Abstract
The Hedgehog proteins are potent organizers of animal development. They carry a cholesterol ester at the C terminus of their signaling domain. The membrane anchoring mediated by this lipophilic modification was studied by means of an approach integrating cell biology, biochemistry, biophysics, and organic chemistry techniques. Sterol-modified and fluorescent-labeled Hedgehog-derived peptides and proteins were synthesized and investigated in biophysical and cell-biological assays. These experiments revealed that cholesterol alone anchors proteins to membranes with significant strength and half-times for spontaneous desorption of several hours. Its membrane anchoring ability is comparable to dual lipidation motifs such as double geranylgeranylation or S-palmitoylation plus S-farnesylation found in other lipidated proteins. The experiments also demonstrate that membrane binding changes dramatically if short lipidated peptides are equipped with a large protein. These data suggest that for Hedgehog release and subsequent signaling an interaction partner such as the Dispatched protein is necessary. In addition to these findings the described approach allows one to correlate biophysical data obtained with model peptides with data determined with fully functional proteins and to combine results from in vitro and in vivo experiments. It should be generally applicable to other membrane anchors and proteins.
Many proteins involved in key processes of cell growth and differentiation embody lipid modifications that are essential for their biological activity. These modifications serve in most cases as anchoring groups for targeting the proteins to a certain membrane or submembrane compartment. In addition, they may mediate controlled release of proteins from membrane regions to form stable gradients. This is particularly true for the Hedgehog protein (Hh), which is among the key players in patterning numerous types of tissues. Mutations in Hh and its downstream signaling molecules are also associated with numerous oncogenic and disease states.
The Hh family of molecules consists of secreted proteins that undergo several posttranslational modifications to gain full activity. In a maturation process they perform an autocatalytic cleavage, generating an N-terminal polypeptide (Hh-Np) containing all of the signaling functions (1–4). During this cleavage process, a cholesterol moiety is attached covalently by an ester function to the C-terminal glycine of the signaling domain (5). The hydrophobicity of the protein is further increased by the addition of a palmitic acid residue to the N terminus of the cleavage product (6, 7).
In Drosophila, forms of Hh in which the C terminus has been deleted have much more potent and seemingly longer-ranging signaling activity than the cholesterol-modified forms. This observation led to the idea that the cholesterol moiety acts as an anchor to the cell membrane and limits the spread of Hh (1–3, 8). However, in vertebrate Sonic Hedgehog (Shh) the cholesterol may be required for correct biological function, e.g., as a long-range morphogen rather than primarily for membrane anchoring (7). Despite intensive studies the roles of cholesterol and palmitic acid in the process of membrane anchoring and release of Hh are still not clear. Thus, the study of fundamental characteristics of these processes in precise molecular detail is of major importance.
To date direct determination of the membrane-binding properties of biologically functional proteins has focused on acylated and isoprenylated peptides and proteins (9–16), whereas there is a gap for sterol-modified peptides and proteins.
Recently we described a strategy for the preparation of lipidated proteins that allows one to quantitatively determine their membrane-binding properties in vitro and in vivo (17, 18). The approach is based on a combination of molecular biology and organic synthesis techniques. A C-terminally truncated oncogenic Ras protein mutant is obtained by means of expression techniques and coupled to peptides incorporating different membrane-anchoring groups whose nature and composition can be altered at will by efficient organic syntheses. The hybrid proteins are then examined on the one hand in biophysical experiments yielding data concerning their ability to bind to model membranes. On the other hand, microinjection of the oncogenic semisynthetic Ras derivatives into PC12 cells induces their differentiation depending on the plasma membrane localization of the proteins, thereby generating a quantifiable in vivo readout.
Here we describe the application of this method to determine quantitatively the membrane-binding characteristics of sterol-modified proteins. To this end, fluorescent-labeled lipidated peptides representing the C terminus of Hh and carrying different sterol esters were synthesized and coupled to an oncogenic Ras mutant. The lipidated peptides and proteins were analyzed in biophysical model membrane-binding experiments based on fluorescence dequenching and surface plasmon resonance (SPR), and the sterol-modified proteins were microinjected into PC12 cells. The results give insights into the membrane-binding characteristics of sterol-anchored peptides and proteins derived therefrom. Also, a correlation of in vitro data with biological in vivo activity was possible for this class of lipid-modified conjugates. The results show that a cholesterol moiety as found at the C terminus of the signaling domain of Hh anchors a protein quasi-irreversibly to a membrane. This finding indicates that in vivo Hh must be actively released from the membrane (e.g., by means of the Dispatched protein) to fulfil its biological function.
