Abstract
Objectives.—
The purpose of this study was to directly compare the pharmacokinetic (PK) profile of 22-mg sumatriptan powder delivered intranasally with a novel Breath Powered™ device (11 mg in each nostril) vs a 20-mg sumatriptan liquid nasal spray, a 100-mg oral tablet, and a 6-mg subcutaneous injection.
Background.—
A prior PK study found that low doses of sumatriptan powder delivered intranasally with a Breath Powered device were efficiently and rapidly absorbed. An early phase clinical trial with the same device and doses found excellent tolerability with high response rates and rapid onset of pain relief, approaching the benefits of injection despite significantly lower predicted drug levels.
Methods.—
An open-label, cross-over, comparative bioavailability study was conducted in 20 healthy subjects at a single center in the USA. Following randomization, fasted subjects received a single dose of each of the 4 treatments separated by a 7-day washout. Blood samples were taken pre-dose and serially over 14 hours post-dose for PK analysis.
Results.—
Quantitative measurement of residuals in used Breath Powered devices demonstrated that the devices delivered 8 ± 0.9 mg (mean ± standard deviation) of sumatriptan powder in each nostril (total dose 16 mg). Although the extent of systemic exposure over 14 hours was similar following Breath Powered delivery of 16-mg sumatriptan powder and 20-mg liquid nasal spray (area under the curve [AUC]0-∞ 64.9 ng*hour/mL vs 61.1 ng*hour/mL), sumatriptan powder, despite a 20% lower dose, produced 27% higher peak exposure (Cmax 20.8 ng/mL vs 16.4 ng/mL) and 61% higher exposure in the first 30 minutes compared with the nasal spray (AUC0-30 minutes 5.8 ng*hour/mL vs 3.6 ng*hour/mL). The magnitude of difference is larger on a per-milligram basis. The absorption profile following standard nasal spray demonstrated bimodal peaks, consistent with lower early followed by higher later absorptions. In contrast, the profile following Breath Powered delivery showed higher early and lower late absorptions. Relative to the 100-mg oral tablet (Cmax 70.2 ng/mL, AUC0-∞, 308.8 ng*hour/mL) and 6-mg injection (Cmax 111.6 ng/mL, AUC0-∞ 128.2 ng*hour/mL), the peak and overall exposure following Breath Powered intranasal delivery of sumatriptan powder was substantially lower.
Conclusions.—
Breath Powered intranasal delivery of sumatriptan powder is a more efficient form of drug delivery, producing a higher peak and earlier exposure with a lower delivered dose than nasal spray and faster absorption than either nasal spray or oral administration. It also produces a significantly lower peak and total systemic exposure than oral tablet or subcutaneous injection.
Keywords: sumatriptan, migraine, bidirectional nasal delivery, Breath Powered nasal delivery, pharmacokinetics, bioavailability
Sumatriptan, a highly selective ligand for 5-HT1B/1D serotonin receptors, was the first registered triptan and remains widely used as an antimigraine drug. Multiple routes of administration for sumatriptan including subcutaneous injection, oral, suppository, and intranasal spray have been shown to be effective in relieving symptoms of migraine in placebo-controlled studies.1–4
Subcutaneous administration typically provides the fastest and most complete migraine symptom relief; however, the high incidence of side effects and patient resistance to the use of injections led to the development of alternative routes of administration.3,5 Oral administration is the most common route for the available triptans but is not satisfactory for many patients. A majority of migraine patients experience gastrointestinal (GI) symptoms, such as nausea and vomiting, which can be a readily apparent barrier to the use of oral medication. Less obviously, it has been shown empirically that migraineurs experience significantly delayed gastric emptying6–8 possibly because of autonomic dysfunction. Delayed gastric emptying can influence the therapeutic effects of orally administered drugs, and evidence specifically suggests that during a migraine attack, absorption of more than 1 class of antimigraine medication is delayed.9 Delayed or inconsistent absorption may reduce early exposure to medication, delay onset of action, and decrease the reliability or predictability of response.
A liquid formulation delivered with a standard nasal spray device was developed as an alternative, seeking benefits such as faster onset of relief than oral dosage forms and fewer adverse effects than the injection. Unfortunately, conventional nasal sprays are suboptimal for true intranasal delivery and have been shown to deposit a large fraction of the delivered dose of a drug in the part of the nasal cavity anterior to the narrow nasal valve located about 2 cm into the nose.10–12 This anterior segment is largely lined with non-ciliated squamous epithelium that is less efficient at medication absorption than the respiratory mucosa beyond the nasal valve.12,13 Anterior deposition following a conventional nasal spray also results in a substantial portion of the delivered dose either dripping out of the nostril or being wiped off. Importantly, a large fraction of the remaining drug that is believed to enter the “deep” nasal cavity following standard nasal spray administration is actually drawn along the floor of the nasal cavity into the pharynx where it is swallowed.12 Swallowing affects such a significant portion of the medication delivered by conventional nasal sprays that a higher plasma peak can be produced via the unintended GI route than by nasal absorption.14 This phenomenon is clearly observed with sumatriptan in the bimodal absorption profile following nasal spray administration: a lower early peak, likely related to intranasal absorption, is produced after 20 minutes and is followed by a higher absorption peak consistent with GI absorption around 90 minutes.14
The OptiNose Breath Powered Bidirectional powder device (OptiNose US, Inc., Yardley, PA, USA) is a new nasal delivery system designed to overcome deficiencies of traditional nasal delivery. The delivery system consists of a device with a mouthpiece and a shaped sealing nosepiece that is designed to take advantage of unique aspects of nasal anatomy and physiology to improve the extent and reproducibility of drug delivery while protecting against the risk of lung inhalation.11,15 First, during oral exhalation against a resistance, a positive pressure is created in the oropharynx, naturally elevating and sealing the soft palate, and completely separating the nasal and oral cavities. Second, because of the sealing nosepiece, a slightly reduced positive oral pressure is transferred into the nasal cavity, where it expands narrow slit-like passages and balances the pressure across the soft palate to avoid over-elevation of the vellum and maintains patency of the communication pathway between the 2 nostrils that is located deep in the nasal cavity posterior to the nasal septum. Under these dynamic circumstances, it is possible for medication to be carried by air flow into 1 nostril, to be deposited throughout the deep nasal cavity, and for the air to escape by the other nostril (bidirectional delivery).
