Abstract
Selective synthesis of particles of angstrom to nanometer size consisting of one to many metal atoms is instrumental in various applications, but it has been hampered by the tendency of the metal atom to form large clusters. We found, as studied by the state-of-the-art electron microscopic technique, a strategy to produce metal-containing nanoparticles isolated from each other by depositing metal atoms in a hydrophilic hole on or in the interior of a carbon nanotube as demonstrated by the reaction of Gd(OAc)3 with oxidized single-wall nanohorns. Besides the potential utilities of the deposited metal clusters, the metal deposition protocol provides a method to control permeation of molecules through such openings.
Keywords: carbon nanotube, metal nanoparticle, self-assembly, metal deposition
As has been amply demonstrated in chemistry by way of metal-catalysis, metal-complexation should immensely widen the scope of carbon cluster science (1–5). Thus, metal-containing hollow carbon clusters, such as endohedral metallofullerenes (6, 7) and carbon nanotube (NT) filled with metal atoms (3, 4, 8–12), have been suggested as promising materials. However, the methodology to rationally control the size and the location of the metal clusters and to ensure high-yield production of the material on a large scale has been lacking. We report here a method for forming a one- to multiatom metal cluster specifically at the hydrophilic hole opening of a NT (8, 13, 14) as demonstrated by deposition of Gd(OAc)3 in single-wall carbon nanohorns (NHs), a new variety of single-wall NTs (15). The hole-selective deposition of the Gd atoms allows atomic-scale detection of the structural defect in the graphitic materials, and, on a bulk scale, controls the permeability of molecules through the holes. The result would find use for modulation of the electronic properties of NTs (16).
Attachment of one atom or a multiatomic cluster onto a selected location of the surface of materials is intellectually challenging and practically useful. Because the oxidized edge of NT is rich in hydrophilic functional groups, such as hydroxy and carboxyl groups (8, 13, 14, 17–19), and hence creates a “locally amphiphilic” structure (20) on a graphene sheet, we considered that selective accumulation of hydrophilic metal ions, one by one through self-assembly, onto a small hole of a partially oxidized graphene sheet should be possible. We report that treatment of single-wall NH possessing hole openings of several angstroms to ≈2-nm diameter with methanolic Gd(OAc)3·(H2O)4 permits selective deposition of one to several Gd(III) atoms in an opening at the tip of the tube, or a cluster of an average 1.6-nm diameter in the interior of an opening on the sidewall. The valence and the number of the deposited metal atoms were determined by high-resolution transmission electron microscopy (TEM) and quantitative electron energy-loss spectrometry (EELS) with atomic sensitivity in a dedicated scanning TEM (STEM) with 0.5-nm spatial resolution. With this analytical method, we show that the Gd(III) ions aggregate in the hydrophilic hole opening and that the number of the metal atoms is controlled by the size and the location of the hole openings.
The nanoporous carbon used in this study is the aggregate of single-wall NHs (15). The NH is a conical tubule closed by caps and assembles radially into dahlia-like aggregates (see Figs. 1, 2, and 3, which is published as supporting information on the PNAS web site), which possess a number of end caps and structural defects on the side of the tubules, through which we can pierce holes by oxidation (21–23). This structural diversity permits us to simultaneously investigate the properties of a variety of hole structures for a single sample, an opportunity unavailable for the studies on structurally homogeneous NT samples. Treatment of the NH aggregates with molecular oxygen at temperatures at 420 and 580°C creates hole openings. The previous gas and fullerene adsorption studies suggested the diameter of the holes in the NH oxidized at 420°C (oxNH420) to be <1 nm (21). The NHs oxidized at 580°C (oxNH580) have larger holes, and the holes in the cap region are <1 nm in diameter and those on the side wall are >1 nm (21).
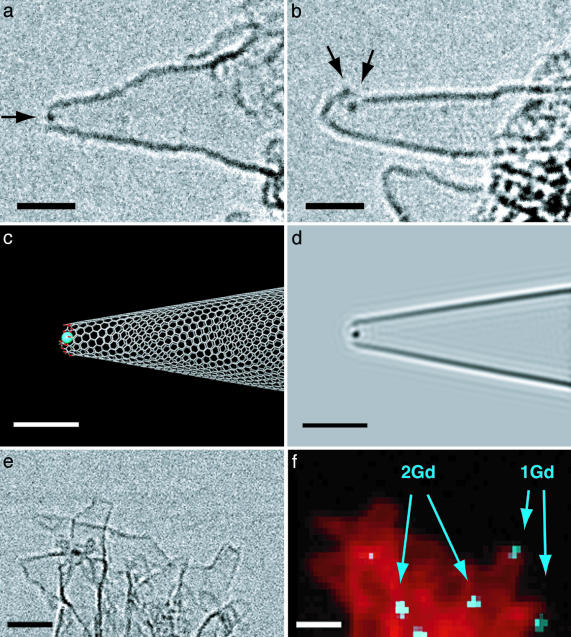
Fig. 1.