Materials and Methods
Fluorescence Spectroscopy. For the generation of vesicles a methanolic solution of the lipid component palmitoyl oleoyl phosphatidylcholine (POPC) was mixed with lipopeptide, fluorescence quencher, or both, yielding a 1% or 2% (mol/mol) solution of lipopeptide and quencher, respectively. From the resulting solution the solvent was removed by using a stream of argon followed by drying overnight in high vacuum. The remaining solid was hydrated with vesicle buffer (10 mM Hepes/150 mM NaCl/8mMKCl/1 mM MgCl2, pH 7.0) to yield a concentration of 10 μM with regard to the lipopeptide, and the suspension was subjected to 50 freeze–thaw cycles for generating vesicles with an average diameter of ≈100 nm. For fluorescence assays this solution was transferred to a cuvette containing vesicle buffer to a final concentration of 50 nM with regard to the lipopeptide. Quencher-free vesicles were prepared in the same way. All measurements were carried out at 20°C in a FluoroMax spectrofluorimeter (Jobin–Yvon, Munich). Excitation was done at 468 nm and emission was observed at 535 nm. When examining lipoproteins, first quencher-containing vesicles were prepared as described above and then they were incubated with an aqueous solution of the corresponding lipoprotein to furnish vesicles containing lipoproteins in the presence of the fluorescence quencher.
For the fast dissociation kinetics of the androstenol-modified protein out of the vesicle membrane a SX16MV stopped-flow system (Applied Photophysics, Surrey, U.K.) was used. The measurements were performed under pseudo-first-order conditions and analyzed by using the Applied Photophysics software. Excitation of the fluorophore was performed at 468 nm, and emission signal was measured by using a cutoff filter 495FG03-25 (Andover, Salem, NH) for wavelengths above 495 nm.
SPR Analysis. SPR experiments were performed in a BIAcore-1000 instrument (BIAcore, Freiburg, Germany) at 20°C. An L1 sensor chip (BIAcore) with a hydrophobically modified dextran layer was loaded with a 0.5 mM solution of POPC vesicles (two injection cycles, 5 μl/min, 25-μl volume). A 30-s injection of buffer (10 mM Hepes/150 mM NaCl/5 mM MgCl2, pH 7.4) at high flow rate (100 μl/min) and a 2-min pulse of 10 mM NaOH (5 μl/min) were performed to remove larger aggregates from the surface. Lipopeptide-N-Ras constructs and unmodified N-Ras were applied at a protein concentration of 50 μg/ml at a flow rate of 5 μl/min in SPR buffer for 7 min. Dissociation was analyzed by washing with SPR buffer for up to 8 hr.
Further materials and methods used are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
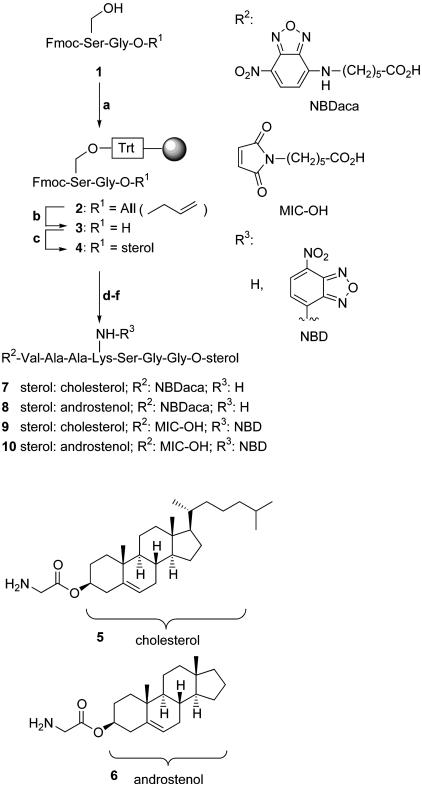
Synthesis of the Lipopeptides. For the synthesis of C-terminally sterol-modified heptapeptides a combined approach consisting of solution and solid-phase chemistry was chosen that allows for a flexible and efficient introduction of different functional and reporter groups, i.e., different sterols, a fluorescent label, and a maleimidocaproyl (MIC) group (see Fig. 1).
Fig. 1.
Synthesis of sterol-modified heptapeptides and overview of synthesized peptides. Step a, 1 (1 eq), N,N-diisopropylethylamine (DIEA, 2 eq), chlorotrityl-resin (0.5 eq), CH2Cl2, 5 hr, room temperature. Step b, Pd(PPh3)4 (0.2 eq), PhSiH3 (24 eq), CH2Cl2, 3 hr, room temperature. Step c, H-Gly-O-sterol (5 or 6, 5 eq), benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP, 5 eq), DIEA (10 eq), dimethylformamide (DMF), 5 hr, room temperature. Step d, DMF/piperidine 1:4 (vol/vol), twice for 10 min, room temperature. Step e, fluorenylmethoxycarbonyl (Fmoc)-(Xaa)x-OH (4 eq), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU, 3.6 eq), 1-hydroxybenzotriazole (HOBt, 4.8 eq), DIEA (8 eq), DMF, 1 hr, room temperature. Step f, repetition of steps d and e, using in the last step N-[6(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproic acid (NBDaca) or MIC-OH, respectively. NBD, 7-nitrobenz-2-oxa-1,3-diazole.