Human in vivo deposition studies using Breath Powered delivery of radio-labeled lactose powder have demonstrated a superior pattern of delivery to the deep nasal regions beyond the nasal valve compared with radio-labeled liquid delivered with a conventional nasal spray pump (Fig. 1), with protection from lung deposition.11,16
Fig 1.
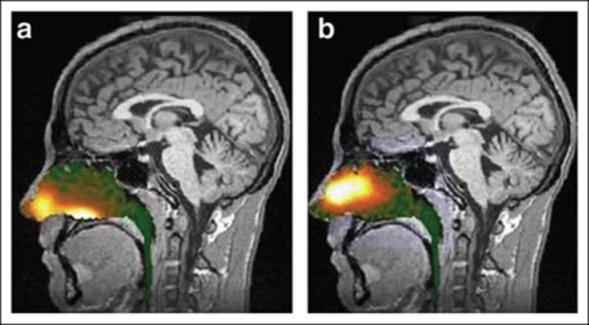
Gamma camera images 2 minutes after delivery using a traditional liquid spray (a) and powder with OptiNose Breath Powered device (b) shown with a logarithmic hot iron intensity scale (reference). Deposition of spray was greatest anterior to the nasal valve and in the lower posterior region of the nose (“floor” of the nasal cavity), whereas deposition of powder was shifted to more superior posterior regions of the nose. The images were from the same subject after each method of administration.
A significantly improved nasal delivery pattern, reducing conversion of nasal delivery to oral delivery, has potential to greatly improve the speed and efficiency of drug absorption with a goal of producing faster onset of migraine pain relief while using lower total doses of medication. Other hypothesized benefits potentially enabled by this form of delivery relate to local activity on deep intranasal nerve structures by both drug and CO2-containing breath, which may further augment treatment response.17
A pilot comparative bioavailability study found that intranasal administration of sumatriptan powder in divided doses using the Breath Powered device resulted in a rapid absorption profile.18 The aim of the current study was to directly compare the single dose bioavailability of 22-mg sumatriptan powder divided into 11 mg per nostril, delivered intranasally using a Breath Powered device vs a commercially approved 20-mg sumatriptan liquid nasal spray, a 100-mg sumatriptan tablet, and a 6-mg sumatriptan subcutaneous injection.
METHODS
This was a randomized, open-label, single-dose, cross-over, comparative bioavailability study in healthy subjects. It was conducted at a single center in the USA.
Subjects.—
The study population included 20 male and female subjects 18-55 years of age, who were judged healthy by the investigator, with no clinically relevant abnormalities as determined by medical history, physical examination, blood chemistry, hematology (including complete blood count), urinalysis, vital signs, and electrocardiogram (ECG). Eligible subjects had a body mass index (BMI) of 18-32 kg/m2 and a bodyweight of not less than 50 kg. Prior to inclusion, subjects agreed to abstain from alcohol intake from 48 hours before each administration of study medication and during the period of confinement, and to limit caffeine/methylxanthine intake to less than 300 mg/day for 7 days prior to and for the duration of the study, with no intake from 24 hours before dosing and throughout confinement. Subjects also agreed not to consume food or beverages containing grapefruit, Seville oranges, or quinine (eg, tonic water) 72 hours prior to study/day 1 until after the last pharmacokinetic (PK) sample had been collected and not to consume food containing poppy seeds during the study. Subjects had verified airflow through both nostrils, an ability to close the soft palate (eg, ability to inflate a balloon), and were able to use the Breath Powered device correctly.
Subjects with a history of migraines, a history of hypersensitivity or allergies to any drug, including sumatriptan or any of its components, or sulphonamides were excluded. Subjects were ineligible if they had a hemoglobin level below the lower limit of normal at screening, had donated blood or experienced significant blood loss (>500 mL) within 3 months prior to screening, or were planning to donate blood within 2 months of completing the study. Use of drug metabolizing enzyme (CYP-450) inducers within 28 days prior to dosing or inhibitors within 14 days prior to dosing, use of any monoamine oxidase inhibitors within 28 days prior to dosing, use of any prescription medications/products, except hormonal contraceptives in female subjects of childbearing potential, and use of any over-the-counter non-prescription preparations (except ibuprofen and acetaminophen used at recommended doses) within 14 days of study entry all resulted in exclusion. Pregnant and lactating females were excluded. The presence of respiratory diseases or known nasal obstruction, including allergic rhinitis, nasal septum deviation, polyposis, severe mucosal swelling, nasal ulcers, nasal trauma, or for any other reason, a history of chronic nose bleeds, current nasopharyngeal illness, and known vellum insufficiency also resulted in exclusion.
