Abstract
Therapeutic hypothermia is commonly used to improve neurological outcomes in patients after cardiac arrest. However, therapeutic hypothermia increases sepsis risk and unintentional hypothermia in surgical patients increases infectious complications. Nonetheless, the molecular mechanisms by which hypothermia dysregulates innate immunity are incompletely understood. We found that exposure of human monocytes to cold (32°C) potentiated LPS-induced production of TNF and IL-6, while blunting IL-10 production. This dysregulation was associated with increased expression of microRNA-155 (miR-155), which potentiates Toll-like receptor (TLR) signaling by negatively regulating Ship1 and Socs1. Indeed, Ship1 and Socs1 were suppressed at 32°C and miR-155 antagomirs increased Ship1 and Socs1 and reversed the alterations in cytokine production in cold-exposed monocytes. In contrast, miR-155 mimics phenocopied the effects of cold exposure, reducing Ship1 and Socs1 and altering TNF and IL-10 production. In a murine model of LPS-induced peritonitis, cold exposure potentiated hypothermia and decreased survival (10 vs. 50%; P < 0.05), effects that were associated with increased miR-155, suppression of Ship1 and Socs1, and alterations in TNF and IL-10. Importantly, miR-155-deficiency reduced hypothermia and improved survival (78 vs. 32%, P < 0.05), which was associated with increased Ship1, Socs1, and IL-10. These results establish a causal role of miR-155 in the dysregulation of the inflammatory response to hypothermia.—Billeter, A. T., Hellmann, J., Roberts, H., Druen, D., Gardner, S. A., Sarojini, H., Galandiuk, S., Chien, S., Bhatnagar, A., Spite, M., Polk, H. C., Jr. MicroRNA-155 potentiates the inflammatory response in hypothermia by suppressing IL-10 production.
Keywords: sepsis, peritonitis, cold exposure
Therapeutic hypothermia has become a standard of care in patients with successful out-of-hospital resuscitation after cardiac arrest due to its beneficial impact on neurological outcomes (1). In the central nervous system, hypothermia has anti-inflammatory properties and it reduces the production of inflammatory cytokines, resulting in reduced cell death and improved neurological recovery (1, 2). Furthermore, hypothermia is commonly used in cardiac and vascular surgery during complicated reconstructions of the aortic arch to diminish the effects of hypoxia during circulatory arrest (3). Because of such clinical utility, therapeutic hypothermia is currently under investigation for its use in other common clinical scenarios in which local tissue hypoxia may occur, such as spinal cord injuries and hemorrhagic shock (4, 5).
Despite these benefits of therapeutic hypothermia, recent results indicate that the beneficial effects of therapeutic hypothermia with respect to neurological function are somewhat offset by a higher risk for infections, mainly nosocomial pneumonia, but also for systemic sepsis (6, 7). Moreover, in patients with brain injury, the beneficial effects of hypothermia are evident only under noninfectious processes (6, 8, 9). Indeed, the use of therapeutic hypothermia in patients with bacterial meningitis results in detrimental outcomes (i.e., higher mortality), especially in patients presenting with sepsis (8). These findings suggest that therapeutic hypothermia alters the immune response in a manner similar to that described for unintentional hypothermia. It has been shown before that unintentional hypothermia increases the rate of surgical site infection (SSI) and cardiac complications in patients undergoing elective surgery (10, 11). Moreover, we have recently found that unintentional hypothermia is associated with a 4-fold increase in mortality in patients undergoing elective surgery (12). The main causes of death in patients with hypothermia are sepsis and cardiovascular complications (11, 12).
Despite compelling evidence for the detrimental effects of hypothermia on host defense, the mechanisms underlying altered immune responses in hypothermia are poorly understood. Previous studies have shown that hypothermia alters the function of innate immune cells, including neutrophils, monocytes, and dendritic cells. For instance, the production of reactive oxygen species (ROS) in neutrophils, which is important for bacterial killing, is reduced in hypothermic conditions in vitro and in patients who became hypothermic during a surgical procedure (13, 14). Furthermore, hypothermia prolongs the production of proinflammatory cytokines, such as TNF, in human monocytes (15–17). Hypothermia also diminishes nitric oxide availability and increases TNF production by dendritic cells, in addition to altering their ability to stimulate T cells (18). Finally, it has been shown that hypothermia prolongs proinflammatory signaling via the NF-κB pathway in human monocytes (15, 16), although the mechanisms leading to prolonged NF-κB activation in hypothermia are not known and the effects of hypothermia on other pathways involved in inflammatory signaling in immune cells have not been elucidated.
Inflammatory signaling pathways in monocytes/macrophages are tightly controlled at multiple levels by a host of regulatory molecules, including ion channels, kinases and phosphatases. In addition, microRNAs (miR) have recently emerged as important regulators of inflammatory signaling pathways, such as the Toll-like receptor 4 (TLR4) pathway (19–25). miRs can both promote the production of proinflammatory cytokines and trigger anti-inflammatory responses by either inhibiting proinflammatory signaling or by increasing anti-inflammatory signaling (i.e., through increased IL-10 production) (22, 24–28). Although several miRs inhibit inflammatory signaling triggered by TLR4 activation, some miRs, such as miR-155, suppress negative regulators of the TLR4 pathway, such as phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 (SHIP1) and suppressor of cytokine signaling 1 (SOCS1), thereby exerting proinflammatory actions (22, 25, 26). Given that hypothermia is associated with impaired host defense in clinical studies and increased proinflammatory responses in vitro, we sought to determine how hypothermia affects the regulation of inflammatory signaling in monocytes.
Our results demonstrate that hypothermia imparts a proinflammatory shift in the monocyte response to TLR activation by increasing miR-155. The results of our gain- and loss-of-function experiments establish a causal role of miR-155 in hypothermia-induced dysregulation of the inflammatory response in which production of IL-10 is reduced whereas expression of proinflammatory cytokines is increased in monocytes and macrophages. Genetic deletion or suppression of miR-155 expression restored the balanced immune response, resulting in improved survival in mice subjected to hypothermia and challenged with LPS.
MATERIALS AND METHODS
This study has been approved by the Institutional Review Board (HSPPO 08.0018), as well as the Institutional Animal Care and Use Committee (protocol 11017) of the University of Louisville. All experiments were conducted in accordance with institutional guidelines.
Isolation and culture of primary human monocytes
Primary human monocytes were isolated from fresh blood of healthy volunteers after collection in EDTA-Vacutainers (Becton Dickinson, Franklin Lakes, NJ, USA) using human CD14 Whole Blood MicroBeads (Miltenyi Biotech, Auburn, CA, USA) according to the manufacturer's instructions. The cells were kept at 37°C during the isolation procedure. The purity of the isolated monocytes was assessed by flow cytometry, and the cell population was found to be >95% pure. The cells (0.25×106 cells/ml) were then stimulated with LPS (100 ng/ml; E. coli 0111:B4, Sigma-Aldrich, St. Louis, MO, USA). After stimulation, the cells were immediately incubated at either 32°C or 37°C. Cells were collected for RNA isolation, while cell culture supernatants were collected for cytokine measurements. RNA was isolated at indicated times using the RNeasy Kit (Qiagen, Valencia, CA, USA) and the concentration and the purity of isolated RNA were determined with a Nanodrop N-1000 (Agilent Biosystems, Santa Clara, CA, USA). All samples fulfilled the quality criteria of an A280/A260 ratio between 1.9 and 2.1.
