Abstract
Purpose
Melanoma, the most aggressive form of skin cancer, accounts for 75% of all skin cancer-related deaths and current therapeutic strategies are not effective in advanced disease. In the current study, we have investigated the efficacy of orally active small-molecule antagonist targeting CXCR2/CXCR1.
Experimental Design
Human A375SM melanoma cells were treated with SCH-479833 or SCH-527123, and their effect on proliferation, motility, and invasion was evaluated in vitro. We examined the downstream signaling events in the cells following treatment with antagonists. For in vivo studies, A375SM cells were implanted subcutaneously into athymic nude mice followed by administration of SCH-479833, SCH-527123, or hydroxypropyl-β-cyclodextrin (20%) orally for 21days and their effect on tumor growth and angiogenesis was evaluated.
Results
Our data show that SCH-479833 or SCH-527123 inhibited the melanoma cell proliferation, chemotaxis, and invasive potential in vitro.Treatment of melanoma cells with SCH-479833 or SCH-527123 also inhibited tumor growth. Histologic and histochemical analyses showed significant (P < 0.05) decreases in tumor cell proliferation and microvessel density in tumors. Moreover, we observed a significant increase in melanoma cell apoptosis in SCH-479833- or SCH-527123-treated animals compared with controls.
Conclusion
Together, these studies show that selectively targeting CXCR2/CXCR1 with orally active small-molecule inhibitors is a promising therapeutic approach for inhibiting melanoma growth and angiogenesis.
Human cutaneous malignant melanoma is the most aggressive form of skin cancer with a very poor prognosis. During 2008, it is estimated that in the United States 62,480 new cases of melanoma will be diagnosed and 8,420 people will die due to this devastating disease (1). Therapy for early disease is primarily surgery with a minor benefit noted with adjuvant therapy; however, there is no effective treatment for advanced disease (2–7). This clearly indicates the need for novel and effective therapeutic measures and a better understanding of the basic molecular mechanisms implicated in disease advancement.
The G-protein-coupled receptors CXCR1 and CXCR2 are important therapeutic targets in malignant melanoma (8). Both CXCR1 and CXCR2 bind to the chemokine, CXCL-8, with high affinity (9–11). Previous studies have shown that both CXCR1 and CXCR2 are expressed by several types of normal cells, such as neutrophils and endothelial cells and various tumor cells (9, 12–14). More importantly, we and others have shown that CXCL-8 is constitutively expressed in malignant melanoma and functions as an autocrine/paracrine growth, invasive, and angiogenic factor (15–18). These multiple functional implications of the CXCL-8-CXCR1/ CXCR2 axis in melanoma pathogenesis underscore its importance as a target for cancer therapy.
Earlier studies from our laboratory have shown that neutralizing antibodies to CXCR1 and CXCR2 inhibit melanoma cell proliferation and invasive potential (18). Small-molecule inhibitors with affinity for CXCR1 such as repertaxin or affinity for CXCR2 such as SB-225002 or SB-332235 have been used against inflammatory diseases (19–21). However, the effectiveness of CXCR1 and/or CXCR2 antagonists in tumor growth and angiogenesis remains unclear. In the present study, we have evaluated the potential of the CXCR2/CXCR1-specific inhibitors, SCH-479833 and SCH-527123, by in vitro and in vivo experiments. Our data show that small-molecule antagonists for CXCR2/CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, invasive potential, and angiogenesis.
Materials and Methods
Cells lines and CXCR2/CXCR1 antagonists
A375SM, a highly metastatic human melanoma cell line, was maintained in culture in DMEM (Mediatech) supplemented with 5% fetal bovine serum (Mediatech), 1% L-glutamine (Mediatech), 1% vitamin solution (Mediatech), and gentamycin (Invitrogen). Cultures were maintained for ≤4 weeks after recovery from frozen stocks. SCH-479833 and SCH-527123 (ref. 22; see structure in Table 1) were synthesized at Schering Plough and formulated in hydroxypropyl-β-cyclodextrin (HPβCD; Acros Organics). The inhibition constant (Ki) of CXCR1 and CXCR2 for SCH-479833 and SCH-527123 was calculated from IC50 value using the Cheng-Prusoff equation (ref. 23; Table 1).