Dipeptide 1 was synthesized from the corresponding monoprotected amino acids and attached to the solid support as a trityl ether that can be cleaved by using 5% trifluoroacetic acid. The C-terminal allyl ester is cleaved by noble-metal-mediated allyl transfer to phenylsilane to yield C-terminally unmasked polymer-bound dipeptide 3. The C terminus was elongated with glycyl-sterol esters 5 or 6 by employing PyBOP as a coupling reagent. The glycyl sterol esters are readily available in high yield by esterification of t-butoxycarbonyl (Boc)-protected glycine and the sterol employing N,N′-diisopropylcarbodiimide (DIC) and 4-(dimethylamino)pyridine (DMAP) followed by selective removal of the Boc protecting group by treatment with 50% trifluoroacetic acid. N-terminal peptide chain elongation was achieved by means of standard solid-phase peptide chemistry to yield differently fluorescent labeled peptides carrying an NBD group at a lysine side chain or the N terminus or MIC-modified peptides 7–10. The N-terminally modified peptides were cleaved from the resin under very mild conditions, furnishing the desired products in high yields without any side reactions. Cholesterol was chosen because of its occurrence in Hh, whereas androstenol was selected because it is the smallest sterol that is able to initiate the Hh autoprocessing step (19).
Membrane-Binding Properties of the Lipopeptides. To evaluate their membrane-binding properties the lipidated peptides 7 and 8 were applied in an assay that allows determination of kinetic values for binding to liposome model membranes (13). For this assay vesicles of POPC were generated by the freeze/thaw technique (see Materials and Methods) containing 1 mol % peptide 7 and 8, respectively, and 2 mol % nonexchangeable fluorescence quencher N-(lissamine rhodamine sulfonyl)phosphatidylethanolamine (Rho-DHPE). The average diameter of the vesicles was measured by dynamic light scattering to be ≈100 nm. The size of the vesicles is relevant because the rate constant determined (see below) may be characteristic of the particular size of the vesicles used here. If in such vesicles the NBD fluorophor of the incorporated lipopetide is excited at 468 nm its fluorescence at 535 nm is absorbed directly by the rhodamine dye of the quencher when the quencher is in close proximity to the lipopeptide. If these vesicles are mixed with an excess of pure POPC vesicles, mobile lipopeptides can desorb from them. After entering the quencher-free vesicles the fluorescence at 535 nm is increased.
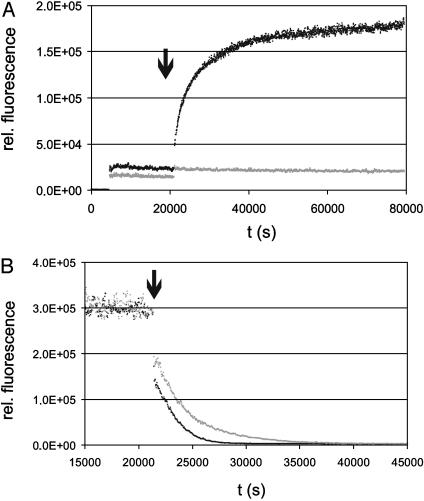
After establishing a stable baseline these doubly loaded vesicles were mixed with a 12-fold excess of pure POPC vesicles. Only lipopeptide 8 with an androstenol moiety gave an increase of fluorescence within the time range of the experiment (Fig. 2A).
Fig. 2.
Membrane binding of lipopeptides. (A) Intervesicle transfer experiments using sterol-modified peptides 7 (gray trace) and 8 (black trace). POPC vesicles containing 2 mol % Rho-DHPE and either 1 mol % peptide 7 or 8 were diluted in buffer to a final concentration of 50 nM NBD-lipopeptide and mixed with a 12-fold excess of pure POPC vesicles (arrow indicating time of addition). The change in fluorescence was monitored at 535 nm. All experiments were carried out at 20°C. (B) Examination of the flip-flop-exchange of the lipopeptides 7 (gray) and 8 (black). For this assay POPC vesicles loaded only with 1 mol % NBD-labeled lipopetide 7 or 8 were diluted to a final concentration of 50 nM lipopeptide. After a stable baseline had been established, 10 mM sodium dithionite was added (arrow). All experiments were carried out at 20°C. The change in fluorescence was monitored at 535 nm.
The transfer of the lipopeptide 8 to the quencher-free vesicles shows that this type of lipid modification is not sufficient for permanent membrane anchoring. In contrast, the NBD emission of the lipopeptide 7 carrying cholesterol was not affected when a 12-fold excess of acceptor vesicles was added. This observation demonstrates that a cholesterol modification that resembles the C-terminal modification of the native Hh protein is sufficient for quasi-irreversible membrane binding of the heptapeptide.