Transfection experiments
Primary human monocytes were transfected using the N-TER Nanoparticle siRNA Transfection System (Sigma-Aldrich) according to the manufacturer's instructions. The monocytes were transfected with miR-155 mimics or antagomirs, SHIP1 or SOCS1 siRNAs (Life Technologies, Foster City, CA, USA) at a concentration of 40 nM/well for 24 h at 37°C. Control cells were transfected with scramble miRs/siRNAs (negative control). After 24 h of transfection, the medium was replaced with fresh medium and the cells were stimulated with LPS (100 ng/0.25×106 cells/ml) for an additional 24 h at the indicated temperatures. Cells were collected for RNA isolation, whereas supernatants were collected for cytokine measurements. Transfection efficiency was confirmed by quantitative RT-PCR and the transfection experiments resulted in an overexpression of miR-155 of 194.3 ± 34.0-fold at 32°C and 36.1 ± 49.0-fold at 37°C compared with samples treated with scramble RNA. The miR-155 antagomirs resulted in a fold change of 0.36 ± 0.13 at 32°C and 0.34 ± 0.1 at 37°C compared with negative control samples. The silencing experiments reduced the expression of SHIP1 mRNA compared with negative control of 0.28 ± 0.13 fold at 32°C and 0.3 ± 0.1-fold at 37°C, respectively. For SOCS1, silencing resulted in a 0.06 ± 0.02-fold change at 32°C and 0.13 ± 0.03 at 37°C compared with the negative control.
Immunofluorescence staining
For immunofluorescence staining, formalin-fixed primary human monocytes were permeabilized with Triton-X (Sigma-Aldrich) and incubated with Protein Block for 1 h at room temperature. The samples were then incubated with the primary anti-human antibody in the dark at 37°C for 2 h. The rabbit anti-SHIP1 (C15C9) antibody was purchased from Cell Signaling (Danvers, MA, USA); the human anti-SOCS1 antibody was purchased from Abcam (Cambridge, M A, USA). After being stained with the primary antibody, the samples were incubated with the secondary antibody [SHIP1: tetramethylrhodamine goat anti-rabbit IgG (H+L); Life Technologies] or SOCS1: Alexafluor donkey anti-goat IgG (Abcam) for 1 h at 37°C in the dark. Nuclei were stained with DAPI (Life Technologies). The slides were left to dry overnight in the dark. Images were acquired with a Nikon Eclipse Ti microscope (Nikon Instruments, Inc., Melville, NY, USA). The Nikon Elements imaging software (Nikon Instruments, Inc.) was used for morphometric analysis.
IL-10 addition/anti-IL-10-receptor experiments
RhIL-10 (5 ng/ml; total of 20 ng; Sigma-Aldrich) was added to monocyte cultures at 32°C 4 h after stimulation with LPS. The rhIL-10 was added after 4 h because our preliminary experiments showed that IL-10 secretion starts 4 h after LPS stimulation. The effects of IL-10 at 37°C were blocked by using an anti-human-IL-10-receptor-α antibody. For this, monocytes were preincubated for 1 h at 37°C with 10 μg of the anti-IL-10-receptor antibody before stimulation. Cells and supernatants were collected at the indicated times for protein and RNA analysis.
Cytokine measurements
Cytokines were measured using ELISAs (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. All samples were measured in duplicate and appropriately diluted for accurate measurements within the standard curve.
miR and messenger RNA expression
Gene expression was assessed with TaqMan Single Gene Assays (Life Technologies) after generating complimentary DNA (cDNA) using the High Capacity cDNA Reverse Transcription kit (Life Technologies). The following genes were assessed: human TNF (Hs01113624_g1), human IL10 (Hs00961622_m1), human SHIP1 (Hs00183290_m1), human SOCS1 (Hs00705164_s1), mouse Tnf (Mm00443260_g1), mouse II6 (Mm00446190_m1), mouse Il10 (Mm00439614_m1), mouse Ship1 (Mm00494987_m1), and mouse Socs1 (Mm00782550_s1). 18s was used for normalization in human (Hs03928990_g1), and mouse (Mm03928990_g1) samples.
miR expression was determined using TaqMan Single microRNA Assays for human and mouse miR-155-5p. miR-specific cDNA was produced using the TaqMan MicroRNA Reverse Transcription Kit (Life Technologies). The housekeeping gene U6 was used for standardization in human and mouse samples (Life Technologies). Fold change was calculated using the ΔΔCt (cycle threshold) method (29).
Animal experiments
Wild-type (WT) male C57BL/6J and miR-155-knockout (KO) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The mice were housed individually during the experiments to prevent the warming of the mice by body contact. During the experiments, rectal temperatures were measured at the indicated intervals. After acclimatization, the mice were either transferred to a cold room (3–5°C) or kept at room temperature (22–24°C). To determine the effect of miR-155 deletion on cytokine expression and survival in hypothermia, both miR-155-KO mice and age- and gender-matched WT mice were exposed to the cold. After 1 h, all mice were injected with LPS (Sigma-Aldrich) intraperitoneally in 0.2 ml of normal 0.9% saline. To study intraperitoneal cytokine and gene expression changes, a nonlethal dose of LPS (4 mg/kg i.p.) was used, and the mice were kept in the cold room or at room temperature for an additional 5 h as indicated. After exposure to cold for a total of 6 h (5 h with LPS), the cold-exposed mice were transferred to room temperature and allowed to recover. No active rewarming measures were used. To measure intraperitoneal cytokines and to collect peritoneal exudate cells (PECs), the mice were euthanized at the indicated time points, and peritoneal lavage fluid was collected. Supernatants were collected and stored at −80°C until further analysis. The PECs were used for RNA isolation using the RNeasy Kit (Qiagen). PECs of unstimulated, naive WT and miR-155 KO mice were used as reference controls for gene expression analysis. In the survival studies, the mice received 20 mg/kg LPS i.p. and were observed for 5 d. This higher LPS dose was chosen due to preliminary experiments showing 50% mortality at room temperature (data not shown).
Sorting of mouse peritoneal cells
To determine macrophage-specific hypothermia-induced changes in gene expression, F4/80hi CD11bhi peritoneal cells were sorted from naive mice, as well as those isolated from mice given LPS for 48 h and housed in the cold room or at room temperature, using a Beckmann Coulter MoFlo (Beckmann Coulter, Brea, CA, USA) (30). The cells were flushed into Trizol LS (Life Technologies), and total RNA was isolated using the organic RNA extraction protocol. RNA expression of the isolated cells was analyzed as described previously.
Statistical analysis
For in vitro experiments, the Wilcoxon ranked-sign test was used for comparing 2 samples of the same donor. For animal experiments, the Student's t test was used. Differences in survival rate between cold-exposed mice and mice kept at room temperature, as well as between WT and miR-155-KO mice, were determined with a log-rank test. Unless otherwise indicated, the data are as means ± se. SPSS 21.0 (IBM, Armonk, NY, USA) was used for statistical analysis. In all cases, a value of P < 0.05 was considered significant.
RESULTS
Cold exposure potentiates inflammatory cytokine production in primary human monocytes
To study the molecular mechanisms leading to alterations in inflammatory signaling in the context of hypothermia, we established a cellular model in which primary human monocytes were cultured at either 37 or 32°C and stimulated with TLR4 agonist, LPS. As shown in Fig. 1A, cold exposure significantly increased the production of the proinflammatory cytokine TNF at both 24 and 36 h post-LPS stimulation. Similarly, IL-6 and IL-12 levels were significantly increased in monocytes maintained at 32°C as compared with those maintained at 37°C (Fig. 1B, C). In contrast to the effects of cold exposure on proinflammatory cytokines, LPS-stimulated production of IL-10 was strongly suppressed at 32°C (Fig. 1D). These findings demonstrate that in human primary monocytes, cold exposure alters the ratio of proinflammatory to anti-inflammatory cytokines.
