Abstract
Background
To assess the clinical and laboratory parameters, response to therapy and development of antituberculosis (TB) drug resistance in pulmonary TB (PTB) patients with diabetes mellitus (DM) and without DM.
Methods
Using a prospective design, 227 of 310 new cases of culture-positive PTB diagnosed at the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital between April 2010 and July 2012 that met the study criteria were selected. Data regarding clinical and laboratory parameters, drug susceptibility and treatment outcomes were compared between PTB patients with DM and those without DM. To control for age, the patients were stratified into two age groups (< 50 and ≥ 50 years) and their data were analysed.
Results
Of the 227 patients, 37 (16.3%) had DM, of which 26 (70.3%) had been diagnosed with DM prior to PTB diagnosis and 11 (29.7%) had developed DM at PTB diagnosis. After controlling for age, no significant differences were found between the two groups regarding mycobacterium burden, sputum-culture conversion rate, evidence of multidrug-resistant tuberculosis, frequency of adverse drug events from anti-TB medications, treatment outcomes and relapse rate. The presenting symptoms of anorexia (p = 0.050) and haemoptysis (p = 0.036) were observed significantly more frequently in PTB patients with DM, while the presenting symptom of cough was observed significantly more frequently in PTB patients without DM (p = 0.047).
Conclusions
Plasma glucose levels should be monitored in all newly diagnosed PTB patients and a similar treatment regimen should be prescribed to PTB patients with DM and those without DM in high TB-burden countries.
What's known
As the incidence of diabetes mellitus (DM), a risk factor for pulmonary tuberculosis (PTB), has been gradually increasing worldwide in high-burden TB countries, it has been increasingly observed in new cases of PTB. However, few data have been collected regarding clinical and laboratory parameters, response to therapy and development of anti-TB drug resistance in PTB patients with DM and PTB patients without DM for comparison of these patient populations.
What's new
Diabetes mellitus was observed in 16.3% of new patients with PTB. Mycobacterium burden, sputum-culture conversion rate, multidrug-resistant tuberculosis rate, treatment outcomes and relapse rates were similar in PTB patients with DM and those without DM. The findings suggest that plasma glucose should be monitored in PTB patients and a similar treatment regimen should be prescribed to PTB patients with DM and those without DM.
Introduction
According to the 2011, World Health Organization (WHO) report, tuberculosis (TB) and human immunodeficiency virus (HIV) are two of the top five causes of death in developing countries 1. Although the estimated incidence of TB in Thailand was 124 per 100,000 populations in 2011, the estimated incidence of TB and HIV coinfection decreased in the same year 2. At the same time, the incidence of diabetes mellitus (DM) has been increasing worldwide, having increased from 153 million to 347 million between 1980 and 2008, because of changes in diet, physical activity, body mass index and ageing patterns 3,4. Previous reports found that patients with DM were two to eight times at higher risk for development of active TB and at approximately three times higher risk for development of pulmonary TB (PTB) as compared with patients without DM 5–8. DM patients with a haemoglobin A1C concentration of > 7 mmol/mol are especially at risk, as elevated A1C concentration is associated with decreased phagocytic activity and T-cell function resulting in impaired cell-mediated immunity 8,9. This phenomenon reflects the fact that cell-mediated immunity plays a pivotal role in defence against intracellular organisms, particularly Mycobacterium tuberculosis 7. Nevertheless, the occurrence of PTB rather than extra-PTB in patients with DM has been attributed to decreased activation of alveolar macrophages 10.
Previous studies found that TB patients with DM experienced higher rates of treatment failure and fatality than those without DM 11–15. These studies, which included patients experiencing different levels of TB severity and HIV coinfection, indicated that coinfection with these diseases might be a possible risk factor for mortality in DM patients 16. However, few data have been collected regarding clinical presentation, severity of disease, response to therapy and development of anti-TB drug resistance in PTB patients with DM and PTB patients without DM. To fill this research gap, this prospective study aimed to determine the incidence of DM in newly diagnosed cases of PTB and to compare the clinical and laboratory parameters, extent of drug susceptibility and treatment outcomes between PTB patients with DM and PTB patients without DM who presented at the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital, Chonburi province, Thailand between April 2010 and July 2012.
Methods
Study site and population
This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University in Bangkok; the Queen Savang Vadhana Memorial Hospital in Chonburi province; and the Chonburi Hospital in Chonburi province, Thailand. Written informed consent was obtained from all patients. A prospective study had been conducted between April 2010 and July 2012 at the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital. All subjects met the subject inclusion criteria of: (i) age ≥ 15 years; (ii) presence of ≥ 2 pulmonary or constitutional symptoms, including cough, dyspnoea, chest pain, haemoptysis, fever, fatigue, malaise, weight loss and/or night sweats; (iii) new diagnosis of PTB according to the 2010 WHO guidelines for TB 17; and (iv) a sputum culture positive for M. tuberculosis. PTB patients were defined as patients with abnormal chest radiographic findings of the reticular, interstitial, nodular infiltrate, or the chest cavity and at least one of three sputum smears testing positive for acid-fast bacilli (AFB) 17. New cases of PTB were defined as PTB patients who had never received treatment for TB or patients who had taken any anti-TB drug for < 1 month 17. None of the subjects met the exclusion criteria of: (i) culture negative for mycobacterium, (ii) culture positive for non-TB mycobacterium, (iii) HIV infection, or (iv) no data were available regarding DM or HIV status.
Data regarding patient demographic characteristics and clinical and laboratory parameters were collected and entered into a predefined case record form. All patients were placed under management and prescribed appropriate anti-TB regimens based on decisions made by clinicians at the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital. Family-based directly observed treatment, defined as the taking of prescribed anti-TB medications in the presence of family members, was incorporated into the overall treatment regimen to improve patient adherence to treatment and prevent the development of drug resistance. All patients were regularly followed up at intervals of 1–4 weeks to assess overall treatment progress as well as document adverse drug events to anti-TB medications and drug adherence until completion of treatment. Non-adherence was defined as the inability to take any prescribed anti-TB medications for at least 7 days 18.
Diagnosis of DM
The criteria used to diagnose DM were the 2010 American Diabetes Association criteria 19 of: (i) fasting plasma glucose levels ≥ 126 mg/dl after fasting for at least 8 h on two occasions and/or (ii) random plasma glucose levels ≥ 200 mg/dl. Patients without DM were defined as patients who did not fulfil the criteria for DM.
