Abstract
Invasion by marine nonindigenous species (NIS) is a spread phenomenon. The tunicate Pyura praeputialis shows pronounced disjoint geographical distribution: along thousands of kilometers in wave-swept headlands on the southeastern coast of Australia, from where it appears to have originated, and exclusively along 60–70 km inside the Bay of Antofagasta, Chile. mtDNA sequences suggested that the species invaded this rocky shore recently. We used field manipulations and juvenile P. praeputialis transplant techniques to test hypotheses regarding the capacity of the tunicate to survive and grow at different sites and tidal heights inside and outside Antofagasta, and its competitive performance for primary space (inside the Bay) against the native mussel Perumytilus purpuratus. We conclude that survival and growth of P. praeputialis showed no significant differences among sites inside and outside the Bay, and suggest that the restrictive distribution of the species in Chile is caused by a specific oceanographic retention mechanism and/or its brief larval dispersal. We demonstrated that, inside the Bay, P. praeputialis outcompetes Perumytilus from the Mid–Low intertidal, constraining Perumytilus to the Upper Mid-Intertidal, modifying the local pattern of intertidal zonation. We show that predation on P. praeputialis juveniles by starfish and snails constitutes a regulatory mechanism for the setting of its low intertidal limit. Major ecological impacts caused by NIS invasions to rocky shores by aggressive primary space users may result in negative aspects, but also may contribute to biodiversity enhancement. We call attention to the need for increment manipulations and testing of ecological hypotheses regarding marine NIS.
Invasion by marine nonindigenous species (NIS) is a wide spread phenomenon (1–8). Marine organisms have been moved around the world accidentally or intentionally. Ports have received for centuries fouled ships, the off-loading of ballast water and “dry” ballast (sand, shingle, rocks, beach debris); aquaculture is now considered one of the major gateways for the introduction of marine NIS (8, 9). Nevertheless, ecological and evolutionary consequences of marine NIS invasions on local communities lags behind that of terrestrial and freshwater communities (10, 11). Invasions by marine NIS may have negative, neutral, or positive impacts on native species, communities, and ecological processes (5, 12–19). For the Southern hemisphere, several marine NIS invasive examples, expanding at fast rates, affecting rocky intertidal and shallow inshore water communities, have been reported. Northern hemisphere barnacles Balanus amphitrite and Balunus glandula invaded (1960–1970) intertidal rocky shores in the southwest Atlantic (Argentina), and ≈30–40 years later have expanded >10° of latitude (7, 20, 21). The Mediterranean mussel Mytilus galloprovincialis arrived on the west coast of South Africa around 1970, and over ≈30 years has spread over thousands of kilometers, becoming the dominant intertidal organism and outcompeting indigenous mussels and limpets for primary space (2, 18, 22). The kelp, Undaria pinnatifida, native to Japan, Korea, and regions of China, is an aggressive invader in the Mediterranean, New Zealand, Tasmania, Spain, United Kingdom, Belgium, and the Netherlands. In 1992, this kelp was recorded, for the first time, attached to wharf pilings in Puerto Madryn, Argentina (23), and ≈8 years later its range had expanded nearly 20 km to the north and south of the port. The dislodged thalli of Undaria are pulled by tides, disturbing the bottom and benthic communities. Codium fragile var tomentosoides, the “broccoli weed,” originally from Japan, has readily expanded to Europe, Australia, New Zealand, Canada, and the United States, and recently invaded Gracilaria chilensis cultures in northern Chile, where it is considered a pest (8).
Pyura praeputialis (Heller 1878) is an intertidal and shallow subtidal, solitary barrel-shaped tunicate, reaching up to 30–35 cm in height in the intertidal, which shows a conspicuous, disjoint geographical distribution, including coasts of Australia, Tasmania, and Chile (24). The species is abundant on wave-swept headlands on the southeastern shores of Australia, from where it appears to have originated (25–29). In Chile, the species is present exclusively along ≈60–70 km of rocky coast inside the Bay of Antofagasta (23° 38′ S; 70° 23′ W) (30–33). Based on cytochrome oxidase I (COI) mitochondrial sequences, P. praeputialis has been suggested as a recent invader, probably having arrived a few hundred years ago from Australia (34). At Antofagasta, P. praeputialis exists as extensive aggregations of cemented individuals that attain a collective unity (packed clumps, matrices; see Fig. 1 A and B) or pseudocoloniality (31, 32, 35). P. praeputialis appears to be an aggressive interspecific competitor for primary space (28, 36). The interspecific competitive capabilities of P. praeputialis and possible factors that may set its upper and lower intertidal limits have been highlighted (31). These authors concluded that because the P. praeputialis upper intertidal limit ended rather abruptly, competition with the native mussel Perumytilus or the barnacle Chthamalus cirratus (=Notochthamalus cirratus) were unlikely factors determining the tunicate intertidal limits (see Fig. 1C). Instead, they proposed that increased physiological stress and/or reduced feeding (both function of immersion/emersion time) were key limiting factors. Also, they suggested that the P. praeputialis matrices were characterized by a set of competitively superior characteristics, enhancing an aggressive displacement of other local intertidal species that use primary substratum.
Fig. 1.
Rocky intertidal shore at Antofagasta Bay. (A) Extended intertidal belts of P. praeputialis covered by Ulva spp. (green). (B) Close-up of P. praeputialis clumps, showing patches created by storms. (C) P. praeputialis (green, low-mid intertidal) and Perumytilus (violet, mid-upper intertidal) belts. (D) P. praeputialis collection for bait and food by an intertidal food-gatherer during low tide.
Here we propose that P. praeputialis invasion at Antofagasta Bay constituted a major perturbation of the original zonation pattern. P. praeputialis probably invaded upon its arrival, displacing to the upper shore an inferior competititor, the mussel, Perumytilus (Fig. 1C). This zonation contrasts with mid-intertidal fringes at other localities along the Chilean coast, where Perumytilus dominates (37, 38). Furthermore, P. praeputialis beds do not end as abruptly toward their lower intertidal limit, where primary space is dominated by crustose lithothamnioid and erect coralline algae (39); and based on field observations, predation has been suggested as the major structuring factor (31). We carried out experiments along ≈200 km of coastline, within an active upwelling zone (40), both inside and outside the Antofagasta Bay and tested (i) whether the exclusive presence of P. praeputialis inside Antofagasta is because the species cannot live outside the bay; (ii) whether there is interspecific competition between Perumytilus and P. praeputialis; and (iii) whether predation by invertebrates affects P. praeputialis survival to a greater extent at their lower limit, where predators tend to be more abundant, than at the center of the tunicate belt.