Figure 1.
Cold exposure alters the balance of proinflammatory and anti-inflammatory cytokines in primary human monocytes stimulated with LPS. Levels of cytokines in supernatants of isolated primary human monocytes incubated at 32 or 37°C and stimulated with LPS (100 ng/ml); n = 7 healthy human donors consisting of 4 independent experiments. A) TNF. B) IL-6. C) IL-12. D) IL-10. Wilcoxon ranked-sign test was used to compare 32 to 37°C. *P < 0.05.
Altered cytokine production in cold-exposed monocytes is associated with suppressed expression of negative regulators of TLR signaling
The increased production of proinflammatory cytokines in monocytes exposed to cold prompted us to investigate whether normally operative negative regulatory checkpoints in TLR signaling were affected by hypothermia. It is well documented that SHIP1 and SOCS1 negatively regulate cytokine production in response to TLR activation by controlling inositol phosphate signaling and the expression of TLR adapter proteins (e.g., MyD88), respectively (31–33). Hence, we measured mRNA for SHIP1 and SOCS1 in monocytes stimulated with LPS and exposed to cold or maintained at 37°C. As shown in Fig. 2A, SHIP1 expression decreased after initial exposure to LPS and this decrease was further potentiated in monocytes exposed to cold, as compared with monocytes at 37°C. Expression of SOCS1 increased in response to LPS stimulation, although its levels were significantly blunted by exposure to cold 24 h post-LPS (Fig. 2B). These results demonstrate that cold exposure suppresses the expression of mediators that negatively regulate TLR signaling, suggesting that enhanced proinflammatory cytokine production in cold-exposed monocytes could be related to altered expression of SOCS1 and SHIP1.
Figure 2.
Role of miR-155 on the inflammatory response in cold-exposed primary human monocytes. A–C) SHIP1 (A), SOCS1 (B), and miR-155 (C) expression in monocytes incubated at 32 and 37°C and stimulated with LPS; n = 7 healthy human donors consisting of 4 independent experiments. Wilcoxon ranked-sign test was used to compare 32 to 37°C. *P < 0.05. D, E) Expression of miR-155 targets, SHIP1 (D) and SOCS1 (E), in monocytes transfected with scramble RNA [negative control (NC)] or miR-155 mimics or antagomirs at 32 and 37°C as indicated; n = 7 healthy human donors consisting of 4 independent experiments. *P < 0.05 comparing negative control with miR-155 mimics or antagomirs. **P < 0.05 comparing 32°C vs. 37°C negative controls. F) Effect of miR-155 overexpression at 37°C on TNF, IL-6, IL-12, and IL-10 protein levels. G) Effect of miR-155 knockdown at 32°C on TNF, IL-6, IL-12, and IL-10 protein levels; n = 6 healthy human donors consisting of 3 independent experiments. Wilcoxon ranked-sign test was used to compare miR-155 mimic/antagomir-treated samples with negative control samples. *P < 0.05.
Increased expression of miR-155 underlies suppressed expression of SOCS1 and SHIP1 in cold-exposed monocytes
To understand how cold exposure suppresses SOCS1 and SHIP1 in monocytes, we evaluated the expression of miR-155, which is known to target these 2 negative regulators of TLR signaling. As shown in Fig. 2C, stimulation of monocytes with LPS led to a robust increase in the expression of miR-155 that peaked at 24 h poststimulation. The levels of miR-155 started to decline by 36 h. Interestingly, exposure of monocytes to cold (32°C) strongly potentiated the expression of miR-155 at both 24 and 36 h post-LPS stimulation (Fig. 2C). Other miRs shown previously to regulate TLR signaling, such as miR-146a and miR-21 (19, 34), were not affected by cold exposure (data not shown). Given that the increase in miR-155 was associated with suppressed levels of SOCS1 and SHIP1, we next evaluated whether expression of miR-155 was causally related to this suppression in cold-exposed monocytes. To this end, we modeled the effects of cold exposure in monocytes kept at 37°C by transfecting monocytes with a miR-155 mimic. As shown in Fig. 2D, E, the miR-155 mimic significantly suppressed the levels of both SHIP1 and SOCS1 protein, as assessed by immunofluorescence microscopy. Conversely, delivery of miR-155 antagomirs to monocytes maintained at 32°C significantly increased levels of SHIP1 and SOCS1, demonstrating that miR-155 plays a causal role in suppressing these mediators in cold-exposed monocytes.
Role of miR-155 in mediating altered cytokine production in cold-exposed monocytes
As miR-155 was increased by hypothermia and this corresponded to decreased expression of its targets, SHIP1 and SOCS1 at 32°C, we next assessed how overexpression or suppression of miR-155 impacts cytokine production in monocytes. Because our results demonstrated that miR-155 expression was low at 37°C, we sought to determine if overexpression of miR-155 at 37°C would mimic the effects of cold exposure. Indeed, we found that overexpression of miR-155 at 37°C increased the production of proinflammatory cytokines TNF, IL-6, and IL-12, while the production of anti-inflammatory cytokine IL-10 was decreased (Fig. 2F). In contrast, inhibition of miR-155 at 32°C suppressed TNF, IL-6, and IL-12 and significantly increased IL-10 production (Fig. 2G). Overall, these data are consistent with the cytokine profile observed in cold-exposed monocytes (see Fig. 1) and demonstrate that miR-155 is causally related to the dysregulated cytokine balance in monocytes exposed to cold.
SHIP1 and SOCS1 have distinct effects on pro- and anti-inflammatory cytokines and together mediate the effects of miR-155 in hypothermia
We have shown that miR-155 potentiates the inflammatory response at 32°C by suppressing IL-10 and increasing TNF, IL-6, and IL-12 and that the changes in miR-155 expression correspond with changes in its targets, SHIP1 and SOCS1. These results indicate that miR-155 may act through these regulators of the TLR4 pathway, enhancing the inflammatory response. Because miRs have several potential target genes, we wanted to determine whether SHIP1 and SOCS1 are the effectors of miR-155's actions in cold-exposed monocytes. For this, we silenced the expression of SHIP1 and SOCS1 at 37°C to mimic the effects of cold exposure (Fig. 3). The targeted knockdown of SHIP1 using siRNA at 37°C did not affect TNF, IL-6, and IL-12 (Fig. 3A–C), but it did suppress IL-10 production (Fig. 3D). The knockdown of SOCS1 at 37°C, on the other hand, increased TNF, IL-6, and IL-12 levels (Fig. 3E–G), while IL-10 expression was not affected (Fig. 3H). Taken together, these results, combined with results presented in Fig. 2, collectively demonstrate that miR-155 likely exerts its effects through a combined action on both SHIP1 and SOCS1.
Figure 3.
Role of SHIP1 and SOCS1 in cytokine production in cold-exposed monocytes. A–D) Production of cytokines in monocytes transfected with scramble RNA (NC) or SHIP1 siRNA and stimulated with LPS and kept at 37°C; n = 7 healthy human donors consisting of 4 independent experiments. Wilcoxon ranked-sign test was used to compare SHIP1 siRNA-treated samples with negative control samples. *P < 0.05. E–H) Production of cytokines in monocytes transfected without or with SOCS1 siRNA and stimulated as described above; n = 7 healthy human donors consisting of 4 independent experiments. Wilcoxon ranked-sign test was used to compare SOCS1 siRNA-treated samples with negative control samples. *P < 0.05.