Sputum smear and culture for mycobacterium
Sputum samples were collected for AFB smears and identification of mycobacterium prior to treatment and at months 2 and 5 of treatment. After collection on 3 different days in sterile, disposable, leak-proof and laboratory-approved containers, three early-morning expectorated sputum samples were sent to the Central Laboratory Unit of the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital for AFB smears using the Ziehl–Neelsen staining, as described elsewhere 20. The results of the AFB smears were interpreted using the 1998 WHO Laboratory Services in TB Control Grading System, according to which AFB < 1+ was defined as 1–9 AFB per 100 oil immersion field (OIF), 1+ as 10–99 AFB per 100 OIF, 2 + as 1–10 AFB per OIF and 3+ as > 10 AFB per OIF 20.
While being maintained at 4–5° C, the sputum sample of each patient found to have the highest AFB grading was transported at each time point to the National Tuberculosis Reference Laboratory of Thailand for mycobacterium culture. The mycobacterium in sputum samples was cultured and drug susceptibility testing was conducted using Lowenstein–Jensen solid medium and liquid medium BACTEC MGIT 960 (Becton Dickinson, MD) according to the manufacturer's instruction. Detection of growth on either one of the two culture media was considered a positive culture.
Sample size calculation
The sample size was estimated based on previous studies conducted in tropical countries that had found that 10–30% of PTB cases examined also had DM 21,22. In this study, the proportion of PTB patients with DM was estimated to be 15%, with a 95% confidence interval, and the precision to be within 5% of the true value and an estimated 10% loss to follow up. A sample size of at least 218 PTB patients was required in our study. In order to compare clinical and laboratory parameters and treatment outcomes between PTB patients with and without DM, data regarding treatment failure were analysed for calculation of sample size. A previous study conducted in Indonesia found that the proportion of PTB patients with and without DM with a positive sputum culture at 6 months was 22% and 6%, respectively 23. Based on these data, the required sample size ratio of PTB patients with DM to PTB patients without DM was estimated at 1:5. To be able to reject the null hypothesis that the failure rates for PTB patients with and without DM are equal at a probability (power) of 0.8 and an alpha level of 0.05, a sample of at least 216 patients that included at least 36 PTB patients with DM and 180 PTB patients without DM needed to be studied. Thus, the minimum sample size was determined to be 218 patients.
Statistical analysis
Data were entered into Microsoft Excel and analysed using the Statistical Package for the Social Sciences for Windows version 18.0 (SPSS Inc., Chicago, IL). Categorical variables were summarised as frequencies and percentages, and then analysed using the χ2 test or the Fisher's exact test where appropriate. Stratified analysis was performed using the Mantel–Haenszel method for binary outcomes to control for potential confounding effect of age. Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Variables with a non-normal distribution were summarised by calculation of their median and interquartile range (IQR) values and compared using the Mann–Whitney U-test for two-group comparison. All tests for significance were two-sided and a p < 0.05 was considered an indication of statistical significance.
Results
A total of 310 new patients with PTB were diagnosed and subsequently managed at the Queen Savang Vadhana Memorial Hospital and the Chonburi Hospital between April 2010 and July 2012. Of these 310 patients, underlying medical illness was observed in 117 (37.9%), including DM (51 patients, 43.6%), HIV infection (33 patients, 28.2%), coronary artery disease (30 patients, 25.6%), liver disease (21 patients, 17.9%), chronic obstructive pulmonary disease (8 patients, 6.8%) and rheumatoid arthritis (4 patients, 3.4%). Testing for presence of mycobacterium from sputum samples revealed that 18 (5.8%) patients with cultures positive for non-tuberculous mycobacterium, 19 (6.1%) with cultures negative for mycobacterium and the remaining 273 (88.1%) with cultures positive for M. tuberculosis. Of these 273 patients, no data regarding DM or HIV status were available for 13, while data regarding a positive result from serological testing for HIV were available for 33.
Based on review of the data collected thus far, 227 new patients with a positive PTB culture were identified and determined eligible for study participation. Of these 227 patients, 37 (16.3%) were also diagnosed with DM and 190 (83.7%) were not diagnosed with DM (Figure1). Among the 37 PTB patients with DM, 26 (70.3%) had been diagnosed with DM prior to being diagnosed with PTB while 11 (29.7%) had developed DM at PTB diagnosis. Regarding the management of DM, 33 (89.2%) PTB patients with DM had undergone administration of hypoglycaemic drugs, including biguanide (28 patients, 84.8%), sulfonylurea (23 patients, 69.7%), insulin (5 patients, 15.2%), thiazolidinedione (4 patients, 12.1%) and dipeptidyl peptidase IV inhibitors (1 patient, 3.0%), while four patients (11.8%) had undergone solely dietary management of DM.
Figure 1.