Table 1.
Structure and inhibition constants (Ki) for CXCR1 and CXCR2
|
| |||
|---|---|---|---|
| KiCXCR2 (nmol/L) | KiCXCR1 (nmol/L) | Ratio | |
| SCH-527123 | 0.05 | 3.7 | 74 |
| SCH-479833 | 0.17 | 7 | 100 |
In vitro cell proliferation
A375SM cells were seeded in 96-well plates at low density (1,000 per well). Following overnight adherence, cells were incubated with medium alone or medium containing serum (1.25%) with SCH-479833, SCH-527123, or HPβCD for 72 h. Four different concentrations (1.0, 0.1, 0.01, and 0.001 μg/mL) of both antagonists were used. Cell proliferation was determined by MTT assay (17, 24). Growth inhibition was calculated as % = [100 - (A / B) × 100], where A and B are the absorbance of treated and untreated cells, respectively.
Cell motility and invasion assay
To investigate the effect of SCH-479833 or SCH-527123 on melanoma cell migration, cells (1 × 106 per well) in serum-free medium were plated in the top chamber of noncoated polyethylene terephthalate membranes (6-well insert, 8 Am pore size; Becton Dickinson), whereas for the invasion assay cells (1,000 per well) were plated onto Matrigel-coated Transwell chambers (24-well insert; 8 Am pore size; Corning Costar) with medium (serum-free). The bottom chamber contained 1.0 mL serum-free medium with or without CXCL-8 (10 ng/mL) and SCH-479833, SCH-527123 (10 ng/mL), or HPβCD was added to the lower chamber. The cells were incubated for overnight at 37°C, and unmigrated cells were removed. Cells that passed through the membrane pores were stained using Hema 3 kit (Fisher Scientific) as per the manufacturer's instructions. Cells were counted in 10 random fields (×200) and expressed as the average number of cells per field of view. Data are represented as the average of three independent experiments.
Western blot analysis
Cells were lysed in Triton X-100 buffer [1% Triton X-100, 50 mmol/L TBS (pH 7.4), 10 mmol/L EDTA with protease inhibitors (Roche Diagnostics) and phosphatase inhibitors (5 mmol/L NaF and 5 mmol/L Na3VO4; Sigma)]. For protein expression analysis, 50 μg lysate was resolved by SDS-PAGE (8-12%). The primary antibodies were against phospho-extracellular signal-regulated kinase (ERK) 1/2 and ERK1/2 (1:1,000; rabbit polyclonal; Cell Signaling Technology) and GAPDH (1:1,000; rabbit monoclonal; Cell Signaling Technology). Secondary antibodies (Santa Cruz Biotechnology) were horseradish peroxidase-conjugated and used at 1:2,000 dilutions.
Tumor growth assay
Female athymic nude mice (6-8 weeks old) were purchased from the National Cancer Institute. All procedures done in mice were in accordance with institutional guidelines and approved by the University of Nebraska Medical Center Institution Animal Care and Use Committee. For in vivo injection, cells were harvested using a brief exposure of 0.25% trypsin in 0.02% EDTA. Trypsinization was stopped with medium-containing serum and cells were washed twice with HBSS. Only single-cell suspensions of >90% to 95% viability (tested by trypan blue exclusion assay) were used for injection. Mice were injected with 1 × 106 cells/0.05 mL HBSS/animal into the subcutis of the lateral flank. Twenty-four hours post-tumor cell injection, mice (n = 6 per group) were given either SCH-479833 or SCH-527123 antagonist at a dose of 100 mg/kg body weight daily in 0.2 mL of 20% HPβCD orally for 21 days. Control group mice received 0.2 mL vehicle (20% HPhCD) alone orally. Tumor growth was monitored with calipers twice a week and animals were sacrificed when moribund. Tumor volume was calculated by the following formula: volume = π / 6 × (smaller diameter)2 × (larger diameter). Animals were sacrificed on day 50 and tumor tissues were harvested and processed for immunohistochemical analysis.
