Abstract
Our purpose was to compare healing characteristics of diabetic foot ulcers treated with dehydrated human amniotic membrane allografts (EpiFix®, MiMedx, Kennesaw, GA) versus standard of care. An IRB‐approved, prospective, randomised, single‐centre clinical trial was performed. Included were patients with a diabetic foot ulcer of at least 4‐week duration without infection having adequate arterial perfusion. Patients were randomised to receive standard care alone or standard care with the addition of EpiFix. Wound size reduction and rates of complete healing after 4 and 6 weeks were evaluated. In the standard care group (n = 12) and the EpiFix group (n = 13) wounds reduced in size by a mean of 32·0% ± 47·3% versus 97·1% ± 7·0% (P < 0·001) after 4 weeks, whereas at 6 weeks wounds were reduced by −1·8% ± 70·3% versus 98·4% ± 5·8% (P < 0·001), standard care versus EpiFix, respectively. After 4 and 6 weeks of treatment the overall healing rate with application of EpiFix was shown to be 77% and 92%, respectively, whereas standard care healed 0% and 8% of the wounds (P < 0·001), respectively. Patients treated with EpiFix achieved superior healing rates over standard treatment alone. These results show that using EpiFix in addition to standard care is efficacious for wound healing.
Keywords: Allograft, Amniotic membrane, Diabetic ulcer, Randomised controlled trial, Wound healing
Introduction
The prevalence of diabetes continues to rise. According to the World Health Organization, by the year 2030 diabetes will be diagnosed in 366 million people worldwide—up from 171 million in the year 2000. Lower extremity ulcers are a serious complication for people with diabetes 1. In diabetic patients, multiple aetiologies contribute to the development of ulcers, but typically neuropathy, trauma and deformity of the foot play key roles 2, 3.
Approximately 25% of diabetic patients will develop a chronic non healing ulcer over their lifetime 1, 4. While some diabetic ulcers may be superficial and heal with conservative treatment, they are often notoriously slow to resolve, taking up to several months to heal 5. Indeed, in one large meta‐analysis, Margolis et al. noted weighted healing rates of 24·2% and 30·9% at 12 and 20 weeks, respectively 6. Rates of healing assessed through planimetric measurement have been shown to be as little as 0·019–0·045 mm/day, in patients receiving standard care 7. Even slower rates of healing may occur in patients with significant vascular disease.
The slow healing nature of diabetic ulcers has led to the development of a number of indicators for experimental purposes that provide more rapid assessment of the effectiveness of various healing modalities. Sheehan et al. noted that subjects with a reduction in ulcer area greater than the 4‐week median had a 12‐week healing rate of 58%, compared with only 9% of those who did not 8. Margolis et al. have similarly noted that surrogate endpoints of wound size reduction measured at 2, 4 or 8 weeks of care were similarly predictive of eventual healing 9. Recently, Warriner et al. have summarised these earlier findings, noting that a 50% reduction in wound size at 4 weeks is a critical cut‐off point for evaluating diabetic foot ulcer treatment success 10. The federally sponsored health insurance program for persons of 65 years or older in the USA, Medicare, currently uses these types of indicators to determine whether a wound has sufficient chronicity to warrant the inclusion of more expensive skin substitute products to the plan of care 11.
The economics of properly treating diabetic ulcers is compelling. Because these ulcers heal slowly, they are often complicated by infection, which in turn leads to more serious complications such as cellulitis or osteomyelitis with subsequent physician visits, hospitalisation and/or amputation. Diabetic foot‐related pathology is the most frequent cause of hospitalisation within this group, and it is estimated that the total cost for treatment ranges from $10 000 to nearly $60 000 depending on ulcer severity and clinical outcomes 4, 12. Approximately 60 of every 10 000 patients with diabetes will undergo a lower extremity amputation. Holzer et al. conducted a retrospective analysis of the costs for lower extremity ulcers in patients with diabetes and concluded that, given the high costs associated with treating these ulcers, the development of better treatment strategies is warranted 13.
One such development in the treatment of chronic wounds is the use of amniotic membrane allografts. In the latter half of the 20th century, natural human amniotic membrane began to be used as a wound covering for the treatment of diabetic neurovascular ulcers, venous stasis ulcers, burns and other various types of post‐surgical and post‐traumatic wound dehiscence 14, 15, 16, 17. Although historically human amniotic membrane has been used successfully in a variety of wounds, issues with obtaining, preparing and storing the material, in addition to concern regarding the potential for infectious disease transmission, have impacted its widespread use.