IL-10 regulates feedback inhibition of TNF and miR-155
The results presented thus far demonstrate that IL-10 is suppressed in hypothermia through the induction of miR-155 (Figs. 1D and 2F, G). McCoy et al. (27) have shown that IL-10 acts as negative regulator of miR-155. Therefore, we hypothesized that cold-exposure dysregulates the naturally occurring feedback loop involving IL-10 and miR-155 (27). Hence, we modulated the levels of IL-10 at 32°C by supplementing rhIL-10 to mimic conditions found at 37°C. To test the reverse scenario, we blocked the biological action of IL-10 at 37°C by inhibiting the α-subunit of the IL-10-receptor. We found that blocking the IL-10 receptor resulted in significantly higher levels of TNF at 12 and 24 h post-LPS stimulation at 37°C (Fig. 4A). The expression of miR-155 was also elevated after blocking the IL-10-receptor at 24 h (Fig. 4B). At 32°C, addition of rhIL-10 suppressed TNF levels significantly at 12 and 24 h (Fig. 4C). Consistent with this observation, the addition of rhIL-10 also suppressed the expression of miR-155 at 24 h (Fig. 4D). These observations confirm previous reports that IL-10 and miR-155 regulate each other and suggest that cold exposure exaggerates the inflammatory response in part by disrupting the normal feedback inhibition of miR-155 by IL-10.
Figure 4.
Role of IL-10 in the dysregulation of inflammatory cytokine production in cold-exposed monocytes. A, B) Effect of IL-10 receptor blockade on TNF (A) and miR-155 (B) expression in monocytes stimulated with LPS and maintained at 37°C. C, D) Effect of addition of rhIL-10 on TNF (C) and miR-155 (D) in monocytes stimulated with LPS and maintained at 32°C. Data are mean of 6 different donors consisting of 3 independent experiments. Wilcoxon ranked-sign test was used to compare between treatment groups. *P < 0.05.
Hypothermia increases the expression of proinflammatory cytokines while reducing IL-10 expression in vivo
Having demonstrated that exposure of monocytes to cold in vitro dysregulates the inflammatory response to TLR4 stimulation, we next established a model to test the in vivo relevance of our findings. For this purpose, we used a model of peritonitis in which LPS is administered by intraperitoneal delivery. To test the effects of cold exposure, we either housed the mice at room temperature or in the cold for 6 h (see scheme in Fig. 5A). On cold exposure, mice were allowed to recover at room temperature. As shown in Fig. 5B, mice exposed to cold for 6 h (3–5°C) and given LPS (4 mg/kg i.p.) showed a progressive and significant decrease in rectal temperature when compared with mice given LPS and kept at room temperature. Mice exposed to cold without LPS treatment only dropped their rectal temperature by 2–3°C and recovered immediately after removal from the cold (data not shown). The decrease in rectal temperature in cold-exposed mice was comparable to changes in body temperature found in patients with hypothermia (35). After transfer to room temperature, the cold-exposed mice recovered within 3 h, after which the mice maintained similar rectal temperatures as mice continuously housed at room temperature. However, this brief duration of hypothermia caused a sustained increase in LPS-induced expression of Tnf and Il6 mRNA in PECs collected at 12 and 48 h, compared with mice kept at room temperature (Fig. 5C, D). Similarly, cold-exposed mice had higher peritoneal TNF protein levels at 48 h (Fig. 5C, bottom panel), whereas protein levels of IL-6 were elevated at 6 h postchallenge but declined thereafter (Fig. 5D, bottom panel). IL-12 was not detectable in the peritoneal lavage fluid at these time points (data not shown). In contrast to the changes in proinflammatory cytokines, Il10 mRNA and protein levels were significantly suppressed at 48 h postchallenge in the cold-exposed mice despite an initial increase at 6 h (Fig. 5E). These results confirm our in vitro studies and establish a model to study the effects of hypothermia on innate immune responses in vivo.
Figure 5.
Hypothermia potentiates the inflammatory response in mice undergoing peritonitis. A) Experimental design. B) Body temperatures of cold-exposed mice and mice kept at room temperature for 48 h after treatment with LPS (4 mg/kg i.p.); n = 11–34/measurement and group; results comprise data from 2–3 independent experiments depending on time point. C–E) Cytokine expression in cold-exposed mice and mice kept at room temperature at 6, 12, and 48 h after LPS administration. Top panels show changes in mRNA-expression in PECs, and the bottom panels show peritoneal exudate cytokine concentrations for TNF (C), IL-6 (D), and IL-10 (E). F–H) Expression of miR-155 (F), Ship1 (G), and Socs1 (H) in PECs of cold-exposed mice and mice kept at room temperature at 6, 12, and 48 h after LPS treatment. 6 h: n = 3 mice/group, 12 h: n = 9 mice/group, 48 h: n = 11 mice/group; each time point represents 2–3 independent experiments. Student's t test was used to compare cold-exposed mice with mice kept at room temperature at each time point.
miR-155 expression is associated with the increased inflammatory response in hypothermic animals
To confirm the pivotal role of miR-155 in hypothermia-related immune dysfunction in our in vivo model, we measured levels of miR-155 in PECs of mice undergoing peritonitis. We found that in comparison with mice kept at room temperature, miR-155 was significantly up-regulated in cold-exposed mice, 12 and 48 h after LPS injection (Fig. 5F). This increase in miR-155 correlated with elevated TNF and suppressed IL-10 levels, as seen in our in vitro studies (Figs. 1 and 2). In addition, the targets of miR-155, Ship1 and Socs1, were significantly down-regulated in cold-exposed mice, 12 and 48 h after LPS, consistent with the suppressive action of miR-155 (Fig. 5G, H). Collectively, these data support our previous observations and are consistent with the notion that the exaggerated inflammatory response in hypothermia could be in part due to an increase in the expression of miR-155.
Given that peritoneal inflammation is associated with the infiltration of multiple leukocyte subsets that could underlie the observed changes in gene expression described above (30, 36–38), we next evaluated how innate immune cell populations were affected by hypothermia during peritonitis. As shown in Fig. 6A–C, hypothermia significantly increased the amount of F4/80+ Gr-1− macrophages 48 h after LPS administration, whereas the infiltration of Gr-1+F4/80− neutrophils was not affected during this time course (data not shown). As peritoneal macrophages recruited during acute inflammation are diverse and comprise both F4/80hiCD11bhi and F4/80loCD11blo populations with different roles in the inflammatory response (30, 39–41), we next determined how these 2 populations were affected by hypothermia. As shown in Fig. 6M, N (with representative dot plots shown in Fig. 6D–L), both populations were identified and hypothermia led to a significant increase in the proportion of F4/80hiCD11bhi macrophages at both 48 and 72 h post-LPS stimulation. A modest decrease in the F4/80loCD11blo cells was observed in the context of hypothermia 72 h after LPS administration. When we sorted the F4/80hiCD11bhi population by FACS and measured expression of TNF, Il6, Il12, Il10, and miR-155, we found that expression of TNF, Il12, and miR-155 was significantly increased in cells isolated from hypothermic mice, relative to mice housed at room temperature and given LPS, whereas the expression of Il10 was significantly suppressed in hypothermic macrophages (Fig. 6O). These observations are consistent with our previous results with total PECs and in human primary monocytes in vitro.
Figure 6.