Gd atom(s) trapped in holes of oxNH420 (GdoxNH420). (a) One Gd atom at an open tip. A conventional TEM image with an arrow indicating the Gd atom. (b) Four Gd atoms (two are overlapping. See Movie 1.) on an open hole on the side of the horn. (c) A model representing the TEM image in a. Color codes for the atoms: C, gray; O, red; Gd, blue. The diameter of the opening (distance between skeletal carbon atoms in the edge) is 0.9 nm. (d) The simulated TEM image based on c.(e) An STEM bright-field image of the cluster. Note the resolution of the STEM is intrinsically lower than the conventional TEM. (f) Element mapping of the STEM image in e from EELS of Gd N-edge (blue) and carbon K-edge (red). The number of Gd atoms was determined by the integrated EELS intensity normalized by the relevant cross section. The high density of Gd atoms in the center bottom is likely due to larger clusters such as those shown in Fig. 2. [Scale bars, 2 nm (a–d) and 5 nm (e and f).]
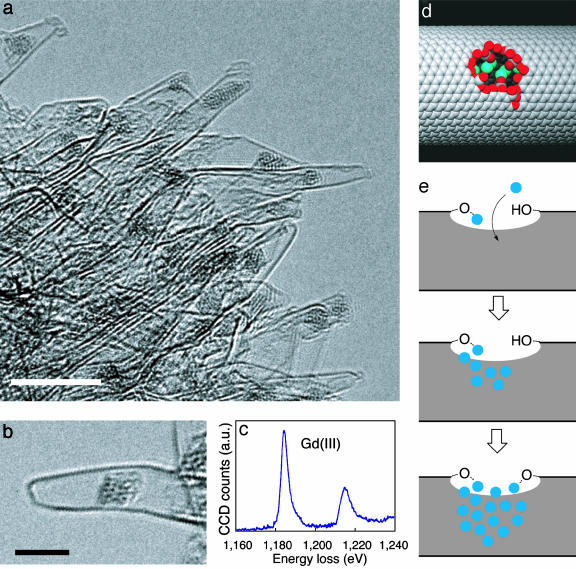
Fig. 2.
Oxygen-bridged Gd(III) cluster trapped in the interior of oxNH580 (GdoxNH580). (a) A low-magnification TEM image of GdoxNH580. (Scale bar, 5 nm.) (b) A cluster in a single-wall tube. (Scale bar, 3 nm.) (c) EELS of the Gd(III) M-edge (1,185 and 1,216 eV) of a cluster in a NH (for the data of carbon and oxygen atoms; see Fig. 4). (d) A model of a 1.5-nm hole opening in a NT of 3.0-nm diameter containing a Gd cluster inside. (e) A scheme of the cluster growth in the interior of NH.
Materials and Methods
GdoxNH420. The NH aggregates were prepared from pure graphite targets by CO2 laser ablation (15). Oxidation of the NHs was performed under 0.1 MPa of oxygen at 420°C for 10 min. The NHs oxidized at 420°C (oxNH420, 100 mg) were placed in a 20-ml round-bottomed flask containing gadolinium triacetate tetrahydrate (20 mg) in methanol (10 ml). The mixture was sonicated for 10 s and stirred for 24 h at 30°C. After filtration through a membrane filter (pore size = 1 μm), the black material on the filter was collected in a vial, and 10 ml of methanol was added. After sonication for 10 s, the NHs were filtered again. The NHs were transferred to a vial and dried under reduced pressure (1 × 102 Pa) at 30°C for 10 h, during which no weight change was observed. The product weighed 96 mg.