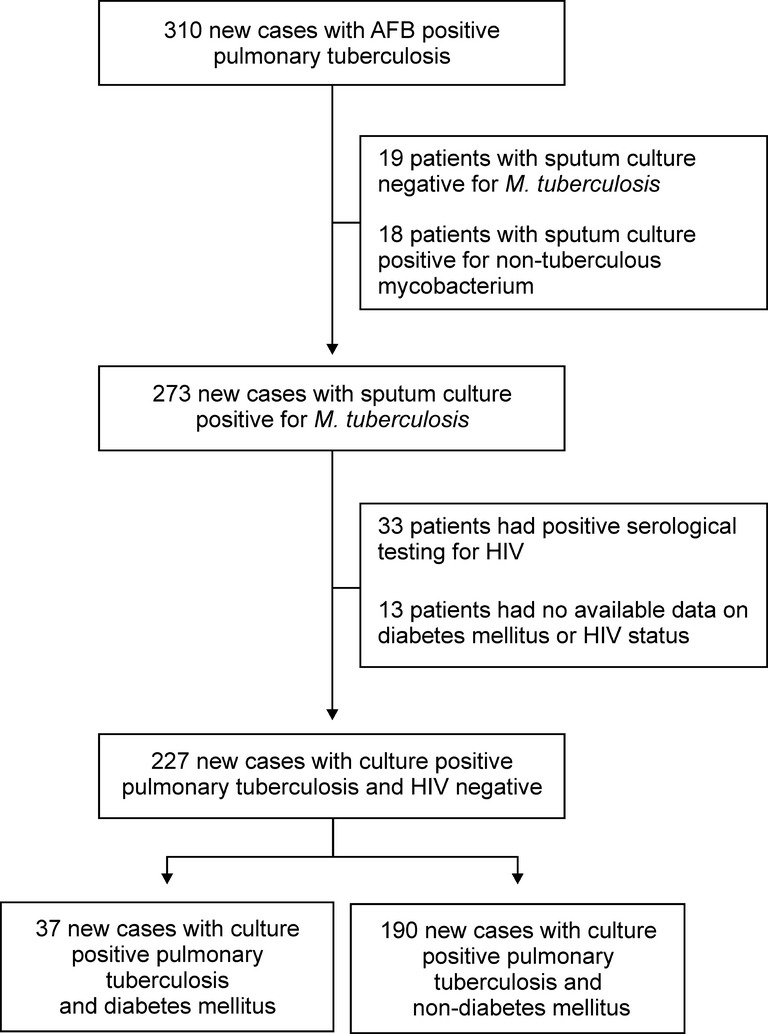
Flow diagram of the study
Baseline characteristics and clinical and laboratory parameters of PTB patients with and without DM
The baseline characteristics of PTB patients with and without DM are presented in Table1. While the data regarding sex (i.e. proportion of males), marital status, smoking history, alcohol consumption and presence of extra-PTB were similar for the two patient groups, the data regarding age (median 51.0 years, IQR: 42.5–60.5 vs. median 36.0, IQR: 27.8–48.0 years, respectively), proportion ≥ 50 years, and proportion either illiterate or with only a primary school education were significantly higher in the group of PTB patients with DM than that of PTB patients without DM (p < 0.001).
Table 1.
Baseline characteristics and clinical and laboratory parameters of new cases of culture-positive pulmonary tuberculosis with and without diabetes mellitus
| Characteristic | n | PTB patients with DM | PTB patients without DM | Crude OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| n | No. (%) | n | No. (%) | DM vs. non-DM | |||
| Baseline characteristic | |||||||
| Age ≥ 50 years | 227 | 37 | 20 (54.0) | 190 | 44 (23.1) | 3.904 (1.883–8.094) | < 0.001 |
| Male sex | 227 | 37 | 28 (75.7) | 190 | 143 (75.3) | 1.023 (0.450–2.322) | 1.000 |
| Married | 227 | 37 | 23 (62.2) | 190 | 125 (65.8) | 0.854 (0.412–1.771) | 0.814 |
| Illiterate or primary school education only | 209 | 33 | 25 (75.8) | 176 | 95 (54.0) | 2.664 (1.139–6.231) | 0.033 |
| Smoking history | 225 | 36 | 24 (66.7) | 189 | 129 (68.3) | 0.930 (0.436–1.984) | 1.000 |
| Alcohol consumption | 226 | 36 | 26 (72.2) | 190 | 137 (72.1) | 1.006 (0.454–2.228) | 1.000 |
| Extra-pulmonary tuberculosis | 227 | 37 | 1 (2.7) | 190 | 5 (2.6) | 1.028 (0.117–9.060) | 1.000 |
| Clinical presentation | |||||||
| Cough | 225 | 36 | 30 (83.3) | 189 | 178 (94.2) | 0.309 (0.106–0.898) | 0.036 |
| Dyspnoea | 225 | 36 | 26 (72.2) | 189 | 124 (65.5) | 1.363 (0.619–2.999) | 0.563 |
| Anorexia | 225 | 36 | 24 (66.7) | 189 | 87 (46.0) | 2.345 (1.108–4.962) | 0.037 |
| Fever | 225 | 36 | 22 (61.1) | 189 | 135 (71.4) | 0.629 (0.300–1.318) | 0.300 |
| Chest pain | 225 | 36 | 17 (47.2) | 189 | 107 (56.6) | 0.686 (0.336–1.401) | 0.392 |
| Haemoptysis | 225 | 36 | 16 (44.4) | 189 | 65 (34.4) | 1.526 (0.741–3.144) | 0.336 |
| Body weight decrease > 5% | 196 | 29 | 22 (75.9) | 167 | 115 (68.9) | 1.421 (0.571–3.536) | 0.590 |
| Laboratory parameter | |||||||
| Haemoglobin level < 12 g/dl | 138 | 22 | 15 (68.2) | 116 | 64 (55.2) | 1.741 (0.661–4.588) | 0.370 |
| WBC count > 10 × 103/μl | 138 | 22 | 9 (40.9) | 116 | 78 (67.2) | 0.337 (0.133–0.858) | 0.035 |
| Sodium level < 135 mmol/l | 85 | 14 | 10 (71.4) | 71 | 35 (49.3) | 2.571 (0.737–8.970) | 0.221 |
| Creatinine level > 1.2 mg/dl | 109 | 17 | 3 (17.8) | 92 | 4 (4.3) | 4.714 (0.952–23.342) | 0.075 |
| Albumin level < 3.5 g/dl | 153 | 22 | 12 (54.5) | 131 | 76 (58.0) | 0.868 (0.350–2.153) | 0.943 |
| AST level > 40 U/l | 157 | 23 | 5 (21.7) | 134 | 34 (25.4) | 0.817 (0.282–2.369) | 0.911 |
| ALT level > 40 U/l | 158 | 24 | 6 (25.0) | 134 | 26 (19.4) | 1.385 (0.500–3.833) | 0.582 |
| Cavity on chest radiograph | 201 | 34 | 12 (35.3) | 167 | 55 (32.9) | 1.111 (0.512–2.408) | 0.947 |
| Sputum AFB grading 3+ | 227 | 37 | 9 (24.3) | 190 | 44 (23.2) | 1.067 (0.468–2.429) | 1.000 |
PTB, pulmonary tuberculosis; DM, diabetes mellitus; OR, odds ratio; CI, confidence interval; IQR, interquartile range; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFB, acid-fast bacilli.
Regarding clinical parameters, cough was the most common presenting symptom of all PTB patients, but the proportion of patients presenting with cough was significantly lower in PTB patients with DM than those without DM. In contrast, the proportion of patients presenting with anorexia was significantly higher in PTB patients with DM than those without DM. The prevalence of other presenting symptoms and signs, including dyspnoea, fever, chest pain, haemoptysis and body weight decrease of > 5% from baseline were similar in both groups (Table1).