Effect of hypothermia on peritoneal macrophage subsets and cytokine expression. A, B) Representative flow scatter plots of mice kept at room temperature or cold-exposed mice 48 h after LPS (4 mg/kg i.p.). C) Changes in F4/80+ GR1− peritoneal macrophages in LPS-treated cold-exposed mice and mice kept at room temperature. D–L) Gating for F4/80hiCD11bhi macrophages and F4/80loCD11blo macrophages in unstimulated as well as LPS-stimulated cold-exposed mice and mice kept at room temperature based on F4/80 and CD11b expression (32). Changes in F4/80hiCD11bhi (M) and F4/80loCD11blo (N) peritoneal macrophage populations over 72 h after LPS treatment in cold-exposed mice and mice kept at room temperature. 6 h: n = 3 mice/group, 12 h: n = 9 mice/group, 48 h: n = 11 mice/group, 72 h: n = 10 mice/group. O) Expression of Tnf, Il6, Il12, Il10, and miR-155 in sorted F4/80hiCD11bhi peritoneal macrophages of cold-exposed mice, mice kept at room temperature after 48 h, and unstimulated naive mice; n = 3 in unstimulated mice, n = 8 in cold-exposed mice, and n = 7 in mice kept at room temperature. Student's t test was used to compare expression changes in F4/80hiCD11bhi macrophages in cold-exposed mice with mice kept at room temperature at the indicated time point. *P < 0.05.
Hypothermia increases LPS-induced mortality in mice
We next sought to determine whether the exaggerated inflammatory response in hypothermia affects LPS-induced mortality. Because LPS was not lethal at a dose of 4 mg/kg, we used a dose of 20 mg/kg. As before, mice exposed to cold and challenged with LPS had significant decreases in body temperature when compared with mice maintained at room temperature (Fig. 7A). Supporting the detrimental effect of hypothermia, cold-exposed mice had a 90% mortality compared with 50% of mice kept at room temperature and given LPS (Fig. 7B). Taken together, these results clearly show that dysregulation of the inflammatory response by hypothermia affects survival after an inflammatory challenge.
Figure 7.
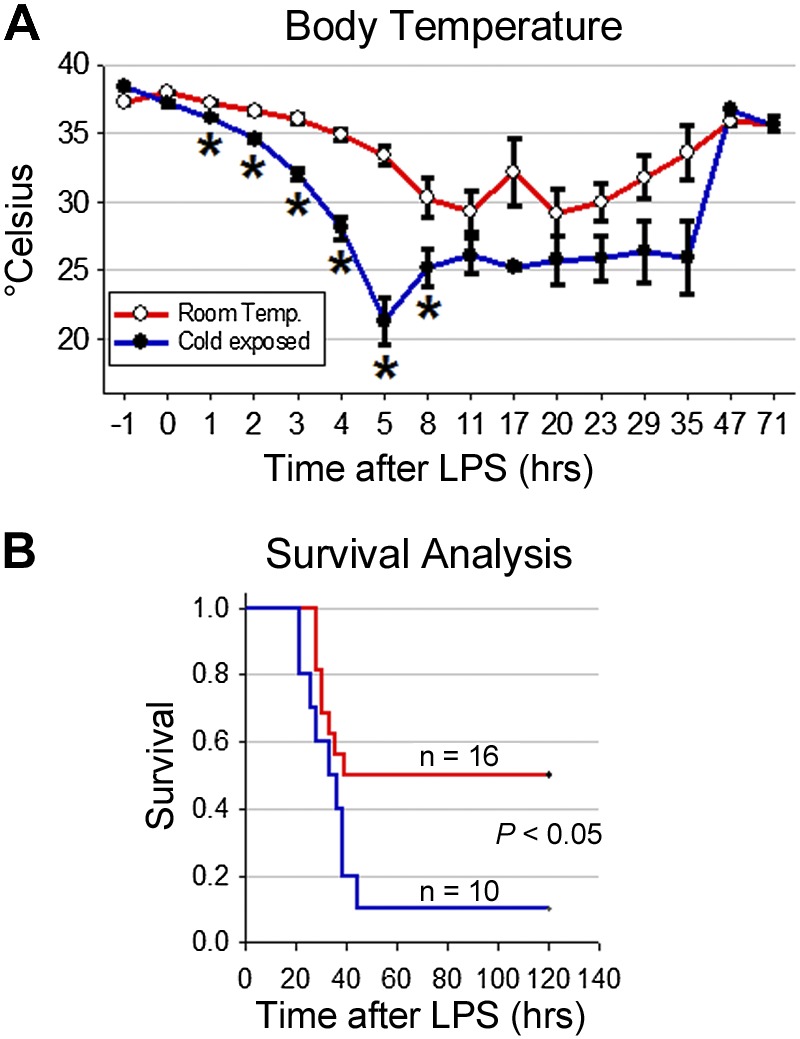
Hypothermia decreases survival in mice undergoing peritonitis. A) Body temperatures of cold-exposed mice and mice kept at room temperature for 72 h after treatment with LPS (20 mg/kg ip). B) Survival curve of LPS-treated cold-exposed mice and mice kept at room temperature; n = 10 for cold-exposed mice and n = 16 for mice kept at room temperature from 2 independent experiments. The log-rank test was used for the analysis of the survival data. *P < 0.05.
miR-155 deficiency protects mice from hypothermia during peritonitis
We have shown above in vitro that miR-155 mediates the effects of cold exposure on the suppression of IL-10 and that the suppression of miR-155 expression increases IL-10 production (see Fig. 2). To validate these findings in vivo, we subjected WT or miR-155-KO mice to 6 h of cold exposure in the presence of LPS (Fig. 8A) and found that the miR-155 KO mice were more resistant to hypothermia than similarly treated WT mice; the recovery period after removal from the cold was similar in miR-155-KO mice and WT mice (Fig. 8B). Notably, the levels of IL-10 were significantly higher in terms of both mRNA and protein in miR-155-KO mice compared with WT mice (Fig. 8C) confirming our previous in vitro and in vivo results that during hypothermia, miR-155 suppresses IL-10 production and mediates the subsequent dysregulation of the inflammatory response. As expected, there was no detectable expression of miR-155 in the KO mice, whereas miR-155 was upregulated 48 h (compared to naive, unstimulated mice) after LPS challenge in WT mice (Fig. 8D). Furthermore, Ship1 and Socs1 mRNA expression was increased in the miR-155-KO mice, again demonstrating that miR-155 is a critical regulator of these mediators in vivo (Fig. 8E, F). As the major differences in the expression of proinflammatory cytokines, TNF and IL-6, were observed at earlier time points (see Fig. 5C, D), no significant differences were observed 48 h post-LPS administration between WT and miR-155-KO mice (data not shown).
Figure 8.
Effects of miR-155 deficiency on body temperature, the inflammatory response and survival in cold-exposed mice. A) Experimental design: WT and miR-155-KO mice were exposed to hypothermia, both groups received LPS. B) Body temperatures of cold-exposed WT and miR-155-KO mice after treatment with LPS (4 mg/kg i.p.); n = 10/measurement and group, data are from 2 independent experiments. C) IL-10 expression in cold-exposed WT and miR-155-KO mice 48 h after LPS treatment. The left panel shows changes in mRNA-expression, the right panel shows peritoneal cytokine concentrations. D–F) Expression of miR-155 (D), Ship1 (E), and Socs1 (F) in cold-exposed WT and miR-155-KO mice 48h after LPS treatment; n = 10 mice per group, data are from 2 independent experiments. G) Body temperatures of cold-exposed WT and miR-155 KO mice after treatment with LPS (20 mg/kg i.p.). H) Survival curve of LPS-treated/cold-exposed WT and miR-155-KO mice; n = 9 for WT mice and n = 10 for miR-155-KO mice; data are from 2 independent experiments. Student's t test was used to compare cold-exposed mice with mice kept at room temperature at each time point, the log-rank test was used for the analysis of the survival data. *P < 0.05.
miR-155 deficiency protects mice against the lethal effects of hypothermia
Based on our results implicating miR-155 as a critical node regulating hypothermia-induced alterations in the inflammatory response, we tested whether genetic deletion of miR-155 would improve survival in hypothermic mice exposed to LPS. Using the conditions described in Fig. 7, we found that the body temperature of miR-155-KO mice was significantly higher during the cold exposure compared with WT mice (Fig. 8G), resulting in a profound protection from the lethal effects of hypothermia. Only 22% mortality was observed in miR-155-KO mice, whereas mortality was nearly 68% in WT mice (Fig. 8H). Collectively, these results demonstrate that miR-155 plays a causal role in the lethal effects of hypothermia in the context on TLR activation.