Protocol Approvals, Registrations, and Subject Consents.—
This study was conducted at Celerion in Neptune, NJ, USA, in accordance with the Declaration of Helsinki, all relevant federal regulations, and in compliance with the International Conference on Harmonization guideline for Good Clinical Practice. The study protocol, informed consent forms, and other appropriate study-related documents were reviewed and approved by an Institutional Review Board. Written informed consent was obtained from each subject prior to any protocol-related activities.
Procedures.—
The study consisted of 6 visits. At visit 1, subjects were screened for eligibility. Following a physical examination, subjects were instructed on the use of the Breath Powered delivery device. Once the subject demonstrated an ability to appropriately use the device, the remaining screening procedures (vital signs, ECG recording, blood and urine sampling for clinical laboratory tests, alcohol and drugs of abuse tests, serum pregnancy test [women only]) were performed.
Eligible subjects attended the clinic for 4 additional visits (visits 2-5). At each visit, subjects checked into the study site the evening before dosing and remained there until after the last blood sample for determining sumatriptan concentration had been drawn. Randomization was generated by Celerion. Subjects were randomly assigned to treatment sequence using a 4 by 4 Latin square design at the first treatment visit (visit 2). The study treatments administered were 22-mg sumatriptan powder administered intranasally with the OptiNose Breath Powered device, 20-mg sumatriptan nasal spray (Imitrex® nasal spray, GlaxoSmithKline, Philadelphia, PA, USA); 100 mg oral tablet (Imitrex tablet, GlaxoSmithKline), and 6-mg subcutaneous injection (Imitrex injection, GlaxoSmithKline). Each subject received each of the 4 treatments on the 4 separate periods at approximately the same time at each visit, with a 7-day washout between treatments. The subjects fasted for at least 8 hours before dosing and up to 4 hours post-dose.
For dosing of sumatriptan powder with the Breath Powered device, subjects first self-administered a 11 mg dose into 1 nostril and then self-administered a second 11-mg dose into the other nostril. For dosing with the nasal spray, subjects were first instructed on appropriate administration, and then subjects self-administered a single dose of 20 mg sumatriptan to 1 nostril. The oral tablet was taken by subjects with 240-mL water. For the subcutaneous injection, the investigator or designee made the injection of the 6-mg dose of sumatriptan in the subjects' abdomen.
Subjects returned at visit 6 for follow-up evaluations between 3 and 10 days after the last blood draw for sumatriptan concentration determination. Safety evaluations were based on reports of adverse events (AEs), physical examination, clinical laboratory tests, and vital signs and ECG measurements.
PK Analysis.—
Blood samples (5 mL) were collected in tubes containing dipotassium ethylene diamine tetraacetic acid (K2EDTA) at pre-dose (time 0), and 2, 5, 10, 15, 20, 25, 30, and 45 minutes, and 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 14 hours post-dose. The plasma fraction was separated by placing the collection tube into a refrigerated centrifuge (2–8°C) for 10 minutes at 1500 × g. All plasma samples were stored frozen at 20°C until shipped to the bioanalytical facility. Plasma samples were analyzed for sumatriptan at the Celerion Bioanalysis Laboratory in Lincoln, NE, USA, using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method. The lower limit of quantitation (LLOQ) was 0.1 ng/mL, and all concentrations below the LLOQ were treated as 0 for the calculations of descriptive statistics and the PK parameters. All PK parameters were calculated using a non-compartmental approach in WinNonlin Professional® Version 5.2 (Mountain View, CA, USA) and SAS® (Release Version 9.1.3, SAS Institute, Inc., Cary, NC, USA). The PK parameters calculated are listed in Table 1.
Table 1.
Pharmacokinetic Parameters Derived
| Parameter | Description |
|---|---|
| Cmax | Maximum observed drug concentration |
| tmax | Time to reach Cmax |
| AUC0-t | Area under the drug concentration time curve from time 0 to time t, where t is the time of the last measurable concentration [Cp], calculated using the linear trapezoidal rule |
| AUC0-∞ | Area under the drug concentration time curve from time 0 to infinity, calculated as AUC0-∞ = AUC0-t + Cp/λZ |
| AUC0-15 min | Area under the drug concentration time curve from time 0 to 15 minutes |
| AUC0-30 min | Area under the drug concentration time curve from time 0 to 30 minutes |
| t | Terminal elimination half-life, calculated as ln(2)/λZ where λZ is the apparent first-order terminal elimination rate constant calculated from a semi-log plot of the concentration vs time curve by linear least-squares regression analysis |
| λZ | Terminal elimination rate constant |
| AUC%extrap | Percentage of AUC0-∞ extrapolated from Cp to infinity, calculated as 100 × (1 − [AUC0-t/ AUC0-∞]) |
AUC = area under the curve.