The intervesicle transfer of lipopeptide 8 consists of two separate processes. The peptides that are attached to the outer phase of the vesicle can directly migrate to the quencher-free vesicle by diffusion. If the acceptor vesicles are present in high concentration this step can be described as an irreversible first-order mechanism. Depending on their distribution between inner and outer face of the vesicles, the intravesicular lipopeptides have to perform a reversible flip-flop diffusion to appear on the outer face of the vesicles. In a first estimation the overall change in fluorescence for the peptide 8 was fitted by a monoexponential function. For vesicles prepared as described above, best fits were obtained for a dissociation rate constant of 2.6 ± 0.9 × 10–4 s–1 (n = 6).
The distribution of the lipopeptides between the inner and outer faces of the POPC vesicles can be monitored by dithionite treatment of POPC vesicles loaded with NBD-labeled lipopetide only (ratio as described above). All accessible NBD groups on the outer phase of the vesicle are readily reduced by an excess of sodium dithionite (10 mM). This first loss of the fluorescence signal was for both of the lipopeptides 7 and 8 in the range of 50–60% of total fluorescence and was followed by a slower decay. This observation indicates that the intravesicular lipopeptides have to perform the flip-flop exchange (Fig. 2B).
The decay after addition was fitted again by a monoexponential function. Best fits were obtained for a flip-flop exchange rate of 3.0 × 10–4 s–1 for lipopetide 8 and 5.0 × 10–4 s–1 for the cholesterol-modified peptide 7. As a control for the sealing quality of the vesicles, we repeated the experiment with lipopeptide 8 for increasing dithionite concentrations. Indeed, the observed rate of fluorescence quenching showed an increase (kobs = 7.2 × 10–4 s–1 for 20 mM and 8.7 × 10–4 s–1 for 30 mM dithionite). Extrapolation from these data gives a real flip-flop exchange rate in the order of 2.6 × 10–4 s–1 for lipopeptide 8.
In any case these values lie in the same range as the data obtained for transvesicular exchange of monofarnesylated and carboxymethylated peptides of the N-Ras C terminus [NBD-GCMGLPC(farn)-OMe], yielding a rate of 8.3 × 10–4 s–1 (13). With regard to the lower temperature (15°C) during these measurements and assuming a moderate influence of the different amino acid of the pentapeptides, one can conclude that farnesylated peptides undergo under comparable conditions (20°C) a significantly faster flip-flop exchange than sterol-modified peptides. This faster exchange may be due to the lower hydrophobicity of the farnesyl modification as compared with the sterol moieties.
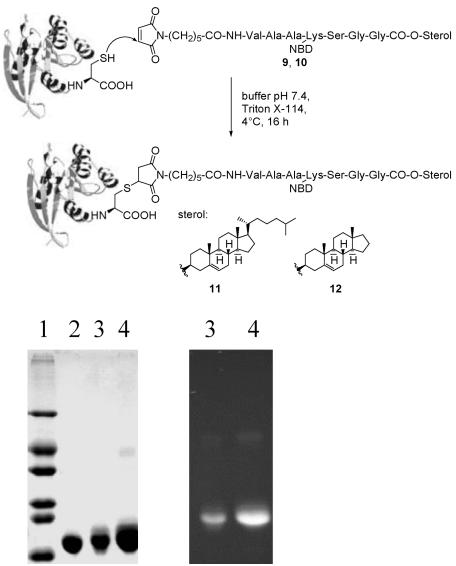
Synthesis of Peptide–Protein Conjugates. To estimate whether the data and conclusions gleaned from the experiments with the lipidated peptides allow for valid extrapolations to proteins, peptide-protein constructs were synthesized consisting of MIC-modified and fluorescently labeled heptapeptides 9 and 10 and truncated N-RasG12V(1–181) to yield the fluorescently labeled protein chimeras 11 and 12 (Fig. 3). Truncated N-Ras proteins with a C-terminal cysteine at position 181 were generated by recombinant expression in Escherichia coli. The removal of the C-terminal MSCKCVLS sequence had no effect on protein expression in E. coli CK600K, nucleotide binding, and the interaction with the Ras-binding domain (RBD) of Raf-kinase (data not shown).
Fig. 3.
Generation of protein chimeras 11 and 12, starting from the lipopeptides 9 and 10. (Upper) Structures. (Lower) Gel electrophoresis of chimeras: after Coomassie-blue staining (Left) and fluorescence (Right). Lane 1, size marker; lane 2, N-RasG12V(1–181); lane 3, N-RasG12V(1–181)-Hh(NBD)-O-cholesterol 11; lane 4, N-RasG12V(1–181)-Hh(NBD)-O-androstenol 12.
Truncated Ras proteins were mixed with the MIC-modified lipopeptides 9 and 10 in a 2:3 stoichiometry, and the reaction products were purified by extraction (20) and ion-exchange chromatography.
N-Ras was chosen as the protein moiety because this system offers the possibility to estimate the membrane binding of different membrane anchors in vivo. Although N-Ras has three additional natural cysteines at positions 51, 80, and 118, previous examinations demonstrated (17, 18) that only the cysteine at position 181 is surface accessible and reacts exclusively with the MIC group to furnish lipid-modified Ras proteins.