DISCUSSION
The major finding of this study is that exposure to cold modulates TLR signaling in innate immune cells by disrupting normally occurring negative feedback loops. We found that cold exposure increases miR-155 expression both in vitro and in vivo and that the expression of miR-155 is causally related to the potentiation of inflammatory signaling induced by hypothermia. Mechanistically, we found that increased expression of miR-155 down-regulates its previously identified targets, SHIP1 and SOCS1; thereby increasing the production of inflammatory cytokines. We also established that hypothermia disrupts feedback regulation of miR-155 expression by IL-10, leading to an imbalance in pro- vs. anti-inflammatory cytokine production, unrestrained TLR signaling and impaired resolution of inflammation. These findings could potentially explain recent findings of clinical studies that hypothermia increases the risk for infections and sepsis in patients treated with therapeutic hypothermia and in patients who became hypothermic unintentionally (6–8, 12). Furthermore, our results indicate that the detrimental effects of hypothermia on the immune response could potentially be alleviated by modulating miR-155 expression.
Hypothermia is increasingly used to reduce hypoxia related brain damage in patients after cardiac arrest resulting in improved neurological outcomes (1, 2). Interestingly, miRs have also been implicated in mediating the neuroprotective effect of therapeutic hypothermia after traumatic brain injury. Truettner et al. (42, 43) demonstrated that therapeutic hypothermia alters the expression of miRs in the brain after traumatic brain injury and that the reduced expression of miR-34a, miR-451, and miR-874 in therapeutic hypothermia leads to reduced vulnerability of neurons by suppression of proinflammatory cytokines such as IL-1β and TNF. Another group additionally found in pigs that hypothermia reduces the expression of typical cerebral proinflammatory mediators after cardiac arrest in the cerebral cortex (44). Based on early clinical work, it was surmised that the use of therapeutic hypothermia is safe and that it does not significantly increase the risk for complications. However, more recent reports demonstrate that the use of therapeutic hypothermia could increase the severity of both pneumonia and sepsis (6, 7). In addition, it has been found that hypothermia could be detrimental, particularly in patients presenting with infections and especially sepsis (8). Several studies have shown that unintentional hypothermia during surgery results in a higher rate of SSI and a higher rate of multiple organ dysfunction syndrome (10, 45–47). In animal models of bacterial sepsis, hypothermia has been shown to increase mortality (48), an observation that provides biological plausibility to correlative human data. Nevertheless, it remains unclear how hypothermia affects host defense and what the underlying mechanisms by which hypothermia affects the immune response are. Clinical studies showing that hypothermic septic patients have higher TNF and IL-6 levels than septic patients with fever and that patients after cardiac arrest treated with hypothermia have higher serum IL-6 levels than normothermic patients (49, 50), suggest significant alterations in the pathways that control cytokine production. This view is supported by the results of both our in vitro and in vivo studies showing profound changes in these cytokines during hypothermia. Moreover, the concordance between the clinical data and our data suggests that our models resemble the clinical situation.
Previous investigations into the effects of hypothermia have shown that ROS production by neutrophils is decreased in patients with hypothermia. In addition, it has also been reported that the activation of NF-κB is prolonged in hypothermic monocytes in vitro (13–16). Nevertheless, the mechanisms underlying these changes have not been examined previously, and key events mediating the effects of hypothermia have not been identified. We found that miR-155 plays a pivotal role in hypothermia-related monocyte/macrophage dysfunction. This view is based on the observation that transfection of primary human monocytes at 37°C with miR-155 increased TNF, IL-6, and IL-12 and suppressed IL-10 production, mimicking the conditions at hypothermia. In contrast, the suppression of miR-155 at 32°C suppressed TNF, IL-6, and IL-12 and increased IL-10, restoring the cytokines to levels observed at 37°C and indicating that miR-155 plays a key role in modulating inflammatory circuits sensitive to temperature. This view is further supported by our in vivo observations showing that the miR-155 deficiency increases IL-10 expression and that this is accompanied by a profound improvement in survival.
Our results also elucidate the mechanism by which miR-155 affects inflammatory signaling. Consistent with previous results, we found that the miR-155 exerts its effects via 2 main targets, SHIP1 and SOCS1, which have been shown to be negative regulators of the TLR4 pathway. However, the roles of these targets in modulating inflammation appear to be distinct. We found that SHIP1 mediates the effects of miR-155 on IL-10, whereas SOCS1 mediates the effects on the proinflammatory cytokines. These results are consistent with previous work showing that miR-155 suppresses SHIP1/SOCS1 production resulting in less inhibition of the TLR4 pathway and thereby prolonging the inflammatory response (26, 31–33). However, in the context of hypothermia, it seems that 2 different mediators working on 2 different aspects of the inflammatory circuit together orchestrate the overall effect of miR-155 observed in our studies.
The mechanisms that lead to an increase in the expression of miR-155 in hypothermia remain unclear. However, the expression of miR-155 is induced by the NF-κB and MAPK-pathway, activation of which has been shown to be prolonged in hypothermic monocytes and dendritic cells (15, 18, 23, 51). Moreover, our results demonstrate that IL-10 and miR-155 interact closely and regulate the expression of one another. IL-10 suppresses the expression of miR-155, whereas, conversely, miR-155 suppresses IL-10 production. This bidirectional relationship is supported by previous studies showing that IL-10 suppresses the transcription of miR-155 (27) and by our finding that miR-155 deficient mice show higher IL-10 levels than WT mice. It appears that the disruption of the regulatory IL-10 feedback loop in hypothermia by miR-155 is important because a balanced IL-10 response is critical for normal host defense (36, 52). Nevertheless, we did not examine how temperature regulates these responses. Furthermore, future studies should address the impact of stress hormones on the inflammatory cascade in hypothermia and in particular on miR-155 expression because this issue has thus far only been marginally investigated and hypothermia likely imparts a significant stress response (53).
We speculate that the effects of hypothermia may be related to changes in the activity of transient receptor potential cation channel subfamily V member 1 (TRPV1) channels. These channels are activated by a variety of mechanical and chemical stimuli such as capsaicin or heat (54), and recent literature suggests that this channel is also expressed on leukocytes and plays an important role in macrophage function (55, 56). TRPV1-KO mice seem to have an enhanced inflammatory response with pronounced multiple organ dysfunction and impaired macrophage function accompanied by increased TNF production (55, 56), which is similar to those observed in our study. However, future studies are required to identify the mechanisms and mediators that impart temperature sensitivity to monocytes/macrophages.