Statistical Analysis.—
The sample size was based on practical considerations rather than statistical power. A sample size of 20 subjects provided at least 5 replications within each sequence using a 4 by 4 Latin square design and was judged to provide a robust evaluation of PK parameters.
The plasma concentrations and PK parameter values were imported into SAS that was used to calculate all descriptive statistics. An analysis of variance (ANOVA) on the ln-transformed PK parameters area under the curve [AUC]0-∞, AUC0-t, AUC0-30 min, and Cmax of sumatriptan was used to compare treatments. The ANOVA model included sequence, treatment, and period as fixed effects and subject nested within sequence as a random effect. Sequence effect was tested using subject (sequence) as the error term at a 5% level of significance. Each ANOVA included calculation of least-squares (LS) means, the difference between treatment LS means, the standard error, and 90% confidence intervals (CIs) associated with this difference. The LS means, difference between LS means, and 90% CI of each difference were exponentiated to the original scale. Two treatments are considered bioequivalent only if the entire 90% CI range of the treatment difference is fully contained within the accepted bounds of 80-125%.19
RESULTS
A total of 20 subjects were randomized. All completed the study and were included in the analysis. The first subject was enrolled on 10 January 2012 and the last subject completed the study on February 7, 2012. Demographic data and other baseline characteristics are presented in Table 2. All 4 study treatments were administered under the supervision of clinic personnel, assuring compliance. Based on the residual analysis of drug capsules and nosepieces on used devices, a mean dose of approximately 8 mg (standard deviation ± 0.9) sumatriptan powder (free base equivalent) was delivered to each nostril by the Breath Powered intranasal delivery device (total dose approximately 16 mg).
Table 2.
Demographics and Other Characteristics of Treated Subjects
| Treated Subjects (n = 20) | |
|---|---|
| Age (years), mean (SD) | 36.8 (9.7) |
| Male, No. (%) | 17 (85) |
| Female, No. (%) | 3 (15) |
| Race, No. (%) | |
| White | 8 (40) |
| Black | 12 (60) |
| Height (cm), mean (SD) | 173 (7) |
| Weight (kg), mean (SD) | 82.7 (10.8) |
| BMI (kg/m2), mean (SD) | 27.5 (2.7) |
BMI = body mass index; SD = standard deviation.
PK and Bioavailability.—
The plasma concentration time profile of sumatriptan was well characterized for each of the 4 treatments (Fig. 2). Overall exposure from both of the intranasally administered sumatriptan treatments was considerably lower than sumatriptan delivered by either the oral or subcutaneous route. The mean plasma concentration time profiles up to 4 hours post-dose for the 2 intranasal treatments demonstrate a clearly differentiated profile following the Breath Powered powder delivery (Fig. 3); in the first 30 minutes following dosing, sumatriptan powder from the Breath Powered device produced a faster rise in plasma sumatriptan concentration and a substantially greater exposure compared with liquid sumatriptan nasal spray.
Fig 2.
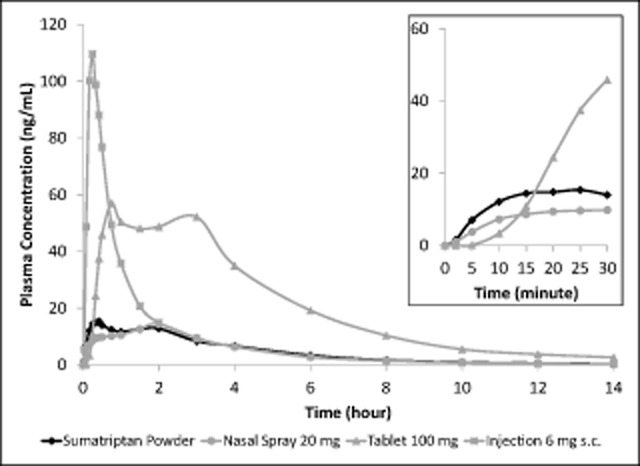
Sumatriptan plasma concentration-time profiles over the entire 14-hour sampling period for intranasal sumatriptan powder, 22-mg nasal spray, 100-mg tablet, and 6-mg subcutaneous injection and Inset for intranasal sumatriptan powder, 22-mg nasal spray, and 100-mg tablet over the first 30 minutes post-dose. The main figure shows that both methods of intranasal delivery resulted in much lower mean plasma sumatriptan concentration time profiles than observed for the tablet and the injection. Inset: In the first 15 minutes post-dose, the rate of rise of plasma sumatriptan concentration was faster for sumatriptan powder than either the 20-mg nasal spray or the 100-mg tablet.
Fig 3.
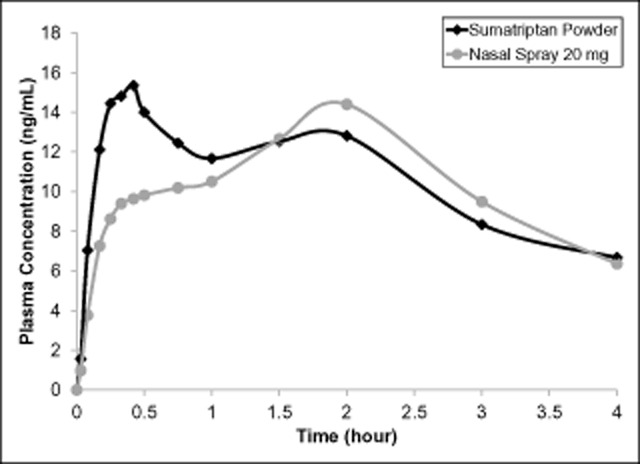
Sumatriptan plasma concentration time profiles over the first 4 hours after administration of 22-mg sumatriptan powder by the Breath Powered device compared with the 20-mg nasal spray.
