Abstract
Detection of circulating tumor cells (CTCs) is advancing as an effective predictor of patient outcome and therapeutic response. Unfortunately, our knowledge of CTC biology remains limited, and the impact of drug treatments on CTC metastatic potential is currently unclear. Improved CTC imaging in vivo and analysis of free-floating tumor cells now demonstrate that cytoskeletal regulation in CTCs contrasts starkly with tumor cells attached to extracellular matrix (ECM). In this review, we examine how persistent microtubule stabilization promotes the formation of microtentacles (McTNs) on the surface of detached breast tumor cells and enhances metastatic potential.
Keywords: microtentacles, circulating tumor cells, metastasis, microtubules, cytoskeleton
INTRODUCTION
To control morphology, cells perform a delicate balancing act of cytoskeletal forces. Termed the cellular tensegrity model, expansion of microtubules is counteracted by tension in the actin cytoskeleton to stabilize cell shape (1). Cytoskeletal aberrations contribute to many characteristics of aggressive tumors, such as increased cell motility, weakened adhesive contacts, and metastatic dissemination. For example, hypoxia-induced signaling can promote an epithelial-to-mesenchymal transition (EMT), which leads to numerous cytoskeletal alterations, including expression of chemotherapeutic-resistant tubulin isoforms and the intermediate filament (IF), vimentin (2, 3).
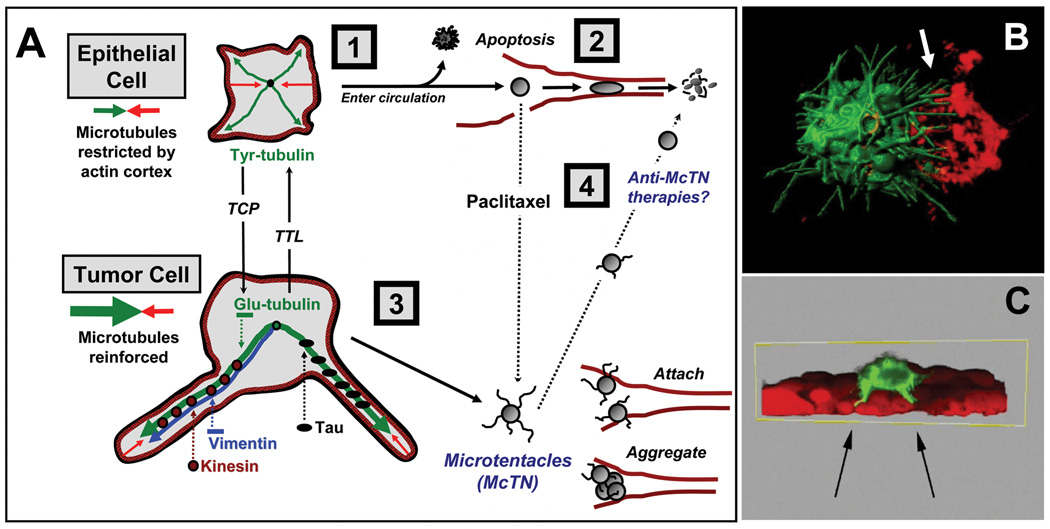
Recent studies demonstrate that altering the balance between microtubules and actin has serious implications for CTC dissemination (4, 5). In vitro CTC modeling reveals that detached cells form McTNs – dynamic, microtubule-enriched plasma membrane extensions that are antagonized by the actin cytoskeleton (4, 6). Microtubule stabilization enhances McTNs and facilitates in vitro tumor cell aggregation and reattachment (7, 8). In vivo, cells forming McTNs are more efficiently retained in the microvasculature (8), reinforcing evidence that CTC attachment to the capillary endothelium is microtubule-dependent (5). Here, we discuss the mechanisms of microtubule stabilization in CTCs and how these alterations influence metastatic efficiency (Figure 1).
Figure 1. Microtubule stabilization in McTNs and metastasis.
