Abstract
Learning from mistakes and prospectively adjusting behavior in response to reward feedback is an important facet of performance monitoring. Dopamine (DA) pathways play an important role in feedback learning and a growing literature has also emerged on the importance of serotonin (5HT) in reward learning, particularly during punishment or reward omission (negative feedback). Cognitive impairments resulting from psychostimulant exposure may arise from altered patterns in feedback learning, which in turn may be modulated by DA and 5HT transmission. We analyzed long-term, off-drug changes in learning from positive and negative feedback and associated striatal DA transporter (DAT) and frontocortical 5HT transporter (SERT) binding in rats pretreated with methamphetamine (mAMPH). Specifically, we assessed the reversal phase of pairwise visual discrimination learning in rats receiving single dose- (mAMPHsingle) vs. escalating-dose exposure (mAMPHescal). Using fine-grained trial-by-trial analyses, we found increased sensitivity to and reliance on positive feedback in mAMPH-pretreated animals, with the mAMPHsingle group showing more pronounced use of this type of feedback. In contrast, overall negative feedback sensitivity was not altered following any mAMPH treatment. In addition to validating the enduring effects of mAMPH on early reversal learning, we found more consecutive error commissions before the first correct response in mAMPH-pretreated rats. This behavioral rigidity was negatively correlated with subregional frontocortical SERT whereas positive feedback sensitivity negatively correlated with striatal DAT binding. These results provide new evidence for the overlapping, yet dissociable roles of DA and 5HT systems in overcoming perseveration and in learning new reward rules.
1. Introduction
Learning from mistakes and prospectively adjusting behavior in response to negative feedback is an important facet of performance monitoring. This cognitive process has been shown to get poorer with age [1,2] and is also suboptimal in youth with a history of disruptive behavior [3]. Recent evidence shows that “high learners” utilize errors (or negative feedback) more optimally to update their future reward choices [4]. A plentitude of rodent and nonhuman primate research shows that such integration of feedback occurs via heterogenous reward signals in the prefrontal cortex, and that learning from both positive and negative feedback depends on dopamine (DA) signaling in areas like the orbitofrontal cortex (OFC) and basal ganglia [5]. Not surprisingly DA drugs, such as those given to Parkinson’s patients, have been shown to modulate learning from reward feedback [6].
Chronic exposure to cocaine or methamphetamine (mAMPH) results in progressive and long-lasting changes in the mesencephalic DA system [7–11]. Repeated administration of high doses of mAMPH results in long-lasting reductions in total DA content [12–14], reduced activity of tyrosine hydroxylase [15,16], decreased DA transporter binding and density [17–21], and compromised DA D2-like receptor availability in the striatum [22]. MAMPH administration also produces enduring impairments in cognitive flexibility, when the inhibition of previously-learned responses is required. Animal models of mAMPH addiction provide evidence that pathological neuroplasticity in prefrontal cortex and striatum underlie compulsive drug seeking and relapse [23–25]. Collectively, the preceding evidence strongly emphasizes the role of DA pathways in feedback-guided learning and suggests that some of the impairments induced by drug exposure as well as the vulnerability to the development of compulsive drug use may arise from altered patterns in feedback monitoring.
Several groups have analyzed how animals use positive and negative trial-by-trial feedback [26–29], however these parameters have not been previously explored in pharmacological studies. Additionally, to our knowledge the effects of different mAMPH administration regimens on animals’ responses to reward feedback have not been previously examined. Both single-dose exposure (mAMPHsingle) and escalating exposure to mAMPH (mAMPHescal) result in cognitive flexibility impairments, as measured by attenuated reversal learning [30]. Though these regimens of mAMPH treatment produce remarkably similar learning impairments, the DA system may be differentially affected and produce such impairments through unique mechanisms. In the present experiment we compared mAMPHescal, mAMPHsingle, and saline (SAL)-treated animals on measures of feedback learning. Specifically, we assessed sensitivity to reward feedback or omission of anticipated reward on the reversal phase of pairwise visual discrimination learning. It should be noted that the trial-by-trial feedback learning we analyzed here occurred well outside of a drug wash out period and do not represent acute effects of mAMPH. Any changes we observed in performance monitoring therefore, represent enduring effects of the drug on this cognitive process.
2. Materials and methods
2. 1. Subjects
Previously-collected and published data [30] were reanalyzed in the present study for trial-by-trial feedback performance. Twenty-one male Long–Evans rats (Charles River Laboratories, Raleigh, NC) weighing between 275 and 300 g at the beginning of the study were individually housed during food restriction, given water ad libitum and maintained at a 12-h light/12-h dark cycle, with the temperature at 22 °C. Body weights were monitored daily. Behavioral testing took place between 0800 and 1600 hours during the rats’ inactive period, consistent with previous studies in our lab [30,31]. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
2. 2. Food restriction and acclimation to food rewards
When rats reached a minimum body weight of 275 g, they were food restricted to no less than 85 % of their free-feeding body weight to ensure motivation to work for food, while water was available ad libitum. On each of the two days prior to the start of testing, rats were fed 20 sucrose pellets in their home cage to accustom them to the food reward.
2. 3 Apparatus
Operant conditioning chambers [35 cm (length) x 28 cm (width) x 34 cm (height)] (#80004, Lafayette Instrument Co.) were housed within sound- and light-attenuating cubicles (#83018DDP, Lafayette Instrument Co.). Each chamber was equipped with an LCD touchscreen (Elo Touch). The houselight was located adjacent to the touchscreen, whereas the tone generator and pellet tray were located next to the pellet dispenser, opposing the touchscreen. The pellet dispenser delivered single 45 mg dustless sucrose pellets (BioServ). Custom software (Ryklin Software Inc.) was used.
