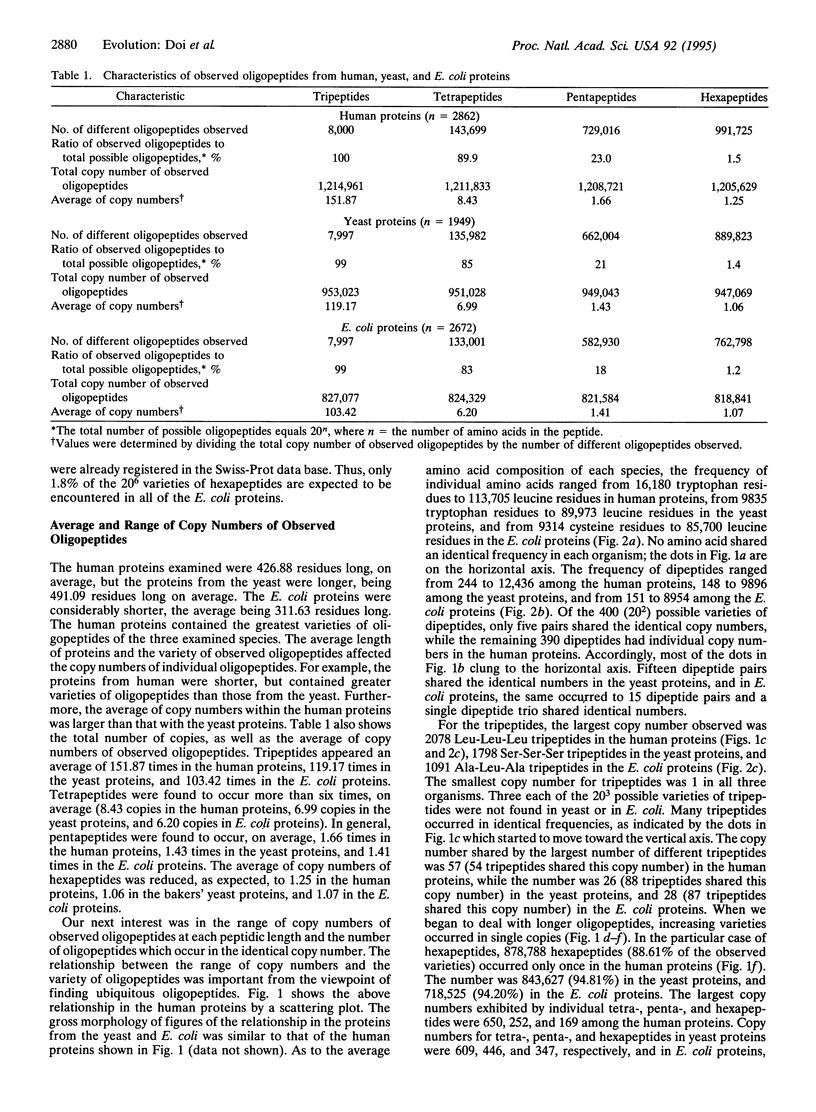
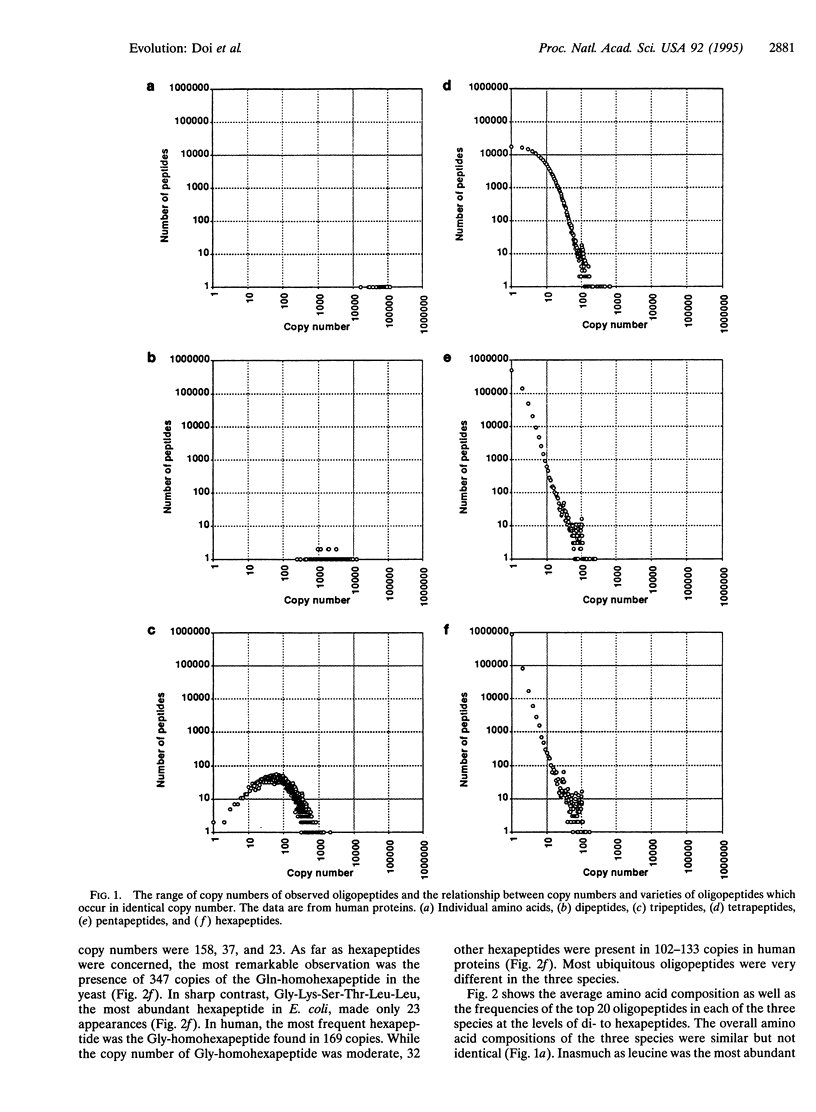
Abstract
Oligopeptidic permutations of the 20 amino acid residues give rise to proteins of diverse functions. Our long-term goal is to produce a lexicon of oligopeptides, classifying them into at least five categories: (i) ubiquitous, (ii) function specific, (iii) group specific, (iv) species specific, and (v) nonexistent. To begin with, we report on the varying frequencies of individual oligopeptides (dipeptidic to hexapeptidic in length) found among 2862 human proteins, 1942 Saccharomyces cerevisiae proteins, and 2672 Escherichia coli proteins registered in the Swiss-Prot data base (version 29.0, released in June 1994). At all lengths (dipeptides to hexapeptides), homooligopeptides were very prominent among the most frequently occurring varieties in proteins of human and bakers' yeast origins. However, this was not the case with E. coli. While all of the expected 20(3) varieties of tripeptides were found among human proteins, three tripeptides (Cys-Cys-Trp, Trp-Trp-Cys, and Trp-Trp-His) were missing from the bakers' yeast proteins. Three tripeptides (Cys-Ile-Trp, Cys-Met-Tyr, and Cys-Trp-Trp) were also absent from E. coli proteins. Inasmuch as the Swiss-Prot data base already contained 67% of the expected total of 4000 E. coli proteins, it is virtually certain that 96,000 varieties of hexapeptides containing at least one or another of the three missing tripeptides noted above shall be nonexistent in E. coli. Furthermore, the observation of missing tripeptides in the bakers' yeast proteins suggests that nonexistent hexapeptides shall be highly phylum specific. Because of the sample size, only a small fraction of the 20(6) varieties of hexapeptides were expected to be encountered in the present survey. Indeed, only 1.2-1.5% of the possible hexapeptides were found, and the average copy number of observed hexapeptides varied between 1.06 and 1.25. Nevertheless, 33 varieties of hexapeptides occurred in 102-169 copies among human proteins. Furthermore, 15 of the 33 varieties contained such rarely used residues as Tyr, His, Cys, and Trp.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ohno S. Active sites of ligands and their receptors are made of common peptides that are also found elsewhere. J Mol Evol. 1995 Jan;40(1):102–106. doi: 10.1007/BF00166601. [DOI] [PubMed] [Google Scholar]
- Ohno S. The cardinal principle of like attracting like generates many ubiquitous oligopeptides shared by divergent proteins. Anim Genet. 1994 Jun;25 (Suppl 1):5–11. doi: 10.1111/j.1365-2052.1994.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]