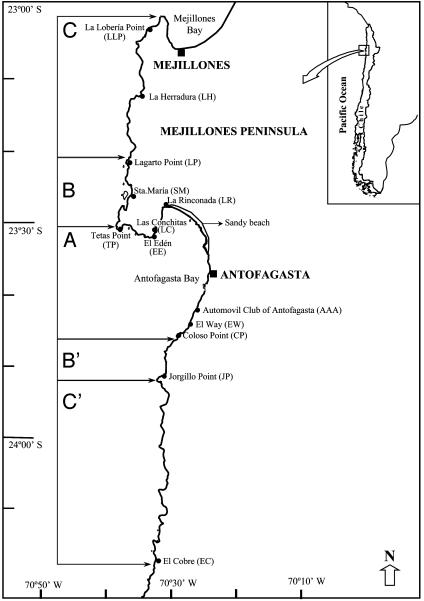
Methods
Transplants of P. praeputialis Juveniles. Tunicate clumps, from the mid-low intertidal fringe (M-LIF, ref. 37) (semidiurnal tides, maximum excursion ≈1.8 m) at El Way (EW, 23° 45′ S; 70° 26′ W, Fig. 2), were gently separated from the substrate and transferred to the laboratory within 3 h. Juveniles (non-mature) were detached from these adults. Juveniles were measured for maximum diameter (36), wet mass was recorded, and juveniles were sorted for transplanting if their diameter was ≈14.5 mm ± 2 mm and their mass was 2.4 ± 1.4 g. At Antofagasta, mature P. praeputialis have a mean wet mass of 259.2 g (SE = 7.3; unpublished results). We avoided transplants of P. praeputialis adults outside the bay to minimize the probability for spreading the tunicate via larvae produced by spawners. Transplants were made to eight intertidal sites. At three of them (inside Antofagasta Bay, Fig. 2 area A), P. praeputialis shows elevated densities (41): Coloso Point (CP: 23° 45′ S; 70° 27′ W), La Rinconada (LR: 23° 27′ S; 70° 30′ W), and Las Conchitas (LC: 23° 31′ S; 70° 32′ W). Two were outside (north) of the bay, within the northern P. praeputialis range boundary area (Fig. 2, area B): Santa María (SM: 23° 24′ S; 70° 35′ W) and Lagarto Point (LP: 23° 22′ S; 70° 36′ W). Two were outside (north) of the bay, but outside the range of distribution of P. praeputialis (Fig. 2, area C): La Herradura (LH: 23° 12′ S; 70° 35′ W), La Lobería Point (LLP: 23° 03′ S; 70′ 31′ W); one site was made outside (south) of the bay, El Cobre (EC: 24° 17′ S; 70° 31′ W) (Fig. 2, area C′). Transplants were made at the M-LIF and low-intertidal fringe (L-IF) at each site. Five experimental units were randomly assigned to each combination of tidal height and site. Each unit consisted of 10 P. praeputialis juveniles arranged within an open PVC cylinder (4-cm diameter × 2.5-cm height). The distance between replicate units was 1–5 m. In the areas with resident tunicate, bare sites (without P. praeputialis) were used (resident P. praeputialis at least 1 m distance from the units). Ten juveniles were randomly assigned to each experimental unit with an average total biomass of 23.8 g (SD = 4.1); before their transplants, they were maintained for 24 h under running seawater. Additionally, 100 extra juveniles were randomly selected (total wet mass and maximum diameter was recorded) and frozen for later dry tunic and visceral mass determinations. We adjusted the relationship of wet mass (win) on dry mass (dm) by simple linear regression: dm = 0.16 + 0.29 win (R2 = 0.70, P < 0.001) [Eq. 1]. We used nonlinear regression to obtain the relationship of diameter (d) on visceral dry mass (vm): vm = 0.000067 d2.448 (R2 = 0.51, P < 0.001) [Eq. 2]. To exclude predators, the units were protected by an external PVC cylinder (9-cm diameter × 3-cm height) and covered with two types of plastic nets (a fine, 2-mm aperture net, “Raschel Marienberg,” 20 cm wide × 20 cm long; and a coarse, 6-mm aperture net, “Tehmco,” 20 cm wide × 20 cm long). Units were screwed to M-LIF rocky platforms (without crevices), on slopes ≤10° using stainless steel bolts. Nets were cleaned of epibionts after 1 month and once predator presence or absence was verified.
Fig. 2.
Map of El Cobre–Antofagasta–Mejillones Peninsula (Chile), divided into five areas: A, inside Antofagasta Bay; B and B′, outside the bay and around the northern and southern distribution range boundary of P. praeputialis; C and C′, outside the range of distribution of P. praeputialis.
Experiments were fully replicated twice: (i) March 17–20 to June 14–16, 1999 (91 days, Austral Winter) and (ii) November 21–24, 1999, to March 21–23, 2000 (122 days, Austral Summer), except at El Cobre, were a single transplant (November 1999 to March 2000) was done. At the end of each transplant we determined: (i) the number of individual P. praeputialis alive and (ii) growth, by measurements of maximum height, maximum diameter, dry mass, and visceral tissue (42). Dry mass was determined in grams (±0.001, Sartorious balance), by oven drying the respective tissues at 70°C for 72 h. A four-way (area, site, date, and tidal height) mixed ANOVA was used for P. praeputialis survival data, and a four-way mixed analyses of covariance (ANCOVA), using survival numbers as covariate for the tunic mass and visceral dry mass data. Area, date, and tidal height were considered as fixed factors, and site nested within area was considered a random factor. For the tunic mass and visceral dry mass analyses, the average final mass (mf) for each replicate was corrected (mc) by the corresponding average initial mass (mi) by using the following formula: mc = [(mf – mi) × 100/n of days]. The initial dry viscera mass for each individual was estimated by using Eq. 2. The initial total dry mass was estimated by using Eq. 1. The initial tunic dry mass was estimated as the difference of initial total dry mass minus initial visceral dry mass (36).