Regarding laboratory parameters, the only parameter that significantly differed between the two groups was the proportion of patients with white blood cell counts > 10 × 103/μl, which was significantly lower in PTB patients with DM than those without DM. The proportions of patients within each group for which specific laboratory parameter results had been obtained, including haemoglobin level > 12 g/dl, serum sodium level < 135 mmol/l, serum creatinine level > 1.2 mg/dl, serum albumin level < 3.5 g/dl, aspartate aminotransferase level > 40 U/l, alanine aminotransferase level > 40 U/l, presence of cavity on chest radiograph and sputum AFB grading 3+, were similar (Table1).
Management, drug susceptibility and treatment outcomes in PTB patients with and without DM
The majority of all PTB patients had undergone a standard treatment regimen of anti-TB medication that included 2 months of isoniazid, rifampicin, ethambutol and pyrazinamide administration during the intensive phase, followed by 4 months of isoniazid and rifampicin administration during the continuation phase. The proportions of patients who had undergone a standard treatment regimen of anti-TB medication and the proportion that had experienced non-adherence and/or adverse drug events were similar in both groups, as was the median duration of treatment during both the intensive and continuation phases (Table2). The adverse drug events experienced by patients in both groups had been rash (93/205 patients, 45.4%), peripheral neuropathy (75/205 patients, 36.6%), visual disturbance (41/205 patients, 20.0%), fatigue (22/205 patients, 10.7%), cholestasis (6/205 patients, 2.9%) and hepatitis (4/205 patients, 2.0%).
Table 2.
Management and treatment outcomes of new cases of culture-positive pulmonary tuberculosis with and without diabetes mellitus
| Characteristic | n | PTB patients with DM | PTB patients without DM | Crude OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|
| n | No. (%) | n | No. (%) | DM vs. non-DM | |||
| Management | |||||||
| Standard regimen | |||||||
| Intensive phase | 227 | 37 | 35 (94.6) | 190 | 188 (98.9) | 0.186 (0.025–1.366) | 0.125 |
| Continuation phase | 206 | 35 | 32 (91.4) | 171 | 168 (98.2) | 0.190 (0.037–0.986) | 0.063 |
| Duration, median (IQR) | |||||||
| Intensive phase, days | 226 | 36 | 62.5 (54.2–70.0) | 190 | 62.0 (53.0–73.0) | − | 0.632 |
| Continuation phase, days | 200 | 33 | 119.0 (109.0–139.0) | 167 | 120.0 (112.0–141.0) | − | 0.589 |
| Non-adherence | |||||||
| Intensive phase | 224 | 36 | 6 (16.7) | 188 | 24 (12.8) | 1.367 (0.515–3.625) | 0.592 |
| Continuation phase | 202 | 32 | 10 (31.3) | 170 | 62 (36.5) | 0.792 (0.352–1.780) | 0.715 |
| Adverse drug events | |||||||
| Intensive phase | 190 | 32 | 26 (81.3) | 158 | 100 (63.3) | 2.513 (0.977–6.465) | 0.079 |
| Continuation phase | 85 | 10 | 8 (80.0) | 75 | 38 (50.7) | 3.895 (0.775–19.568) | 0.100 |
| Treatment outcome | |||||||
| Sputum-culture conversion | |||||||
| At month 2 | 208 | 34 | 29 (85.3) | 174 | 146 (83.9) | 1.112 (0.396–3.121) | 1.000 |
| At month 5 | 197 | 32 | 30 (93.8) | 165 | 161 (97.6) | 0.373 (0.065–2.127) | 0.252 |
| Treatment success | 227 | 37 | 30 (81.1) | 190 | 156 (82.1) | 0.934 (0.379–2.303) | 1.000 |
| Cured | 21 (70.0) | 116 (71.6) | |||||
| Treatment completed | 9 (30.0) | 40 (28.4) | |||||
| Death | 223 | 37 | 2 (5.4) | 186 | 4 (2.2) | 2.600 (0.458–14.747) | 0.260 |
PTB, pulmonary tuberculosis; DM, diabetes mellitus; OR, odds ratio; CI, confidence interval; IQR, interquartile range.
At PTB diagnosis, drug susceptibility testing revealed no significant difference in isoniazid resistance [1/37 (2.7%) patients vs. 14/184 (7.8%) patients, p = 0.476] and rifampicin resistance [0/37 (0%) patients vs. 2/184 (1.1%) patients, p = 1.000] between PTB patients with DM and those without DM, but none had ethambutol resistance in both groups. The presence of primary multidrug-resistant TB (MDR-TB) was similar between PTB patients with DM and those without DM [0/37 (0%) patients vs. 2/184 (1.1%) patients, p = 1.000]. At 2 months of treatment, the presence of isoniazid resistance [0/33 (0%) patients vs. 4/173 (2.3%) patients, p = 1.000] and rifampicin resistance [0/33 (0%) patients vs. 1/173 (0.6%) patients, p = 1.000] was similar between PTB patients with DM and those without DM, but none had ethambutol resistance in both groups. The presence of MDR-TB was similar between PTB patients with DM and those without DM [0/33 (0%) patients vs. 1/173 (0.6%) patients, p = 1.000]. At 5 months of treatment, the presence of isoniazid resistance [0/31 (0%) patients vs. 2/165 (1.2%) patients, p = 1.000], rifampicin resistance [0/31 (0%) patients vs. 2/165 (1.2%) patients, p = 1.000] and ethambutol resistance [0/31 (0%) patients vs. 1/165 (0.6%) patients, p = 1.000] was also similar between PTB patients with DM and those without DM. The presence of MDR-TB was similar between PTB patients with DM and those without DM [0/31 (0%) patients vs. 2/165 (1.2%) patients, p = 1.000].
The treatment outcomes evaluated were sputum-culture conversion rate; treatment success rate, which included consideration of cure rate and rate of patients who completed treatment according to the 2010 WHO Guidelines for TB 17; and case fatality rate. The treatment outcomes of sputum-culture conversion rate at 2 months, sputum-culture conversion rate at 5 months, treatment success rate and case fatality rate were found to be similar for PTB patients with and without DM (Table2). Evaluation of other treatment outcomes described in the 2010 WHO Guidelines for TB 17 revealed that among the 37 PTB patients with DM, 2 (5.4%) patients had experienced treatment failure, 2 (5.4%) patients had defaulted and 1 (2.7%) patient had been observed to have transferred out. Among the 190 PTB patients without DM, 4 (2.1%) had experienced treatment failure, 19 (10.0%) had defaulted and 7 (3.7%) had been transferred out.