Immunohistochemical analysis
Immunohistochemical analysis was done as described previously (25). Briefly, 6 Am thick sections were deparaffinized, and nonspecific binding was blocked by incubation in antibody diluent (BD Pharmingen). The sections were then immuno-stained using mouse monoclonal anti-proliferating cell nuclear antigen (PCNA; 1:40; Santa Cruz Biotechnology) or mouse biotinylated GS-IB4 (isolectin from Griffonia simplicifolia; 1:50; Vector Laboratories). Immunoreactivity was detected using the ABC Elite kit and DAB substrate (Vector Laboratories) as per the manufacturer's instructions. A reddish brown precipitate in the cytoplasm indicated a positive reaction. Negative controls had all reagents included, except the primary antibody. The number of microvessels was quantitated microscopically with a 5 × 5 reticle grid (Klarmann Rulings) using × 400 objective (250 μm total area).
Apoptotic cells in tumor samples were identified by terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay according to the manufacturer's instructions (In situ Cell Death Detection Kit, fluorescein; Roche). The number of apoptotic cells was evaluated by counting the positive (brown-stained) cells in 10 arbitrarily selected fields at ×200 magnification in a double-blinded manner and expressed as average number of cells per field view.
Statistical analysis
All the values are expressed as mean ± SE. Differences between the groups were compared using unpaired two-tailed t test. In vivo analysis was done using Mann-Whitney U test of significance. P < 0.05 was deemed significant. All statistical analyses were done using SPSS software (SPSS).
Results
Orally active CXCR1 and CXCR2 antagonists inhibited human melanoma growth
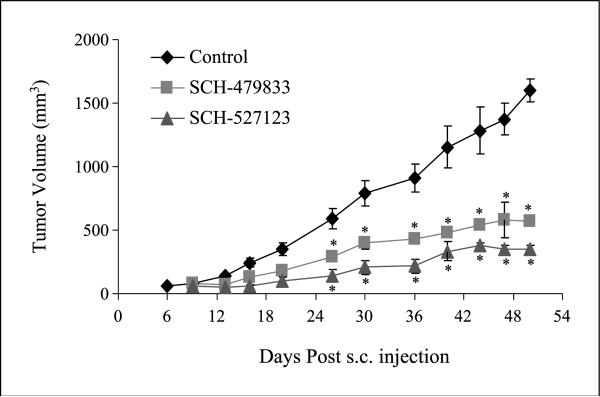
To examine the effect of antagonists on tumor growth, we injected human melanoma A375SM cells subcutaneously into athymic nude mice, and after 24 h, 100 mg/kg SCH-479833 or SCH-527123 antagonist in HPβCD CD or HPβCD alone (control) was given orally once a day for 21 days. A remarkable decrease in tumor size was observed in both SCH479833- and SCH527123-treated animals compared with control group (Fig. 1). Administration of SCH-479833 and SCH-527123 antagonists resulted in a significant (P < 0.05; 2.9- to 4.5-fold) reduction in tumor volume compared with HPβCD-treated animals. In the control group, tumors started appearing within 6 days post-injection compared with 9 days post-injection in SCH-479833- and SCH-527123-treated groups.
Fig. 1.
SCH-479833 and SCH-527123 inhibit melanoma growth. Eight-week-old mice were injected with highly metastatic human melanoma A375SM cells subcutaneously.Twenty-four hours after injection, mice were orally fed with SCH-479833 or SCH-527123 antagonist (100 mg/kg in 0.2 mL of 20% HPβCD) once a day for 21d. Control mice were fed with 0.2 mL vehicle (20% HPβCD) alone. Growth curve of A375SM cells in athymic nude mice treated with vehicle, SCH-479833, and SCH-527123. Average ± SE tumor volume.