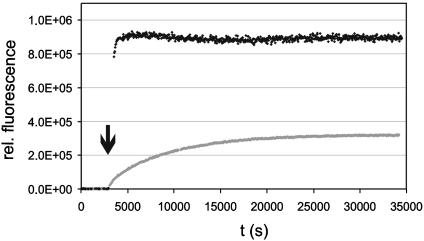
Membrane-Binding Properties of the Lipoproteins. With the semisynthetic sterol-modified proteins, intervesicle exchange experiments analogous to the experiments above were carried out. Solutions of quencher containing POPC vesicles in buffer were incubated with solutions of the lipoprotein to yield vesicles that contain NBD-labeled lipoprotein and quencher at a ratio of 1:2 while the lipid component is present in 100-fold excess with regard to the lipoprotein. After establishing a stable baseline of fluorescence, a 12-fold excess of quencher-free vesicles was applied (Fig. 4).
Fig. 4.
Intervesicle transfer experiments using sterol-modified proteins 11 (black) and 12 (gray). Buffer solutions containing 5 μM POPC vesicles doped with 2 mol % Rho-DHPE were incubated with 50 nM protein–lipid chimera to generate lipoprotein-loaded POPC vesicles. After a stable baseline had been established a 12-fold excess of pure POPC vesicles was added (arrow) and the fluorescence change was monitored. All experiments were carried out at 20°C.
For both lipoproteins an increase in fluorescence signal could be observed, demonstrating that intervesicle transfer takes place. Due to the very fast exchange in the case of the androstenol-modified lipoprotein 12 the kinetics of the fluorescence change could not be resolved in a standard fluorescence spectrometer. Thus, experiments were performed in a stopped-flow instrument mixing solutions of quencher and NBD-labeled lipoprotein-containing vesicles with pure POPC vesicles at the ratio described above. On the basis of the fluorescence assay the exchange of the cholesterol-modified protein 11 was fitted by a monoexponential function. Best fits were obtained for a dissociation rate of 5.9 ± 0.9 × 10–5 s–1. From the stopped-flow experiments the rate of dissociation of the androstenol-modified lipoprotein was estimated to be 9.2 ± 0.2 × 10–1 s–1.
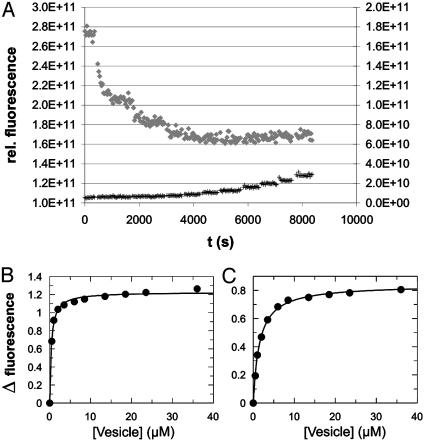
Approaches to titrate the lipopeptides directly with lipid vesicles failed for solubility reasons. However, binding curves were obtained for the corresponding lipoproteins. Both the cholesterol- and the androstenol-modified protein were diluted in buffer to a final concentration between 50 and 100 nM and titrated with POPC vesicles doped with Rho-DHPE. Fig. 5A shows the change in the NBD fluorescence signal for the cholesterol-modified lipoprotein and a buffer control. In Fig. 5 B and C the specific fluorescence changes were plotted against the efficient lipid concentration and fitted as a 1:1 binding curve. The equilibrium partition coefficients were 0.38 μM for the cholesterol construct and 1.5 ± 0.2 μM (n = 3) for the androstenol protein. The coefficient for the cholesterol chimera represents the upper limit of the real value. Half-maximal binding was established with the first titration point, at which the accessible lipid concentration was not in sufficient excess over protein concentration.
Fig. 5.
Titration of lipoproteins 11 and 12 with POPC/Rho-DHPE vesicles. Androstenol- and cholesterol-modified lipoproteins were diluted in buffer to a final concentration between 50 and 100 nM and the change in NBD emission at 535 nm was monitored after stepwise addition of POPC vesicles containing 4 mol % Rho-DHPE. (A) Raw data for titration of lipoprotein 11 (♦, left axis). Nonspecific increase in signal due to the addition of Rho-DHPE-containing vesicles was measured in a cuvette filled with buffer only (+, right axis). (B and C) Binding curves for lipoprotein 11 and 12, respectively.
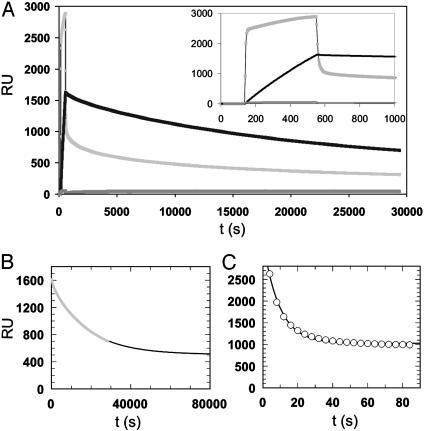
As an independent readout system for membrane binding the hydrophobic surface of an L1 sensor chip of a commercial SPR system (BIAcore 1000) was coated with a POPC lipid bilayer (Fig. 6).