Competition Experiments. To test the hypothesis that P. praeputialis competitively displaces Perumytilus, we transplanted adult mussels inside P. praeputialis matrices in PVC ring units (14-cm diameter; 3-cm height; covered with a plastic 5-mm pore net), bolted to rocks. Three experimental units of Perumytilus were transplanted at each of five intertidal flat platforms (<10° slope, separated by ≈25–30 m), at the Automovil Club de Antofagasta (AAA, Fig. 2). There were three competition treatments: (i) M-LIF transplants very close (≈5 cm) to the P. praeputialis matrix border; (ii) M-LIF transplants separated (15–50 cm) from the P. praeputialis matrix border; and (iii) Mid-upper intertidal fringe (M-UIF, ref. 37) transplants in the Perumytilus matrices (control). Fifty-five mussels were collected from El Way (Fig. 2): n = 5 >30 mm, n = 20 between 25 and 30 mm, and n = 30 between 10 and 24.9 mm, were placed in each unit. We used mixed size structure for our mussel transplants to represent the size structure of mussel matrices (43). Units were transplanted in August 2002 and covered with plastic nets, surrounded by 14-cm-diameter plastic rings, and bolted to rocks. To ensure mussel attachment, the units remained fixed to the rocks for 90 days; every 30 days, algae attached to nets were removed. After the removal of the ring and plastic nets (November 2002), mussels surviving were photographed (digital camera Kodak DC-280) every 30 days until April 2003 and counted, and the percent encroachment on Perumytilus by P. praeputialis was estimated (cm2). To avoid predation on P. praeputialis and transplanted Perumytilus, the sunstar Heliaster helianthus, common in the area and by far the most frequent P. praeputialis predator (44), was manually removed from the experimental platforms at the beginning of the experiment and at 7- to 14-day intervals thereafter. Heliaster removal (initial density of ≈0.25 ind × m–2) had a success of 95%. It was not possible to control for fish, bird, and crab predation. Failure-time analysis (45, 46) was used to analyze mussel survival pattern over time and to determine the effect of competition treatments. The analysis accommodates “censored” data corresponding to live mussels at the end of the experiment, or lost during the experiments by catastrophic events (e.g., predation and/or wave impact). Survival curves were tested for homogeneity between the three competition treatments by the SAS LIFETEST procedure (47) using a log-rank test. Sequential Bonferroni procedure was used to adjust for multiple comparisons.
Predation Experiments. PVC cylinder experimental units (diameter, 3.8 cm; height, 3.0 cm), bolted to rocks, were used in full exclusion, semiexclusion, and no exclusion (control) predator treatments. Full exclusion treatments also had the PVC base painted with either antifouling (to exclude herbivores) and non-antifouling paint (as a paint control). For full predator exclusion, the entrance of the cylinder was covered with an inner fine net (2-mm pore) and an external coarser net (6-mm pore). For semiexclusion, the entrance of the cylinder was covered only with the coarse net, permitting access by small predators, such as juveniles of the snail Thais haemastoma, but excluding large predators, such as starfishes and the muricid gastropod Concholepas concholepas (loco). Nets appear to be effective in preventing bird predation, because during ≈540 h of observations (low tides; accumulated time by four observers), we never detected bird predation in the experimental units. For no exclusion, the entrance to the cylinder were set without net protection (also, bird predation was never observed). Experiments were done at Coloso Point and La Rinconada at two intertidal heights: M-LIF and L-IF. At each site, six random blocks were installed on bare intertidal rocks, but with P. praeputialis at least 1.5 m from the experimental units. The distance between blocks was 3–10 m. Ten P. praeputialis juveniles (13- to 17-mm diameter and 19- to 24-mm maximum height; mean total biomass = 20 ± 2 g) were randomly assigned to each unit; units were assigned at random to the different treatments within each block: predation, antifouling, tidal height, and site. Experimental units were installed August 10–11, 1999, and kept in place for 2 months, covered with a 2-mm pore net, allowing the tunicate to adhere firmly to the cylinders. No P. praeputialis removals due to waves (physical forces) were observed. On October 22–27, 1999, the covering was removed. On January 22–23, 2000, the surviving tunicates and number of predators recorded on, inside, and 1 m around the experimental units were counted. For P. praeputialis survival data, a four-way, blocked, mixed-factor ANOVA was done: site, predation, antifouling, and tidal height, with a blocking factor within site. Also, a reduced three-way, blocked, mixed ANOVA was used: site, predation, and tidal height. P. praeputialis survival was used as the dependent variable, and we assumed no interaction effects between blocks and the other factors.
Statistical Analyses. For statistical analyses (see models above), we used proc glm ss3 for unbalanced raw data (47). If criteria for homoscedasticity were not met, the data were rank-transformed (48). For mixed models, we declared random variables in the RANDOM/TEST procedure of PROC GLM and computed the Satterthwaite correction for unbalanced designs (47). When the rank-transformed and untransformed raw data showed the same trends and significance, we only show the analyses for untransformed data. When interaction terms in factorial designs were significant, we compared cell means by using the SLICE procedure in proc glm (47).