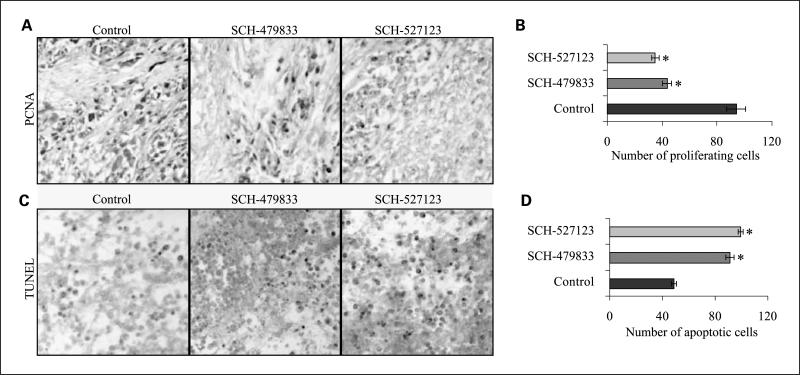
Next, we examined whether inhibition in tumor growth was due to reduced melanoma cell proliferation and/or survival by PCNA and TUNEL immunostaining, respectively. PCNA is a cell cycle-associated protein and an important biomarker used frequently to measure the proliferative activity of tumor tissues (26), whereas TUNEL is a common method for detecting DNA fragmentation that results from apoptotic signaling cascades (27). PCNA immunostaining of tumor sections showed a significant decrease in PCNA-positive cells in SCH-479833- and SCH-527123-treated groups (Fig. 2A). The average numbers of PCNA-positive cells were 43.5 ± 3.22 and 34.9 ± 2.74 in SCH-479833- and SCH-527123-treated tumors compared with 94.0 ± 6.97 in control-treated tumors (Fig. 2B). TUNEL staining of tumor sections showed a significant increase in apoptotic cells in SCH-479833- and SCH-527123-treated groups (Fig. 2C). The average numbers of TUNEL-positive cells were 91.3 ± 2.93 and 99.3 ± 2.08 in SCH-479833- and SCH-527123-treated tumors compared with 48.5 ± 1.97 in control tumors (Fig. 2D).
Fig. 2.
SCH-479833 and SCH-527123 inhibit proliferation and survival in human melanoma tumors. Immunohistochemical staining for PCNA (A) andTUNEL (C) were analyzed based on DAB staining as described in Materials and Methods. Control (left), SCH-479833 (middle), and SCH-527123 (right). Pictures at ± 200. Proliferating (B) and apoptotic (D) cells were counted in 10 arbitrarily selected fields at ×200 magnification in a double-blinded manner and expressed as average ± SE number of cells per field view. *, P < 0.05, significantly different from control HPβCD-treated animals.
Tumor angiogenesis was decreased in SCH-479833 or SCH-527123 antagonist-treated animals
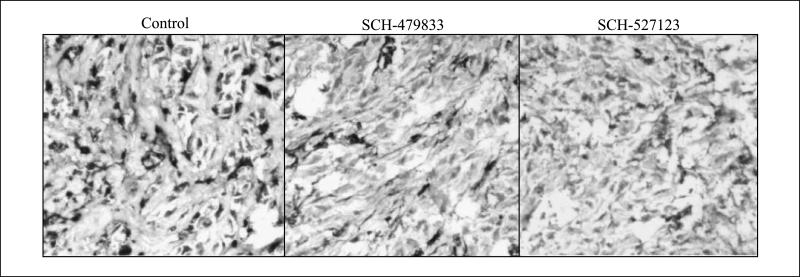
Most solid tumors, including melanoma, require new blood vessel formation to grow beyond a few millimeters in diameter (28). In previous studies, it has been reported that CXCL-8, a ligand for CXCR1 and CXCR2, acts as an angiogenic factor (16–18). Therefore, we examined whether inhibition of human melanoma growth by SCH-479833 or SCH-527123 antagonist involved an effect on angiogenesis. The microscopic examination of tumor tissue for neovascularization showed a significantly lower number of microvessels in SCH-479833 or SCH-527123 antagonist-treated groups compared with the control group (Fig. 3; Table 2). On average, treatment with SCH-479833 and SCH-527123 resulted in a 35% and 45% reduction in the number of blood vessels (Fig. 3; Table 2).
Fig. 3.
SCH-479833 and SCH-527123 inhibit microvessel density in human melanoma tumors. Immunohistochemical staining for microvessel using anti-GS-IB4 was analyzed as described in Materials and Methods. Control (left), SCH-479833 (middle), and SCH-527123 (right). Representative pictures at ×200.
Table 2.
