observed with multiple dosing indicating non-corneal route of drug delivery to posterior ocular tissues [106]. No drug was detected in blood circulation (Table 2) [107]. The authors suggested that due to minimal amount of drug availability in the ocular chambers (aqueous humor, lens and vitreous humor) chances of developing drug induced side effects such as increased intraocular pressure or cataract formation may be avoided. This novel mixed nanomicellar formulation approach established a platform technology to deliver therapeutics non-invasively to retina/choroid with topical drop instillation.
Table 2.
Pharmacokinetic Parameters of 14C-Voclosporin Following Single or Repeat (QD for 7 Days) Dose of Mixed Nanomicellar Voclosporin Formulation in Female New Zealand Albino Rabbits
| Ocular Tissue(s)/Fluids & Blood |
Cmax (ng eq./g) | AUC (hr* ng eq./g) | Tmax (hr) | t1/2 (hr) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SD | RD | Ratio | SD | RD | Ratio | SD | RD | SD | RD | |
| Aqueous Humor | 6 | 13 | 2.3 | 45 | 96 | 2.1 | 0.5 | 0.5 | - | 14 |
| Choroid/Retina | 48 | 76 | 1.6 | 472 | 897 | 1.9 | 1.0 | 2 | 23 | - |
| Cornea | 1203 | 3382 | 2.8 | 23166 | 54624 | 2.4 | 8.0 | 0.5 | - | - |
| Iris/Ciliary body | 20 | 119 | 5.8 | 382 | 1952 | 5.1 | 24.0 | 1 | - | - |
| Lacrimal Gland | 31 | 120 | 3.9 | 416 | 1109 | 2.7 | 2.0 | 4 | - | 6 |
| Lens | 4 | 26 | 6.7 | 47 | 356 | 7.5 | 24.0 | 0.5 | - | - |
| Lower Bulbar | 1810 | 2929 | 1.6 | 12029 | 16585 | 1.4 | 0.5 | 0.5 | 10 | 7 |
| Conjunctiva Lower Eyelid | 20814 | 41635 | 2.0 | 207630 | 358791 | 1.7 | 1.0 | 0.5 | - | - |
| Nictitating membrane | 1716 | 2468 | 1.4 | 12135 | 15964 | 1.3 | 0.5 | 0.5 | 7 | 8 |
| Optic Nerve | 83 | 164 | 2.0 | 569 | 1805 | 3.2 | 0.5 | 0.5 | - | 16 |
| Sclera | 223 | 367 | 1.6 | 2646 | 3825 | 1.4 | 0.5 | 0.5 | - | 16 |
| Submandibular | 74 | 120 | 1.6 | 893 | 1190 | 1.3 | 2.0 | 2 | - | - |
| Lymph Node Tear | 20246 | 30904 | 1.5 | 168259 | 230878 | 1.4 | 0.5 | 0.5 | - | 7 |
| Upper Bulbar | 2235 | 3170 | 1.4 | 14782 | 19944 | 1.3 | 0.5 | 0.5 | 7 | 7 |
| Conjunctiva Upper Eyelid | 9896 | 17500 | 1.8 | 114651 | 98656 | 0.9 | 1.0 | 0.5 | - | 4 |
| Vitreous Humor | 2 | 2 | 1 | 27 | 23 | 0.9 | 8.0 | 4 | - | - |
| Blood | BQL | BQL | NC | NC | NC | NC | NC | NC | NC | NC |
Time needed to achieve peak IOP increase.
Duration of IOP increase response (the time interval needed for the IOP to return to its normal pretreatment value).
Approximate values calculated from the slope ln (% increase in IOP)/Δt for the terminal points of % increase in IOPt versus t curves.
Ratio of AUC to the value for the commercial DEX (0.1, w/v, %) eye drops.
Statistically significant difference as compared with commercial DEX (0.1, w/v, %) eye drops (p < 0.05).