(A) In detached epithelial cells [1], microtubules (green) outwardly expanding from the cell center are counterbalanced by contraction of the cortical actin cytoskeleton (red). Such cells die by apoptosis or fragmentation in capillaries [2] when circulating. In breast tumor cells [3], McTNs overcome the restrictive forces of the actin cortex by associating with tau. Similarly, removal of the COOH-terminal tyrosine of Tyr-tubulin by TCP yields Glu-tubulin that associates with vimentin until it is retyrosinated by TTL. Microtubule-stabilizing drugs [4], like paclitaxel, increase McTNs and tumor cell reattachment. (B) Confocal microscopy of live GFP-labeled breast tumor cells shows that McTNs stimulate aggregation by encircling adjacent cells labeled with a red lipophillic dye (B, white arrow). McTNs also promote tumor cell reattachment to mCherry-labeled endothelial cell layers (C, black arrows). Movies of 3-D reconstructions from the imaged time point in (B) and (C) are available as supplementary data (rendered with Bitplane Imaris6.4).
MECHANISMS OF MICROTUBULE STABILIZATION IN CANCER
Formation of detyrosinated tubulin
Microtubules are polarized and linear polymers composed of α- and β-tubulin heterodimers, which can be regulated through post-translational modification, such as the cyclical removal and ligation of the COOH-terminal tyrosine on α-tubulin. An unidentified tubulin carboxypeptidase (TCP) removes this tyrosine from α-tubulin, exposing a glutamic acid residue to form detyrosinated microtubules (Glu-tubulin) (9). This modification is reversed by tubulin tyrosine ligase (TTL), which replaces the tyrosine on free tubulin heterodimers to regenerate tyrosinated α-tubulin (Tyr-tubulin). In vivo, glu-microtubules persist for hours, whereas tyr-microtubules turnover within 3-5 minutes (9). This stabilization effect is not observed with purified tubulin in vitro, demonstrating that cellular mechanisms stabilize glu-microtubules, including capping of the plus end and crosslinking with IFs (9).
Tubulin detyrosination is clinically associated with tumor aggressiveness and poor prognosis. In a study of 134 breast cancer patients, 65.4% of grade 3 primary breast tumors stained strongly for glu-microtubules, compared to 3.4% of grade 1 tumors (10). This glu-microtubule enrichment upon tumor progression may result from TTL inactivation, which is commonly observed in human carcinomas and sarcomas (10). Unlike TTL, the contribution of TCP to cancer progression is unknown. Despite over 30 years of effort, TCP protein remains unidentified. What little is known about TCP stems from cell extract studies of chick brain embryos or cell lines, which have demonstrated that the enzyme detyrosinates α-tubulin only in polymerized microtubules. In general, carboxypeptidases operate in a variety of mechanisms, using serine-, cysteine-, or metal ion-containing active sites. Others possess affinities for terminal amino acids that have specific side chains. Phosphatase inhibitor studies indicate that TCP association with microtubules is regulated by serine/threonine phosphorylation, and not tyrosine phosphorylation, but it is not known if TCP, tubulin or an intermediate is the phosphorylation-regulated component (11). In addition, unlike other carboxypeptidases, TCP remains unaffected by inhibitors of carboxypeptidase A, a pancreatic carboxypeptidase with catalytic affinity for COOH-terminal amino acids with aliphatic or aromatic side chains, such as tyrosine (12).
Tubulin detyrosination may also be associated with invasion and metastatic dissemination. Glu-microtubules orient in the direction of cell migration, implying their involvement in tumor invasiveness (13). In models of the hematogenous dissemination of CTCs, detachment of breast carcinoma cell lines increases glu-tubulin, which concentrates in McTNs (4, 6). Like the glu-microtubules from which they are formed, McTNs are persistent and are observed hours and days following cell detachment, especially in apoptosis-resistant cells (4). Furthermore, McTN formation and tumor cell reattachment are enhanced upon weakening of the actin cortex (4, 7), illustrating how cytoskeletal force imbalance can impact the metastatic potential of CTCs.
Crosslinking of microtubules to vimentin intermediate filaments
The cellular tensegrity model predicts that microtubules resist compressive buckling through a number of lateral interactions (1). Most notable is the association with IFs, non-polarized ropelike structures that impart mechanical stability to animal cells through their association with other cytoskeletal structures and sites of cell-cell and cell-ECM adhesion. For example, microinjection of non-polymerizable and non-tyrosinatable glu-tubulin causes collapse of IFs to the perinuclear region, demonstrating how these two cytoskeletal systems coordinate to provide structural stability to the cell (9).