2. 4. General
The animals were given one testing session per day until the learning criterion was reached and were restricted to a maximum of 60 correct responses or 120 total trials per testing session. Each session of training and testing lasted a maximum of 45 min. Only a small area (2.5 cm diameter circle) on the touchscreen was sensitive to nosepoking, while all other areas were programmed to be unresponsive. The primary parameter considered for advancement in learning was performance accuracy, defined as percent correct (correct/total trials). Criterion in each phase of pretraining was 60 correct nosepokes at 85% correct responses to the stimulus within 45 min, on each of two consecutive days. The testing session was terminated for one of the three reasons: 1) allotted time had elapsed, 2) maximum number of trials had been reached, or 3) maximum number of correct responses had been reached.
2. 5. Autoshaping and pretraining
Autoshaping began with the display of white graphic stimuli on the black background of the touchscreen, the disappearance of which coincided with the onset of a “reward event”: a sucrose pellet, a 1 s tone, and a 1 s illumination of the house light. An ITI of 20 s was used, while stimuli remained on the screen for 8 s. At any time, rats could nosepoke the stimuli on the touchscreen and initiate the reward event. Criterion for autoshaping occurred when rats ate 60 sucrose pellets within 30 min for each of two consecutive days. After autoshaping, the pretraining phase commenced and consisted of four different stages previously outlined in detail [30].
2. 6. Visual discrimination learning
Rats were presented with two novel, white, equiluminescent stimuli that differed only in shape [32,33] with predetermined reinforcement contingencies. The software enabled either a reward event as a result of nosepoking the correct stimulus (S+), or a punishment as a result of nosepoking the incorrect stimulus (S−); the latter consisting of a 5 s “houselight off” and “time out” wherein rats were unable to initiate the next trial. If the rat committed an error and received a punishment, a correction trial was administered: this consisted of the same left/right presentation of the stimulus until the rat nosepoked correctly. These were consecutive errors and were tallied independently of “first” errors. Stimuli presentation (i.e., left/right presentation of the S+) occurred pseudorandomly according to a Gellerman schedule. Stimulus assignment (SA+SB− or SA−SB+) was counterbalanced across treatment groups.
2. 7. Drug treatment
Rats were given injections of mAMPH (Sigma, St. Louis, MO; s.c.) or physiological SAL solution (1 ml/kg, s.c.) on a clean room procedure table in their housing room, five times per week for 4 weeks, between 1200 and 1500 hours. A 4- week treatment regimen was chosen for its similarity to other mAMPH- and amphetamine-escalating protocols [34–37]. There were three treatment groups: (1) mAMPHescal group received mAMPH, starting at 0.3 mg/kg and escalating in 0.3 mg/kg increments per day, culminating in 6 mg/kg (n=9), (2) mAMPHsingle dose group received SAL for 4 weeks with a single dose of 6 mg/kg mAMPH only on the last day of treatment (n=6), and (3) the SAL group received saline for the duration of treatment (n=6). The largest dose of 6 mg/kg was chosen because it was found to be well-tolerated by our intermittently food-restricted animals.
2. 8. Retention and reversal learning
Following 3–5 days of rest after treatment with mAMPH, rats were tested for retention of the initial discrimination contingencies (unimpaired, as reported in [30]), using procedures identical to the visual discrimination learning phase, above. Upon reaching criterion on this phase (85 % correct trials for each of two consecutive days), the rats were tested on a reversal of the reward contingencies. Parameters for the reversal phase were identical to the visual discrimination learning (above), with the exception that the reward contingencies were reversed.
2. 9. [125I]RTI-55 binding to DAT and SERT
Rats were euthanized between 6 to 8 weeks after mAMPH or SAL treatment with an overdose of sodium pentobarbital (250 mg/kg, i.p.) and decapitated, and their brains were removed and frozen at −20 °C by immersion in isopentane. Twenty-micrometer-thick coronal sections were cut on a cryostat at the level of the anterior striatum (AP coordinates +1.9 to +1.0 mm, relative to bregma) and prefrontal cortex (AP coordinates +3.7 to +2.8 mm, according to [38], thaw-mounted on Vectabond-treated glass slides and stored at −20 °C until used for autoradiographic determination of DAT or SERT binding, respectively. For the determination of striatal DAT binding, warmed slides removed from the −20 °C freezer were preincubated in a solution of assay buffer (10 mM NaPO4, 120 mM NaCl, and 100 mM sucrose) for 5 min to remove endogenous ligands that could interfere with subsequent radioligand binding. After preincubation, the sections were incubated in a solution of assay buffer containing 25 pM [125I]RTI-55 for 2 h. The preincubation and incubation media contained 100 nM fluoxetine to block [125I]RTI-55 binding to SERT [39]. The sections were then rinsed twice for 2 min each at 4 °C in assay buffer then once for 10 s in 4 °C distilled water. The rinsed slides were then rapidly dried under a stream of heated air. Determinations of frontoparietal cortex SERT were performed in much the same way as those for striatal DAT except that fluoxetine was omitted. The dried slides and [14C]-containing autoradiographic standards were apposed to Hyperfilm MP (GE Healthcare) for 48 h before development. Our analyses were limited to quantification of DAT in striatum and SERT in frontoparietal cortex because the binding of [125I]RTI-55 to SERT in striatum and DAT in frontoparietal cortex was below threshold for accurate measurements. Under the assay conditions used, the binding of [125I]RTI-55 to striatal DAT constitutes >99 % of its total binding, while the binding of [125I]RTI-55 to frontoparietal SERT constitutes >94 % of the total in that region.