Among the group of 192 PTB patients who had experienced treatment success, which included 30 patients with DM and 162 patients without DM, 0 (0%) of patients with DM and only 1 (0.6%) patient without DM experienced relapse after completion of treatment for PTB. The median durations of follow up after completion of PTB treatment for patients with and without DM were 337.5 days (IQR: 247.5–431.0) and 359.0 days (IQR: 266.5–478.3), respectively, the difference between which did not reach a level of statistical significance (p = 0.333).
Comparison of PTB patients with and without DM after adjustment for age
Two previous studies reported that while type 2 DM mostly occurred in adults aged > 40 years, the majority (88%) of patients with smear-positive PTB in the study samples had been aged 15–64 years 2,4. As analysis of the data collected in this study revealed the median age of PTB patients with and without DM to be 51.0 (IQR: 42.5–60.5) and 36.0 (27.8–48.0) years, respectively (p < 0.001), the patients were stratified into two groups by age (< 50 and ≥ 50 years) in order to control for confounding variables. Subsequent analysis revealed that baseline characteristics, including sex (i.e. proportion of males), marital status, education level, smoking history, alcohol consumption and incidence of extra-PTB, as well as clinical parameters, including presence of dyspnoea, fever, chest pain and body weight decrease > 5%, were similar for both the groups. However, a significantly higher proportion of PTB patients with DM had presented with anorexia and haemoptysis, while a significantly higher proportion of PTB patients without DM had presented with cough. Despite these differences, the results of testing of laboratory parameters were similar for both groups (Table3), as was the proportion of patients who had undergone a standard treatment regimen of anti-TB medications and had experienced non-adherence and/or adverse drug events. Moreover, both the groups had undergone treatment regimens of a similar duration and had experienced similar treatment outcomes, including sputum-culture conversion rate, treatment success rate and case fatality rate, subsequent to completion of treatment (Table4).
Table 3.
Baseline characteristics and clinical and laboratory parameters of new cases of culture-positive pulmonary tuberculosis with and without diabetes mellitus after adjustment for age
| Characteristic | PTB patients < 50 years | PTB patients ≥ 50 years | DM vs. non-DM patients (all ages) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | Non-DM | p-Value | DM | Non-DM | p-Value | OR* (95% CI) | p-Value | |||||
| n | No. (%) | n | No. (%) | n | No. (%) | n | No. (%) | |||||
| Baseline characteristic | ||||||||||||
| Male sex | 17 | 17 (100) | 146 | 102 (69.9) | 0.007 | 20 | 11 (55.0) | 44 | 41 (93.2) | 0.001 | 1.129 (0.492–2.594) | 0.774 |
| Married | 17 | 9 (52.9) | 146 | 90 (61.6) | 0.665 | 20 | 14 (70.0) | 44 | 35 (79.5) | 0.526 | 0.657 (0.303–1.425) | 0.288 |
| Illiterate or primary school education only | 15 | 8 (53.3) | 135 | 61 (45.2) | 0.743 | 18 | 17 (94.4) | 41 | 34 (82.9) | 0.414 | 1.742 (0.683–4.446) | 0.245 |
| Smoking history | 16 | 13 (81.3) | 145 | 91 (62.8) | 0.233 | 20 | 11 (55.0) | 44 | 38 (86.4) | 0.010 | 1.306 (0.601–2.839) | 0.501 |
| Alcohol consumption | 16 | 14 (87.5) | 146 | 106 (72.6) | 0.244 | 20 | 12 (60.0) | 44 | 31 (70.5) | 0.566 | 1.137 (0.490–2.638) | 0.765 |
| Extra-pulmonary tuberculosis | 17 | 0 | 146 | 4 (2.7) | 1.000 | 20 | 1 (5.0) | 44 | 1 (2.3) | 0.531 | 0.941 (0.088–10.094) | 0.960 |
| Clinical presentation | ||||||||||||
| Cough | 17 | 14 (82.4) | 145 | 137 (94.5) | 0.093 | 19 | 16 (84.2) | 44 | 41 (93.2) | 0.355 | 0.324 (0.106–0.986) | 0.047 |
| Dyspnoea | 17 | 11 (64.7) | 145 | 98 (67.6) | 1.000 | 19 | 15 (78.9) | 44 | 26 (59.1) | 0.219 | 1.416 (0.638–3.142) | 0.392 |
| Anorexia | 17 | 9 (52.9) | 145 | 66 (45.5) | 0.746 | 19 | 15 (78.9) | 44 | 21 (47.7) | 0.043 | 2.148 (1.001–4.607) | 0.050 |
| Fever | 17 | 10 (58.8) | 145 | 107 (73.8) | 0.251 | 19 | 12 (63.2) | 44 | 28 (63.6) | 1.000 | 0.697 (0.326–1.489) | 0.352 |
| Chest pain | 17 | 7 (41.2) | 145 | 88 (60.7) | 0.199 | 19 | 10 (52.6) | 44 | 19 (43.2) | 0.678 | 0.789 (0.381–1.636) | 0.525 |
| Haemoptysis | 17 | 11 (64.7) | 145 | 58 (40.0) | 0.091 | 19 | 5 (26.3) | 44 | 7 (15.9) | 0.485 | 2.388 (1.061–5.376) | 0.036 |