Recently, development of a dehydrated form of human amniotic membrane that has preserved the properties of the natural membrane, yet has a stable shelf life of 5 years at room temperature, has become commercially available. Although several case studies and clinical reports on its use are available, this study seeks to formally investigate the use of this material in a randomised controlled trial 18, 19, 20. The objective was to compare healing characteristics (wound reduction and rates of complete healing) when the amniotic membrane product was included in the standard of care versus the standard of care only, without the amniotic membrane product, in patients with indolent diabetic foot ulcers.
Patients and methods
A prospective, stratified, randomised, comparative, parallel group, non blinded clinical trial comparing the proportion of ulcers completely healed with the use of dehydrated human amniotic membrane allograft (EpiFix®, MiMedx, Kennesaw, GA) versus a standard protocol of wound care (moist wound therapy) in diabetic patients with a foot ulcer was conducted. The single‐centre trial was conducted in Southwest Virginia under the direction of a senior clinician with expertise in diabetic foot care with continuous enrolment of all eligible patients who wished to participate. Patients read and signed an IRB‐approved informed consent form prior to any study involvement. The study design conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was reviewed and approved by Western IRB and preregistered in ClinicalTrials.gov (NCT01552499). The study population was comprised of patients with a history of type 1 or type 2 diabetes presenting for care of a diabetic ulcer located anywhere on the foot. Study inclusion criteria were as follows: age 18 or older; able and willing to provide consent and agree to comply with study procedures and follow‐up evaluations; ulcer size >1 and <25 cm2; ulcer duration of ≥4 weeks; no clinical signs of infection; serum creatinine < 3·0 mg/dl; glycosylated haemoglobin (HbA1c) < 12%; adequate circulation to the affected extremity as demonstrated by dorsum transcutaneous oxygen test (TcPO2) ≥ 30 mmHg, ankle‐brachial index (ABI) between 0·7 and 1·2 or triphasic or biphasic Doppler arterial waveforms at the ankle of affected leg. Patients were excluded if any of the following were present: participating in another clinical trial; charcot foot; index ulcer probing to bone; currently receiving radiation or chemotherapy; known or suspected malignancy of current ulcer; diagnosis of autoimmune connective tissue disease; received a biomedical or topical growth factor for their wound within the previous 30 days; pregnant or breast feeding; taking medications considered to be immune system modulators and allergy to gentamicin or streptomycin. Screening evaluations consisted of a medical history and physical examination, an infection assessment, wound site measurement, serum creatinine, HbA1c and a vascular assessment including circulation to the affected extremity (dorsum TcPO2, ABIs or Doppler arterial waveforms) within the last 60 days. Eligible patients meeting criteria were then randomised to receive either the EpiFix allograft material in addition to the standard regimen of wound care or a standard regimen of wound care alone in a 1:1 ratio. The randomisation schedule was balanced and permuted in blocks of 10.
Study procedures
Patients randomised to the standard regimen of wound care (SOC group) were treated with wound debridement, appropriate moist wound therapy adhering to standardised guidelines with the use of Silvasorb gel and Aquacel AG at the discretion of the attending clinician and a compression dressing. Patients were instructed on how to perform their daily dressing changes and were provided with all necessary supplies on a weekly basis.
Patients randomised to the amniotic membrane group (EpiFix group) received an application of the dehydrated amniotic membrane allograft (EpiFix) following surgical debridement of all necrotic tissue. A non adherent dressing (Adaptic®) was used to cover the EpiFix, followed by a moisture‐retentive dressing (hydrogel bolster) and a compression dressing. Dressing changes in the EpiFix group took place weekly during the office visit. Per study protocol, an additional piece of EpiFix was applied at weeks 2, 4, 6, 8 and 10, if the ulcer had not completely epithelialised at those time points.
The wound dressing techniques described were consistently used for all patients and across time points. For both treatment groups, a record was kept of each dressing change performed. All wounds were offloaded using a removable cast walker (Active Offloading Walker; Darco, Huntington, WV).