SCH-479833 and SCH-527123 inhibit microvessel density in human melanoma tumors
| Treatments | MVD (mean ± SE) | % Inhibition | t test |
|---|---|---|---|
| HPβCD | 8.00 ± 0.33 | ||
| SCH-479833 | 5.22 ± 0.33 | 35 | P < 0.05 |
| SCH-527123 | 4.44 ± 0.22 | 45 | P < 0.05 |
NOTE: Immunohistochemical staining for microvessel using anti-GS-IB4 was analyzed as described in Materials and Methods. Microvessel density was quantitated microscopically with a 5 × 5 reticle grid at ×400 magnification.
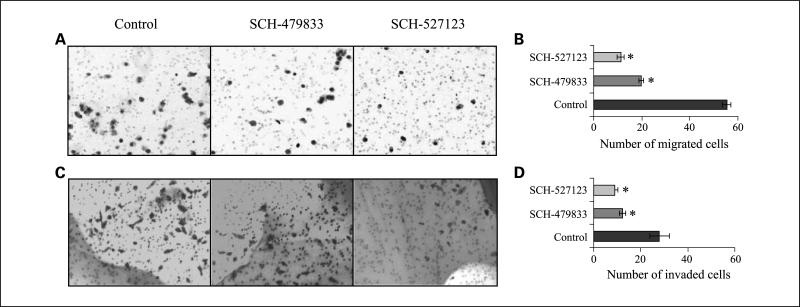
SCH479833 and SCH527123 treatment decreased chemotaxis and invasion of human melanoma cells in vitro
Overall growth of the tumor is determined by the interaction of tumor cells with the host microenvironment. In previous studies, it has been reported that chemokine signaling not only helps in the migration of endothelial cells, and thus angiogenesis, but also in the motility and invasion of the tumor cells (29). Therefore, we investigated whether treatment with CXCR2/CXCR1 antagonists would affect tumor cell chemotaxis and invasion. Our data showed a significant (P < 0.05) inhibition in CXCL-8-induced chemotaxis (2.8- to 5.0-fold decrease) of SCH-479833-or SCH-527123-treated cells compared with the control-treated cells (Fig. 4A and B). Cells treated with SCH-479833 or SCH-527123 also showed a significant inhibition (P < 0.05) in the number of invading cells (2.3- to 3.1-fold decrease) compared with control in an in vitro invasion assay (Fig. 4C and D).
Fig. 4.
SCH-479833 and SCH-527123 inhibit melanoma cell motility and invasion. Cells were plated on noncoated or Matrigel-coated membranes for motility (A and B) and invasion (C and D) assays and incubated for overnight. Serum-free medium containing 10 ng/mL CXCL-8 and SCH-479833 or SCH-527123 (10 ng/mL) or HPβCD was added to the lower chamber.The cells that did not migrate through the Matrigel and/or pores in the membrane were removed, and cells on the other side of the membrane were stained and photographed at ×200 magnification. Cells were counted in 10 random fields (×200) and expressed as the average number of cells per field of view. Number ± SE of migrated cells. Representative of three experiments done in triplicate. *, P < 0.05,×significantly different from control CXCL-8-treated cells.
SCH-479833 and SCH-527123 treatment inhibited melanoma cell proliferation in a dose-dependent manner and repressed the ERK1/2 signaling pathway
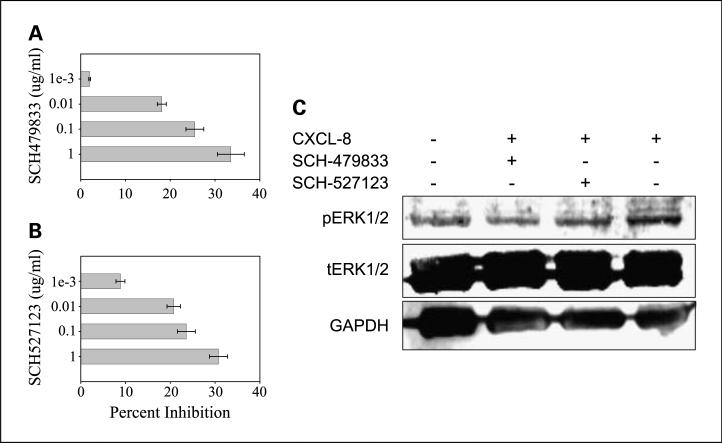
Treatment of human melanoma cells with increasing doses of SCH-479833 and SCH-527123 antagonists (0.001-1.00 μg/mL) resulted in a progressive inhibition of proliferation as examined by MTT in vitro proliferation assays (Fig. 5A and B). At the 1 Ag/mL dose of SCH-479833 and SCH-527123 antagonists, a 33% and 31% inhibition of melanoma cell proliferation was observed.