Quantification of [125I]RTI-55 binding was done using an MCID image analyzer (InterFocus Imaging; Cambridge, England). Image densities were converted to [125I]RTI-55 binding levels using a calibration curve based on images of the standard slides packed with each film. Regional densities of binding were obtained by outlining the desired structures on their respective [125I]RTI-55 images. Values obtained represented mean measurements taken from both hemispheres in a total of four sections per animal. For DAT analysis, the images were first divided into caudate–putamen (CP) and whole nucleus accumbens septi samples. The CP was then subdivided into four subregions: dorsomedial (dmCP), dorsolateral (dlCP), ventromedial (vmCP), and ventrolateral (vlCP) parts, which were separately quantified for binding. For SERT analysis, samples encompassing all cortical layers were taken in infralimbic (IL), prelimbic (PrL), cingulate (Cing), motor (Mtr), somatosensory (SS), insular (Ins), and orbitofrontal (OFC) cortical regions.
2. 10. Data Analyses
An alpha level of less than or equal to 0.05 was required to denote significance. Reversal learning performance data were analyzed using SPSS Statistics software. Overall learning patterns were analyzed according to: (1) number of trials required to overcome initial perseverative responding and reach 50% performance accuracy within each session, and (2) number of trials required to reach criterion after the animals overcome the “at chance” level (50% accuracy) performance. Feedback sensitivity was analyzed according to: (1) the probability of an animal responding correctly following positive feedback (preceding trial rewarded; (Correct + 1)/Correct Total) (2) a “difference score” measuring the difference in the percentage of correct trials that resulted from the use of positive (the correct response on the preceding trial was repeated) and negative (the error response was corrected) feedback [(Correct + 1)/Correct Total − (Error +1)/Correct Total)], (3) number of incorrect responses (errors) the animals made at the beginning of the session before switching to a correct response, and (4) probability of switching to a correct response following negative feedback (the incorrect stimulus was chosen on a previous trial; (Error + 1)/Error Total). “+1” in all the formulas was used to denote the correct trial immediately following feedback [26–29].
All the feedback learning parameters were calculated within-session. To account for differences in learning rates and better access the feedback sensitivity the sessions were organized based on animals’ performance accuracy, defined as percent Correct within session (Correct/Total Trials), instead of the order in which they occurred. If the rat’s performance accuracy was not different on several sessions, the last value was carried forward for parameter analyses.
First, omnibus repeated-measure ANOVAs (rmANOVAs) were conducted for all the feedback response measurements. When significant interactions were found, post-hoc simple effects were reported. Additionally, for each level of accuracy, ANOVAs comparing SAL, mAMPHsingle and mAMPHescal groups followed by Fisher’s least significant difference (LSD) post hoc tests were performed to assess whether different treatment regimens produced distinct effects on learning patterns. Pearson product-moment correlation coefficients were generated for regional DAT and SERT levels and post-treatment behavioral measures.
3. Results
We performed fine-grained, trial-by-trial analyses analogous to previous reports on reversal leaning data [28,29] to examine the effect of positive (rewarded choices) and negative (unrewarded choices) feedback on subsequent choices in mAMPH-treated animals. Analyses were conducted on all trials from only the reversal learning phase of the experiment, following the drug treatment and wash out period. The overall learning data are shown in Table 1. Treatment groups were not different in the number of testing sessions [F(2,19)=1.325, p=0.29] or trials [F(2,19)= 2.08, p=0.15] necessary to advance to criterion after overcoming the “at chance” level of accuracy. Despite no between-group difference in the number of trials that the animals required to suppress the previously reinforced response and advance to the “at chance” level of performance [F(2,19) =0.07, p= 0.93], groups were significantly different in the number of sessions required to reach 50% correct responses in each session [F(2,19) = 3.576, p italic> 0.05], with both mAMPHsingle and mAMPHescal requiring significantly more sessions compared to SAL treated animals.
Table 1.
Performance during the reversal learning by treatment group
| Treatment Group | Number of Sessions | Number of Trials | ||
|---|---|---|---|---|
| To 50% correct | 50% correct to Criterion | To 50% correct | 50% correct to Criterion | |
|
| ||||
| SAL | 7.83±0.99 | 5.17±1.7 | 357.17±54.75 | 318.06±129.85 |
| mAMPHsingle | 17.17±3.1* | 3.67±2.25 | 384.67±56.57 | 345.83±77.75 |
| mAMPHescal | 16.1±2.5* | 2.77±1.85 | 391.89±72.26 | 383.33±113.24 |
Number of sessions and trials ± SEM for SAL, mAMPHsingle and mAMPHescal treatment groups.