A summary of the PK parameters for the 4 treatments is presented in Table 3. There were no first point tmax values and the mean residual area (defined as AUC%extrap) was approximately 5% or less for all treatments. The extent of systemic exposure as measured by AUC0-t and AUC0-∞ over 14 hours was similar for Breath Powered powder and nasal spray liquid sumatriptan. In contrast, the sumatriptan powder delivered with the Breath Powered device produced a substantially lower peak and overall systemic exposure relative to both the 100-mg oral tablet and the 6-mg subcutaneous injection. Intranasal administration of sumatriptan powder using the Breath Powered device resulted in a 27% higher peak exposure (Cmax), and a 75% higher early exposure (AUC0-15 min) relative to the sumatriptan nasal spray despite a 20% lower delivered dose. On a dose-adjusted basis, this represents a 59% higher peak exposure and 119% higher early exposure. Although the absorption profile curve for both intranasal products was characterized by bimodal peaks consistent with a combination of early nasal absorption followed by late GI absorption, these products did not show the same pattern (Fig. 3). The early peak was higher with Breath Powered delivery, while the later peak was higher with nasal spray delivery. The high early peak supports results obtained with Breath Powered delivery of powder sumatriptan in patients suffering a migraine, where tmax at 20 minutes was previously demonstrated.18
Table 3.
Sumatriptan Pharmacokinetic (PK) Results for Breath Powered Intranasal Delivery of Sumatriptan Powder Compared With 20-mg Nasal Spray, 100-mg Tablet, and 6-mg Subcutaneous Injection
| PK Parameters | Sumatriptan Powder† | 20 mg Nasal Spray | 100 mg Oral Tablet | 6 mg S.C. Injection |
|---|---|---|---|---|
| Mean ± SD (n = 20) | Mean ± SD (n=20) | Mean ± SD (n=20) | Mean ± SD (n = 20) | |
| Cmax (ng/mL) | 20.8 ± 12.2 | 16.4 ± 5.7 | 70.2 ± 25.3 | 111.6 ± 21.6 |
| AUC0-t (ng*hour/mL) | 63.0 ± 20.3 | 59.2 ± 17.7 | 292.6 ± 87.5 | 127.3 ± 17.3 |
| AUC0-∞ (ng*hour/mL) | 64.9 ± 20.6 | 61.1 ± 17.8 | 308.8 ± 92.4 | 128.2 ± 17.4 |
| AUC0-15 min (ng*hour/mL) | 2.1 ± 1.6 | 1.2 ± 0.7 | 0.7 ± 0.7 | 16.2 ± 4.0 |
| AUC0-30 min (ng*hour/mL) | 5.8 ± 4.1 | 3.6 ± 1.9 | 8.1 ± 5.0 | 39.7 ± 7.1 |
| t (hour) | 3.1 ± 0.6 | 3.3 ± 0.9 | 3.8 ± 1.8 | 2.3 ± 0.4 |
| λZ | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.0 |
| AUC%extrap (%) | 3.0 ± 1.4 | 3.4 ± 2.3 | 5.2 ± 4.5 | 0.7 ± 0.3 |
Sumatriptan powder delivered using the Breath Powered device, mean delivered dose 16 mg.
AUC = area under the curve; SD = standard deviation.
The apparent terminal elimination half-life, at approximately 3-4 hours, was comparable following the 2 intranasal treatments and the oral tablet but was shorter for the subcutaneous injection at approximately 2 hours. PK parameters were similar for both races represented in the study.
Statistical comparisons of the plasma sumatriptan PK parameters using geometrical means are summarized in Table 4. Although the overall extent of systemic exposure (not dose adjusted) was similar for Breath Powered delivery of sumatriptan powder and nasal spray, the peak exposure and cumulative exposure in the first 30 minutes post-dose was approximately 20% and 52%, respectively, higher for sumatriptan powder suggesting that more sumatriptan reaches the systemic circulation early after dosing despite the delivery of an approximately 20% lower dose (16 mg vs 20 mg). Relative to both oral tablet and subcutaneous injection, the peak and overall exposure following sumatriptan powder delivered intranasally by the Breath Powered device were substantially lower.
Table 4.