The findings of our study could potentially lead to the development of therapeutic interventions in patients with hypothermia, independent of the cause of hypothermia. The central role of miR-155 in hypothermia-related innate immune dysfunction identifies miR-155 as a potential therapeutic target. We found that the genetic deletion of miR-155 provides robust protection against the deleterious effects of hypothermia. Hence, the knockdown of miR-155 in vivo using antagomirs might be of therapeutic benefit. Although this would require in vivo administration of this miR, this procedure has been successfully used to alter miR-expression in the liver, heart, and lungs (57–60). Further studies are required to test whether systemic administration of miR-155 antagomirs could protect against the effects of hypothermia. Alternatively, based on the central role of IL-10 in regulating miR-155, preventive administration of IL-10 before induction of therapeutic hypothermia may also be able to abbreviate the prolonged proinflammatory response.
In summary, the results of this study provide strong in vitro and in vivo evidence for a profound dysregulation of the monocyte/macrophage response in hypothermia and may explain the increased susceptibility to infections and sepsis in patients treated with hypothermia. Hypothermia reduces the ability of monocytes/macrophages to produce IL-10, which disrupts the anti-inflammatory feedback loop. This in turn is associated with increased expression of miR-155, prolonging the inflammatory response. These findings suggest that the effects of hypothermia are mediated in part by the up-regulation of miR-155 and that specific modulation of miR-155 expression may be able to reduce exaggerated inflammation not only in unintentionally hypothermic patients but especially in patients subjected to therapeutic hypothermia.
Acknowledgments
The authors thank Donald E. Fry for insight, advice, and productive discussions and Dr. James McCracken for help with the FACS analysis of the peritoneal exudate cells.
This work was funded by the Price Trust Fund, by the Joint Royal College of Surgeons of Edinburgh (RCSEd)/James and Emmeline Ferguson Research Fellowship Trust and by U.S. National Institutes of Health grants HL-106173 (to M.S.) and GM-103492 (to M.S. and A.B.). A.T.B. holds the Joint RCSEd/James and Emmeline Ferguson Research Fellowship and currently works at the Department of Surgery at the University of Heidelberg (Heidelberg, Germany). J.H. is the recipient of a National Research Service Award from the U.S. National Institutes of Health (grant HL-116186).
The funding sources had no role in the production of the manuscript.
Footnotes
- IL-6
- interleukin-6
- IL-10
- interleukin-10
- IL-12
- interleukin-12
- miR/miRNA
- microRNA
- ROS
- reactive oxygen species
- SHIP1
- phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1
- siRNA
- silencing RNA
- SOCS1
- cuppressor of cytokine signaling 1
- SSI
- surgical site infection
- TLR4
- Toll-like receptor 4
- TNF
- tumor necrosis factor alpha
- TRPV1
- transient receptor potential cation channel subfamily V member 1
REFERENCES
- 1. Arrich J., Holzer M., Havel C., Mullner M., Herkner H. (2012) Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 9, CD004128. [DOI] [PubMed] [Google Scholar]
- 2. Matsui T., Yoshida Y., Yanagihara M., Suenaga H. (2014) Hypothermia at 35 degrees C reduces the time-dependent microglial production of pro-inflammatory and anti-inflammatory factors that mediate neuronal cell death. Neurocrit. Care 20, 301–310 [DOI] [PubMed] [Google Scholar]
- 3. Tian D. H., Wan B., Bannon P. G., Misfeld M., Lemaire S. A., Kazui T., Kouchoukos N. T., Elefteriades J. A., Bavaria J., Coselli J. S., Griepp R. B., Mohr F. W., Oo A., Svensson L. G., Hughes G. C., Yan T. D. (2013) A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2, 148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang S. Y., Zhao Y. Y., Zhao X. G. (2013) Potential role of therapeutic hypothermia in the salvage of traumatic hemorrhagic shock. Crit. Care 17, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmad F. U., Wang M. Y., Levi A. D. (2014) Hypothermia for acute spinal cord injury–a review. World Neurosurg. 82, 207–214 [DOI] [PubMed] [Google Scholar]
- 6. Geurts M., Macleod M. R., Kollmar R., Kremer P. H., van der Worp H. B. (2014) Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit. Care Med. 42, 231–242 [DOI] [PubMed] [Google Scholar]
- 7. Perbet S., Mongardon N., Dumas F., Bruel C., Lemiale V., Mourvillier B., Carli P., Varenne O., Mira J. P., Wolff M., Cariou A. (2011) Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am. J. Respir. Crit. Care Med. 184, 1048–1054 [DOI] [PubMed] [Google Scholar]
- 8. Mourvillier B., Tubach F., van de Beek D., Garot D., Pichon N., Georges H., Lefevre L. M., Bollaert P. E., Boulain T., Luis D., Cariou A., Girardie P., Chelha R., Megarbane B., Delahaye A., Chalumeau-Lemoine L., Legriel S., Beuret P., Brivet F., Bruel C., Camou F., Chatellier D., Chillet P., Clair B., Constantin J. M., Duguet A., Galliot R., Bayle F., Hyvernat H., Ouchenir K., Plantefeve G., Quenot J. P., Richecoeur J., Schwebel C., Sirodot M., Esposito-Farese M., Le Tulzo Y., Wolff M. (2013) Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA 310, 2174–2183 [DOI] [PubMed] [Google Scholar]
- 9. Bukur M., Kurtovic S., Berry C., Tanios M., Ley E. J., Salim A. (2012) Pre-hospital hypothermia is not associated with increased survival after traumatic brain injury. J. Surg. Res. 175, 24–29 [DOI] [PubMed] [Google Scholar]
- 10. Kurz A., Sessler D. I., Lenhardt R. (1996) Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 334, 1209–1215 [DOI] [PubMed] [Google Scholar]
- 11. Frank S. M., Fleisher L. A., Breslow M. J., Higgins M. S., Olson K. F., Kelly S., Beattie C. (1997) Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 277, 1127–1134 [PubMed] [Google Scholar]
- 12. Billeter A. T., Hohmann S. F., Druen D., Cannon R., Polk H. C., Jr. (2014) Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. [E-pub ahead of print] Surgery 10.1016/j.surg.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 13. Akriotis V., Biggar W. D. (1985) The effects of hypothermia on neutrophil function in vitro. J. Leukoc. Biol. 37, 51–61 [DOI] [PubMed] [Google Scholar]
- 14. Wenisch C., Narzt E., Sessler D. I., Parschalk B., Lenhardt R., Kurz A., Graninger W. (1996) Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth. Analg. 82, 810–816 [DOI] [PubMed] [Google Scholar]
- 15. Fairchild K. D., Singh I. S., Carter H. C., Hester L., Hasday J. D. (2005) Hypothermia enhances phosphorylation of IκB kinase and prolongs nuclear localization of NF-κB in lipopolysaccharide-activated macrophages. Am. J. Physiol. Cell Physiol. 289, C1114–C1121 [DOI] [PubMed] [Google Scholar]
- 16. Fairchild K. D., Singh I. S., Patel S., Drysdale B. E., Viscardi R. M., Hester L., Lazusky H. M., Hasday J. D. (2004) Hypothermia prolongs activation of NF-kappaB and augments generation of inflammatory cytokines. Am. J. Physiol. Cell Physiol. 287, C422–C431 [DOI] [PubMed] [Google Scholar]
- 17. Fairchild K. D., Viscardi R. M., Hester L., Singh I. S., Hasday J. D. (2000) Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J. Interferon Cytokine Res. 20, 1049–1055 [DOI] [PubMed] [Google Scholar]
- 18. Hammarfjord O., Wallin R. P. (2010) Dendritic cell function at low physiological temperature. J. Leukoc. Biol. 88, 747–756 [DOI] [PubMed] [Google Scholar]
- 19. O'Neill L. A., Sheedy F. J., McCoy C. E. (2011) MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 11, 163–175 [DOI] [PubMed] [Google Scholar]