All patients were seen by the investigator at time zero and at least once every 7 days (±3 days) for up to 12 weeks or until complete healing, whichever occurred first. Additionally, patients were exited from the study and allowed to seek alternative treatment if the index ulcer did not achieve 50% area reduction at 6 weeks. During each weekly visit, ulcer cleansing with a sterile normal saline solution (rinsing, swabbing or irrigating), ulcer measurement with a graded centimetre ruler (length, width and depth) and a dressing change were conducted. When applicable, measurements were done after debridement. The wound area was calculated by multiplying the width and length measurements.
Study outcomes and data analysis
The purpose of this investigation was to determine the efficacy of EpiFix (dehydrated human amniotic membrane) versus standard of care in the treatment of indolent diabetic lower extremity ulcers. Primary study outcomes included reduction of wound size and the proportion of ulcers completely healed after 4 and 6 weeks. For the purposes of this study, healing was defined as complete epithelialisation of the open area of the wound. The 4‐week surrogate endpoint has been well established as an industry marker by Margolis et al. in evaluating the prognosis for eventual healing 5, 8, 9, 10. A final evaluation of study outcomes occurred at 12 weeks for those patients with continued enrolment.
The percent of ulcers healed at 4‐ and 6‐week time points was evaluated with a χ 2 test with odds ratio and 95% confidence interval. Wound surface area reduction was evaluated using a Mann–Whitney U‐test. In addition, the healing characteristic of each modality as a function of time to complete healing was assessed among patients healing within the overall 12‐week study period. The level of statistical significance was set at P < 0·05.
Results
The study was comprised of individuals who were representative of the types of patients typically seen in the community environment. Eligible patients were those with a history of type 1 or type 2 diabetes receiving treatment for a chronic diabetic foot ulcer of at least 4‐week duration. All eligible patients were offered enrolment as long as they met the IRB‐approved study inclusion and exclusion criteria described above. Twenty‐five subjects were enrolled and randomly assigned to the SOC group (n = 12) or EpiFix group (n = 13) between March and August of 2012. Characteristics of patients in the SOC and EpiFix groups are described in Table 1. Clinical characteristics were similar between the study groups (all P > 0·05).
Table 1.
Patient characteristicsa
| Variable | Intervention group | P value | |
|---|---|---|---|
| SOC (n = 12) | EpiFix (n = 13) | ||
| Gender, M (%)/F (%) | 7 (58%)/5 (42%) | 9 (69%)/4 (31%) | 0·571 |
| Age (years) | 61·7 ± 10·3 | 56·4 ± 14·7 | 0·307 |
| 59·5 (46, 81) | 55·0 (31, 80) | ||
| Body mass index (kg/m2) | 35·4 ± 6·6 | 30·4 ± 5·7 | 0·057 |
| 34·4 (27·0, 51·6) | 28·5 (23·1, 41·1) | ||
| History of index ulcer (week) | 16·4 ± 15·5 | 14·1 ± 13·0 | 0·687 |
| 11·0 (4·0, 48·0) | 10·0 (5·0, 51·0) | ||
| Baseline wound size (cm2) | 3·4 ± 2·9 | 2·6 ± 1·9 | 0·477 |
| 2·7 (1·1, 9·6) | 2·0 (1·1, 7·6) | ||
| Ulcer location | |||
| Forefoot or digital | 7 (58%) | 7 (54%) | 1·00 |
| Heel or midfoot | 5 (42%) | 6 (46%) | 1·00 |
SOC, standard of care.
Data presented as mean ± SD, median (min, max) or number (percent) as indicated.
Study outcomes are presented in Table 2. At 4 weeks, the average ulcer surface area reduction was 32·0% ± 47·3% for the 12 subjects of the SOC group and 97·1% ± 7·0% for the 13 subjects of the EpiFix group (P < 0·001). At 6 weeks, the average ulcer surface area reduction was −1·8% ± 70·3% for the 12 subjects of the SOC group and 98·4% ± 5·8% for the 13 subjects of the EpiFix group (P < 0·001). Mean wound area reduction by week is presented in Figure 1. In the SOC group there was a mean reduction in wound size of 20% at week 1, compared to a mean reduction in wound size of over 80% in those in the EpiFix group. Mean wound size reduction by patient is presented in Figure 2 for those in the EpiFix and SOC groups, respectively. Note that the SOC patients showed the typical pattern of irregular wound size variation over time, whereas the EpiFix patients demonstrated a consistently rapid reduction in wound size over time with less variation.