p<0.05
3.1. mAMPH-pretreated animals show greater sensitivity to positive feedback
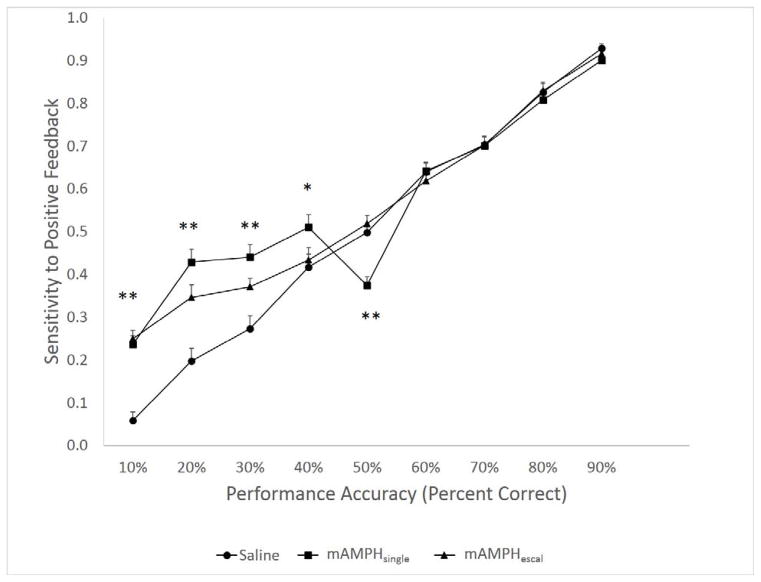
A rmANOVA was conducted to test for differences in feedback sensitivity by treatment group (mAMPHescal vs. mAMPHsingle vs. SAL) across the levels of performance accuracy (percent correct within a single session) at the reversal learning phase of the experiment. A significant main effect of treatment group was found [F(2,18)= 7.958, pbold>0.01], as well as a significant treatment group x performance accuracy interaction [F(16, 144)= 7.92, p<0.01], and a significant within-subject effect of performance accuracy [F(8,144)=360.94, p<0.01]. One-way ANOVAs followed by Fisher’s least significant difference (LSD) post hoc tests conducted separately for each level of performance accuracy further revealed significant differences between mAMPHescal, mAMPHsingle and SAL groups at all performance levels until animals reached 50% correct choices in a session. Both mAMPHescal and mAMPHsingle animals showed greater sensitivity to positive feedback compared to SAL animals at 10%, 20% and 30% performance accuracy levels (p<0.01). Whereas, at the 40% and 50% accuracy levels, only the mAMPHsingle animals were significantly different from mAMPHescal and SAL groups. Between-group differences in sensitivity to positive feedback disappeared after the animals bypassed the “at chance” level of performance (Figure 1).
Figure 1. Sensitivity to positive feedback.
as a probability of an animal responding correctly following positive feedback (preceding trial was rewarded). Significant differences between mAMPHescal, mAMPHsingle and SAL groups were observed at all performance levels until animals reached 50% correct choices in one session. Both mAMPHescal and mAMPHsingle-treated animals showed greater sensitivity to positive feedback compared to SAL animals at 10%, 20% and 30% performance accuracy levels. However, at the 40% and 50% correct the mAMPHsingle-treated animals were significantly different from mAMPHescal and SAL groups. Between-group differences in sensitivity to positive feedback disappeared after the animals bypassed the “at chance” level of performance. Group means + SEM, *p<0.05, **p<0.01.
3.2.1. mAMPH-treated animals rely more on positive feedback to improve performance on the reversal learning task than SAL animals
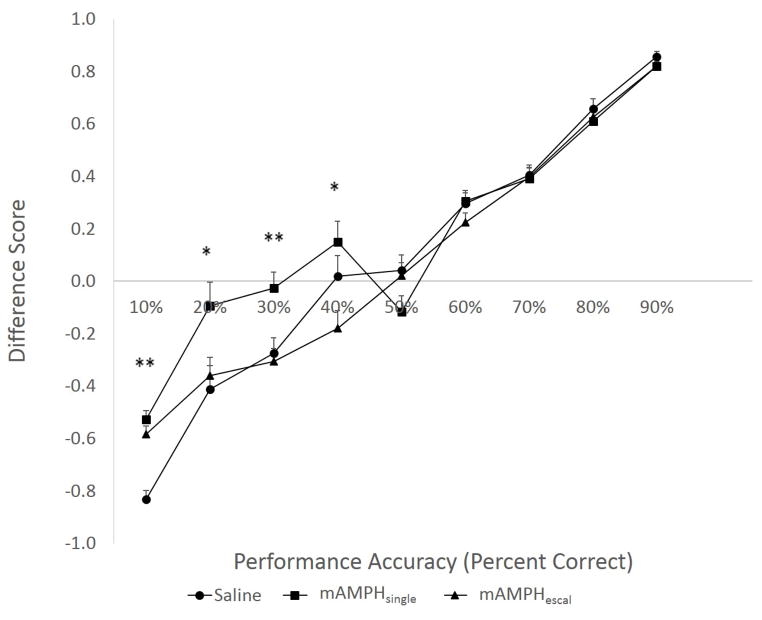
A similar trend was observed when the animals’ performance was analyzed for the difference in the percentage of correct trials that were immediately preceded by positive or negative feedback. Most animals used more negative than positive feedback early in learning, as evidenced by the negative difference scores before the 50% performance accuracy level in the reversal phase of the experiment was reached. Subsequent performance improvement was associated with a greater positive feedback use. Although a similar learning trend was observed for all groups, a rmANOVA revealed significant between-group differences in the relative impact of positive and negative feedback on learning at the beginning of learning [within-subject effect of performance accuracy: F(8, 144)=256.95, p<0.01, main effect of treatment group: F(2, 18)=16.64, p<0.01, interaction F(16, 144)=5.31, p=0.01]. A one-way ANOVA further showed that both mAMPH-treated groups failed to adopt the same strategy as SAL-treated animals. mAMPH-treated animals relied more on positive feedback to improve performance compared to SAL group at the 10% performance accuracy level (p<0.01) as evidence by the difference scores being closer to 0 in mAMPH-treated groups compared to SAL. The mAMPHsingle group was significantly different from both mAMPHescal and SAL groups at the 20% (p<0.05) and 30% (p<0.01) performance accuracy levels, and from mAMPHescal group only at the 40% (p<0.05) accuracy level. Between-group differences disappeared as animals’ performance on the reversal learning task improved (Figure 2).