In malignancies, vimentin expression increases during the EMT associated with tumor invasion and metastasis, especially among breast cancers and melanomas. We have shown that vimentin expression enhances McTN formation (6). Metastatic breast carcinoma cell lines with elevated vimentin also exhibit increased McTNs. Conversely, tumor cell lines that express epithelial cytokeratins demonstrate fewer McTNs and decreased metastatic potential. Promoting vimentin phosphorylation and disassembly through protein phosphatase inhibition also reduces McTN formation (6). Furthermore, exogenous expression of dominant-negative vimentin decreases McTN frequency in metastatic cell lines that natively express full-length vimentin (6). Vimentin associates preferentially with detyrosinated microtubules in a kinesin-dependent manner, suggesting a mechanism by which the disruption of this association can lead to collapse of IFs (9). Both vimentin (14) and detyrosinated tubulin (10) predict poor breast cancer survival, but the mechanism responsible is currently unknown. It remains possible that the independent influences of vimentin (14) and detyrosinated tubulin (10) on tumor metastasis could result from their interdependent contributions to the microtubule stabilization underlying McTNs (4, 6).
Microtubule stabilization through binding of structural microtubule-associated proteins
Microtubule-associated proteins (MAPs) have functionally diverse roles. Here, we focus on the structural MAPs – non-enzymatic filamentous proteins that promote tubulin polymerization and microtubule stabilization. The MAP1 and the MAP2/tau protein families are the largest and best characterized structural MAPs; however, tau has been the most extensively studied MAP with respect to human malignancy.
Clinically, tau expression provides resistance to microtubule-based chemotherapeutics in breast, gastric, and pancreatic cancers. Tau was identified as the most differentially expressed gene during neoadjuvant paclitaxel chemotherapy, associated with both estrogen receptor status and residual disease following treatment (15). While a subsequent randomized clinical trial revealed that patients with tau-positive primary tumors had significantly better disease-free and overall survival, with no tau-associated benefit from paclitaxel, our recent report indicates that tau contributes to the metastatic efficiency of breast tumor cells (8). We demonstrated that tau directly induces McTNs in detached mammary epithelial and breast carcinoma cell lines, which significantly increases their ability to reattach following release from ECM (8). In vivo, tau-overexpressing MCF-7 cells are more efficiently trapped and retained in lung capillaries compared to tau-deficient cells in experimental metastasis assays (8). In 102 breast cancer patients, tau expression was significantly increased in 26% of lymph node metastases compared to matched primary tumors. An additional 26% displayed elevated tau expression that was maintained between primary and metastatic tumors. In only 12% of patients did a loss of tau expression occur between primary and metastatic tumors (from pathological scores +1 to 0). These data suggest that tau could provide a selective advantage during metastasis (8).
Similar to microtubule strengthening by IF association, tau tips the force balance between microtubules and actin by providing structural strength to microtubules. Tau promotes microtubule bundling, and individual tau-decorated microtubules can withstand greater deforming forces compared to naked microtubules (16, 17). Similarly, actin depolymerization in BT-20 breast carcinoma cells is not sufficient to induce McTNs, but combining tau expression and actin depolymerization significantly enhanced McTN formation (8). These data indicate that, even when the actin cortex is disrupted, strengthening of microtubules may still be required for McTNs to emerge.
IMPLICATIONS FOR CANCER PROGRESSION
Deformation of CTCs in transit
Optical deformability of cells is an emerging marker of cancer progression (18). A central determinant of metastatic efficiency is tumor cell survival during hematogenous dissemination. Metastasis of carcinoma CTCs is limited by the ability of the relatively large epithelial tumor cells to deform within the narrow size restrictions of the microvasculature. If CTC membranes are deformed beyond the limits of their surface area, the cells perish through shear-induced fragmentation. In contrast, metastatically-efficient CTCs have been observed to undergo sphere-to-cylinder shape transformations within capillaries (19). These observations are supported by experimental deformations of suspended tumor cells with microfluidic optical stretchers, which indicate that tumor cells are more deformable than normal epithelial cells (18). The degree of actin crosslinking is directly implicated in CTC deformation., As malignancy progresses, the F-to-G actin ratio decreases, indicating a less polymerized actin cytoskeleton (20). Mutation in or altered expression of mediators of actin polymerization can also disrupt actin organization and crosslinking (21), which may weaken the cortical integrity. When microtubules are stabilized in concert with reduced actin integrity, CTCs produce increased McTNs (7, 8).