Results
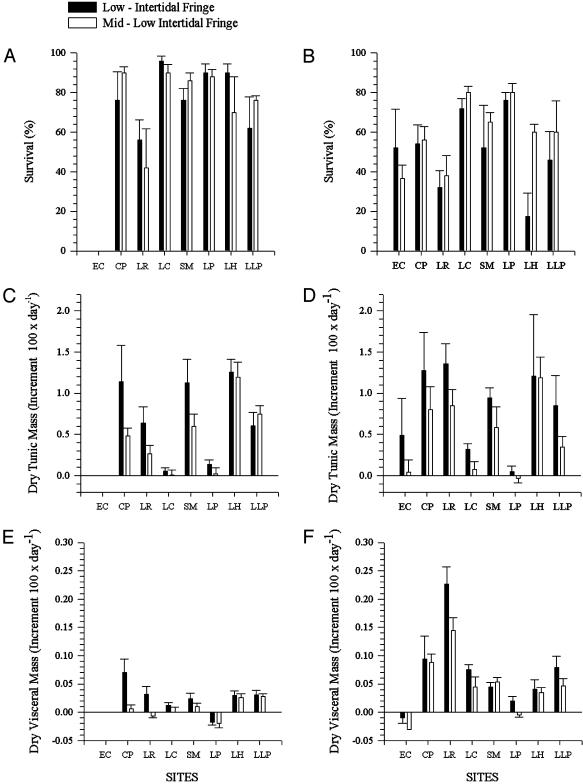
Transplants. The data did not meet the criterion of homoscedasticity; however, the rank-transformed and raw data showed the same trends and significance and we show results from nontransformed data. The survival of P. praeputialis juveniles was significantly greater (P = 0.006, Table 1 and Fig. 3 A and B) during the 1999 autumn–winter period than during the 1999–2000 spring–summer period. There was no significant difference (P = 0.54) between area A, which P. praeputialis typically inhabits, area B (its northern boundary range), and areas C and C′ (outside its distribution range in the Antofagasta Bay, Fig. 2). None of the interaction terms of the ANOVA were significant (Table 1). The analysis of covariance showed that growth, expressed as dry tunic biomass, was significantly affected by tidal height (P = 0.038, Table 2). Juveniles grew significantly faster at the L-IF than at the M-IF (Table 2 and Fig. 3 C and D), and there were significant differences among sites, within areas (P = 0.06, Table 2 and Fig. 3 C and D). Dry visceral biomass was significantly affected by tidal height (P = 0.018, Table 2), and variation among sites depended on the date of sampling (P = 0.026, Table 2). The dry visceral biomass was greater for the L-IF than for the M-IF (Fig. 3 E and F). The significance of the date × site (area) interaction is explained by the higher tunicate visceral growth rate at La Rinconada during the spring–summer period than at any other site in any other area (Fig. 3 E and F; P < 0.05, Tukey test).
Table 1. Four-way (area, site, date, and tidal height) mixed ANOVA for survival in the transplant experiment.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Area | 2 | 27.8417 | 0.70 | 0.539 |
| Site (area) | 5 | 39.3718 | 8.60 | 0.339 |
| Date | 1 | 146.4407 | 25.25 | 0.006 |
| Tidal height | 1 | 3.6343 | 0.58 | 0.481 |
| Area × date | 2 | 3.5859 | 0.62 | 0.582 |
| Area × tidal height | 2 | 0.6035 | 0.10 | 0.910 |
| Date × tidal height | 1 | 15.5561 | 2.07 | 0.221 |
| Date × site (area) | 4 | 5.7994 | 0.77 | 0.598 |
| Tidal height × site (area) | 5 | 6.2829 | 0.84 | 0.582 |
| Area × date × tidal height | 2 | 5.2577 | 0.70 | 0.547 |
| Date × tidal height × site (area) | 4 | 7.5563 | 1.31 | 0.269 |
| Error | 112 | 5.7528 |
MS, mean squares value; F, F ratio. Boldface indicates statistical significance.
Fig. 3.
Survival and growth of transplanted P. praeputialis juveniles at eight sites and two intertidal fringes: LIF (black bars), and M-LIF (white bars). Shown are1999 autumn–winter (A) and 2000 summer–spring (B) survival of P. praeputialis (+1 SE); 1999 autumn–winter (C) and 2000 spring–summer (D) mean growth dry tunic mass (+1 SE); 1999 autumn–winter (E) and 2000 spring–summer (F) mean growth (dry visceral mass) (+1 SE). EC, El Cobre; CP, Coloso Point; LR, La Rinconada; LC, Las Conchitas; SM, Santa María; LP, Lagarto Point; LH, La Herradura; LLP, La Lobería Point. Sites are as in Fig. 2.
Table 2. Four-way (area, site, date, and tidal height) mixed analysis of covariance for incremental dry tunics biomass and incremental dry visceral biomass.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Incremental dry tunic biomass | ||||
| Survival | 1 | 8.0028 | 54.81 | <0.001 |
| Area | 2 | 0.2290 | 0.10 | 0.907 |
| Site (area) | 5 | 2.2654 | 4.73 | 0.060 |
| Date | 1 | 0.9963 | 3.350 | 0.124 |
| Tidal height | 1 | 2.5000 | 8.01 | 0.038 |
| Area × date | 2 | 0.8127 | 2.40 | 0.202 |
| Area × tidal height | 2 | 0.0249 | 0.08 | 0.924 |
| Date × tidal height | 1 | 0.0140 | 0.09 | 0.777 |
| Date × site (area) | 4 | 0.3500 | 2.26 | 0.224 |
| Tidal height × site (area) | 5 | 0.3086 | 1.99 | 0.252 |
| Area × date × tidal height | 2 | 0.0106 | 0.07 | 0.934 |
| Date × tidal height × site (area) | 4 | 0.1549 | 1.06 | 0.380 |
| Error | 100 | 0.1460 | ||
| Incremental dry visceral biomass | ||||
| Survival | 1 | 0.0179 | 21.09 | <0.001 |
| Area | 2 | 0.0286 | 3.20 | 0.132 |
| Site (area) | 5 | 0.0090 | 0.78 | 0.613 |
| Date | 1 | 0.0274 | 4.19 | 0.164 |
| Tidal height | 1 | 0.0128 | 12.45 | 0.018 |
| Area × date | 2 | 0.0178 | 1.46 | 0.333 |
| Area × tidal height | 2 | 0.0021 | 2.07 | 0.225 |
| Date × tidal height | 1 | 0.0001 | 0.08 | 0.792 |
| Date × site (area) | 4 | 0.0127 | 9.39 | 0.026 |
| Tidal height × site (area) | 5 | 0.0010 | 0.77 | 0.616 |
| Area × date × tidal height | 2 | 0.0001 | 0.05 | 0.949 |
| Date × tidal height × site (area) | 4 | 0.0013 | 1.60 | 0.180 |
| Error | 100 | 0.0008 |
Boldface indicates statistical significance. The covariate used was survival number. See Table 1 for abbreviations.