GdoxNH580. The NHs oxidized at 580°C (oxNH580, 25 mg) were treated similarly with gadolinium triacetate tetrahydrate (25 mg) in refluxing methanol (10 ml) for 24 h. After filtration, the NHs were sonicated in methanol (10 ml) for 10 s. The suspension was filtered again, and the NHs were dried under reduced pressure (1 × 102 Pa) at 30°C for 10 h to afford the sample (23 mg).
Treatment of GdoxNH580 with [60]Fullerene. To a sample of GdoxNH580 placed on the carbon grid disk for TEM was added a saturated solution of [60]fullerene in toluene (10 μl). The sample was sucked dry quickly by filtration paper and analyzed by TEM. Details of the method have been described (22, 23).
Analyses by TEM. Each sample of NH (GdoxNH420 and GdoxNH580) was dispersed in methanol and sonicated for 30 s. Each dispersed suspension was then dropped onto holey-carbon grid disks, sucked dry, and analyzed by TEM and STEM. The samples were analyzed by a TEM (JEOL 2010F, 120 kV) and STEM (Hitachi HD2000-UHV, 120 kV) equipped with an EELS spectrometer. Identification of the element and element mapping were achieved by the spectrum-imaging method with a dedicated STEM and EELS instrument (24–26). STEM images were obtained by using a highly focused electron beam (0.3–0.5 nm in diameter). EELS spectra were recorded at 150 eV (Gd N-edge) and 300 eV (C K-edge) with 0.5-nm spatial resolution, which then converted to the element-mapping images. The number of Gd atoms were determined by integrated electron counts of the Gd N-edge spectra normalized by the inelastic cross section.
Results and Discussion
Gadolinium(III) was chosen for this first study because of its high oxygen affinity, large ionic radius (1.07 Å), the ability to accept as many as ten ligands, and the magnetic moment to be of interest in the future studies (27). Gadolinium also enjoys technical merits for ready detection by TEM and EELS (24). The oxidized sample (oxNH420 and oxNH580) and gadolinium acetate tetrahydrate [Gd(OAc)3·4H2O] were mixed in methanol under air, on a 20-mg scale, sonicated for 10 sec, and then at 30–60°C for 24 h to give NHs containing Gd(III) atoms (denoted as GdoxNH420 and GdoxNH580, respectively).
Thermogravimetric analysis (TGA) indicated that the amount of Gd-doping qualitatively reflects the oxidation temperature and likely the number of oxygen-rich sites. Thus, GdoxNH420 and GdoxNH580 contain <2.7 and 13 wt% of Gd, respectively, the values corresponding to Gd/C atomic ratios of 0.21% and 1.1% (assuming the Gd metal to be in the Gd2O3 form). We could not dope intact NH with Gd(OAc)3 to any significant extent.
In the sample of GdoxNH420, we observed one to several Gd atoms trapped in a hole opening. Fig. 1 a and b and also Movie 1 (which is published as supporting information on the PNAS web site) show two illustrative examples of a single NH, where one and four Gd atoms are observed in a hole opening at the tip or on the side of NH. The diameter of the hole in the tip in Fig. 1a is estimated to be 0.9 nm based on the molecular models in Fig. 1c and its simulation (Fig. 1d). During the TEM observation at room temperature, we found that the Gd atoms attached to the holes move around on the edge of the hole without being detached from it.
The spatial distribution and the number of Gd atoms were determined by the state-of-the-art spectrum-imaging by 0.5 × 0.5 nm area by the use of a dedicated STEM and EELS instrument (24–26). The quantitative EELS analysis provides us with the first opportunity to quantify the number of Gd(III) atoms in a large area of a TEM image (Fig. 1 e and f). Wherever an individual hole in a GdoxNH420 sample was identified to hold Gd atom(s), the cluster size did not exceed more than several atoms. As is seen in Fig. 1 a, b, and f, Gd atoms are not attached to an intact graphene surface.
Possessing holes of >1 nm in diameter on the side wall (23), the behavior of oxNH580 toward Gd(OAc)3 is markedly different from that of oxNH420. In all the dahlia-like NH aggregates, except a few of those that are considered to be incompletely oxidized, we found one or sometimes two clusters of 1.6 ± 0.5 (4)-nm average diameter fitted in the interior of each NH (Fig. 2 a and b). An average cluster contains about 30 metal atoms. Whereas the metal atoms in the cluster exhibit Brownian-like movement under the TEM observation conditions, the cluster does not move away from the region where it is attached (Movie 2, which is published as supporting information on the PNAS web site). The EELS elemental profiles (Figs. 2c and 4, which is published as supporting information on the PNAS web site) suggested that the cluster comprises Gd(III) atoms bridged by oxygen anions (EELS essentially the same as that of Gd2O3).