Figure 2. Relative impact of positive and negative feedback on learning.
shown as a “difference score” in percentage of correct trials that were immediately preceded by positive (+) or negative (−) feedback (shown as deviation from zero). Most rats used more negative than positive feedback before the 50% performance accuracy level, whereas performance improvement above chance was associated with greater positive feedback use. Both mAMPH-treated groups relied more on positive than negative feedback to improve performance compared to SAL in early learning, and specifically at the 10% performance accuracy level. The mAMPHsingle group used significantly more positive feedback compared to both mAMPHescal and SAL groups at the 20% and 30% performance accuracy levels, and differed only from the mAMPHescal group at the 40% accuracy level. Between-group differences disappeared as animals’ performance on the task improved. Group means + SEM, *p<0.05, **p<0.01.
3.2.2. Difference scores are correlated with striatal DAT binding
Significant inverse correlations were found between difference score and DAT binding in CP [r(21)= − 0.452 p<0.05], dlCP [r(21)= − 0.452 p<0.05], and vlCP [r(21)= − 0.486 p<0.05], but not vmCP, dmCP or NAc (Table 2). No significant relationships were found between “difference scores” and striatal DAT binding when mAMPH and SAL groups were analyzed separately.
Table 2.
Correlation matrix of striatal DAT and performance
| CP subregion DAT | Nac DAT | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| CP | dmCP | dlCP | vlCP | vmCP | ||
| Sessions to 50% accuracy | ns | ns | ns | ns | ns | ns |
| Trials to 50% accuracy | ns | ns | ns | ns | ns | ns |
| Positive feedback sensitivity | ns | ns | ns | ns | ns | ns |
| Negative feedback sensitivity | ns | ns | ns | ns | ns | ns |
| Difference score | − .452 * | ns | − .452 * | − .486 * | ns | ns |
| Errors to first correct | ns | ns | ns | − .508 * | ns | ns |
Scores are reported as r values showing levels of DAT binding in regions of the CP and NAc and their association with post-treatment performance measures. DAT dopamine transporter, CP caudate putamen, dmCP dorsomedial caudate putamen, dlCP dorsolateral caudate putamen, vlCP ventrolateral caudate putamen, vmCP ventromedial caudate putamen, NAc nucleus accumbens.
p<0.05,
p<0.01.
3.3.1 mAMPH-treated animals commit more errors before switching response in early reversal learning
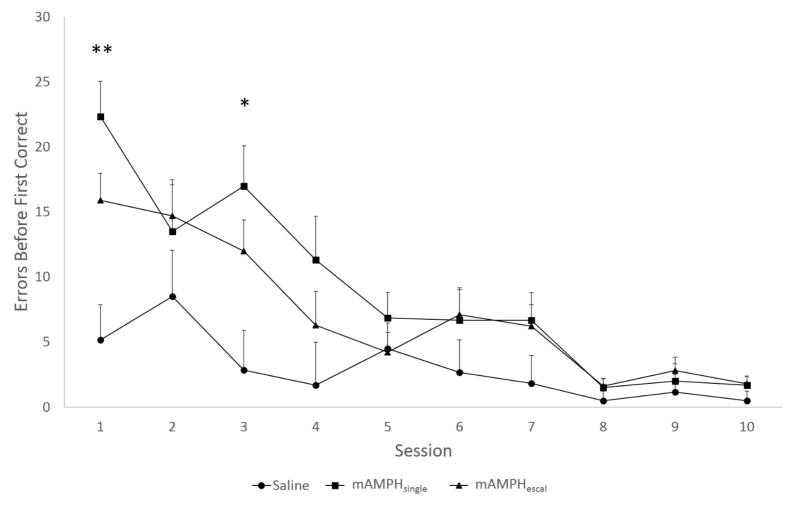
A rmANOVA was conducted to test for differences in the number of errors before the first correct response between treatment groups (mAMPHescal vs. mAMPHsingle vs. SAL), analyzed across the first ten sessions of reversal learning. A significant main effect of treatment group was found [F(2,19)= 4.47, p<0.05], as well as a significant treatment group x session interaction [F(18, 171)= 2.33, p<0.01]. A significant within-subject effect of session was also found [F(9,171)=18.62, p<0.01], indicating that all animals improved their performance with experience on the task. One-way ANOVAs followed by Fisher’s least significant difference (LSD) post hoc tests conducted separately for session further showed significant differences between groups at the beginning of reversal learning. Both mAMPHescal and mAMPHsingle groups committed more errors before making the first correct response compared to SAL animals on session 1 (p<0.01) and session 3 (p<0.05) (Figure 3).
Figure 3. Number of errors before the first correct response.
All animals made fewer errors at the beginning of each session as learning progressed. Significant group differences in the number of errors before switching response were found at the beginning of the reversal learning phase. Both mAMPHescal and mAMPHsingle groups committed more errors before making their first correct response compared to SAL animals on sessions 1 and 3. Group means + SEM, *p<0.05, **p<0.01.
3.3.2. Number of errors committed before switching to a correct response is correlated with frontoparietal SERT and striatal DAT binding
We examined the relationship between frontoparietal SERT and striatal DAT binding and post-treatment performance measures. The total number of errors before the first correct response was inversely correlated with SERT binding in Cg [r(21)= −0.584, p<0.01], Ins [r(21)= − 0.477, p<0.05] and OFC [r(21)= − 0.571 p<0.01] cortices. No significant correlations were found between SERT levels in the remaining cortical regions examined with this or other performance measures (Table 3). When the relationship between the total number of errors before the first correct response was analyzed in SAL-, mAMPHsingle- and mAMPHescal-treated groups separately no significant correlations were observed. However, when mAMPHsingle and mAMPHescal groups were collapsed for within-group correlation analyses an inverse relationship was observed between the performance measure and SERT binding in Cg [r(16)=−0.629, p<0.01] and OFC [r(16)=−0.561, p<0.05] in mAMPH-treated animals. A trend toward significance was found between the number of errors before the first correct and SERT in Cg [r(5)=−0.86, p=0.06] in SAL animals.