| Body weight decrease > 5% | 14 | 9 (63.4) | 135 | 95 (70.4) | 0.761 | 15 | 13 (86.7) | 32 | 20 (62.5) | 0.170 | 1.420 (0.569–3.543) | 0.452 |
| Laboratory parameter | ||||||||||||
| Haemoglobin level < 12 g/dl | 11 | 6 (54.5) | 88 | 48 (54.5) | 1.000 | 11 | 9 (81.8) | 28 | 16 (57.1) | 0.266 | 1.601 (0.599–4.274) | 0.348 |
| WBC count > 10 × 103/μl | 11 | 5 (45.5) | 88 | 63 (71.6) | 0.094 | 11 | 4 (36.4) | 28 | 15 (53.6) | 0.541 | 0.399 (0.153–1.038) | 0.059 |
| Sodium level < 135 mmol/l | 7 | 4 (57.1) | 52 | 22 (42.3) | 0.688 | 7 | 6 (85.7) | 19 | 13 (68.4) | 0.629 | 2.112 (0.572–7.792) | 0.262 |
| Creatinine level > 1.2 mg/dl | 9 | 0 | 67 | 2 (3.0) | 1.000 | 8 | 3 (37.5) | 25 | 2 (8.0) | 0.078 | 3.873 (0.710–21.131) | 0.118 |
| Albumin level < 3.5 g/dl | 10 | 4 (40.0) | 102 | 55 (53.9) | 0.513 | 12 | 8 (66.7) | 29 | 21 (72.4) | 0.721 | 0.649 (0.245–1.716) | 0.383 |
| AST level > 40 U/l | 11 | 2 (18.2) | 104 | 23 (22.1) | 1.000 | 12 | 3 (25.0) | 30 | 11 (36.7) | 0.719 | 0.665 (0.221–2.000) | 0.468 |
| ALT level > 40 U/l | 11 | 3 (27.3) | 104 | 16 (15.4) | 0.387 | 13 | 3 (23.1) | 30 | 10 (33.3) | 0.720 | 1.073 (0.382–3.016) | 0.893 |
| Cavity on chest radiograph | 16 | 7 (43.8) | 129 | 45 (34.9) | 0.674 | 18 | 5 (27.8) | 38 | 10 (26.3) | 1.000 | 1.282 (0.572–2.870) | 0.546 |
| Sputum AFB grading 3+ | 17 | 5 (29.4) | 146 | 32 (21.9) | 0.542 | 20 | 4 (20.0) | 44 | 12 (27.3) | 0.755 | 1.026 (0.443–2.377) | 0.952 |
Mantel–Haenszel (adjusted) common odds ratio estimate. PTB, pulmonary tuberculosis; DM, diabetes mellitus; OR, odds ratio; CI, confidence interval; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFB, acid-fast bacilli.
Table 4.
Management and treatment outcomes of new cases of culture-positive pulmonary tuberculosis with and without diabetes mellitus after adjustment for age
| Characteristic | PTB patients < 50 years | PTB patients ≥ 50 years | DM vs. non-DM (all ages) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | Non-DM | p-Value | DM | Non-DM | p-Value | OR* (95% CI) | p-Value | |||||
| n | No. (%) | n | No. (%) | n | No. (%) | n | No. (%) | |||||
| Management | ||||||||||||
| Standard regimen | ||||||||||||
| Intensive phase | 17 | 17 (100) | 146 | 144 (98.6) | 1.000 | 20 | 18 (90.0) | 44 | 44 (100) | 0.094 | 0.152 (0.013–1.722) | 0.128 |
| Continuation phase | 16 | 16 (100) | 130 | 127 (97.7) | 1.000 | 19 | 16 (84.2) | 41 | 41 (100) | 0.028 | 0.160 (0.023–1.132) | 0.066 |
| Duration, median (IQR) | ||||||||||||
| Intensive phase, days | 17 | 64.0 (56.5–70.0) | 146 | 62.0 (54.0–73.5) | 0.499 | 19 | 57.0 (51.0–83.0) | 44 | 58.0 (17.8–72.0) | 0.595 | − | − |
| Continuation phase, days | 16 | 62.0 (54.0–73.5) | 126 | 119.0 (112.0–140.0) | 0.387 | 17 | 121.0 (115.0–144.5) | 41 | 124.0 (115.0–176.5) | 0.533 | − | − |
| Non-adherence | ||||||||||||
| Intensive phase | 17 | 2 (11.8) | 144 | 19 (13.2) | 1.000 | 19 | 4 (21.1) | 44 | 5 (11.4) | 0.434 | 1.361 (0.490–3.782) | 0.555 |
| Continuation phase | 16 | 8 (50.0) | 130 | 51 (39.2) | 0.577 | 16 | 2 (12.5) | 40 | 11 (27.5) | 0.308 | 0.968 (0.418–2.242) | 0.939 |
| Adverse drug events | ||||||||||||
| Intensive phase | 16 | 12 (75.0) | 123 | 76 (61.8) | 0.450 | 16 | 14 (87.5) | 35 | 24 (68.6) | 0.185 | 2.262 (0.867–5.906) | 0.095 |
| Continuation phase | 3 | 2 (66.7) | 57 | 26 (45.6) | 0.594 | 7 | 6 (85.7) | 18 | 12 (66.7) | 0.626 | 2.708 (0.501–14.649) | 0.247 |
| Treatment outcome | ||||||||||||
| Sputum-culture conversion | ||||||||||||
| At month 2 | 16 | 14 (87.5) | 133 | 113 (85.0) | 1.000 | 18 | 15 (83.3) | 41 | 33 (80.5) | 1.000 | 1.225 (0.422–3.552) | 0.709 |
| At month 5 | 14 | 12 (85.7) | 125 | 122 (97.6) | 0.079 | 18 | 18 (100) | 40 | 39 (97.5) | 1.000 | 0.324 (0.058–1.809) | 0.199 |
| Treatment success | 17 | 14 (82.4) | 146 | 118 (80.8) | 1.000 | 20 | 16 (80.0) | 44 | 38 (86.4) | 0.712 | 0.859 (0.335–2.201) | 0.751 |
| Cured | 12 (85.7) | 88 (74.6) | 9 (56.3) | 28 (73.7) | ||||||||
| Treatment completed | 2 (14.3) | 30 (25.4) | 7 (43.7) | 10 (26.3) | ||||||||
| Death | 17 | 0 | 142 | 3 (2.1) | 1.000 | 20 | 2 (10.0) | 44 | 1 (2.3) | 0.228 | 2.232 (0.322–15.454) | 0.416 |
Mantel–Haenszel (adjusted) common odds ratio estimate. PTB, pulmonary tuberculosis; DM, diabetes mellitus; OR, odds ratio; CI, confidence interval; IQR, interquartile range.