Fig. 6.
Examination of membrane insertion of lipoproteins 11 and 12 by using SPR. Solutions containing 50 μg/ml of lipoprotein 11 (black), 12 (light gray), or nonmodified N-Ras (dark gray) protein were applied to a POPC-coated surface of an L1 biochip. (A) Complete time trace showing injection of (lipo-)protein and washing with buffer. (Inset) Expanded view of the injection and early dissociation phase. (B and C) Monoexponential fits for the dissociation phases of lipoproteins 11 (B, fast phase) and 12 (C).
Solutions of the different lipoproteins were applied to this surface, and by rinsing the surface with buffer desorption of the lipoproteins from the membrane was studied. The results demonstrated that both membrane insertion and desorption of the cholesterol-modified protein 11 correlate well with the results obtained from the fluorescence assay. For the cholesterol-modified protein 11 the desorption process was fitted by using again an exponential function giving a dissociation rate constant of 5.8 × 10–5 s–1. This value fits exceptionally well with the results obtained from the intervesicle transfer assay. The androstenol construct showed a biphasic pattern for both binding and dissociation. The initial fast phase of the dissociation was fitted monoexponentially, resulting in a kdiss of 0.11 s–1. The apparent dissociation rate constant for the SPR experiment was almost one order of magnitude smaller than in the corresponding stopped-flow experiment. We attribute this difference to the fast rebinding of the androstenol lipoprotein to the membrane. For the cholesterol-modified Ras this behavior is less pronounced because of slower association and dissociation. For both lipoproteins fits were performed with an offset, indicating that part of the surface-bound protein was absorbed irreversibly.
Membrane Binding in Vivo. To evaluate the biological activity of the lipoproteins in vivo we chose a system based on microinjection into PC12 cells. In this approach a protein composed of a truncated oncogenic N-Ras protein and a lipopeptide tail is injected into the cells. The Ras protein head manifests and displays its biological activity, depending on the efficiency of the membrane anchoring caused by the lipid modifications in the lipopeptide tail. Thus, this method offers a general approach to estimate the membrane-inserting ability of different lipid modifications in vivo and to examine the distribution of the lipid-modified proteins between different endomembranes.
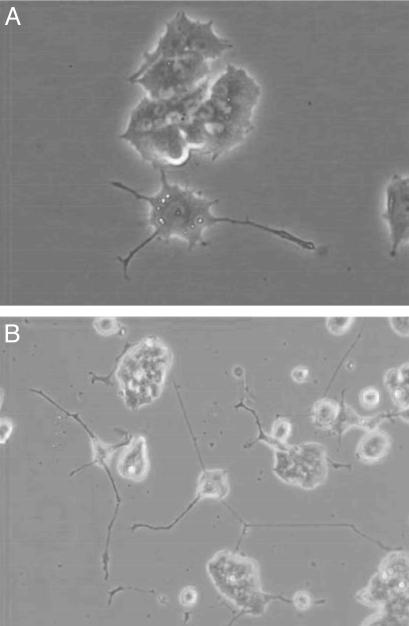
After injection of the coupling product 11 of the cholesterol-modified peptide with N-RasG12V(1–181) at a concentration of 60 μM, 56 ± 4% of the injected cells developed neurites (Fig. 7B). Injection of the androstenol-modified conjugate 12 led to a differentiation rate of 27 ± 7% (Fig. 7A). Thus, the cholesterol-modified protein conjugate 11 shows a higher differentiation efficiency than the androstenol construct, probably due to an increased proportion of the lipoprotein that is anchored in cellular membrane structures. Remarkably, the cholesterol-modified protein qualitatively behaves like the full-length recombinant oncogenic N-RasG12V protein and a Ras protein equipped with an S-farnesyl and an S-hexadecyl anchor (17, 18).
Fig. 7.
Transformation of PC12 cells by protein chimera 11 (B) and 12 (A). Proteins were injected at a concentration of 60 μM in PBS.
Discussion
Secreted signaling proteins of the Hh family are potent organizers of animal development (21). They carry lipid modifications that anchor them to the plasma membrane and limit their range of action. Despite the fact that intense research activities have accumulated a substantial body of knowledge about the Hedgehog proteins many questions concerning the molecular details governing Hh insertion in and release from membranes and thereby the subsequent signaling processes remain open. We have used an approach that combines techniques of cell biology, biochemistry, biophysics, and organic chemistry to gain quantitative insight into the membrane-anchoring ability of one of the two membrane anchors of Hh, the C-terminal cholesterol ester. Sterol-modified and fluorescent-labeled Hh-derived lipidated peptides and proteins were synthesized by solid-phase chemistry techniques and a maleimido (MIC) group for coupling to an expressed Ras mutant.