Statistical Comparisons of Plasma Sumatriptan Pharmacokinetic Parameters: Sumatriptan Powder vs 20-mg Nasal Spray
| Parameter | Geometric LS Means | |||
|---|---|---|---|---|
| Sumatriptan Powder (n = 20) | 20 mg Nasal Spray (n = 20) | % Geometric Mean Ratio | 90% Confidence Intervals | |
| Cmax | 18.4 | 15.4 | 119.4 | (98.9-144.1)* |
| AUC0-t | 60.1 | 56.5 | 106.4 | (93.8-120.7) |
| AUC0-30 min | 4.8 | 3.1 | 151.9 | (117.1-197.0)* |
| AUC0-∞ | 61.9 | 58.4 | 106.0 | (93.6-120.0) |
| Parameter | Geometric LS Means | |||
|---|---|---|---|---|
| Sumatriptan Powder (n = 20) | 100 mg Oral Tablets (n = 20) | % Geometric Mean Ratio | 90% Confidence Intervals | |
| Cmax | 18.4 | 66.4 | 27.7 | (23.0-33.4)* |
| AUC0-t | 60.1 | 280.9 | 21.4 | (18.9-24.3)* |
| AUC0-30 min | 4.8 | 6.9 | 68.5 | (52.8-88.8)* |
| AUC0-∞ | 61.9 | 296.5 | 20.9 | (18.5-23.7)* |
| Parameter | Geometric LS Means | |||
|---|---|---|---|---|
| Sumatriptan Powder (n = 20) | 6 mg S.C. Injection (n = 20) | % Geometric Mean Ratio | 90% Confidence Intervals | |
| Cmax | 18.4 | 109.6 | 16.8 | (13.9-20.2)* |
| AUC0-t | 60.1 | 126.2 | 47.6 | (42.0-54.0)* |
| AUC0-30 min | 4.8 | 39.1 | 12.2 | (9.4-15.8)* |
| AUC0-∞ | 61.9 | 127.1 | 48.7 | (43.1-55.2)* |
Outside the boundary for bioequivalence (entire 90% confidence interval within the range of 80-125%).
Parameters were ln-transformed prior to analysis.
Values for sumatriptan powder, 20-mg nasal spray, 100-mg tablet, and 6 mg injection are the exponentiated LS means from the ANOVA. % Geometric mean ratio = 100*exp(LS mean test – LS mean reference). % Intrasubject CV = 100*sqrt(exp(s2) – 1), where s2 is the residual variance component from the ANOVA.
ANOVA = analysis of variance; AUC = area under the curve; LS = least-squares.
Safety and Tolerability.—
There were no serious AEs reported in this study, and no subject was discontinued due to an AE. The most frequently reported AEs were nausea reported by 3 subjects each following the tablet and the injection, and flushing reported by 4 subjects following the injection. The only AE considered to be related to Breath Powered administration of sumatriptan powder was dysgeusia in 1 subject. No clinically significant changes in clinical laboratory tests, vital signs, or ECG results were recorded.
DISCUSSION
Different routes of administration and formulations of sumatriptan offer alternatives not only for addressing dose reliability or patient convenience but also for altering the PK profile to maximize the balance between efficacy and tolerability. It has been suggested that the rate of absorption of sumatriptan is an important factor in the level of efficacy produced, while the level of exposure (both peak and total) is likely associated with reduced tolerability.20 The current study allows for a comparative characterization of the PK profile of a new migraine therapy, OptiNose Breath Powered intranasal sumatriptan powder, to 3 distinct and commonly utilized sumatriptan products.
The PK characteristics of sumatriptan powder in the present study were consistent with those previously reported with this delivery system; a prior study showed rapid absorption but significantly lower Cmax and total exposure than injection in patients suffering a migraine episode.18 In this study, the initial rate of rise in plasma concentration was faster following Breath Powered administration of sumatriptan powder than following either the 20-mg sumatriptan nasal spray or the 100-mg oral tablet.
Comparison of various oral and parenteral formulations of sumatriptan indicate that the rate of rise of plasma concentrations during the initial period of absorption gives a good indication of efficacy4,20 and may explain the similar clinical efficacy of a 20-mg conventional nasal spray to that of 100-mg oral tablets despite significant differences in plasma levels.4 Rate of rise in concentration may also contribute to the relatively high efficacy at 60 minutes reported with the Breath Powered sumatriptan powder device in migraine patients.21
Evaluation of the mean absorption profile for the 2 forms of intranasal administration revealed some key differences. Unlike the range of currently available sumatriptan injection products, which are bioequivalent, PK profiles demonstrate that these intranasal products are not bioequivalent. With the liquid nasal spray, there is a pronounced hybrid absorption pattern with a dual peak (Fig. 3), suggesting proportionately lower intranasal absorption followed by a higher degree of what is most likely GI absorption, consistent with a large portion of the delivered dose being swallowed.14 In contrast, the early peak is more pronounced after sumatriptan powder, suggesting a larger proportion of the delivered dose is intranasally absorbed. As presented in Table 3, differences between Breath Powered intranasal powder and the standard liquid nasal spray, respectively, are also evident in several metrics characterizing the absorption profiles even before performing dose adjustment for delivered dose, including Cmax (20.8 vs 16.4 ng/mL), AUC0-30 (5.8 ng*hour/mL vs 3.6 ng*hr/mL) and AUC0-15 (2.1 ng*hour/mL vs 1.2 ng*hr/mL). The delay in time to maximum concentration associated with the nasal spray relative to sumatriptan powder (blended values for tmax(median):1.5 hour vs 0.75 hour, respectively) is also consistent with Breath Powered delivery producing a higher proportion of early nasal absorption. However, blended values for tmax must be interpreted with caution in the context of bimodal absorption profiles. Blended tmax values do not necessarily reflect the time to peak concentration for most individual patients, do not inform the distinct nasal and GI absorption profiles, and blended values are likely delayed relative to intranasal absorption.4 Caution is also needed when interpreting PK curves of healthy volunteers rather than from migraine patients experiencing a migraine attack. In migraine patients, the GI component of absorption is significantly delayed possibly because of autonomic dysfunction, as discussed further later. Further research is necessary to define the proportionate contributions of nasal and GI absorption following administration with the Breath Powered formulation.