Homo- and heterotypic cell aggregation
CTC survival in the circulation requires protection from mechanical damage due to shear and collision forces and from destruction by immune survelliance. Such protections are granted by the ability of metastatically-efficient CTCs to homotypically and heterotypically aggregate. In vitro selection of tumor cell lines that efficiently cluster homotypically exhibited a greater ability to metastasize in vivo than non-aggregating parental cells (22). Similarly, our own studies demonstrate that McTNs enhance homotypic cell aggregation (4). While formation of homotypic CTC aggregates increases the survival of disseminating cells by mechanical trapping, evidence suggests that CTC reattachment to the endothelium may also be activated through heterotypic aggregation of CTCs with platelets (22). Association of CTCs with platelets and other coagulation factors is implicated in venous thromboembolism, which correlates with CTC burden and poor prognosis in metastatic breast cancer patients (23). The CTC-platelet association involves activation of integrins and clotting factors to induce a fibrin coat that enhances CTC spreading following attachment to organ microvasculature. In addition, immune-mediated destruction of CTCs can be avoided through the direct association of CTCs with leukocytes. Given their ability to encircle adjacent cells during homotypic aggregation (Figure 1B), McTNs may also facilitate heterotypic association of CTCs with platelets or with white blood cells.
Increased intravascular retention
Currently, two models describe the successful reattachment of CTCs during bloodborne metastasis: a passive mechanism, whereby CTCs are mechanically trapped within the microvasculature; or an active mechanism, whereby specific adhesive interactions of CTCs permit microvasculature retention (24). In vivo studies by Korb et al. used intravital microscopy to demonstrate that circulating HT-29 colon carcinoma cells adhere to liver sinusoidal capillaries in a microtubule-dependent manner (5). Specifically, pretreatment of these cells with the microtubule depolymerizer, nocadazole, significantly reduced CTC attachment. Surprisingly, inhibition of actin polymerization with cytochalasin-D significantly enhanced CTC reattachment (5). The cytoskeletal mechanisms supporting McTN formation (4, 6, 7) and McTN-dependent microvascular retention (8) match identically with these in vivo observations (5). As described above, tau stabilizes microtubules and increases McTNs in breast tumor cells, while whole-animal bioluminescence imaging shows that tau-expressing CTCs are more efficiently retained in the lung microvasculature compared to tau-deficient controls (8). These data (5, 8) and the ability of McTNs to penetrate endothelial layers (Figure 1C) support an active role for microtubule stabilization during CTC reattachment within the microvasculature. Given that cells with McTNs attach more efficiently to ECM in vitro (4), further investigation is needed to determine whether McTNs are enriched in distinct adhesion proteins that may mediate CTC-endothelial attachment, such as integrins or selectins. While selectin-mediated endothelial attachment permits tumor cell rolling, integrin-mediated ECM attachment is required for CTCs to arrest against the force of blood flow (25). These data support a model where contact with the underlying ECM through transendothelial penetration is necessary to promote the earliest stages of CTC extravasation, and McTNs are capable of such transendothelial penetration (Figure 1C).
MICROTUBULE-TARGETED DRUGS AND CTCs
Stabilizing drugs, like taxanes, prevent microtubule disassembly, stalling tumor cells in metaphase and initiating cell death through spindle checkpoint activation or mitotic catastrophe. While microtubule-targeted drugs are highly successful in primary tumor treatment, their success is limited for metastatic disease. The effects of microtubule-targeted chemotherapies on CTCs have not been thoroughly investigated. Evidence that taxanes enhance McTN formation and tumor cell reattachment (7) emphasizes that the effects of existing cancer drugs on CTCs should be examined to ensure that anti-mitotic therapies do not inadvertently increase metastatic potential. Emerging evidence that paclitaxel treatment actually increases lung colonization of CTCs in mice has been connected to the tumor stem cell phenotype (26), but a direct role for microtubule stabilization in CTC retention has not yet been excluded in this model.