With the lipopeptides, membrane-anchoring characteristics of two different sterols were examined by using intervesicle transfer experiments on liposomes. To our knowledge this is the first study to determine membrane-binding properties of this type of lipophilic modification. In a previous study the effect of cholesterol in combination with a palmitoyl group for the association with lipid raft-like structures was examined (22). We could show that a single cholesterol moiety is sufficient for quasi-irreversible membrane anchoring of a heptapeptide to a model membrane. The anchoring properties of the cholesterol anchor are comparable to the dual-anchor motifs found in doubly geranylgeranylated and carboxymethylated or doubly palmitoylated peptides (13, 23). Comparison of the rate constant for the intervesicle transfer of the androstenol-modified lipopeptide 8 with data obtained for lipopeptides carrying one lipid modification (palmitoyl thioester) shows that androstenol confers a significantly enhanced stability of membrane insertion (one order of magnitude) with regard to a palmitoyl group (23).
To draw a direct comparison of the membrane-binding properties of lipopeptides with the corresponding lipoproteins, protein chimeras were generated from sterol-modified heptapeptides and a truncated Ras protein to mimic the hydrophilicity of the protein part. These semisynthetic proteins were subjected to the same biophysical experiments carried out with the lipopeptides. These revealed that the cholesterol moiety, which is able to incorporate peptides almost irreversibly to membranes, leads in the case of an attached 20-kDa protein to dissociation with half-times of about 3 h. For the androstenol moiety a relatively stable membrane insertion of peptides can be observed, whereas the corresponding protein is bound to the membrane in a rapidly reversible manner.
Silvius and co-workers (13) described coupling with polyethylene glycol (PEG) chains of different size as a mimicry for a soluble protein moiety in intervesicle transfer experiments with doubly geranylgeranylated lipopeptides. Dissociation rates of the corresponding lipopeptides from donor vesicles increased continuously with the size of the PEG chain, reducing the half-time from 117 h for the lipopeptide itself to 14.4 h for the largest PEG-modified lipopeptide of ≈2 kDa size. McLaughlin and colleagues found that the binding of myristoylated Src peptides (10) and proteins (15) with phosphatidylcholine/phosphatidylserine vesicles resulted in an increase of the apparent Kd by one order of magnitude. Compared with these data and apart from differences in the experimental details (e.g., lipid compositions, temperatures) the increase in dissociation rate of the androstenol-anchored probe by three orders of magnitude after attachment of a 21-kDa protein domain is unexpected.
For Hh our findings indicate that the cholesterol ester alone anchors the protein to the membrane with significant strength and a high half-time too long for spontaneous transfer. The available structural data of the hedgehog signaling domain (24) do not indicate that Hh has a hydrophobic binding pocket that could bury the cholesterol anchor temporary as described for, e.g., the small GTP-binding protein Arf (25). Given the fact that the protein carries a palmitic acid as a second strong membrane anchor, spontaneous release of the protein to generate a signaling gradient can be ruled out on the basis of biophysical considerations alone. Rather, and in accordance with current knowledge about Hh signaling, an interaction partner such as the Dispatched protein appears to be necessary (26). These conclusions are additionally backed by cell-biological experiments with the Ras conjugates carrying the sterol anchors.
Different efficiencies of the cholesterol and the androstenol group in anchoring peripheral membrane proteins in the plasma membrane became evident by inducing PC12 cell differentiation with oncogenic Ras lipoproteins. The protein part of these conjugates serves as a probe indicating the efficiency of membrane incorporation in vivo. Thus, microinjection of 60 μM peptide-protein conjugates into PC12 cells led to differentiation of 56% in the case of the cholesterol-modified construct 11, whereas the androstenol-modified conjugate 12 initiated a transformation rate of 27%. This biological activity reflects in a convincing manner the different membrane-binding properties determined by the biophysical examinations in vitro.
Beyond the conclusions drawn for membrane anchoring of Hh and (in earlier investigations) of the Ras proteins we would like to point out that the combined chemical-biological approach applied by us has the potential to serve as a fairly general biological readout system. In this system on the one hand the oncogenic Ras protein can be used to generate in the PC12 cell assay a biological readout quantifying the plasma-membrane-anchoring ability of different membrane anchors whether they are natural or not. On the other hand, the typical Ras membrane anchor can be coupled to different proteins directing them to the plasma membrane so that their activity dependent on this specific localization can be studied.
Supplementary Material
Acknowledgments
We thank C. Nowak for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. C.P. is grateful to the Hermann-Schlosser-Stiftung of the Degussa AG for financial support.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Hh, Hedgehog protein; SPR, surface plasmon resonance; POPC, palmitoyl oleoyl phosphatidylcholine; MIC, maleimidocaproyl; NBD, 7-nitrobenz-2-oxa-1,3-diazole; Rho-DHPE, N-(lissamine rhodamine sulfonyl)phosphatidylethanolamine.