Discussion
Tuberculosis is an infectious disease caused by the bacillus M. tuberculosis, which typically affects the lungs 2. The burden of TB is highest in Asia, particularly in Southeast Asia and the Western Pacific region, where TB cases account for 60% of the cases reported worldwide. A recent study ranked Thailand, a Southeast Asian nation, 18th in a list of countries experiencing the highest incidence of TB 2. Among the major risk factors for developing TB, which include contraction of immunodeficiency diseases, such as HIV or AIDS; poverty; illiteracy; smoking; and development of DM 2,24, the last has been gradually increasing worldwide, especially in Southeast Asia 4, raising concern regarding TB and DM as comorbid conditions 7.
Despite knowledge of the concurrent increase in TB and DM incidence, collection of data regarding the characteristics of TB patients with DM has been limited to the incidence of DM in TB patients and the differences between the clinical and laboratory parameters, MDR-TB incidence and treatment outcomes of TB patients with and without DM. Previous studies have reported that patients experiencing different degrees of TB infection severity with HIV coinfection were 21–34 times at higher risk of developing active TB, leading to increased risk of death 25. In order to clarify the role of DM in the treatment and management of TB patients, no patients with HIV coinfection were included in the current prospective study, which aimed to determine the proportion of newly diagnosed cases of PTB presenting with DM at two hospitals in Thailand and compare clinical and laboratory parameters, drug susceptibility and treatment outcomes in PTB patients with DM and without DM.
Among the 227 new cases of culture-positive PTB identified, 16.3% were found to have DM, a percentage similar to that found in other tropical countries 2. Among the PTB patients with DM, 70.3% had been diagnosed with DM prior to PTB diagnosis and 29.7% had developed DM at PTB diagnosis. While no significant differences were found between PTB patients with DM and PTB patients without DM regarding the majority of the baseline characteristics and clinical parameters examined, PTB patients with DM were found to be significantly older and have a significantly lower level of education, which accorded with previous reports of advanced age and lower level of education as risk factors for DM 4,26. After adjustment for age as a confounding factor, a significantly higher proportion of PTB patients without DM was found to have presented with the symptom of cough, whereas a significantly higher proportion of PTB patients with DM was found to have presented with anorexia and haemoptysis, findings similar to those of a previous study reporting that the clinical presentation of PTB differs little between patients with and without DM 27. However, other studies have also reported differences in the results of chest radiography in PTB patients with and without DM because of differences in duration of illness and host immune status 7,14,28–34. While some studies have reported a higher incidence of multi-lobar disease and multiple cavities on chest radiography in PTB patients with DM 14,28,30,32–34, other studies have reported no differences in chest radiography between PTB patients with and without DM 29,31. The current study, which found no significant differences in the chest radiography results of PTB patients with and without DM, accords with the latter group of studies.
Previous studies have reported sputum-culture conversion duration of 42–67 days in PTB patients with DM, compared with a shorter duration of 37–39 days in PTB patients without DM 13,35,36. On the other hand, previous studies have also reported that the sputum-culture conversion rate at 2 months after anti-TB therapy did not significantly differ in PTB patients with DM and without DM (82–86% and 90–99%, respectively) 23,37, a finding in accordance with the results of this study. While similar proportions of PTB patients with DM and PTB patients without DM were observed to have a high mycobacterium burden in sputum in this study, it is probable that the prolonged sputum-culture conversion duration in PTB patients with DM was because of factors other than high mycobacterium burden in sputum. Previous studies have hypothesised that the lower level of rifampicin observed in the serum of TB patients with DM may have been because of decreased absorption and protein binding of the drug, which could have led to increased sputum-culture conversion duration, MDR-TB rate, treatment failure rate and/or mortality in these patients 7,15,38,39.
Previous studies have also raised concerns regarding drug interactions between anti-TB and hypoglycaemic drugs, with one reporting a decrease in serum concentrations of sulphonylurea and thiazolidinedione with simultaneous administration of rifampicin. In this study, similar adverse drug events were observed in PTB patients with and without DM, including rash (45.4%), peripheral neuropathy (36.6%), visual disturbance (20.0%), fatigue (10.7%), cholestasis (2.9%) and hepatitis (2.0%). In our clinical practice, pyridoxine is commonly prescribed to prevent peripheral neuropathy in PTB patients at risk of developing malnutrition because of anorexia or ageing. MDR-TB incidence, treatment success rate and mortality were likewise found to be similar for PTB patients with and without DM. After completion of treatment, PTB patients with DM who had experienced treatment success did not experience relapse of PTB during the 12-month follow-up period, a finding that contrasts with those of previous studies reporting a higher risk of MDR-TB, mortality, treatment failure and relapse for PTB patients with DM 11–15,40. This discrepancy may have been because of the inclusion of only newly diagnosed PTB patients and exclusion of patients with HIV coinfection in this study. Another discrepancy in findings was the identification of a treatment success rate of approximately 80% for both PTB patients with DM and PTB patients without DM, a percentage slightly lower than that (88%) reported by previous studies conducted in other high TB-burden countries 2, which may have been because of higher incidence of non-adherence during the intensive and continuation phases.
In conclusion, the incidence of DM in the newly diagnosed PTB patients examined in this study was 16.3%, of whom 70.3% had been diagnosed with DM prior to PTB diagnosis and 29.7% had developed DM at PTB diagnosis. The majority of clinical and laboratory parameters, as well as the MDR-TB incidence and treatment outcomes, were similar in PTB patients with DM and those without DM. These findings, particularly that 30% of PTB patients with DM may have developed DM at PTB diagnosis and that many patients later diagnosed with DM presented with a high plasma glucose level during PTB diagnosis, suggest that plasma glucose levels should be monitored during PTB diagnosis. The findings also suggest that similar treatment for newly diagnosed PTB patients with DM and without DM should be provided in high TB-burden countries.
Author contributions
Duangjai Duangrithi developed the study design, enrolled the study participants, collected clinical data for analysis, performed statistical analysis and data interpretation, wrote the manuscript for publication and approved the manuscript for submission.
Vipa Thanachartwet developed the study design, performed statistical analysis and data interpretation, wrote and reviewed the manuscript for publication and approved the manuscript for submission.
Varunee Desakorn developed the study design, performed statistical analysis and data interpretation, wrote and reviewed the manuscript for publication and approved the manuscript for submission.
Pasakorn Jitruckthai enrolled the study participants, collected clinical data for analysis, helped organise the study and approved the manuscript for submission.
Kamol Phojanamongkolkij enrolled the study participants, collected clinical data for analysis, helped organise the study and approved the manuscript for submission.