Table 3.
Correlation matrix of cortical SERT and performance
| Brain region SERT | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| IL | PrL | Cing | Mtr | SS | Ins | OFC | |
| Sessions to 50% accuracy | ns | ns | ns | ns | ns | ns | ns |
| Trials to 50% accuracy | ns | ns | ns | ns | ns | ns | ns |
| Positive feedback sensitivity | ns | ns | ns | ns | ns | ns | ns |
| Negative feedback sensitivity | ns | ns | ns | ns | ns | ns | ns |
| Difference score | ns | ns | ns | ns | ns | ns | ns |
| Errors to first correct | ns | ns | − .584 ** | ns | ns | − .477 * | − .571 ** |
Scores are reported as r values showing levels of SERT binding within regions of frontoparietal cortex and their association with post-treatment performance measures. SERT serotonin transporter, IL infralimbic, PrL prelimbic, Cing cingulate, Mtr motor, SS somatosensory, Ins insular, OFC orbitofrontal cortex.
p<0.05,
p<0.01.
As above, when the relationship between the total number of errors before the first correct response was analyzed for individual treatment groups separately, no significant correlations were observed. However, when mAMPHsingle and mAMPHescal groups were collapsed for within-group correlation analyses, an inverse relationship was found between the number of errors before the first correct response and DAT levels in vlCP [r(21)= − 0.508 p<0.05] (Table 2). The analyses revealed that the correlation was primarily driven by mAMPHsingle group since a significant inverse relationship between DAT binding in vlCP and this behavioral measure was detected in mAMPH-treated [r(16)=−0.513, p<0.05], but not the SAL group.
3.4. mAMPH-treated animals do not differ from SAL in their responses to negative feedback
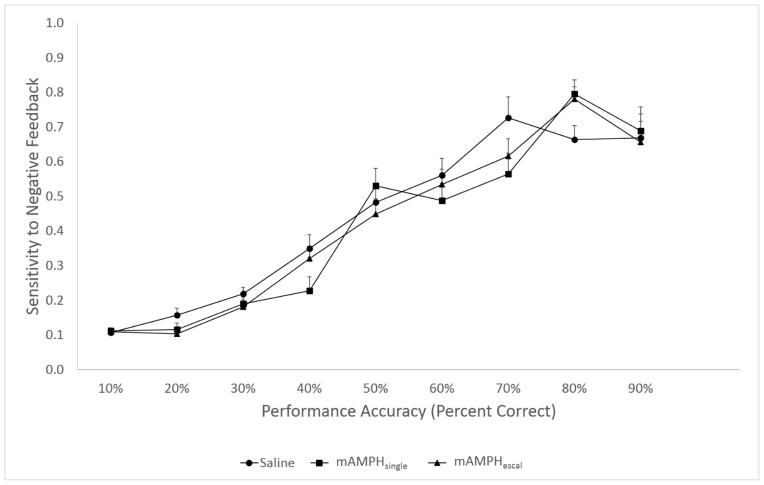
A rmANOVA conducted to test for the differences in negative feedback sensitivity revealed only a within-subject effect of performance accuracy [F(8,144)=106.785, p<0.01] providing evidence that all the animals learned to use negative feedback as their performance on the reversal task improved, but there was no main effect of treatment group [F(2, 18)=0.587, p=0.57] or interaction [F(16,144) =1.33, p=0.19) (Figure 4).
Figure 4. Sensitivity to negative feedback.
as a probability of correct response on the subsequent trial following negative feedback (the incorrect stimulus was chosen on a previous trial). All the animals learned to use the negative feedback as their performance on the reversal task improved; no between-group differences were observed. Group means + SEM.
4. Discussion
Impairments in cognitive flexibility and alterations in learning patterns following mAMPH exposure are well documented [30,31][40–42], yet our knowledge of the performance monitoring and feedback learning mechanisms by which these impairments may manifest remains relatively unexplored. Using a fine-grained trial-by-trial analysis of performance in reversal learning, we provide evidence here that 1) drug exposure leads to long-lasting differences in feedback processing in early learning and 2) important and dissociable facets of this feedback learning correlate with DAT and SERT binding (described below). Importantly, these alterations represent enduring changes as a consequence to even brief drug exposure, and may contribute to poor decision making in the long-term.
4.1 Increased positive feedback sensitivity and altered learning strategies in mAMPH-pretreated animals: Involvement of DAT
All animals progressively learned to direct their responses to rewarded stimuli. Trial-by-trial analysis revealed that both mAMPH-pretreated groups benefited more from positive feedback than SAL animals early in learning. One feature of the discrimination reversal learning tasks employed in the present study is that it allows examination of responses to rewards not associated with psychostimulant drugs, i.e. natural reward. Our results indicate that following mAMPH administration, rats may become more sensitive even to natural rewards, consistent with previous findings that long-term drug abuse changes basal reward valuation [43].
Notably, all groups adopted a similar strategy of feedback use: animals used more negative than positive feedback before the 50% performance accuracy level, whereas subsequent performance improvement was associated with a stronger impact of positive relative to negative feedback. Despite the similar learning trajectory, both mAMPHsingle and mAMPHescal groups relied on positive feedback to improve performance to a greater degree than the SAL group at the beginning of reversal learning, with mAMPHsingle animals showing more pronounced alterations in feedback response. Although other factors contributing to learning cannot be ruled out, these data suggest that the performance improvement in mAMPH-pretreated animals at least in early reversal learning is primarily accounted for by the use of positive feedback.