Fig. 5.
SCH-479833 and SCH-527123 inhibit A375SM cell proliferation and ERK1/2 signaling. A375SM cells (1,000 per well) in a 96-well plate were cultured in medium with or without serum (1.25%) and SCH-479833 (A) or SCH-527123 (B) in four different concentrations. Cellular proliferation was determined at 72 h by MTTassay. Mean ± SE percent inhibition of proliferation. C, A375SM cell were treated with the antagonists for 1h and then stimulated with CXCL-8 for 30 min. Cell lysates (50 μg) were fractionated by SDS-PAGE and subjected to Western blotting using pERK1/2 or ERK1/2 antibody, and GAPDH was used as a loading control.
Next, we examined the effect of antagonist treatment on ERK1/2 phosphorylation, which is an important signaling pathway involved in the control of growth signals, cell survival, and invasion (30). Treatment of human melanoma cells with 1 μg/mL of either SCH-479833 or SCH-527123 significantly decreased the phosphorylation of ERK1/2, suggesting it as a mechanism in the antiproliferative, anti-invasive, and anti-angiogenic activity of SCH-479833 and SCH-527123 (Fig. 5C).
Discussion
In the present study, we showed that oral administration of the CXCR2/CXCR1 antagonists (SCH-479833 or SCH-527123) in athymic nude mice inhibited the growth of implanted human melanoma tumors. Furthermore, our studies showed that the antitumor efficacy of SCH-479833 and SCH-527123 resulted from a decrease in cell proliferation and angiogenesis and enhanced apoptosis. In addition, we also observed reduced chemotaxis and invasion of human A375SM cells on treatment with the antagonists.
The use of small-molecule inhibitors represents an attractive targeted therapeutic approach. Previously, we showed that CXCL-8-elicited melanoma growth was mediated through the chemokine receptors, CXCR1 and CXCR2 (18). Therefore, targeting of these receptors seemed an obvious choice. Our in vivo study, where mice were given either SCH-479833 or SCH-527123 antagonist orally, exhibited a reduction in tumor volume in the treatment groups compared with the control group. This finding clearly indicated the potential of SCH-479833 and SCH-527123 antagonists as novel therapeutics for melanoma and was in accordance with our previous observations on the association of CXCR1 and CXCR2 with melanoma aggressiveness (25).
The overall growth of the tumor in vivo depends on several factors including the rate of tumor cell proliferation, survival, and vascularization of the tumor tissue along with the invasive capacity of the tumor cells (31, 32). While evaluating the effect of SCH-479833 or SCH-527123 antagonists on proliferation and apoptosis, we observed that xenografts derived from treated mice were associated with a decrease in cell proliferation and an increase in apoptosis as detected by PCNA and TUNEL immunostaining. The in vitro studies also supported the above finding, thus showing the antiproliferative effect of the CXCR2/CXCR1 antagonists SCH-479833 and SCH-527123. These results provide further support to our previous work that showed inhibition of the proliferation of melanoma cells on neutralization of CXCR1 and CXCR2 (18).