Somsak Rienthong performed identification of mycobacterium and drug susceptibility testing and approved the manuscript for submission.
Charoen Chuchottaworn developed the study design, reviewed the manuscript critically for publication and approved the manuscript for submission.
Punnee Pitisuttithum supervised and organised the study, commented on the manuscript for publication and approved the manuscript for submission.
Acknowledgments
We would like to thank the doctors, nurses and staff at the Queen Sawang Vadhana Memorial Hospital and the Chonburi Hospital, Chonburi province, Thailand for their help with this study. We extend special thanks to Associate Professor Pratap Singhasivanon, Dean of the Faculty of Tropical Medicine for his assistance with this manuscript; to Duangjai Sahassananda for her assistance with data management; to Dr Ongard Kosintarajit, Chonburi Hospital, Dr Apichart Chinnawan, Chonburi Hospital, Dr Arun Lerdworawiwat, Chonburi Hospital, Dr Natcha Laopichainpong, Muang Hospital, and Dr Poonlarb Panjaluk, Aowudom Hospital, for their assistance with enrolment of study participants; and to Mr Chavapon Oudkla, Microbiology Unit at the Queen Sawang Vadhana Memorial Hospital, Ms Wacharee Joraka, Microbiology Unit at the Chonburi Hospital, Mr Sermsak In-u-dom, Microbiology Unit at the Muang Hospital, and Mr Boonrod Notavean, Microbiology Unit at the Aowudom Hospital, for performing smearing, storing and packing of AFB samples.
Funding
This study was supported by the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
References
- 1.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–61. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2012. Geneva: WHO; 2012. http://www.who.int/tb/publications/global_report/en/ (accessed November 2012) [Google Scholar]
- 3.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Tuberculosis and Diabetes 2011. Geneva: WHO; 2011. , September http://www.who.int/tb/publications/diabetes_tb.pdf/ (accessed May 2012) [Google Scholar]
- 6.Brostrom RJ. Summary of the Impact of Diabetes on Tuberculosis Control and Submission of Draft Standards for Diabetes and Tuberculosis in the US-affiliated Pacific Islands. Nadi: WHO; 2010. , May http://www.spc.int/tb/en/publications/doc_download/73-summary-of-the-impact-of-diabetes-on-tuberculosis-control/ (accessed May 2012) [Google Scholar]
- 7.Dooley K, Chaisson R. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–94. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 10.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tubercle Lung Dis. 1999;79:235–42. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 11.Mboussa J, Monabeka H, Kombo M, Yokolo D, Yoka-Mbio A, Yala F. Course of pulmonary tuberculosis in diabetics. Rev Pneumol Clin. 2003;59:39–44. [PubMed] [Google Scholar]
- 12.Morsy AM, Zaher HH, Hassan MH, Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J. 2003;9:689–701. [PubMed] [Google Scholar]
- 13.Dooley K, Tang T, Golub J, Dorman S, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CS, Yang CJ, Chen HC, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–10. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 15.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes 2010. Geneva: WHO; 2010. http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf/ (accessed November 2012) [Google Scholar]
- 18.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. BJCP. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Laboratory Services in Tuberculosis Control Part II, 1998. Geneva: WHO; 1998. http://www.phppo.cdc.gov/dls/ila/documents/lstc2.pdf/ (accessed October 2012) [Google Scholar]
- 21.Alisjahbana B, van Crevel R, Sahiratmadja E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 22.Mugusi F, Swai AB, Alberti KG, McLarty DG. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tuberculosis. 1990;71:271–6. doi: 10.1016/0041-3879(90)90040-f. [DOI] [PubMed] [Google Scholar]
- 23.Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 24.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. TB/HIV Facts 2012-2013. Geneva: WHO; 2012. http://www.who.int/tb/publications/factsheet_tbhiv.pdf/ (accessed December 2012) [Google Scholar]
- 26.Sacerdote C, Ricceri F, Rolandsson O, et al. Lower educational level is a predictor of incident type 2 diabetes in European countries: the EPIC-InterAct study. Int J Epidemiol. 2012;41:1162–73. doi: 10.1093/ije/dys091. [DOI] [PubMed] [Google Scholar]
- 27.Faurholt-Jepsen D, Range N, PrayGod G, et al. The role of diabetes on the clinical manifestations of pulmonary tuberculosis. Trop Med Int Health. 2012;17:877–83. doi: 10.1111/j.1365-3156.2012.03002.x. [DOI] [PubMed] [Google Scholar]
- 28.Ikezoe J, Takeuchi N, Johkoh T, et al. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: comparison with patients who had no underlying disease. Am J Roentgenol. 1992;159:1175–9. doi: 10.2214/ajr.159.6.1442377. [DOI] [PubMed] [Google Scholar]
- 29.Morris JT, Seaworth BJ, McAllister CK. Pulmonary tuberculosis in diabetics. Chest. 1992;102:539–41. doi: 10.1378/chest.102.2.539. [DOI] [PubMed] [Google Scholar]
- 30.Umut S, Tosun GnA, Yildirim N. Radiographic location of pulmonary tuberculosis in diabetic patients. Chest. 1994;106:326. [PubMed] [Google Scholar]
- 31.Bacakoglu F, Basoglu OK, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration. 2001;68:595–600. doi: 10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Guzman C, Tottes-Cruz A, Villarreal-Velarde H, Vargas M. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med. 2000;162:1738–40. doi: 10.1164/ajrccm.162.5.2001040. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Salazar-Lezama MA, Vargas MH. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis. 2001;5:455–61. [PubMed] [Google Scholar]
- 34.Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–83. [PubMed] [Google Scholar]
- 35.Guler M, Unsal E, Dursun B, Aydin O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–5. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 36.Restrepo BI, Fisher-Hoch SP, Smith B, Jeon S, Rahbar MH, McCormick JB. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79:541–4. [PMC free article] [PubMed] [Google Scholar]
- 37.Singla R, Khan N, Al-Sharif N, Al-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–9. [PubMed] [Google Scholar]
- 38.Nijland HM, Ruslami R, Stalenhoef JE, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848–54. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 39.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 40.Jiménez-Corona ME, Cruz-Hervert LP, García-García L, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68:214–20. doi: 10.1136/thoraxjnl-2012-201756. . doi:10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]