SD = Single dose;
RD = Repeat dose;
Ratio = Repeat dose/Single Dose.;
- = Insufficient tissue concentration to determine t1/2;
BQL = Below Quantifiable Limit (<0.1 ng/mL);
NC = Not calculated.
Recently, attempts were made to deliver dexamethasone and rapamycin to posterior ocular tissues non-invasively with mixed nanomicellar eye drop [109]. Dexamethasone and rapamycin have an aqueous solubility of 159µg/ml (2) and 2.6 µg/ml [110] respectively. These two hydrophobic drugs were encapsulated in mixed nanomicellar formulation resulting in 1 and 2 mg/ml dexamethasone and rapamycin formulations respectively. Solubility of dexamethasone and rapamycin in the nanomicellar formulation was improved by about 6.7 and 1000 fold. These two novel formulations were used to non-invasively deliver therapeutics to posterior ocular tissues (retina/choroid). Ocular tissue distribution studies for dexamethasone and rapamycin mixed nanomicellar formulation revealed that 50 and 370 ng/g of tissue drug levels [106] were detected in the posterior ocular tissues (retina/choroid). Minimal/no drug levels were detected in the aqueous ocular chambers indicating possibly a non-corneal or conjunctival/scleral pathway of drug absorption to the posterior segment.
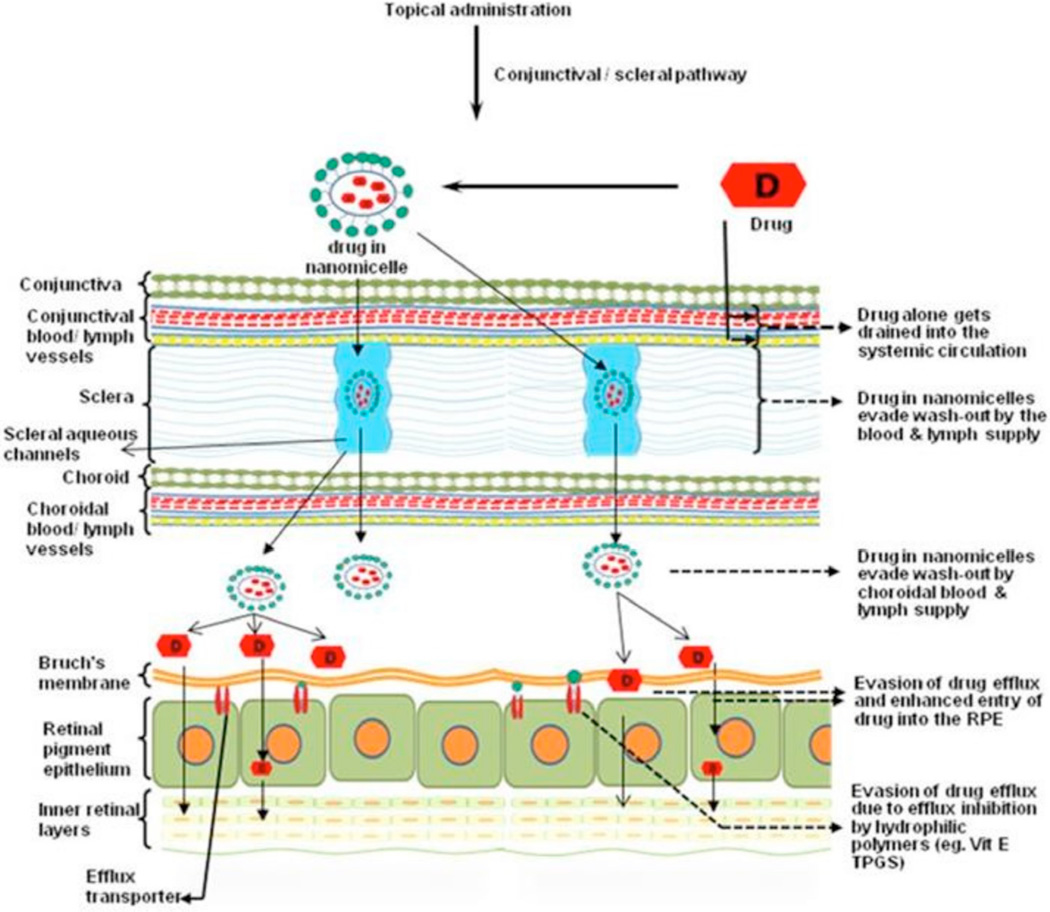
For a topically applied eye drop formulation two pathways are expected for the drug to reach the posterior ocular tissues (i) corneal and (ii) non-corneal or conjunctival/scleral pathway. Topically applied hydrophobic drugs suffer from limited intraocular entry due to the presence of hydrophilic (i) corneal stroma which behaves as rate limiting barrier and (ii) reverse direction of fluid flow which further hinders intraocular drug penetration. For such hydrophobic drugs to reach the posterior ocular tissues, both the static and dynamic ocular barriers need to be circumvented. An alternative approach, conjunctival-scleral pathway, is hypothesized for the mixed nanomicellar formulation. Hydrophobic drugs are encapsulated in the hydrophobic core of mixed nanomicelles which form spherical structures with outer hydrophilic corona (Fig. 4). Also, it is hypothesized that extremely small size and hydrophilic nanomicellar corona aids scleral transport through the aqueous pores/channels. In addition, the hydrophilic nanomicellar corona can minimize drug wash out into systemic circulation by the conjunctival/choroidal blood circulation and lymphatics. After reaching the basolateral side these mixed