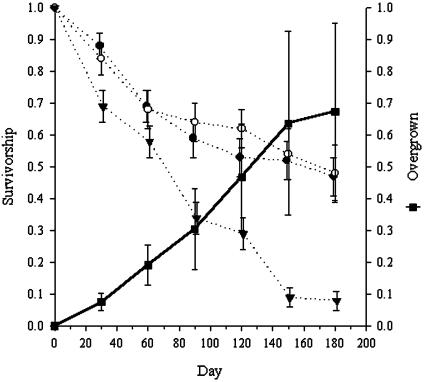
P. praeputialis–Perumytilus Competition. Two units from treatment 2 and two from treatment 1 were lost between 90 and 120 days after the initiation of the experiment. One experimental unit, originally from treatment 2, was completely overgrown by P. praeputialis after 60 days of initiating the experiment, so it was considered as belonging to treatment 1 (competition effect). Mussels from treatment 1 were systematically overgrown by P. praeputialis, and they showed lower survivorship than mussels not overgrown at the M-LIF and at the M-UIF (Fig. 4). Mussel survival analysis shows that survival time differed significantly among competition treatments (log-rank χ2 = 126.6, P < 0.001). Multiple paired comparisons between the three different treatments showed that mussels not subjected to competition did not differ significantly between tidal height (log-rank adjusted P = 0.41). Nevertheless, mussels overgrown showed a reduced and significantly different survival than mussels transplanted to the M-LIF (log-rank adjusted P < 0.001) and to the M-UIF (log-rank adjusted P < 0.001). The difference (non-overgrown minus overgrown survivorship) in survival of mussels under no competition (at both intertidal fringes) increased linearly, as did the percentage increase of overgrowth (regression analysis, P < 0.001, Fig. 4).
Fig. 4.
Perumytilus purpuratus mean survival in competition with P. praeputialis. Inverted filled triangles, mussel survival, overgrown by tunicate, at the M-LIF (treatment 1). Open circles, mussel survival, no overgrown by tunicate, at the M-LIF (treatment 2). Filled circles, mussel survival at the M-UIF (treatment 3, control). Error bars are confidence limits at 95%. The right Y axis (filled square) shows the proportion (mean ± 1 SE) of mussels overgrown by P. praeputialis in treatment 1.
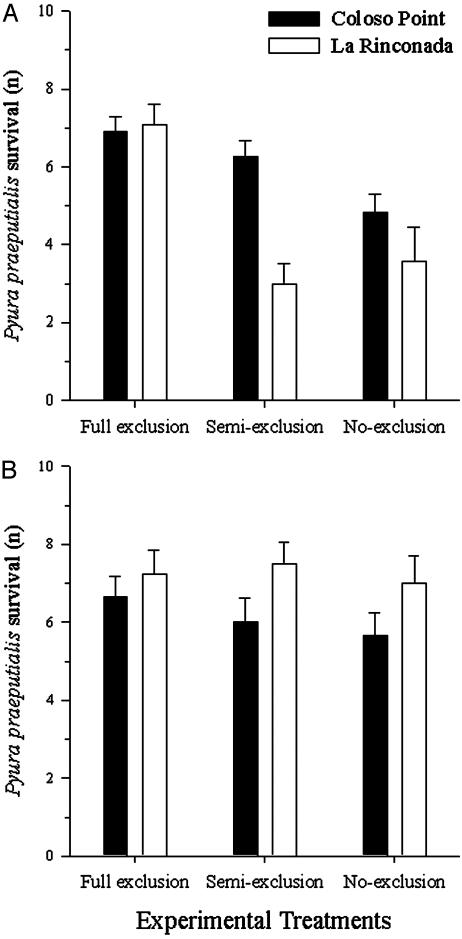
Predation. The data did not meet the criterion of homoscedasticity. However, the rank-transformed and raw data showed the same trend and significance and we show results from nontransformed data. There were no effects of antifouling paint, neither as a main effect, nor through its interactions with all of the other factors (P > 0.28); therefore, antifouling paint was not included in the statistical analysis and data were pooled. P. praeputialis survival was significantly affected by the third-order interaction of site × tidal height × treatment (P = 0.024, Table 3 and Fig. 5). This precludes an analysis of main effects and second order interactions. Therefore, in each combination of site and tidal heights, we compared the differential effects among predation treatment by using the SLICE option of proc glm (47). At Coloso Point and La Rinconada, there were significant differences among predation treatments at the L-IF (P < 0.027, Table 4 and Fig. 5A), but not at the M-LIF (P > 0.456, Table 4 and Fig. 5B). Also at Coloso Point, the full exclusion and semiexclusion predator treatments did not differ significantly (P = 0.50, Tukey's test adjusted for multiple comparisons). Nevertheless, both sites showed significantly greater survival for P. praeputialis than the control (i.e., no predator exclusion, P < 0.05, Tukey's test adjusted for multiple comparisons). At La Rinconada, only the predator full exclusion was significantly greater (P = 0.017, Tukey's test) than the other treatments, which did not differ significantly (P = 0.80)
Table 3. Three-way (site, tidal height, and treatment) ANOVA with blocking factor for P. praeputialis survival for predation experiment.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Site | 1 | 0.8403 | 0.11 | 0.744 |
| Tidal height | 1 | 70.8403 | 19.42 | <0.001 |
| Treatment | 2 | 38.0833 | 10.44 | <0.001 |
| Site × tidal height | 1 | 60.0625 | 16.47 | <0.001 |
| Site × treatment | 2 | 5.0278 | 1.38 | 0.256 |
| Treatment × tidal height | 2 | 18.7778 | 5.14 | 0.007 |
| Site × tidal height × treatment | 2 | 14.0833 | 3.86 | 0.024 |
| Block (site) | 10 | 7.4347 | 2.04 | 0.035 |
| Error | 122 | 3.6467 |
Boldface indicates statistical significance. See Table 1 for abbreviations.
Fig. 5.
P. praeputialis mean survival (+1 SE) under three predator-exclusion treatments (full exclusion, semi-exclusion, and no-exclusion) at Coloso Point (black bars) and La Rinconada (white bars) in two tidal heights: L-IF (A) and M-LIF (B).
Table 4. Site × tidal height × treatment effect sliced by site × tidal height for P. praeputialis survival (see Methods).
| Site | Tidal height | df | MS | F | P |
|---|---|---|---|---|---|
| Coloso Point | L-IF | 2 | 13.5833 | 3.72 | 0.027 |
| Coloso Point | M-LIF | 2 | 3.1111 | 0.85 | 0.456 |
| La Rinconada | L-IF | 2 | 58.5278 | 16.05 | <0.001 |
| La Rinconada | M-LIF | 2 | 0.7500 | 0.21 | 0.814 |
Boldface indicates statistical significance. See Table 1 for abbreviations.