References
- 1.Mullor, J. L., Sanchez, P. & Ruiz i Altaba, A. (2002) Trends Cell Biol. 12, 562–569. [DOI] [PubMed] [Google Scholar]
- 2.Roelink, H., Porter, J. A., Chiang, C., Tanabe, Y., Chang, D. T., Beachy, P. A. & Jessell, T. M. (1995) Cell 81, 445–455. [DOI] [PubMed] [Google Scholar]
- 3.Porter, J. A., Vonkessler, D. P., Ekker, S. C., Young, K. E., Lee, J. J., Moses, K. & Beachy, P. A. (1995) Nature 374, 363–366. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe, J., Pierani, A., Jesell, T. M. & Ericson, J. (2000) Cell 101, 435–445. [DOI] [PubMed] [Google Scholar]
- 5.Porter, J. A., Young, K. E. & Beachy, P. A. (1996) Science 274, 255–259. [DOI] [PubMed] [Google Scholar]
- 6.Pepinsky, R. B., Zeng, C. H., Wen, D. Y., Rayhorn, P., Baker, D. P., Williams, K. P., Bixler, S. A., Ambrose, C. M., Garber, E. A., Miatkowski, K., et al. (1998) J. Biol. Chem. 273, 14037–14045. [DOI] [PubMed] [Google Scholar]
- 7.Zeng, X., Goetz, J. A., Suber, L. M., Scott, W. J., Schreiner, C. M. & Robbins, D. J. (2001) Nature 411, 716–720. [DOI] [PubMed] [Google Scholar]
- 8.Bumcrot, D. A., Takata, R. & McMahon, A. P. (1995) Mol. Cell. Biol. 15, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peitzsch, R. M. & McLaughlin, S. (1993) Biochemistry 32, 10436–10443. [DOI] [PubMed] [Google Scholar]
- 10.Buser, C. A., Sigal, C. T., Resh, M. D. & McLaughlin, S. (1994) Biochemistry 33, 13093–13101. [DOI] [PubMed] [Google Scholar]
- 11.Silvius, J. R. & l'Heureux, F. (1994) Biochemistry 33, 3014–3022. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian, S. & Silvius, J. R. (1995) Biochemistry 34, 3813–3822. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder, H., Leventis, R., Rex, S., Schelhaas, M., Nagele, E., Waldmann, H. & Silvius, J. R. (1997) Biochemistry 36, 13102–13109. [DOI] [PubMed] [Google Scholar]
- 14.Silvius, J. R. (1999) in Protein Lipidation Protocols, Methods in Molecular Biology, ed. Gelb, M. E. (Humana, Totowa, NJ), Vol. 116, pp. 177–186. [Google Scholar]
- 15.Sigal, C. T., Zhou, W., Buser, C. A., McLaughlin, S. & Resh, M. D. (1994) Proc. Natl. Acad. Sci. USA 91, 12253–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silvius, J. R. (2002) in Peptide–Lipid Interactions, Currents Topics in Membranes, eds. Simon, S. & McIntosh, T. (Academic, New York), Vol. 52, pp. 371–395. [Google Scholar]
- 17.Bader, B., Kuhn, K., Owen, D. J., Waldmann, H., Wittinghofer, A. & Kuhlmann, J. (2000) Nature 403, 223–226. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn, K., Owen, D. J., Bader, B., Wittinghofer, A., Kuhlmann, J. & Waldmann, H. (2001) J. Am. Chem. Soc. 123, 1023–1035. [DOI] [PubMed] [Google Scholar]
- 19.Mann, R. K. & Beachy, P. A. (2000) Biochim. Biophys. Acta 1529, 188–202. [DOI] [PubMed] [Google Scholar]
- 20.Bordier, C. (1981) J. Biol. Chem. 256, 1604–1607. [PubMed] [Google Scholar]
- 21.Nybakken, K. & Perrimon, N. (2002) Curr. Opin. Genet. Dev. 12, 503–511. [DOI] [PubMed] [Google Scholar]
- 22.Wang, T. Y., Leventis, R. & Silvius, J. R. (2001) Biochemistry 40, 13031–13040. [DOI] [PubMed] [Google Scholar]
- 23.Eisele, F., Kuhlmann, J. & Waldmann, H. (2001) Angew. Chem. Int. Ed. 40, 369–373. [PubMed] [Google Scholar]
- 24.Tanaka Hall, T. M., Porter, J. A., Beachy, P. A. & Leahy, D. J. (1995) Nature 378, 212–216. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg, J. (1998) Cell 95, 237–248. [DOI] [PubMed] [Google Scholar]
- 26.Burke, R., Nellen, D., Bellotto, M., Hafen, E., Senti, K.-A., Dickson, B. J. & Basler, K. (1999) Cell 99, 803–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.