nanomicelles may be engulfed by RPE cells by endocytosis [111–113]. A hypothetical diagrammatic representation of mixed nanomicellar formulation translocating from topical drop to posterior segment ocular tissues is depicted in Fig. (5). The cargo is expected to release the load after coming into contact with the lipophilic cell membrane. It is also hypothesized that during the translocation of drug encapsulated formulation, the hydrophilic mixed nanomicellar corona may help to circumvent drug entry into systemic circulation and thereby prevent systemic toxicity. Mixed nanomicellar approach appears to be a promising strategy for non-invasive drug, protein, peptide, DNA, RNA delivery to posterior ocular tissues with minimal entry into systemic circulation.
Fig. (5).
Schematic representation of back of the eye drug delivery for drugs entrapped in mixed nanomicelles (utilization of scleral aqueous pores and evasion of conjunctival/choroidal blood vessels and lymphatics).
CURRENT & FUTURE DEVELOPMENTS
Delivery of drugs to the targeted ocular tissues is a challenging task. Nanoscale carrier systems are being developed to overcome static and dynamic barriers. Novel micellar approach appears to be highly promising for posterior segment ocular drug delivery. The hydrophobic core and hydrophilic corona of the micelles can produce clear aqueous solutions which are very attractive for topical ocular drug delivery. Improved drug solubility in the micelle core is long known but their utilization to deliver drugs to posterior target tissue in the eye has recently been pursued and is still emerging. Highly hydrophobic drugs such as voclosporin, rapamycin, dexamethasone, could be efficiently delivered by nanomicellar formulations to anterior and posterior ocular tissues. Selected polymers could be utilized to deliver drugs to the anterior segment. Due to their small size, EPR effect is observed which helps in selective accumulation of micelles in the targeted tissues with minimal/no drug accumulation in normal tissues. Proper selection of surfactant/polymers, design and engineering techniques can produce nanomicelles which could be utilized to deliver drugs to both anterior and posterior ocular tissues i.e., retina/choroid, non-invasively in therapeutic levels. Emerging nanomicellar topical drop approach, in the near future, may replace the patient non-compliant route of drug administration to posterior ocular tissues such as intravitreal and periocular injections to the globe.