Discussion
Survival and growth of juvenile transplanted P. praeputialis showed no significant differences among sites inside Antofagasta Bay, in the distributional range boundary areas, or sites outside the Bay, although P. praeputialis growth was significantly greater at the L-IF than at the M-LIF (Table 2 and Fig. 3 C–F). Even though P. praeputialis showed higher visceral growth rates at La Rinconada (spring–summer period), the general result confirms that juveniles of P. praeputialis can live and grow inside as well as outside Antofagasta Bay (Tables 1 and 2 and Fig. 3). This result suggests that the plastic cylinder transplant devices may be equivalent to protection by matrix tunicates and/or similar to natural rock crevices or upright structures (coralline algae, plastic brushes) (44), where newly arriving P. praeputialis tend to settle and establish.
The arrival of P. praeputialis to Antofagasta has resulted in a unique rocky intertidal seascape with P. praeputialis densities of >1,800 individuals × m–2 and average total dry biomass up to 20.45 k × m–2 at the center of the bay (41). We experimentally tested hypotheses regarding the capacity of P. praeputialis to survive and grow at different sites and tidal heights inside and outside the bay of Antofagasta, and of its competitive performance regarding the abundant native mussel Perumytilus. Perumytilus matrices transplanted to the M-LIF, and almost in contact with P. praeputialis, were systematically overgrown by the tunicate. Mussels overgrown by P. praeputialis showed reduced survival relative to mussels not overgrown (Fig. 4). The P. praeputialis encroaching mechanism, based on observations done inside mussel matrices completely overgrown by P. praeputialis, showed that juvenile and adult tunicate encroach and grow successfully on Perumytilus shells. Mussels subsequently became detached from the rock substratum, demonstrating that P. praeputialis outcompetes Perumytilus at this tidal height. At sites outside the bay, without P. praeputialis, the mussel occupies fully the mid-intertidal fringe (unpublished results, also see refs. 37 and 38), supporting our initial hypothesis that the NIS P. praeputialis is responsible for a major ecological impact on the Antofagasta rocky shore. Furthermore, preliminary evidence strongly suggests that, at the M-LIF, Perumytilus and/or mixed Perumytilus/P. praeputialis matrices enhance the recruitment of the tunicate probably via the influence of adults on the retention of the free-swimming P. praeputialis larvae, by adding space and surface area for recruitment (see ref. 49).
Predation has been suggested as the main factor governing the lower intertidal limit of P. praeputialis, probably in the same way that, for instance, Pisaster ochraceus regulates the lower intertidal limit of Mytilus californianus along the northwest coast of the United States (31, 50–52). Our predation experiments showed that, in the center of the P. praeputialis belt, there were no significant differences in P. praeputialis (juveniles) survival, suggesting that predation on juvenile tunicates does not play an important role in the dynamics of P. praeputialis at this tidal height. Nevertheless, L-IF predation treatments (Table 4 and Fig. 5) showed that tunicate survival was significantly greater for the full and semiexclusion predator treatments, as compared with controls. This suggests that predation on juveniles of P. praeputialis by starfish and snails may constitute a regulatory mechanism for tunicate population structure at this tidal height. At La Rinconada (a site characterized by small sized predators) the survival of juvenile P. praeputialis was significantly greater only in the full predator exclusion treatment. However, food-gathering by artisanal fishers on the muricid C. concholepas (53), a P. praeputialis predator, may be an important factor explaining reducing potential impact in the studied sites. Preliminary results at the Coloso Point rocky intertidal, inside Minera Escondida Limitada coastal reserve (44), suggest that high densities of C. concholepas (i.e., > 20 individuals × m–2) gathering in crevices under clumps of P. praeputialis may contribute to their eventual destruction, via predation on juvenile and adult tunicates. Furthermore, predation on intertidal P. praeputialis is not exclusively restricted to invertebrates. At Antofagasta, there are reports of predation on M-ILF attached P. praeputialis by the oystercatchers Haematopus palliatus pitanay and Haematopus ater (for Chile see ref. 54, and for Australia see ref. 55). Oystercatchers mainly feed at the center and upper sector of the P. praeputialis belts, selecting specific size classes (56). H. palliatus pitanay shows a mean consumption rate of 2.3 P. praeputialis × 5 min–1, and its foraging tends to be concentrated around packed P. praeputialis individuals. These foraging activities on P. praeputialis must be added to gathering for food and bait (Fig. 1D and refs. 32 and 53). Therefore, contrary to reports for the same tunicate in Australia (57), our results suggest that predation (mainly on juvenile tunicates, but also on adults by C. concholepas) and environmental disturbance by waves and storms (Fig. 1B and refs. 57 and 58) play important ecological roles in the structure and dynamics of P. praeputialis.
Worldwide, there is a lack of experimental manipulations on marine competitively dominant NIS (invertebrates), many of which cause major ecological impacts in coastal systems (but see ref. 19). This may be because few such cases exist or they have not been properly documented (18, 59). Alternatively, their impacts may be rare due to negative biotic interactions with native (resident) species and/or abiotic factors preventing this type of NIS from becoming established (16). The restricted distribution of P. praeputialis in Chile, exclusively inside the Bay of Antofagasta, is puzzling. Nevertheless, it is known that the oceanographic characteristics of this Bay are unique: (i) it is one of the few bays in Chile facing southward, (ii) there exists an upwelling-shadow water lens showing surface water temperature 2–4°C higher than outside water masses, (iii) and a retentive circulation is present (60), which, together with the tunicate short-lived larvae (33), may explain its retention inside the Bay. Tunicates are known to disperse widely via anthropogenic mechanisms (ship fouling, ballast water, “dry” ballast), and because port facilities and ship traffic in Antofagasta have existed since ≈1868 (34), it may appear odd that the species has not expanded its distribution. We are not aware of any special biotic conditions outside the Bay of Antofagasta in northern or central Chile intertidal systems (i.e., predation intensification) preventing the expansion of P. praeputialis. Therefore, a reduced dispersal distance (i.e., due to a brief larval interval: ≈2 h in the plankton, 33) coupled to a requirement for dense intertidal tunicate matrices, which facilitate P. praeputialis recruitment (58), may require special biological and oceanographic conditions, not commonly met.