It is worth noting that the sumatriptan powder was administered to 2 nostrils, while the nasal spray was administered to a single nostril. The impact of administering liquid sumatriptan nasal spray in divided doses between both nostrils on the PK profile has been previously investigated5 and found not to improve either the rate or extent of absorption over administration to a single nostril. Therefore, it is unlikely that this difference in administration procedure explains the findings of the current study.
One limitation of this study is that 85% of the participants were men, whereas the therapeutic target population of migraineurs is majority female. However, the influence of gender on the PK of sumatriptan has been previously evaluated, and it has been shown that there is no significant difference between men and women after adjustment for bodyweight.22 Therefore, the results observed in this study are likely applicable to the intended population. Another limitation of this study was that the proportion of drug absorbed via the nasal mucosa was not directly measured experimentally for either of the intranasal delivery methods (eg, by use of charcoal to isolate from GI absorption). This has been done with a triptan previously,23 and such an experiment with Breath Powered delivery of sumatriptan powder would be informative.
The dose of sumatriptan powder loaded into the pair of drug capsules delivered using the Breath Powered device was approximately 22 mg. However, the measured mean delivered dose was 16 mg, which is 20% lower than the 20 mg of sumatriptan delivered with the nasal spray. This further accentuates the differences in both the rate and extent of absorption observed between the 2 different intranasal delivery approaches.
Sumatriptan liquid nasal spray has not been widely used.24 This may in part reflect a lack of motivation because of few significant perceived benefits associated with the nasal spray, which is limited by the inherent inadequacies of nasal spray delivery. Given that in many subjects, a large portion of drug is absorbed from the GI tract,14 the difference between intranasal delivery (using a nasal spray) and oral delivery may not be observable in many patients. Breath Powered delivery of sumatriptan powder with the OptiNose device avoids many of the delivery inadequacies of a typical spray by distributing powder to the area beyond the nasal valve, producing an absorption profile consistent with proportionately more intranasal and less GI absorption. The resulting large difference in speed and extent of absorption at the earliest time points after treatment is likely due to a more extensive absorption from the nasal cavity. This study evaluated healthy volunteers; however, a shift towards proportionately greater nasal absorption may be especially important in the clinical context of a migraineur, where the differences between oral dosing and Breath Powered dosing may be more pronounced than in healthy volunteers. Multiple studies have shown delayed gastric emptying in patients with migraine headache, suggesting risks to reliability and speed of medication absorption after oral dosing9 and a “rightward shift” of the oral PK curve in such patients. Because rapid rate of rise in sumatriptan blood levels has been hypothesized to produce a faster speed of onset or higher magnitude of treatment efficacy,4 it is important to note that Breath Powered delivery was associated with a more rapid initial rate of rise than either oral or nasal spray. Additional theoretical benefits associated with achieving true deep intranasal deposition, augmented by positive pressure exhaled breath, include delivery of drug and CO2 to the first branch of the trigeminal nerve and the parasympathetic sphenopalatine ganglion.25 The higher efficacy per milligram of intranasal delivery relative to all other routes of delivery has been previously noted when attempting to predict efficacy on the basis of sumatriptan PKs,4 but the presence or absence of such unique benefits should be better elucidated in future clinical trials designed to test the efficacy of Breath Powered sumatriptan powder.
Tolerability or safety concerns are sometimes associated with use of injected and oral triptans.26 This study found significantly lower peak and overall systemic exposure following use of the Breath Powered sumatriptan powder device compared with either the tablet or the injection. Reduced exposure may translate into a better safety and tolerability profile. This study found Breath Powered delivery of sumatriptan powder to be safe and well tolerated by healthy subjects, with no systemic AEs and only a single subject reporting dysgeusia. In contrast, 4 subjects experienced flushing following the subcutaneous injection, and 3 subjects each reported nausea following the tablet and the injection. No clinically significant findings were evident in the other safety assessments made.
It is concluded that Breath Powered intranasal delivery of sumatriptan powder produced a faster and more efficient absorption profile when compared with nasal spray and a substantially lower level of exposure than either the tablet or injection. Further research to characterize the potential clinical benefits of this novel form of delivery in the treatment of migraine headache is warranted.