ACKNOWLEDGEMENTS
This work was supported by NIH (grants R01 EY 09171-16 and R01 EY 010659-14.
Footnotes
CONFLICTS OF INTERESTS
There is no conflict of interest.
REFERENCES
- 1.Forrest ML, Won C-Y, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(ε-caprolactone) micelles. J Controlled Release. 2006;110(2):370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Loftsson T, Hreinsdottir D. Determination of aqueous solubility by heating and equilibration: A technical note. AAPS Pharm Sci Tech. 2006;7(1):E4. doi: 10.1208/pt070104. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: The past, the present, and the future. Surv Ophthalmol. 2008;53(2):139–149. doi: 10.1016/j.survophthal.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond) 2008;22(4):590–591. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 5.Michael S, Ip M. Intravitreal injection of triamcinolone. An emerging treatment for diabetic macular edema. Diabetes Care. 2004;27(7):1794–1797. doi: 10.2337/diacare.27.7.1794. [DOI] [PubMed] [Google Scholar]
- 6.Schoenwald RD. Ocular drug delivery. Pharmacokinetic considerations. Clin Pharmacokinet. 1990;18(4):255–269. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lee VH, Robinson JR. Topical ocular drug delivery: Recent developments and future challenges. J Ocul Pharmacol. 1986;2(1):67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 8.Mishima S, Gasset A, Klyce SD, Jr, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 1966;5(3):264–276. [PubMed] [Google Scholar]
- 9.Chrai SS, Patton TF, Mehta A, Robinson JR. Lacrimal and instilled fluid dynamics in rabbit eyes. J Pharm Sci. 1973;62(7):1112–1121. doi: 10.1002/jps.2600620712. [DOI] [PubMed] [Google Scholar]
- 10.Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63(3):333–338. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed I. The noncorneal route in ocular drug delivery. In: Mitra AK, editor. Ophthalmic drug delivery systems. 2nd edition ed. New York: Marcel Dekker, Inc; 2003. pp. 335–363. [Google Scholar]
- 12.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60(2):207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents III: In vitro-in vivo correlations. J Pharm Sci. 1983;72(11):1279–1281. doi: 10.1002/jps.2600721110. [DOI] [PubMed] [Google Scholar]
- 14.Jorge F. The Corneal Endothelium. In: Fischbarg J, editor. Advances in Organ Biology. Elsevier; 2005. pp. 113–125. [Google Scholar]
- 15.Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336(1):12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34(1):1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey S, Patel J, Anand BS, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44(7):2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J Physiol. 1996;491(3):743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holash JA, Stewart PA. The relationship of astrocyte-like cells to the vessels that contribute to the blood-ocular barriers. Brain Res. 1993;629(2):218–224. doi: 10.1016/0006-8993(93)91323-k. [DOI] [PubMed] [Google Scholar]
- 20.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58(11):1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Arto U. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Delivery Rev. 2006;58(11):1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res. 2001;73(5):581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- 23.Bill A. The blood-aqueous barrier. Trans Ophthalmol Soc U K. 1986;105(2):149–155. [PubMed] [Google Scholar]
- 24.Saha P, Kim KJ, Lee VH. A primary culture model of rabbit conjunctival epithelial cells exhibiting tight barrier properties. Curr Eye Res. 1996;15(12):1163–1169. doi: 10.3109/02713689608995151. [DOI] [PubMed] [Google Scholar]
- 25.Saha P, Yang JJ, Lee VH. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1998;39(7):1221–1226. [PubMed] [Google Scholar]