The “difference scores” were inversely correlated with DAT binding in vlCP and dlCP. Previous evidence from our laboratories has demonstrated that only the mAMPHsingle regimen produces a pronounced decrease in vlCP DAT binding [30]. Taken together with alterations in positive feedback reported here, this implicates DAT as an important moderator of feedback use early in learning, when deviations from expected outcomes are highest.
However, DAT levels were not correlated with our measure of overall positive feedback sensitivity in accord with a recent observation that the DAT1 genotype does not alter trial-by-trial win-stay behavior or the likelihood of repeating the correct response, a measure analogous to positive feedback sensitivity in our study [44].
4.2 mAMPH-pretreated animals are not impaired in learning from negative feedback, but exhibit behavioral rigidity
MAMPH pretreated animals’ demonstrated a different pattern of learning before they reached 50% correct performance, providing evidence that exposure to the drug affected rats’ ability to adapt to changes in reward contingency when proactive interference strength and levels of ambiguity were the highest. This may result in difficulties overcoming interference during reversal learning. MAMPH-treated animals in the present study showed increased number of incorrect responses before the first correct response in early reversal learning. Due to the nature of the task, with only two mutually exclusive options, it is difficult to define the nature of that impairment. Ongoing projects in our laboratory employing 3-choice tasks with a neutral stimulus are aimed at addressing this question. One plausible explanation is general insensitivity to negative feedback in the form of omission of the expected reward. This is unlikely since the mAMPH-treated animals were not different from controls in the probability of switching to a correct response following negative feedback. More plausible explanations are that animals are unable to integrate newly learned associations into long-term decision-making, and/or greater perseveration resulting from disruptions in normal reward valuation. These explanations, however, require direct testing.
The number of errors before the first correct response was inversely correlated with DAT binding in vlCP, a subregion that we have shown previously to exhibit decreased binding following single-dose mAMPH treatment [30]. Thus, lesser DAT binding in this region was associated with a greater number of “perseverative” type errors in early learning. These findings add to strong support for adaptations in CP predicting habit learning and behavioral rigidity. The negative correlation between DAT binding and the degree of behavioral flexibility also accords well with the results of a recent study on human DAT1 allelic variation on choice behavior [44]. Specifically, there is a greater influence of choice history on perseveration (e.g. a larger weight of past experience at the beginning of reversal) with an increasing number of 9R alleles of DAT1, which in turn is associated with decreased expression.
It is noteworthy that the significant relationship between DAT and the number of errors before the first correct response occurred only when both SAL and mAMPH groups or when combined mAMPH groups were included in the analysis, and was not present when the SAL group was analyzed independently. Thus, mAMPH’s damaging effects in the treatment group likely drove this relationship. Though we provide no in vivo measures to support a mechanism here, this may result from partial damage to DA terminals by mAMPH in the animals with reduced binding (mAMPHsingle, as reported in [30]). Thus, DA tone may be associated with increased reward sensitivity and decreased behavioral flexibility. MAMPH pretreatment, and not endogenous variations in DAT in all animals, explained the relationship between striatal DAT and errors in early learning.
Our data raises the intriguing question as to why, if mAMPH-pretreated rats show enhanced sensitivity to positive feedback and intact responses to negative feedback, they are not performing at a comparable level to SAL-treated animals on reversal learning. Other factors may contribute to their performance and decrease the learning rate. For example, we show here that they commit more errors at the beginning of a session. It appears mAMPH-treated rats learn as efficiently as controls within a session, but have difficulty with maintaining or integrating this learning across sessions. Whether this represents behavioral rigidity or aberrant reward valuation processes is unknown, and either could be an explanation for mAMPH rats displaying different learning trajectories.
4.3 SERT and learning from negative feedback
Impairments in cognitive flexibility associated with psychostimulant exposure have been traditionally attributed to changes in DA function. The results of the present study also implicate serotonin (5HT) in feedback sensitivity and learning from punishment (an incorrect response was always accompanied by reward omission, a “lights-out” and a “time-out”). This role for 5HT aligns well with growing evidence and a recent postulation that, unlike DA involvement in behavioral activation and reinforcement, 5HT is critical for aversive processing and behavioral inhibition, complementary cognitive processes [45].
In support of this, in the present study, we observed the number of errors before the first correct response to be inversely correlated with SERT binding in Cg, Ins and OFC, but not associated with SS, Mtr, IL or PrL SERT. We have previously reported that mAMPH pretreatment does not result in decreases binding of frontocortical SERT [30], yet the present results suggest that behavioral rigidity varies with individual differences in SERT binding. Unlike the relationship between striatal DAT and errors before the first correct response, the relationship between SERT and consecutive errors may be driven by individual variation in SERT binding since the association was observed even when groups were assessed separately. The most robust inverse correlation was between Cg SERT binding and consecutive errors. This finding adds to accumulated evidence for this subregion in performance monitoring and strategy shifting.
Our results also concur with those of a recent study demonstrating a negative correlation between SERT binding in OFC and degree of perseveration, defined in that study as the number of extra lever presses in both SAL and mAMPH-treated animals [46]. Reduced 5HT signaling has also been associated with an increased number of perseverative errors on a reversal learning task [47], and 5HT receptor antagonists affect the number of errors during reversal before criterion is reached [48,49]. In sum, the literature implicates 5HT in overcoming perseveration and in learning new reward rules [33], and the present data support that view.