Table 2.
Study outcomes at 4 and 6 weeksa
| Outcome | Intervention group | P value | |
|---|---|---|---|
| Wound size reduction | SOC (n = 12) | EpiFix (n = 13) | |
| 4 Weeks | 32·0 ± 47·3% | 97·1 ± 7·0% | <0·001 |
| 23·8 (−66·7, 96·9) | 100 (76·0, 100) | ||
| 6 Weeks | −1·8 ± 70·3% | 98·4 ± 5·8% | <0·001 |
| 17·1 (−131·7, 100) | 100 (79·0, 100) | ||
| Ulcers healed | SOC (n = 12) | EpiFix (n = 13) | P value |
| 4 Weeks | 0 (0%) | 10 (77%) | <0·001 |
| 6 Weeks | 1 (8%) | 12 (92%) | <0·001 |
SOC, standard of care.
Data presented as mean ± SD and median (min, max).
Figure 1.
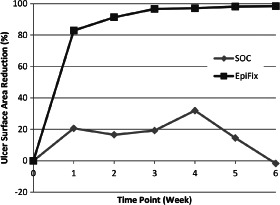
Mean percent reduction of ulcer surface area by week for patients treated with EpiFix or standard of care (SOC).
Figure 2.
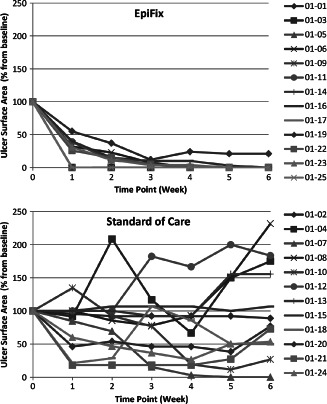
Mean percent reduction of ulcer surface area by week for each patient receiving EpiFix or standard of care.
Patients randomised to receive EpiFix had higher healing rates than those receiving standard of care without EpiFix (Table 2). At 4 weeks, none of the subjects from the SOC group (0%) was healed, whereas 10 of the 13 subjects in the EpiFix group (77%) had wounds that had completely epithelialised (P < 0·001). At 6 weeks, 1 of the 12 subjects from the SOC group (8%) was healed and 12 of the 13 subjects in the EpiFix group (92%) were healed (P < 0·001). Rates of healing by week are illustrated in Figure 3. Interestingly, over 50% of patients in the EpiFix group were healed (defined as complete epithelialisation of the open area of the wound) within 1 week of study enrolment.
Figure 3.
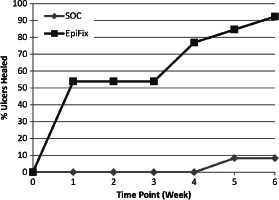
Percent of ulcers completely healed at weeks 1–6.
For those patients that healed, mean time to complete healing was 5 weeks in the SOC group (n = 1) versus 2·5 ± 1·9 weeks in the EpiFix group (n = 12), representing a 50% faster healing rate for those patients in the EpiFix treatment group.
Study completion
Per study protocol, patients failing to achieve at least a 50% reduction in wound size within 6 weeks of study enrolment had the option of exiting the study in order to seek alternative wound care treatment. By week 6, 1 of the 12 subjects from the SOC group had healed completely, 1 had healed >50% and 10 exited the study as the index ulcer had not achieved 50% area reduction. At the final endpoint of 12 weeks, one patient exited without achieving complete wound closure. In the EpiFix group, all of the patients achieved >50% healing by 6 weeks. Indeed, at the 6‐week evaluation, 12 of the 13 subjects in the EpiFix group had healed completely. Only one patient in the EpiFix group did not achieve complete healing and opted to exit at week 11 for alternative treatment.
Adverse events
During the study period, four patients in the SOC group and one patient in the EpiFix group experienced adverse events. Two patients in the SOC group developed cellulitis of the effected extremity, which was treated with sharp debridement and antibiotics. Other events in the SOC group included one patient with a gastrointestinal bleed and one with acute pyelonephritis. One patient in the EpiFix group experienced pneumonia, respiratory distress and acute renal failure during the study period, although this was not believed to be related to the use of the amniotic membrane allograft.