On the basis of the high oxygen affinity and the hydrophilicity of the Gd ion, we suggest an equilibrium model of the cluster growth in the interior of the NH (Fig. 2e). When the opening is small, the number of Gd atoms is proportionally small, because only a few atoms chelated at the edge are enough to close the hole opening. Once the opening is larger than a certain threshold size, it becomes possible for the Gd atoms to go into the NH. The Gd(OAc)3 molecules in methanol thus migrate through the hole, undergo anion exchange with the oxygenated edge of the hole, and start to grow an oxygen-bridged cluster first in the opening then within the interior of the tube until the cluster touches the hydrophobic internal wall of the tube and hence stops growing any further. The size of the larger clusters is limited by the tube diameter of the NH (generally 1–2 nm), and such clusters do not grow in the pointed tip of a NH, since the immediate interior is highly hydrophobic (compare Fig. 1c).
Bulk solution experiments indicated that we can fill in most of the hole openings in oxNH580 by the cluster formation and, hence, can greatly reduce permeation of molecules through the holes. [60]Fullerene quickly enters the internal space of oxNH580 through the holes (22, 23), and we exploited this property of fullerene to show that the clusters form on most of the hole openings. Thus, oxNH580 was first treated with methanolic Gd(OAc)3, as described above, followed by a toluene solution of [60]fullerene: TEM observation of several of the dahlia aggregates (Fig. 3) indicated that almost all of the cluster-bearing NHs in this GdoxNH580 sample remain empty, the remainder of the cluster-bearing NHs are sparingly filled, and the NHs not bearing any clusters (which are a few in number) are filled with many fullerene molecules. This bulk experiment also indicates that the TEM-observed cluster formation is not an artifact of the TEM imaging.
In summary, we have devised a “ship-in-bottle” synthesis of a metal cluster in the interior of a hollow tube by supplying the metal atoms through a hole opening. Given the flexibility of the approach, we expect that the method will allow the construction of semiconductor nanoparticles and of mixed metal clusters of high chemical or magnetic activities within the tube. To fully exploit the potential of this method for modulation of the properties of NTs, for instance, magnetic modulation of electronic properties, one needs to selectively create hole openings, which can be achieved by focused electron irradiation of the tube (28). Finally, because even fullerene molecules fuse together to form NTs (29), we speculate that the hole openings can likely be mended under suitable conditions after introduction of the metal clusters.
Supplementary Material
Acknowledgments
E.N. was supported by Monbukagakusho, Japan (Grant-in-Aid for Scientific Research, Specially Promoted Research, and the 21st Century Center of Excellence Program for Frontiers in Fundamental Chemistry), and H.I. was supported by Suntory Institute for Bioorganic Research (Osaka, Japan). A.H. and H.Y. received postdoctorial fellowships from Japan Society for the Promotion of Science. Work on electron microscopy was supported by New Energy and Industrial Technology Development Organization Nano-Carbon Project.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NT, carbon nanotube; NH, carbon nanohorn; TEM, transmission electron microscopy/microscope; STEM, scanning TEM; oxNH, oxidized carbon nanohorn; EELS, electron energy-loss spectrometry.
References
- 1.Nakamura, E. & Sawamura M. (2001) Pure Appl. Chem. 73, 355–359. [Google Scholar]
- 2.Balch, A. L. & Olmstead, M. M. (1998) Chem. Rev. 98, 2123–2165. [DOI] [PubMed] [Google Scholar]
- 3.Ajayan, P. M. & Iijima, S. (1993) Nature 361, 333–334. [Google Scholar]
- 4.Lee, J., Kim. H., Kahng, S. J., Kim, G., Son, Y. W., Ihm, J., Kato, H., Wang, Z. W., Okazaki, T., Shinohara, H. & Kuk, Y. (2002) Nature 415, 1005–1008. [DOI] [PubMed] [Google Scholar]
- 5.Odom, T. W., Huang, J.-L., Cheung, C. L. & Lieber, C. M. (2000) Science 290, 1549–1552. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara, H. (2000) in Fullerenes: Chemistry, Physics and Technology, eds. Kadish, K. M. & Ruoff, R. S. (Wiley, New York), pp. 357–393.