Camara et al. demonstrated that a potential consequence of taxane administration is the mobilization of CTCs. Reports indicate that newly diagnosed breast cancer patients receiving either combination or single-agent therapy, each with subsequent taxane administration, experienced as much as a 10,000-fold increase in CTCs during the taxane arm (27). These cell numbers remain elevated pre- and post-surgery, indicating persistent CTCs that resisted taxane treatment or new CTCs that were mobilized during surgery (27).
Growing evidence indicates that the ability of CTCs to successfully metastasize may increase due to detrimental effects of chemotherapeutics. For example, cyclophosphamide has been observed to cause microvascular damage and to increase intravascular proliferation and extravasation of tumor cells. Likewise, anti-angiogenic therapies can also increase metastatic potential (28). Enhancement of metastasis could be a consequence of taxanes, which can alter the balance between microtubules and actin. Balzer et al. demonstrated that jasplakinolide, an in vitro actin-stabilizing peptide that causes abnormal nucleation and aggregation of actin in vivo, increased McTN formation (7, 29). This effect was enhanced by paclitaxel-mediated microtubule stabilization, which additionally increased tumor cell reattachment and spreading on ECM (7). These data indicate that CTCs may be stimulated to adhere to blood vessel walls or to the underlying ECM after taxane-mediated microtubule stabilization or actin disruption. While microtubule-targeted therapies are a common strategy to reduce the growth of attached tumor cells, proteins that alter actin polymerization are emerging as potential targets for chemotherapy (30).
Taxane resistance with tau-expression may heighten the risk of successful metastasis due to an increased mobilization of McTN-forming CTCs. Tau contributes to taxane resistance by competing with paclitaxel for microtubule binding. Pulse-chase experiments measuring tritiated-paclitaxel incorporation into microtubules revealed that microtubules assembled in the presence of tau incorporate less paclitaxel compared to those assembled with tubulin alone. Consequently, the IC50 of paclitaxel required to kill tau-expressing breast tumor cells increases (15).
Further studies are required to evaluate the effects of microtubule-targeted chemotherapeutics on CTCs and metastatic risk. As new therapeutic targets are identified and new therapies developed, any possible detrimental effects of microtubule-based chemotherapies could be counteracted or avoided by defining a specific risk window during the course of treatment.
FUTURE CONSIDERATIONS
Metastasis remains the leading cause of death among cancer patients. CTCs pose a particular challenge as our knowledge of the interaction between CTCs and the circulatory microenvironment is not well-defined. It is now understood that tipping the cytoskeletal force balance toward microtubule stabilization and actin depolymerization causes CTCs to acquire characteristics that promote metastatic efficiency. Through stabilization by detachment-induced post-translational modifications and interactions with accessory proteins, microtubules can overcome the restrictive forces of cortical actin to protrude the plasma membrane; thus mediating McTN formation, cell-cell aggregation and endothelial attachment of CTCs.
Considering the detrimental effects of chemotherapeutics on CTC mobilization and McTN induction, it raises the question of whether our anti-cancer effort is missing an important target. It remains critical to develop methods that distinguish whether drug-induced tumor shrinkage arises from tumor cell death or tumor cell dissemination, since these two effects have dramatically different consequences in a neoadjuvant setting. While current drug development efforts and clinical endpoints focus on limiting the growth of either primary or metastatic tumors, it will be important to understand the effects of existing cancer drugs on CTCs in order to improve treatment strategies and develop novel therapies aimed at reducing the metastatic potential of CTCs.
Supplementary Material
Acknowledgments
Grant support to S.S.M.: R01-CA124704, National Cancer Institute; Breast Cancer Idea Award, USA Medical Research and Materiel Command (BC061047); MD Stem Cell Exploratory Award (MSCRFE-0081); Clinical Innovator Award, Flight Attendant Medical Research Institute (CIA-062497).