CXCR1 and CXCR2 have also been implicated in the haptotactic migration/chemotaxis and angiogenic response of melanoma cells (33–35). Nonetheless, a report from Addison et al. (33) described the importance of CXCR2 in mediating angiogenesis. We have observed significant inhibition in tumor neovascularization in CXCR2/CXCR1 antagonist-treated animals compared with control-treated group. Our earlier data show that endothelial cells express CXCR1 and CXCR2 and CXCL-8 enhanced their survival and proliferation (36). In our antagonist-treated group, the decrease in vascular density could be due to low expression of angiogenic factors or apoptosis of endothelial cells. We have observed decrease in CXCL-8-induced endothelial cells survival following treatment with CXCR2/CXCR1 antagonists.3 Furthermore, we have observed an inhibition in the levels of CXCL-8 and vascular endothelial growth factor protein in SCH-527123 and SCH-479833 tumors compared with control.3
Previously, CXCL-8 expression by endothelial cells was shown to elicit a chemotactic response for melanoma cells through CXCR1 (37). Similarly, the chemotaxis of neutrophils was shown to be mediated primarily by CXCR1 despite similar affinities of CXCL-8 for CXCR1 and CXCR2 and their similar expression level (38, 39). Despite these studies indicating somewhat distinctive functions for CXCR1 and CXCR2, we observed an inhibition of melanoma cell chemotaxis on treatment with either of the antagonists; the effect was more pronounced with SCH-527123. Both SCH-527123 and SCH-479833 are fairly selective for CXCR2 compared with CXCR1 (74 and 100 times). Their major difference is in their affinity for CXCR2, with SCH-527123 being over 3 times more potent. Thus, SCH-527123 improved activity is probably due to its lower Ki for CXCR2.
The mitogen-activated protein kinase signaling pathway controls various cellular functions such as growth, proliferation, invasion, and differentiation (40). There is a growing body of evidence suggesting that activation of the ERK1/2 pathway may be involved in the oncogenic behavior and invasive potential of melanoma cells (30, 41). We showed that, on treatment with either of the antagonists in vitro, A375SM cells exhibited a significant reduction in ERK1/2 phosphorylation. This finding, therefore, may suggest a role of the ERK pathway in CXCL-8-CXCR1/2 signaling in proliferation and invasion of melanoma cells.
The inhibition of tumor growth and angiogenesis by these antagonists has also been observed in a colon cancer mouse model,4 indicating that these observations were not limited to the tumor model chosen. Similar to our observations, recently SCH-527123 has also been shown to inhibit neutrophil recruitment, mucus production, and goblet cell hyperplasia in an animal model of pulmonary inflammation (42). This indicates that these antagonists may also have clinical utility in diseases implicating CXCR1 and/or CXCR2 directly or indirectly such as rheumatoid arthritis, respiratory distress syndrome, psoriasis, and pulmonary disease (42–45).
In conclusion, our data show that CXCR2/CXCR1 antagonists are effective against a melanoma tumor model. The inhibitory effects of these antagonists resulted from decreased proliferation, survival, and invasion of melanoma cells. Furthermore, angiogenesis was also inhibited, which is extremely important in maintaining the supply of nutrients and oxygen to the tumor cells. Based on these preclinical findings, SCH-479833 and SCH-527123 represent potential novel therapeutic agents for human malignant melanoma.
Translational Relevance.
Human cutaneous malignant melanoma is the most aggressive form of skin cancer with a very poor prognosis and current therapeutic strategies are not effective in advanced disease. Our data show that orally active small-molecule antagonists for CXCR2/CXCR1 are effective in inhibiting melanoma tumor growth and angiogenesis. Based on these preclinical findings, SCH-479833 and SCH-527123 represent potential novel therapeutic agents for human malignant melanoma.
Acknowledgments
We thank Dr. Ajay P. Singh (University of Nebraska Medical Center) for careful reading of this article.
Grant support: Grant CA72781, National Cancer Institute, NIH Cancer Center Support Grant P30CA036727, Nebraska Research Initiative Molecular Therapeutics Program, and Shering-Plough Research Institute (R.K. Singh).
The costs of publication of this article were defrayed in part by the payment of page charges.This article must therefore be hereby marked advertisement in accordance with18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Unpublished data.