- 7.Akasaka, T. & Nagase, S., eds. (2002) Endofullerenes. A New Family of Carbon Clusters (Kluwer Academic, Dordrecht, The Netherlands).
- 8.Ajayan, P. M., Ebbesen, T. W., Ichihashi, T., Iijima, S., Tanigaki, K. & Hiura, H. (1993) Nature 362, 522–524. [Google Scholar]
- 9.Guerret-Plécourt, C., Le Bouar, Y., Lolseau, A. & Pascard, H. (1994) Nature 372, 761–765. [Google Scholar]
- 10.Tsang, S. C., Chen, Y. K., Harris, P. J. F. & Green, M. L. H. (1994) Nature 372, 160–162. [Google Scholar]
- 11.Ajayan, P. M., Stephan, O., Redlich, P. & Colliex, C. (1995) Nature 375, 564–567. [Google Scholar]
- 12.van Bommel, K. J. C., Friggeri, A. & Shinkai, S. (2003) Angew. Chem. Int. Ed. Engl. 42, 980–999. [DOI] [PubMed] [Google Scholar]
- 13.Tsang, S. C., Harris, P. J. F. & Green, M. L. H. (1993) Nature 362, 520–522. [Google Scholar]
- 14.Liu, J., Rinzler, A. G., Dai, H. J., Hafner, J. H., Bradley, R. K., Boul, P. J., Lu, A., Iverson, T., Shelimov, K., Huffman, C. B., et al. (1998) Science 280, 1253–1256. [DOI] [PubMed] [Google Scholar]
- 15.Iijima, S., Yudasaka, M., Yamada, R., Bandow, S., Suenaga, K., Kokai, F. & Takahashi, K. (1999) Chem. Phys. Lett. 309, 165–170. [Google Scholar]
- 16.Baughman, R. H., Sakhidov, A. A. & de Heer, W. A. (2002) Science 297, 787–792. [DOI] [PubMed] [Google Scholar]
- 17.Chen, J., Hamon, M. A., Hu, H., Chen, Y. S., Rao, A. M., Eklund, P. C. & Haddon, R. C. (1998) Science 282, 95–98. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, A. (2002) Angew. Chem. Int. Ed. Engl. 41, 1853–1859. [DOI] [PubMed] [Google Scholar]
- 19.Bahr, J. L. & Tour, J. M. (2002) J. Mater. Chem. 12, 1952–1958. [Google Scholar]
- 20.Nakamura, E. & Isobe, H. (2003) Acc. Chem. Res. 36, 807–815. [DOI] [PubMed] [Google Scholar]
- 21.Murata, K., Kaneko, K., Kanoh, H., Kasuya, D., Takahashi, K., Kokai, F., Yudasaka, M. & Iijima, S. (2002) J. Phys. Chem. B 106, 12668–12669. [Google Scholar]
- 22.Yudasaka, M., Ajima, K., Suenaga, K., Ichihashi, T., Hashimoto, A. & Iijima S. (2003) Chem. Phys. Lett. 380, 42–46. [Google Scholar]
- 23.Ajima, K., Yudasaka, M., Suenaga, K., Kasuya, D., Azami, T. & Iijima, S. (2004) Adv. Mater. 16, 397–401. [Google Scholar]
- 24.Suenaga, K., Tence, T., Mory, C., Colliex, C., Kato, H., Okazaki, T., Shinohara, H., Hirahara, K., Bandow, S. & Iijima, S. (2000) Science 290, 2280–2282. [DOI] [PubMed] [Google Scholar]
- 25.Colliex, C., Imhoff, D., Perez-Omil, J. A., Stephan, O., Suenaga, K. & Tence, M. (2000) J. Electron Microsc. 48, 995–1003. [Google Scholar]
- 26.Kadavanich, A. V., Kippeny, T. C., Erwin, M. M., Pennycook, S. J. & Rosenthal, S. J. (2001) J. Phys. Chem. B 105, 361–369. [Google Scholar]
- 27.Aime, S., Botta, M., Fasano, M. & Terreno, E. (1999) Acc. Chem. Res. 32, 941–949. [Google Scholar]
- 28.Banhart, F. (1999) Rep. Prog. Phys. 62, 1181–1221. [Google Scholar]
- 29.Bandow, S., Takizawa, M., Hirahara, K., Yudasaka, M. & Iijima, S. (2001) Chem. Phys. Lett. 337, 48–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.