- 26.Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39(5):244–254. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 27.Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38(3):627–634. [PubMed] [Google Scholar]
- 28.Cruysberg LP, Nuijts RM, Geroski DH, Koole LH, Hendrikse F, Edelhauser HF. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J Ocul Pharmacol Ther. 2002;18(6):559–569. doi: 10.1089/108076802321021108. [DOI] [PubMed] [Google Scholar]
- 29.Ambati J, Canakis CS, Miller JW, et al. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41(5):1181–1185. [PubMed] [Google Scholar]
- 30.Maurice DM, Polgar J. Diffusion across the sclera. Exp Eye Res. 1977;25(6):577–582. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 31.Dunlevy JR, Rada JA. Interaction of lumican with aggrecan in the aging human sclera. Invest Ophthalmol Vis Sci. 2004;45(11):3849–3856. doi: 10.1167/iovs.04-0496. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Richard FS. Age-Related Choroidal Atrophy. Am J Ophthalmol. 2009;147(5):801–810. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857–2864. [PubMed] [Google Scholar]
- 36.Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(Suppl 1):S10–S32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 37.Hewitt AT, Newsome DA. Altered synthesis of Bruch's membrane proteoglycans associated with dominant retinitis pigmentosa. Curr Eye Res. 1985;4(3):169–174. doi: 10.3109/02713688509000846. [DOI] [PubMed] [Google Scholar]
- 38.Hewitt AT, Nakazawa K, Newsome DA. Analysis of newly synthesized Bruch's membrane proteoglycans. Invest Ophthalmol Vis Sci. 1989;30(3):478–486. [PubMed] [Google Scholar]
- 39.Gardner TW, Lieth E, Khin SA, et al. Astrocytes increase barrier properties and ZO-1 expression in retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 1997;38(11):2423–2427. [PubMed] [Google Scholar]
- 40.Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci. 1997;38(9):1653–1666. [PubMed] [Google Scholar]
- 41.Steuer H, Jaworski A, Elger B, et al. Functional characterization and comparison of the outer blood-retina barrier and the blood-brain barrier. Invest Ophthalmol Vis Sci. 2005;46(3):1047–1053. doi: 10.1167/iovs.04-0925. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis. 2002;8:422–430. [PubMed] [Google Scholar]
- 43.Greenwood J. Characterization of a rat retinal endothelial cell culture and the expression of P-glycoprotein in brain and retinal endothelium in vitro . J Neuroimmunol. 1992;39(1–2):123–132. doi: 10.1016/0165-5728(92)90181-j. [DOI] [PubMed] [Google Scholar]
- 44.Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Expression of ATP-binding cassette transporters at the inner blood-retinal barrier in a neonatal mouse model of oxygen-induced retinopathy. Brain Res. 2009;1283:186–193. doi: 10.1016/j.brainres.2009.05.095. [DOI] [PubMed] [Google Scholar]
- 45.Mannermaa E, Vellonen KS, Ryhanen T, et al. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26(7):1785–1791. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- 46.la Cour M, Ehinger B. The Retina. In: Fischbarg J, editor. Advances in Organ Biology. Elsevier; 2005. pp. 195–252. [Google Scholar]
- 47.Candiello J, Balasubramani M, Schreiber EM, et al. Biomechanical properties of native basement membranes. FEBS J. 2007;274(11):2897–2908. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 48.Pederson JE. In: Fluid physiology of the subretinal space. Fourth edition ed. Ryan SJ, editor. Philadephia, PA: Elsevier Inc; 2006. pp. 1909–1920. Retina. [Google Scholar]
- 49.Lee B, Litt M, Buchsbaum G. Rheology of the vitreous body: Part 3. Concentration of electrolytes, collagen and hyaluronic acid. Biorheology. 1994;31(4):339–351. doi: 10.3233/bir-1994-31404. [DOI] [PubMed] [Google Scholar]