Statement of Authorship
Category 1
-
(a) Conception and Design
Elliot Offman, John Messina, Ramy A. Mahmoud, Jennifer Carothers
-
(b) Acquisition of Data
Jennifer Carothers
-
(c) Analysis and Interpretation of Data
Mohammad Obaidi, Elliot Offman, John Messina, Per Djupesland
Category 2
-
(a) Drafting the Manuscript
John Messina, Ramy A. Mahmoud
-
(b) Revising It for Intellectual Content
Elliot Offman, Mohammad Obaidi, Per Djupesland, Jennifer Carothers
Category 3
-
(a) Final Approval of the Completed Manuscript
Mohammad Obaidi, Elliot Offman, John Messina, Jennifer Carothers, Per Djupesland, Ramy A. Mahmoud
Glossary
- AE
adverse event
- ANOVA
analysis of variance
- AUC
area under the curve
- BMI
body mass index
- CI
confidence intervcal
- ECG
electrocardiogram
- GI
gastrointestinal
- LLOQ
lower limit of quantification
- LS
least-squares
- PK
pharmacokinetic(s)
References
- 1.Erlichson K, Waight J. Therapeutic applications for subcutaneous triptans in the treatment of migraine. Curr Med Res Opin. 2012;28:1231–1238. doi: 10.1185/03007995.2012.674501. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin 5-HT1B/1D agonists) in migraine: Detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–658. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahlöf C. Sumatriptan nasal spray in the acute treatment of migraine: A review of clinical studies. Cephalalgia. 1999;19:769–778. doi: 10.1046/j.1468-2982.1999.1909769.x. [DOI] [PubMed] [Google Scholar]
- 4.Fox AW. Onset of effect of 5-HT1B/1D agonists: A model with pharmacokinetic validation. Headache. 2004;44:142–147. doi: 10.1111/j.1526-4610.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 5.Salonen R, Ashford E, Dahlöf C. Intranasal sumatriptan for the acute treatment of migraine. J Neurol. 1994;241:463–469. doi: 10.1007/BF00919706. [DOI] [PubMed] [Google Scholar]
- 6.Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63. doi: 10.1111/j.1526-4610.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 7.Aurora S, Kori S, Barrodale P, Nelsen A, McDonald S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443–1446. doi: 10.1111/j.1526-4610.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405–409. doi: 10.1111/j.1365-2125.1990.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aurora SK, Papapetropoulos S, Kori SH, Kedar A, Abell TL. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia. 2013;33:408–415. doi: 10.1177/0333102412473371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal R, Cardozo A, Homer JJ. The assessment of topical nasal drug distribution. Clin Otolaryngol. 2004;29:201–205. doi: 10.1111/j.1365-2273.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 11.Djupesland PG, Skretting A, Winderen M, Holand T. Breath actuated device improves delivery to target sites beyond the nasal valve. Laryngoscope. 2006;116:466–472. doi: 10.1097/01.MLG.0000199741.08517.99. [DOI] [PubMed] [Google Scholar]
- 12.Djupesland PG. Nasal drug delivery devices: Characteristics and performance in a clinical perspective – A review. Drug Deliv Transl Res. 2013;3:42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc. 2011;8:31–39. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 14.Duquesnoy C, Mamet JP, Sumner D, Fuseau E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur J Pharm Sci. 1998;6:99–104. doi: 10.1016/s0928-0987(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 15.Djupesland PG, Skretting A, Winderen M, Holand T. Bi-directional nasal delivery of aerosols can prevent lung deposition. J Aerosol Med. 17:249–259. doi: 10.1089/jam.2004.17.249. [DOI] [PubMed] [Google Scholar]
- 16.Djupesland PG, Skretting A. Nasal deposition and clearance in man: Comparison of a bidirectional powder device and a traditional liquid spray pump. J Aerosol Med Pulm Drug Deliv. 2012;25:280–289. doi: 10.1089/jamp.2011.0924. [DOI] [PubMed] [Google Scholar]
- 17.Vause C, Bowen E, Spierings E, Durham P. Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons. Headache. 2007;47:1385–1397. doi: 10.1111/j.1526-4610.2007.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luthringer R, Djupesland PG, Sheldrake CD. Rapid absorption of sumatriptan powder and effects on glyceryl trinitrate model of headache following intranasal delivery using a novel bi-directional device. J Pharm Pharmacol. 2009;61:1219–1228. doi: 10.1211/jpp/61.09.0012. [DOI] [PubMed] [Google Scholar]
- 19.2003. U.S. Food and Drug Administration Guidance for Industry: Bioavailability and bioequivalence studies for orally administered drug products – General considerations http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf.
- 20.Goadsby PJ. A triptan too far. J Neurol Neurosurg Psychiatry. 1998;64:143–147. doi: 10.1136/jnnp.64.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djupesland PG, Dočekal P the Czech Migraine Investigators Group. Intranasal sumatriptan powder delivered by a novel breath-actuated bi-directional device for the acute treatment of migraine: A randomised, placebo-controlled study. Cephalalgia. 2010;30:933–942. doi: 10.1177/0333102409359314. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. 1997. Summary basis of approval for NDA 20-626. August 26, http://www.accessdata.fda.gov/drugsatfda/nda/97/020626_imitrex_toc.cfm.
- 23.Kågedal M, Zingmark P-H, Hedlund C. True nasopharyngeal absorption of zolmitriptan after administration via nasal spray in healthy volunteers. Am J Drug Deliv. 2005;3:133–140. [Google Scholar]
- 24. IMS National Prescription Audit, May 2010–April 2011.
- 25.Tzabazis A, Niv SH, Manering NA. Trigeminal antihyperalgesic effect of intranasal carbon dioxide. Life Sci. 2010;87:36–41. doi: 10.1016/j.lfs.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders, 2nd edn. Cephalalgia. 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. (Suppl. 1): [DOI] [PubMed] [Google Scholar]