Successful marine invasions on rocky shores by NIS, such as the tunicate P. praeputialis, altering the ecology of an intertidal system, may also cause positive impacts on habitat structure, bioarchitecture, and species diversity. P. praeputialis can be characterized as an ecosystem bioengineer (61) NIS, providing habitat for 116 species of macroinvertebrates and algae at the M-ILF in Antofagasta Bay (17); this is ≈50% higher than equivalent rocky intertidal fringes outside of the Bay (62). Experimental manipulations, as presented here, may contribute to a better understanding of the structure, dynamics, and resilience, or lack thereof, of rocky intertidal systems to marine NIS invaders over relatively short time periods, particularly, in view of possible facilitation of marine NIS invasions by future ocean warming (63). P. praeputialis invasion of the Bay of Antofagasta, seemingly within an historical time interval, continues to provide exceptional opportunities to explore the impact of an aggressive invader. Ecological invasions are continuing, even at an accelerating pace. Whether an established NIS can spread, and how far, remain critical and minimally explored issues. The presence of P. praeputialis in coastal Chile is permitting this challenge, important to both ecology and conservation biology, to be explored experimentally.
Acknowledgments
We acknowledge logistic support through the Universidad de Antofagasta, Facultad de Recursos del Mar, by Dean H. Baeza and Profesora M. Clarke. We sincerely thank M. Clarke, M. Uribe, R. Pinto, J. Alvarado, C. Pacheco, M. Cerda, and M. Varas for field and laboratory assistance. S. Navarrete, P. Neill, and B. Kelaher suggested modifications to different versions of the manuscript. We sincerely acknowledge R. T. Paine for important suggestions to the last version of the manuscript. We acknowledge financial support from the 1998 Cátedra Presidencial en Ciencias (to J.C.C.); Minera Escondida Limitada–Pontificia Universidad Católica de Chile grant; the Mellon Foundation–Pontificia Universidad Católica de Chile grant (to S. Navarrete and J.C.C.), and from the Center for Advanced Studies in Ecology & Biodiversity, Comisión Nacional de Investigación Científica y Tecnológica–Fondo Nacional de Desarrollo Cientifíco y Tecnológico Project 1501-0001, Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: NIS, nonindigenous species; M-LIF, mid-low intertidal fringe; L-IF, low intertidal fringe; M-UIF, mid-upper intertidal fringe.
See accompanying Biography on page 8514.
References
- 1.Carlton, J. (1996) Ecology 77, 1653–1655. [Google Scholar]
- 2.Vermeij, G. J. (1996) Biol. Conserv. 78, 3–9. [Google Scholar]
- 3.Ruiz, G. M., Carlton, J. T., Grosholz, E. D. & Hines, A. H. (1997) Am. Zool. 37, 621–632. [Google Scholar]
- 4.Ruiz, G. M., Grosholz, E. D., Hines, A. H. & Grosholz, E. D. (1999) Limnol. Oceanogr. 44, 950–972. [Google Scholar]
- 5.Ruiz, G. M., Fofonoff, P. W., Carlton, J. T., Wonham, M. J. & Hines, A. H. (2000) Annu. Rev. Ecol. Syst. 31, 481–531. [Google Scholar]
- 6.Cranfield, H. J., Gordon, D. P., Willian, R. C., Marshall, B. A., Battershill, C. N., Francis, M. P., Nelson, W. A., Glasby, C. J. & Read, G. B. (1998) Nat. Inst. Water Atmos. Res. Tech. Rep. 34, 1–48. [Google Scholar]
- 7.Orensanz, J. M., Schwindt, E., Pastorino, G., Bortolus, A., Casas, G., Darrigran, G., Elias, R., Lopez, G. J. J., Obenat, S., Pascual, M., et al. (2002) Biol. Invasions 4, 115–143. [Google Scholar]
- 8.Castilla, J. C., Uribe, M., Bahamonde, N., Clarke, M., Desqueyroux-Faúndez, R., Kong, I., Moyano, H., Rozbaczylo, N., Santelices, B., Valdovinos, C. & Zavala, P., Biol. Invasions, in press.
- 9.Naylor, R., Williams, S. L. & Strong, D. R. (2001) Science 294, 1655–1656. [DOI] [PubMed] [Google Scholar]
- 10.Grosholz, E. D. (2002) Trends Ecol. Evol. 17, 22–27. [Google Scholar]
- 11.Mooney, H. A. & Cleland, E. E. (2001) Proc. Natl. Acad. Sci. USA 98, 5446–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert, W. J., Levin, P. S. & Berman, J. (1992) Mar. Ecol. P.S.Z.N.I. 88, 303–307. [Google Scholar]
- 13.Grosholz, E. D. & Ruiz, G. M. (1995) Mar. Biol. 122, 239–247. [Google Scholar]
- 14.Baskin, Y. (1998) BioScience 48, 788–792. [Google Scholar]
- 15.Bryant, R. T. (1999) in Invasive Species and Biodiversity Management, eds. Sandlund, O. T., Schei, P. J. & Viken, A. (Kluwer Academic, Dordrecht, The Netherlands), pp. 163–175.
- 16.Reusch, T. B. H. & Williams, S. L. (1999) Oikos 84, 398–416. [Google Scholar]
- 17.Cerda, M. & Castilla, J. C. (2002) Rev. Chil. Hist. Nat. 74, 841–853. [Google Scholar]
- 18.Steffani, C. N. (2001) Ph.D. thesis (Univ. of Cape Town, Cape Town, South Africa).
- 19.Crooks, J. A. (2002) Oikos 97, 153–166. [Google Scholar]
- 20.Bastida, R. (1971) Rev. Mus. Argentina Cienc. Nat. Bernardino Rivadavia 3, 203–285. [Google Scholar]
- 21.Bastida, R., Trivi de Mandri, M., Lichtschein de Bastida, V. & Stupak, M. (1980) in V Congreso Internacional de Corrosión Marina e Incrustaciones, Madrid (Garsi, Madrid), pp. 229–320.