REFERENCES
- 1.Ingber DE, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 2.Raspaglio G, Filippetti F, Prislei S, Penci R, De Maria I, Cicchillitti L, et al. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3' flanking region. Gene. 2008;409:100–108. doi: 10.1016/j.gene.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 4.Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313:1326–1336. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korb T, Schluter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, et al. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–247. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68:5678–5688. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzer EM, Whipple RA, Cho EH, Matrone MA, Martin SS. Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells. Breast Cancer Res Treat. 2010;121:65–78. doi: 10.1007/s10549-009-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29:3217–3227. doi: 10.1038/onc.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell. 1999;10:1105–1118. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–5027. [PubMed] [Google Scholar]
- 11.Contin MA, Purro SA, Bisig CG, Barra HS, Arce CA. Inhibitors of protein phosphatase 1 and 2A decrease the level of tubulin carboxypeptidase activity associated with microtubules. Eur J Biochem. 2003;270:4921–4929. doi: 10.1046/j.1432-1033.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- 12.Contin MA, Sironi JJ, Barra HS, Arce CA. Association of tubulin carboxypeptidase with microtubules in living cells. Biochem J. 1999;339(Pt 2):463–471. [PMC free article] [PubMed] [Google Scholar]
- 13.Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domagala W, Striker G, Szadowska A, Dukowicz A, Harezga B, Osborn M. p53 protein and vimentin in invasive ductal NOS breast carcinoma--relationship with survival and sites of metastases. Eur J Cancer. 1994;30A:1527–1534. doi: 10.1016/0959-8049(94)00288-g. [DOI] [PubMed] [Google Scholar]
- 15.Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 17.Schaap IA, Hoffmann B, Carrasco C, Merkel R, Schmidt CF. Tau protein binding forms a 1 nm thick layer along protofilaments without affecting the radial elasticity of microtubules. J Struct Biol. 2007;158:282–292. doi: 10.1016/j.jsb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Remmerbach TW, Wottawah F, Dietrich J, Lincoln B, Wittekind C, Guck J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009;69:1728–1732. doi: 10.1158/0008-5472.CAN-08-4073. [DOI] [PubMed] [Google Scholar]
- 19.Weiss L. Biomechanical interactions of cancer cells with the microvasculature during hematogenous metastasis. Cancer Metastasis Rev. 1992;11:227–235. doi: 10.1007/BF01307179. [DOI] [PubMed] [Google Scholar]
- 20.Katsantonis J, Tosca A, Koukouritaki SB, Theodoropoulos PA, Gravanis A, Stournaras C. Differences in the G/total actin ratio and microfilament stability between normal and malignant human keratinocytes. Cell Biochem Funct. 1994;12:267–274. doi: 10.1002/cbf.290120407. [DOI] [PubMed] [Google Scholar]
- 21.Kelley LC, Shahab S, Weed SA. Actin cytoskeletal mediators of motility and invasion amplified and overexpressed in head and neck cancer. Clin Exp Metastasis. 2008;25:289–304. doi: 10.1007/s10585-008-9154-6. [DOI] [PubMed] [Google Scholar]
- 22.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 23.Mego M, De Giorgi U, Broglio K, Dawood S, Valero V, Andreopoulou E, et al. Circulating tumour cells are associated with increased risk of venous thromboembolism in metastatic breast cancer patients. Br J Cancer. 2009;101:1813–1816. doi: 10.1038/sj.bjc.6605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–329. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Haier J, Nicolson GL. Tumor cell adhesion under hydrodynamic conditions of fluid flow. Apmis. 2001;109:241–262. doi: 10.1034/j.1600-0463.2001.d01-118.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camara O, Rengsberger M, Egbe A, Koch A, Gajda M, Hammer U, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol. 2007;18:1484–1492. doi: 10.1093/annonc/mdm206. [DOI] [PubMed] [Google Scholar]
- 28.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 30.Rao J, Li N. Microfilament actin remodeling as a potential target for cancer drug development. Curr Cancer Drug Targets. 2004;4:345–354. doi: 10.2174/1568009043332998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.