- 50.Lee B, Litt M, Buchsbaum G. Rheology of the vitreous body: Part 2. Viscoelasticity of bovine and porcine vitreous. Biorheology. 1994;31(4):327–338. doi: 10.3233/bir-1994-31403. [DOI] [PubMed] [Google Scholar]
- 51.Mitra AK, Anand BS, Duvvuri S. Drug delivery to the eye. In: Fischbarg J, editor. Advances in Organ Biology. Elsevier; 2005. pp. 307–351. [Google Scholar]
- 52.Clarke S. J Chem Educat. 8. 2nd edition. Vol. 58. Tanford: Charles; 1981. The hydrophobic effect: Formation of micelles and biological membranes; p. A246. [Google Scholar]
- 53.Chevalier Y, Zemb T. The structure of micelles and microemulsions. Rep Prog Phys. 1990;(53):279–371. [Google Scholar]
- 54.Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine. 2010;5(3):485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 56.Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine. 2010;5(3):485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rangel-Yagui CO, Pessoa A, Jr, Tavares LC. Micellar solubilization of drugs. J Pharm Pharm Sci. 2005;8(2):147–165. [PubMed] [Google Scholar]
- 58.Rosen MJ. Micelle Formation by Surfactants. Surfactants and Interfacial Phenomena: John Wiley & Sons, Inc; 2004. pp. 105–177. [Google Scholar]
- 59.Jones MN, Chapman D. Micelles, monolayers and biomembranes. New York: Wiley-Liss; 1995. [Google Scholar]
- 60.Puvvada S, Blankschtein D. Molecular-thermodynamic approach used to predict micellization, phase behavior, and phase separation of micellar solutions. Langmuir. 1990;6(5):894–895. [Google Scholar]
- 61.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 62.Maturana R, Subiabre V. Search for parasitic elements in gallstones. Bol Chil Parasitol. 1982;37(1–2):31. [PubMed] [Google Scholar]
- 63.Hidenori Otsukaa, Nagasakib Y, Kazunori Kataoka. Self-assembly of poly(ethylene glycol)-based block copolymers for biomedical applications. Curr Opinion in Collid & Interface Science. 2001;6:3–10. [Google Scholar]
- 64.Liu J, Zeng F, Allen C. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J Control Release. 2005;103(2):481–497. doi: 10.1016/j.jconrel.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 65.Kang N, Leroux J-C. Triblock and star-block copolymers of N-(2-hydroxypropyl)methacrylamide or N-vinyl-2-pyrrolidone and d,l-lactide: synthesis and self-assembling properties in water. Polymer. 2004;45(26):8967–8980. [Google Scholar]
- 66.Zhang L, Eisenberg A. Multiple morphologies of "crew-cut" aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science. 1995;268(5218):1728–1731. doi: 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]
- 67.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2–3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 68.Batrakova EV, Kabanov AV. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.La SB, Okano T, Kataoka K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J Pharm Sci. 1996;85(1):85–90. doi: 10.1021/js950204r. [DOI] [PubMed] [Google Scholar]
- 70.Müller RH. Colloidal carriers for controlled drug delivery and targeting. Boca Raton: CRC Press; 1990. [Google Scholar]
- 71.Kwon GS, Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Delivery Rev. 1995;16(2):295–309. [Google Scholar]
- 72.Jones M, Leroux J. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 73.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids and Surfaces B: Biointerfaces. 1999;16(1–4):3–27. [Google Scholar]
- 74.Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: Drug loading and release. Curr Pharm Des. 2006;12(36):4685–4701. doi: 10.2174/138161206779026263. [DOI] [PubMed] [Google Scholar]