Our unpublished data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA CancerJ Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Lee JA. Trends in melanoma incidence and mortality. Clin Dermatol. 1992;10:9–13. doi: 10.1016/0738-081x(92)90052-z. [DOI] [PubMed] [Google Scholar]
- 4.Lee JE. Factors associated with melanoma incidence and prognosis. Semin Surg Oncol. 1996;12:379–85. doi: 10.1002/(SICI)1098-2388(199611/12)12:6<379::AID-SSU2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Clark WH, Jr., Elder DE, Guerry D, Epstein MN, Greene MH, Van HM. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–65. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 6.Clark WH. Tumour progression and the nature of cancer. BrJ Cancer. 1991;64:631–44. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herlyn M. Molecular targets in melanoma: strategies and challenges for diagnosis and therapy. Int J Cancer. 2006;118:523–6. doi: 10.1002/ijc.21605. [DOI] [PubMed] [Google Scholar]
- 8.Pease JE, Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am J Respir Med. 2002;1:19–25. doi: 10.1007/BF03257159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1andTNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 10.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 11.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflamma-tory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–8. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Dubey S, Varney ML, Singh RK. Interleukin-8-induced proliferation, survival, and MMP production in CXCR1 and CXCR2 expressing human umbilical vein endothelial cells. Microvasc Res. 2002;64:476–81. doi: 10.1006/mvre.2002.2442. [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Varney ML. IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol. 2000;15:843–9. doi: 10.14670/HH-15.843. [DOI] [PubMed] [Google Scholar]
- 15.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 16.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–7. [PubMed] [Google Scholar]
- 17.Singh RK, Varney ML. Regulation of interleukin 8 expression in human malignant melanoma cells. Cancer Res. 1998;58:1532–7. [PubMed] [Google Scholar]
- 18.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different meta-static potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723–31. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 19.Bertini R, Allegretti M, Bizzarri C, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791–6. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JR, Lee JM, Young PR, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–8. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 21.Thatcher TH, McHugh NA, Egan RW, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L322–8. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonsiorek W, Fan X, Hesk D, et al. Pharmacological characterization of Sch527123, a potent allosteric CXCR1/CXCR2 antagonist. J Pharmacol Exp Ther. 2007;322:477–85. doi: 10.1124/jpet.106.118927. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 24.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–304. [PubMed] [Google Scholar]
- 25.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125:209–16. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 26.Hall PA, Levison DA, Woods AL, et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–94. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 27.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–67. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? IntJCancer. 2003;104:527–32. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998;95:10820–5. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oapos;Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the micro-environment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 33.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 34.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 35.Peiper SC, Wang ZX, Neote K, et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals wholack the erythrocyte receptor. JExp Med. 1995;181:1311–7. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 37.Ramjeesingh R, Leung R, Siu CH. Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1. FASEB J. 2003;17:1292–4. doi: 10.1096/fj.02-0560fje. [DOI] [PubMed] [Google Scholar]
- 38.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–94. [PubMed] [Google Scholar]
- 39.Quan JM, Martin TR, Rosenberg GB, Foster DC, Whitmore T, Goodman RB. Antibodies against the N-terminus of IL-8 receptor A inhibit neutrophil chemo-taxis. Biochem Biophys Res Commun. 1996;219:405–11. doi: 10.1006/bbrc.1996.0246. [DOI] [PubMed] [Google Scholar]
- 40.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 41.Zhuang L, Lee CS, Scolyer RA, et al. Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma. J Clin Pathol. 2005;58:1163–9. doi: 10.1136/jcp.2005.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman RW, Minnicozzi M, Celly CS, et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol ExpTher. 2007;322:486–93. doi: 10.1124/jpet.106.119040. [DOI] [PubMed] [Google Scholar]
- 43.Kurdowska A, Noble JM, Grant IS, Robertson CR, Haslett C, Donnelly SC. Anti-interleukin-8 autoanti-bodies in patients at risk for acute respiratory distress syndrome. Crit Care Med. 2002;30:2335–7. doi: 10.1097/00003246-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Nickoloff BJ, Karabin GD, Barker JN, et al. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-a in psoriasis. Am J Pathol. 1991;138:129–40. [PMC free article] [PubMed] [Google Scholar]
- 45.Woods JM, Katschke KJ, Jr., Tokuhira M, et al. Reduction of inflammatory cytokines and prostaglandin E2 by IL-13 gene therapy in rheumatoid arthritis synovium. J Immunol. 2000;165:2755–63. doi: 10.4049/jimmunol.165.5.2755. [DOI] [PubMed] [Google Scholar]