Discussion
This is the first randomised controlled trial in the USA comparing standard of care versus standard of care plus advanced therapy using a dehydrated human amniotic membrane product (EpiFix) in patients with a long‐standing diabetic ulcer. Patients in the study sample treated with EpiFix had significantly greater rates of healing and healed in a more rapid fashion than those patients receiving standard of care alone.
Human amniotic membrane has been scientifically studied to examine how it contributes to the healing process in wounds. Amniotic membrane is composed principally of three types of material: structural collagen and extracellular matrix, biologically active cells and a large number of important regenerative molecules 21. Collagen types IV, V and VII provide an important substrate, which is not only important for the structural integrity of the membrane but also facilitates wound healing and cellular ingrowth. There is clear evidence that many of these molecules interact with one another in a highly complex milieu of bioregulation that requires the presence of membranes, individual growth factors and interactions that upregulate and downregulate the various regenerative processes of healing 22.
Human amniotic membrane has been found to have a number of characteristics that make it uniquely suited to wound healing. For example, previous studies have shown that amniotic membrane:
provides a matrix for cellular migration and proliferation 15,
promotes increased healing and enhancement of the wound healing process 23,
is non immunogenic 24,
reduces inflammation 25,
reduces scar tissue 26,
contains a number of essential growth factors and cytokines 32.
Overall, amniotic membrane has potential uses in a variety of other wound healing applications in addition to cutaneous wounds including applications in periodontology, otorhinolaryngology and general surgery. The material appears to be safe in its overall use and contributes significantly to the regeneration of various soft tissues 31, 33, 34.
Models of cost‐effectiveness in wound care have been postulated and developed by a number of authors. Data from registries often are used to calculate the health care claims costs of wound care, and are expressed in terms of cost to heal, hospitalisation costs or overall medical care costs 13, 35. While health economists focus on these tangible costs of wound management, the true economic analyses also develop from productivity, quality of life and related issues as well 36. In order for an advanced therapy such as EpiFix to be seen as an acceptable treatment option it must be clinically efficacious, operationally efficient for the clinician, improve the patients' quality of life and be cost‐effective. A treatment that reduces healing time while requiring minimal dressing changes with a low need for reapplication meets these requirements.
Dehydrated amniotic membrane material is operationally efficient in that it can be transported and stored at room temperature for long periods of time up to 5 years, thus minimising the need for complex policies for receiving and storing the material. In addition, the added expense of subzero refrigeration or short storage life that often leads to wasted product is no longer a consideration or expense. Handling characteristics of the EpiFix material permit easy retrieval and use and minimise time for application, allowing for more efficient utilisation of the clinicians' time and effort. The material can be provided in a number of different sizes, minimising the amount of waste when used on varying size ulcers and at various stages in healing.
In this study, the overall comparison of the standard of care with the standard of care plus dehydrated amniotic membrane allograft reflected an unprecedented ability of the material to assist in the resolution of diabetic neurotrophic ulcers. The use of the EpiFix material obviated at least six dressing changes by the patient per week and any associated home health care visits. The EpiFix material, placed on an every other week regimen, aggressively closed the wounds under consideration in a far shorter time than standard wound treatment. Therefore, as the allograft material is placed less frequently, and the wounds heal more quickly than alternative therapies, the potential for reduced cumulative as well as episode costs can be assumed.
Limitations of this study are those inherent to small sample size. Our findings should be confirmed and expanded with subsequent clinical trials. As the EpiFix allograft was applied biweekly we do not know if weekly application would result in even more expeditious healing. Studies are currently underway to address this question. Also, as our comparative group did not receive other advanced therapies we are unable to assess if the EpiFix allograft is as good as, or better, than other available advanced wound care products. Additional comparative effectiveness studies are required to address those questions. As this study was limited to patients with chronic diabetic foot ulcers we are unable to comment on how the EpiFix product performs in other patient populations and for other medical or surgical indications.
In conclusion, the application of dehydrated human amniotic membrane has demonstrated superior clinical effectiveness when compared with standard of care in the treatment of indolent neurotrophic ulcers of the lower extremity in diabetic patients. The results show that over a 6‐week period, healing occurred in 92% of chronic diabetic foot ulcers treated with dehydrated human amniotic membrane allografts (EpiFix) as an addition to standard of care (conservative wound management) versus only 8% of wounds healed with standard of care alone. Therefore, EpiFix appears to be a clinically viable and economically feasible treatment option that should be considered by clinicians that treat diabetic pedal ulcers.
Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers.
References
- 1. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the ADA, with endorsement by the AACE. Diabetes Care 2008;8:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frykberg R, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV; American College of Foot and Ankle Surgeons. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45(5 Suppl):S1–66. [DOI] [PubMed] [Google Scholar]
- 3. Snyder RJ, Kirsner RS, Warriner RA 3rd, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56(4 Suppl):S1–24. [PubMed] [Google Scholar]
- 4. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 5. Snyder R. Wound percent area reduction and making decisions about utilizing advanced therapies. Podiatry Manage 2010;29:197–201. [Google Scholar]
- 6. Margolis D, Kantor J, Berlin J. Healing of neuropathic ulcers: results of a meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 7. Zimny S, Schatz H, Pfohl M. Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complications 2002;16:327–32. [DOI] [PubMed] [Google Scholar]
- 8. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 9. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–700. [DOI] [PubMed] [Google Scholar]
- 10. Warriner RA, Snyder RJ, Cardinal MH. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J 2011;8:632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. http://www.trailblazerhealth.com/Tools/LCDs.aspx?ID=3332 [accessed on 1 November 2012].
- 12. Boulton AJM, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55. [DOI] [PubMed] [Google Scholar]
- 13. Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther 1998;20:169–81. [DOI] [PubMed] [Google Scholar]
- 14. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am 2003;16:43–65. [DOI] [PubMed] [Google Scholar]
- 15. Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 2009;26:507–23. [DOI] [PubMed] [Google Scholar]
- 16. Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J 1978;118:1237–46. [PMC free article] [PubMed] [Google Scholar]
- 17. Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns 1989;15:339–42. [DOI] [PubMed] [Google Scholar]
- 18. Forbes J, Fetterolf D. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care 2012;21:290–6. [DOI] [PubMed] [Google Scholar]
- 19. Serena T, Fetterolf D. Clinical research: dehydrated human amniotic membrane (dHAM) treatment of lower extremity venous ulceration (CR23). Poster Presented at SAWC Annual Spring Meeting, Atlanta, 2012.
- 20. Ennis W, Sui A, Papineau E, et al. Clinical experience with a novel regenerative template for hard to heal wounds. SAWC Annual Spring Meeting, Atlanta, 2012.
- 21. Schmidt W. The amniotic fluid compartment: the fetal habitat. Berlin: Springer‐Verlag, 1992:1–98. [DOI] [PubMed] [Google Scholar]
- 22. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 2000;70:329–37. [DOI] [PubMed] [Google Scholar]
- 24. Akle C, Adinolfi M, Welsh K, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981;318:1003–5. [DOI] [PubMed] [Google Scholar]
- 25. Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000;19:348–52. [DOI] [PubMed] [Google Scholar]
- 26. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor‐beta isoforms, TGF‐beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999;179:325–35. [DOI] [PubMed] [Google Scholar]
- 27. King AE, Paltoo A, Kelly RW, et al. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007;28:161–9. [DOI] [PubMed] [Google Scholar]
- 28. Talmi Y, Sigler U, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta 1991;12:285–8. [DOI] [PubMed] [Google Scholar]
- 29. Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 2007;15:459–64. [DOI] [PubMed] [Google Scholar]
- 30. Adly OA, Moghazy AM, Abbas AH. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010;36:703–10. [DOI] [PubMed] [Google Scholar]
- 31. Niknejad H, Peirovi H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 32. Parolini O, et al. Human term placenta as a therapeutic agent: from the first clinical applications to future perspectives. In: Berven E, editor. Human placenta: structure and development. Hauppauge, New York: Nova Science Publishers, 2010:1–48. [Google Scholar]
- 33. Chen E, Tofe A. A literature review of the safety and biocompatibility of amnion tissue. J Impl Adv Clin Dent 2010;2:67–75. [Google Scholar]
- 34. Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion‐derived cells for regeneration of various tissues. J Pharmacol Sci 2007;105:215–28. [DOI] [PubMed] [Google Scholar]
- 35. Fife CE, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds 2012;24:10–7. [PubMed] [Google Scholar]
- 36. Snyder RJ, Hanft JR. Diabetic foot ulcers‐‐effects on QOL, costs, and mortality and the role of standard wound care and advanced‐care therapies. Ostomy Wound Manage 2009;55:28–38. [PubMed] [Google Scholar]