- 75.Yokoyama M, Satoh A, Sakurai Y, et al. Incorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle size. J Control Release. 1998;55(2–3):219–229. doi: 10.1016/s0168-3659(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 76.Le Garrec D, Gori S, Luo L, et al. Poly(N-vinylpyrrolidone)-block-poly(D,L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release. 2004;99(1):83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Jette KK, Law D, Schmitt EA, Kwon GS. Preparation and drug loading of poly(ethylene glycol)-block-poly(epsilon-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharm Res. 2004;21(7):1184–1191. doi: 10.1023/b:pham.0000033005.25698.9c. [DOI] [PubMed] [Google Scholar]
- 78.Kohori F, Yokoyama M, Sakai K, Okano T. Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. J Control Release. 2002;78(1–3):155–163. doi: 10.1016/s0168-3659(01)00492-8. [DOI] [PubMed] [Google Scholar]
- 79.Fournier E, Dufresne MH, Smith DC, Ranger M, Leroux JC. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm Res. 2004;21(6):962–968. doi: 10.1023/b:pham.0000029284.40637.69. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Jackson JK, Burt HM. Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int J Pharm. 1996;132(1–2):195–206. [Google Scholar]
- 81.Yokoyama M, Opanasopit P, Okano T, Kawano K, Maitani Y. Polymer design and incorporation methods for polymeric micelle carrier system containing water-insoluble anti-cancer agent camptothecin. J Drug Target. 2004;12(6):373–384. doi: 10.1080/10611860412331285251. [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Xiao Y, Allen C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J Pharm Sci. 2004;93(1):132–143. doi: 10.1002/jps.10533. [DOI] [PubMed] [Google Scholar]
- 83.Letchford K, Zastre J, Liggins R, Burt H. Synthesis and micellar characterization of short block length methoxy poly(ethylene glycol)-block-poly(caprolactone) diblock copolymers. Colloids Surf B Biointerfaces. 2004;35(2):81–91. doi: 10.1016/j.colsurfb.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 84.M.H. Dufresne EF, Jones MC, Ranger M, Leroux JC. Gurny R, editor. Block copolymer micelles-engineering versatile carriers for drugs and biomacromolecules. BT Gattefossé. 2003;96:87–102. (Gattefossé, Saint-Priest,) [Google Scholar]
- 85.Teagarden DL, Baker DS. Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur J Pharm Sci. 2002;15(2):115–133. doi: 10.1016/s0928-0987(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 86.Gupta AK, Madan S, Majumdar DK, Maitra A. Ketorolac entrapped in polymeric micelles: Preparation, characterisation and ocular anti-inflammatory studies. Int J Pharm. 2000;209(1–2):1–14. doi: 10.1016/s0378-5173(00)00508-1. [DOI] [PubMed] [Google Scholar]
- 87.Giammona G, Cavallaro G, Licciardi M, Civiale C, Paladino GM, Mazzone MG. Ophthalmic pharmaceutical composition containing amphiphilic polyaspartamide copolymers. 2009 US20090221545. [Google Scholar]
- 88.Civiale C, Licciardi M, Cavallaro G, Giammona G, Mazzone MG. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int J Pharm. 2009;378(1–2):177–186. doi: 10.1016/j.ijpharm.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 89.Di Tommaso C, Torriglia A, Furrer P, Behar-Cohen F, Gurny R, Moller M. Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int J Pharm. 2011;416(2):515–524. doi: 10.1016/j.ijpharm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Pepic I, Jalsenjak N, Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int J Pharm. 2004;272(1–2):57–64. doi: 10.1016/j.ijpharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 91.Pepic I, Hafner A, Lovric J, Pirkic B, Filipovic-Grcic J. A nonionic surfactant/chitosan micelle system in an innovative eye drop formulation. J Pharm Sci. 2010;99(10):4317–4325. doi: 10.1002/jps.22137. [DOI] [PubMed] [Google Scholar]
- 92.Usui T, Sugisaki K, Amano S, Jang WD, Nishiyama N, Kataoka K. New drug delivery for corneal neovascularization using polyion complex micelles. Cornea. 2005;24(8 Suppl):S39–S42. doi: 10.1097/01.ico.0000178738.29459.59. [DOI] [PubMed] [Google Scholar]