- 22.Griffiths, C. L., Hockey, P. A. R., Schurink, C. V. & Le Roux, P. J. (1992) S. Afr. J. Mar. Sci. 12, 713–722. [Google Scholar]
- 23.Piriz, M. & Casas, G. (1994) Appl. Phycol. Forum 10, 4. [Google Scholar]
- 24.Castilla, J. C. & Guiñez, R. (2000) Rev. Chil. Hist. Nat. 73, 585–603. [Google Scholar]
- 25.Kott, P. (1985) Mem. Q. Mus. 23, 1–440. [Google Scholar]
- 26.Kott, P. (1997) in Marine Invertebrates of South Australia, eds. Shepperd, S. A. & Davies, M. (South Australian Research and Development Inst. Aquatic Sciences, Adelaide), Part III, pp. 1092–1255.
- 27.Fairweather, P. G. (1991) Ocean Coast. Manage. 15, 125–142. [Google Scholar]
- 28.Dalby, J. E. (1995) Mar. Freshwater Res. 46, 1195–1199. [Google Scholar]
- 29.Monteiro, S. M. (2002) Ph.D. thesis (Univ. of Sydney, Sydney).
- 30.Guiller, E. R. (1959) Pap. Proc. R. Soc. Tasman. 93, 33–58. [Google Scholar]
- 31.Paine, R. T. & Suchanek, T. H. (1983) Evolution (Lawrence, Kans.) 37, 821–831. [DOI] [PubMed] [Google Scholar]
- 32.Castilla, J. C. (1998) in Minería del Cobre, Ecología y Ambiente Costero: El Caso de Minera Escondida Ltda, ed. Arcos, D. (Editorial Anibal Pinto, Concepción, Chile), pp. 191–214.
- 33.Clarke, M., Ortiz, V. & Castilla, J. C. (1999) B. Mar. Sci. 65, 745–754. [Google Scholar]
- 34.Castilla, J. C., Collins, A. G., Meyer, C. P., Guiñez, R. & Lindberg, D. R. (2002) Mol. Ecol. 11, 1579–1584. [DOI] [PubMed] [Google Scholar]
- 35.Castilla, J. C. & Camaño, A. (2001) in Sustentabilidad de la Biodiversidad, eds. Alveal, K. & Antezana, A. (Editorial Universidad de Concepción, Concepción, Chile), pp. 719–729.
- 36.Guiñez, R. & Castilla, J. C. (2001) Ecology 82, 2331–2341. [Google Scholar]
- 37.Castilla, J. C. (1981) Medio Ambiente Chile 5, 190–215. [Google Scholar]
- 38.Navarrete, S. A. & Castilla, J. C. (2003) Oikos 100, 251–262. [Google Scholar]
- 39.Meneses, I. C. (1993) Hydrobiologia 260/261, 121–129. [Google Scholar]
- 40.Escribano, R. & Hidalgo, P. (2001) Rev. Biol. Mar. Oceanogr. 36, 43–60. [Google Scholar]
- 41.Castilla, J. C., Guiñez, R., Alvarado, J. L., Pacheco, C. & Varas, M. (2000) Mar. Ecol. P.S.Z.N.I. 21, 161–174. [Google Scholar]
- 42.Astorga, M., Guiñez, R., Ortiz, J. C. & Castilla, J. C. (2002) Rev. Chil. Hist. Nat. 75, 515–526. [Google Scholar]
- 43.Alvarado, J. L. & Castilla, J. C. (1996) Mar. Ecol. P.S.Z.N.I. 133, 135–141. [Google Scholar]
- 44.Alvarado, J. L. (2004) Ph.D. thesis (Pontificia Universidad Católica de Chile, Santiago).
- 45.Muenchow, G. (1986) Ecology 67, 246–250. [Google Scholar]
- 46.Fox, G. A. (1993) in Design and Analysis of Ecological Experiments, eds. Scheiner, S. & Gurevitch, J. (Chapman and Hall, New York), pp. 253–289.
- 47.SAS Institute (1996) SAS/STAT User's Guide (SAS Institute, Cary, NC), Release 6.3.
- 48.Conover, W. J. & Iman, R. L. (1981) Am. Stat. 35, 124–133. [Google Scholar]
- 49.Osman, R. W. & Whitlatch, R. B. (1995) J. Exp. Mar. Biol. Ecol. 190, 199–220. [Google Scholar]
- 50.Paine, R. T. (1976) Ecology 57, 858–873. [Google Scholar]
- 51.Paine, R. T. (1977) in Changing Scenes in Natural Sciences, ed. Clyde., E. G. (Acad. Nat. Sci., Philadelphia), Vol. 12, pp. 245–270. [Google Scholar]
- 52.Paine, R. T. (1984) Ecology 65, 1339–1348. [Google Scholar]
- 53.Varas, M. (1996) Ph.D. thesis (Universidad Arturo Prat, Iquique, Chile).
- 54.Pacheco, C. J. & Castilla, J. C. (2001) J. Ethol. 19, 23–26. [Google Scholar]
- 55.Chafer, C. J. (1992) Stilt 20, 20–21. [Google Scholar]
- 56.Pacheco, C. J. & Castilla, J. C. (2000) Rev. Chil. Hist. Nat. 73, 533–542. [Google Scholar]
- 57.Underwood, A. J. & Fairweather, P. G. (1985) Proc. Ecol. Soc. Aust. 14, 7–16. [Google Scholar]
- 58.Alvarado, J. L., Pinto, R., Marquet, P., Pacheco, C., Guiñez, R. & Castilla, J. C. (2002) Mar. Ecol. P.S.Z.N.I. 224, 93–101. [Google Scholar]
- 59.Crooks, J. A. & Khim, H. S. (1999) J. Exp. Mar. Biol. Ecol. 240, 53–75. [Google Scholar]
- 60.Castilla, J. C., Lagos, N., Guiñez, R. & Largier, J. L. (2002) in The Oceanography and Ecology of the Nearshore and Bays in Chile, eds. Castilla, J. C. & Largier, J. L. (Ediciones Universidad Católica de Chile, Santiago), pp. 179–203.
- 61.Jones, C. G., Lawton, J. H. & Shachak, M. (1994) Oikos 69, 373–386. [Google Scholar]
- 62.Castilla, J. C., Lagos, N. A. & Cerda, M. (2004) Mar. Ecol. P.S.Z.N.I. 268, 119–130. [Google Scholar]
- 63.Stachowicz, J. J., Fried, H., Osman, R. W. & Whitlatch, R. B. (2002) Ecology 83, 2575–2590. [Google Scholar]