5. Conclusion
In addition to validating the enduring effects of mAMPH on learning, the present findings demonstrate long-lasting differences in feedback processing in early learning as a consequence of even brief drug exposure. Both mAMPH groups showed increased sensitivity to and reliance on positive feedback, with mAMPHsingle animals showing more pronounced alterations in feedback learning. We provide evidence that negative feedback sensitivity is not altered following mAMPH exposure. Animals’ ability to integrate newly learned associations into long-term decision-making is altered after drug exposure, leading to behavioral rigidity. Importantly, different facets of this feedback learning are correlated with striatal DAT and frontocortical SERT binding, providing further support for the roles of 5HT and DA systems in overcoming perseveration and in learning new reward rules.
Highlights.
Long-term positive and negative feedback learning was studied after methamphetamine
Drug pretreatment resulted in increased use of positive feedback in learning
Despite intact negative feedback learning, pretreated rats made more early errors
Striatal DAT binding was inversely correlated with positive feedback learning
Frontocortical SERT binding was inversely correlated with measures of perseveration
Acknowledgments
Funding and Disclosure
This work was supported by the NIH Minority Biomedical Research Support program at California State University, Los Angeles (CSULA). Partial support also came from NIMH (Grant SC2 MH087974 to AI) and NIDA (Grants 1RO1DA012204 and 1R21 DA033572 to JFM).
We acknowledge Alisa Kosheleff and Danilo Rodriguez for behavioral testing and drug treatment and the Jentsch lab for valuable feedback on an early version of the manuscript.
Footnotes
There is nothing to disclose nor are there any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eppinger B, Hammerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Ann N Y Acad Sci. 201(1235):1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietschmann M, Endrass T, Czerwon B, Kathmann N. Aging, probabilistic learning and performance monitoring. Biol Psychol. 2011;86:74–82. doi: 10.1016/j.biopsycho.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, Williams WC, et al. Disrupted expected value and prediction error signaling in Youths with disruptive behavior disorders during a passive avoidance task. American Journal of Psychiatry. 2013;170(3):315–23. doi: 10.1176/appi.ajp.2012.12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luft CD, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. J Neurosci. 2013;33(5):2029–38. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 6.Rutledge RB, Lazzaro SC, Lau B, Myers CE, Gluck MA, Glimcher PW. Dopaminergic drugs modulate learning rates and perseveration in Parkinson’s patients in a dynamic foraging task. J Neurosci. 2009;29(48):15104–14. doi: 10.1523/JNEUROSCI.3524-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. Plos ONE. 2010;5(1):1–9. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. Journal of Pharmacology and Experimental Therapeutics. 2009;331:555–62. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TE, Yew J, Paulson PE, Camp DM. The longterm effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neuroscience Letters. 1990;110:193–8. doi: 10.1016/0304-3940(90)90810-v. [DOI] [PubMed] [Google Scholar]
- 13.Gibb JW, Johnson M, Hanson GR. Neurochemical basis of neurotoxicity. Neurotoxicology. 1990;11:317–21. [PubMed] [Google Scholar]
- 14.Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug And Alcohol Dependence. 1976;1(3):215–9. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- 15.Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. European Journal of Pharmacology. 1976;36:363–71. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sciences. 1979;25:1373–8. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- 17.Krasnova I, Cadet J. Methamphetamine toxicity and messengers of death. Brain Research Reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama M, Koyama T, Yamashita I. Long-lasting decrease in dopamine uptake sites following repeated administration of methamphetamine in the rat striatum. Brain Research. 1993;601:209–12. doi: 10.1016/0006-8993(93)91712-2. [DOI] [PubMed] [Google Scholar]
- 21.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181:151–60. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American Journal of Psychiatry. 2001;158(12):2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 26.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 27.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–7. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 28.Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28(33):8338–43. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, et al. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J Neurosci. 2013;33(9):4105–9. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and escalating methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012;224(3):459–67. doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–14. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izquierdo A, Belcher AM. Rodent models of adaptive decision making. In: Kobeissy FH, editor. Psychiatric Disorders: Methods and Protocols. New York: Humana Press; 2012. pp. 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izquierdo A, Carlos K, Ostrander S, Rodriguez D, McCall-Craddolph, Yagnik G, et al. Impaired reward learning and intact motivation after serotonin depletion in rats. Behav Brain Res. 2012;233(2):494–9. doi: 10.1016/j.bbr.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res. 2008;189:170–9. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–32. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- 36.Madden LJ, Flynn CT, Zandonatti MA, May M, Parsons LH, Katner SN, et al. Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30:350–9. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- 37.Segal DS, Kuczenski R. Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther. 1997;282(2):561–73. [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 39.Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse (N Y) 2010;12:27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- 40.White IM, Minamoto T, Odell JR, Mayhorn J, White W. Brief exposure to methamphetamine (METH) and phencyclidine (PCP) during late development leads to long-term learning deficits in rats. Brain Res. 2009;1266:72–86. doi: 10.1016/j.brainres.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007;1186:255–66. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dezfouli A, Piray P, Keramati MM, Ekhtiari H, Lucas C, Mokri A. A neurocomputational model for cocaine addiction. Neural Computation. 2009;21:2869–93. doi: 10.1162/neco.2009.10-08-882. [DOI] [PubMed] [Google Scholar]
- 44.den Ouden HEM, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, et al. Dissociable Effects of Dopamine and Serotonin on Reversal Learning. Neuron. 2013;80(4):1090–100. doi: 10.1016/j.neuron.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son JH, Kuhn J, Keefe KA. Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology. 2013;67:95–103. doi: 10.1016/j.neuropharm.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–82. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–19. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 49.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–8. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]