- 93.Liaw J, Chang SF, Hsiao FC. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001;8(13):999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 94.Tong YC, Chang SF, Liu CY, Kao WW, Huang CH, Liaw J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J Gene Med. 2007;9(11):956–966. doi: 10.1002/jgm.1093. [DOI] [PubMed] [Google Scholar]
- 95.Tong YC, Chang SF, Kao WW, Liu CY, Liaw J. Polymeric micelle gene delivery of bcl-xL via eye drop reduced corneal apoptosis following epithelial debridement. J Control Release. 2010;147(1):76–83. doi: 10.1016/j.jconrel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Ideta R, Yanagi Y, Tamaki Y, Tasaka F, Harada A, Kataoka K. Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS Lett. 2004;557(1–3):21–25. doi: 10.1016/s0014-5793(03)01315-2. [DOI] [PubMed] [Google Scholar]
- 97.Katoaka K, Tamaki Y, Harada A, Takasa F. Ophthalmic drug delivery system using polymer micelle. 2006 US20060110356. [Google Scholar]
- 98.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 99.Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54(2):203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 100.Ideta R, Tasaka F, Jang WD, et al. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Lett. 2005;5(12):2426–2431. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- 101.Alba AK RM, Takano R, Kobayashi C, Nakajima A. The effect on the cornea of various vehicles for cyclosporine eye drops. Folia Ophthalmol Jpn. 1989;(40):902–908. [Google Scholar]
- 102.BenEzra D, Maftzir G. Ocular penetration of cyclosporine A in the rat eye. Arch Ophthalmol. 1990;108(4):584–587. doi: 10.1001/archopht.1990.01070060132063. [DOI] [PubMed] [Google Scholar]
- 103.Pleyer U, Elkins B, Ruckert D, et al. Ocular absorption of cyclosporine A from liposomes incorporated into collagen shields. Curr Eye Res. 1994;13(3):177–181. doi: 10.3109/02713689408995775. [DOI] [PubMed] [Google Scholar]
- 104.Le Bourlais CA, Chevanne F, Turlin B, et al. Effect of cyclosporine A formulations on bovine corneal absorption: ex-vivo study. J Microencapsul. 1997;14(4):457–467. doi: 10.3109/02652049709033830. [DOI] [PubMed] [Google Scholar]
- 105.Kuwano M, Ibuki H, Morikawa N, Ota A, Kawashima Y. Cyclosporine A formulation affects its ocular distribution in rabbits. Pharm Res. 2002;19(1):108–111. doi: 10.1023/a:1013671819604. [DOI] [PubMed] [Google Scholar]
- 106.Poonam R, Velagaleti EA, John Khan, Brian C Gilger, Ashim K. Mitra Topical delivery of hydrophobic drugs using a novel mixed nanomicellar technology to treat diseases of the anterior & posterior segments of the eye. Drug Delivery Technol. 2010:42–47. [Google Scholar]
- 107.Mitra AK, Velagaleti PR, Natesan S. Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors. 2009 US20090092665. [Google Scholar]
- 108.Earla R, Boddu SH, Cholkar K, Hariharan S, Jwala J, Mitra AK. Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids-quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2010;52(4):525–533. doi: 10.1016/j.jpba.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitra AK, Velagaleti PR, Grau UM. Topical drug delivery systems for ophthalmic use. 2010 US20100310642. [Google Scholar]
- 110.Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213(1–2):25–29. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- 111.Allen C, Yu Y, Eisenberg A, Maysinger D. Cellular internalization of PCL(20)-b-PEO(44) block copolymer micelles. Biochim Biophys Acta. 1999;1421(1):32–38. doi: 10.1016/s0005-2736(99)00108-x. [DOI] [PubMed] [Google Scholar]
- 112.Luo L, Tam J, Maysinger D, Eisenberg A. Cellular internalization of poly(ethylene oxide)-b-poly(epsilon-caprolactone) diblock copolymer micelles. Bioconjug Chem. 2002;13(6):1259–1265. doi: 10.1021/bc025524y. [DOI] [PubMed] [Google Scholar]
- 113.Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300(5619):615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]