- 20. Ceppi M., Pereira P. M., Dunand-Sauthier I., Barras E., Reith W., Santos M. A., Pierre P. (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 106, 2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. (2009) MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 183, 2150–2158 [DOI] [PubMed] [Google Scholar]
- 22. Kurowska-Stolarska M., Alivernini S., Ballantine L. E., Asquith D. L., Millar N. L., Gilchrist D. S., Reilly J., Ierna M., Fraser A. R., Stolarski B., McSharry C., Hueber A. J., Baxter D., Hunter J., Gay S., Liew F. Y., McInnes I. B. (2011) MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. U. S. A. 108, 11193–11198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tili E., Michaille J. J., Cimino A., Costinean S., Dumitru C. D., Adair B., Fabbri M., Alder H., Liu C. G., Calin G. A., Croce C. M. (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 179, 5082–5089 [DOI] [PubMed] [Google Scholar]
- 25. Zhu Q. Y., Liu Q., Chen J. X., Lan K., Ge B. X. (2010) MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J. Immunol. 185, 7435–7442 [DOI] [PubMed] [Google Scholar]
- 26. Cremer T. J., Ravneberg D. H., Clay C. D., Piper-Hunter M. G., Marsh C. B., Elton T. S., Gunn J. S., Amer A., Kanneganti T. D., Schlesinger L. S., Butchar J. P., Tridandapani S. (2009) MiR-155 induction by F. novicida but not the virulent F. tularensis results in SHIP down-regulation and enhanced pro-inflammatory cytokine response. PLoS One 4, e8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCoy C. E., Sheedy F. J., Qualls J. E., Doyle S. L., Quinn S. R., Murray P. J., O'Neill L. A. (2010) IL-10 inhibits miR-155 induction by toll-like receptors. J. Biol. Chem. 285, 20492–20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheedy F. J., Palsson-McDermott E., Hennessy E. J., Martin C., O'Leary J. J., Ruan Q., Johnson D. S., Chen Y., O'Neill L. A. (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 11, 141–147 [DOI] [PubMed] [Google Scholar]
- 29. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 30. Ghosn E. E., Cassado A. A., Govoni G. R., Fukuhara T., Yang Y., Monack D. M., Bortoluci K. R., Almeida S. R., Herzenberg L. A. (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. U. S. A. 107, 2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Connell R. M., Chaudhuri A. A., Rao D. S., Baltimore D. (2009) Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. U. S. A. 106, 7113–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trotta R., Chen L., Ciarlariello D., Josyula S., Mao C., Costinean S., Yu L., Butchar J. P., Tridandapani S., Croce C. M., Caligiuri M. A. (2012) miR-155 regulates IFN-gamma production in natural killer cells. Blood 119, 3478–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang P., Hou J., Lin L., Wang C., Liu X., Li D., Ma F., Wang Z., Cao X. (2010) Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 185, 6226–6233 [DOI] [PubMed] [Google Scholar]
- 34. Billeter A. T., Druen D., Kanaan Z. M., Polk H. C., Jr. (2012) MicroRNAs: new helpers for surgeons? Surgery 151, 1–5 [DOI] [PubMed] [Google Scholar]
- 35. Inaba K., Berg R., Barmparas G., Rhee P., Jurkovich G. J., Recinos G., Teixeira P. G., Demetriades D. (2012) Prospective evaluation of ambient operating room temperature on the core temperature of injured patients undergoing emergent surgery. J. Trauma Acute Care Surg. 73, 1478–1483 [DOI] [PubMed] [Google Scholar]
- 36. Ajuebor M. N., Das A. M., Virag L., Flower R. J., Szabo C., Perretti M. (1999) Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 162, 1685–1691 [PubMed] [Google Scholar]
- 37. Goldszmid R. S., Caspar P., Rivollier A., White S., Dzutsev A., Hieny S., Kelsall B., Trinchieri G., Sher A. (2012) NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36, 1047–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jenkins S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E. (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. (2005) Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 40. Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ariel A., Serhan C. N. (2012) New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Truettner J. S., Alonso O. F., Bramlett H. M., Dietrich W. D. (2011) Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 31, 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Truettner J. S., Motti D., Dietrich W. D. (2013) MicroRNA overexpression increases cortical neuronal vulnerability to injury. Brain Res. 1533, 122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meybohm P., Gruenewald M., Zacharowski K. D., Albrecht M., Lucius R., Fosel N., Hensler J., Zitta K., Bein B. (2010) Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit. Care 14, R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beilman G. J., Blondet J. J., Nelson T. R., Nathens A. B., Moore F. A., Rhee P., Puyana J. C., Moore E. E., Cohn S. M. (2009) Early hypothermia in severely injured trauma patients is a significant risk factor for multiple organ dysfunction syndrome but not mortality. Ann. Surg. 249, 845–850 [DOI] [PubMed] [Google Scholar]
- 46. Flores-Maldonado A., Medina-Escobedo C. E., Rios-Rodriguez H. M., Fernandez-Dominguez R. (2001) Mild perioperative hypothermia and the risk of wound infection. Arch. Med. Res. 32, 227–231 [DOI] [PubMed] [Google Scholar]
- 47. Seamon M. J., Wobb J., Gaughan J. P., Kulp H., Kamel I., Dempsey D. T. (2012) The effects of intraoperative hypothermia on surgical site infection: an analysis of 524 trauma laparotomies. Ann. Surg. 255, 789–795 [DOI] [PubMed] [Google Scholar]
- 48. Xiao H., Remick D. G. (2005) Correction of perioperative hypothermia decreases experimental sepsis mortality by modulating the inflammatory response. Crit. Care Med. 33, 161–167 [DOI] [PubMed] [Google Scholar]
- 49. Arons M. M., Wheeler A. P., Bernard G. R., Christman B. W., Russell J. A., Schein R., Summer W. R., Steinberg K. P., Fulkerson W., Wright P., Dupont W. D., Swindell B. B. (1999) Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit. Care Med. 27, 699–707 [DOI] [PubMed] [Google Scholar]
- 50. Fries M., Stoppe C., Brucken D., Rossaint R., Kuhlen R. (2009) Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J. Crit. Care 24, 453–457 [DOI] [PubMed] [Google Scholar]
- 51. Monk C. E., Hutvagner G., Arthur J. S. (2010) Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One 5, e13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Der Poll T., Marchant A., van Deventer S. J. (1997) The role of interleukin-10 in the pathogenesis of bacterial infection. Clin. Microbiol. Infect. 3, 605–607 [DOI] [PubMed] [Google Scholar]
- 53. Pu J., Bai D., Yang X., Lu X., Xu L., Lu J. (2012) Adrenaline promotes cell proliferation and increases chemoresistance in colon cancer HT29 cells through induction of miR-155. Biochem. Biophys. Res. Commun. 428, 210–215 [DOI] [PubMed] [Google Scholar]
- 54. Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 55. Clark N., Keeble J., Fernandes E. S., Starr A., Liang L., Sugden D., de Winter P., Brain S. D. (2007) The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 21, 3747–3755 [DOI] [PubMed] [Google Scholar]
- 56. Fernandes E. S., Liang L., Smillie S. J., Kaiser F., Purcell R., Rivett D. W., Alam S., Howat S., Collins H., Thompson S. J., Keeble J. E., Riffo-Vasquez Y., Bruce K. D., Brain S. D. (2012) TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 188, 5741–5751 [DOI] [PubMed] [Google Scholar]
- 57. Brock M., Samillan V. J., Trenkmann M., Schwarzwald C., Ulrich S., Gay R. E., Gassmann M., Ostergaard L., Gay S., Speich R., Huber L. C. (2012) AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. [E-pub ahead of print] Eur. Heart J. 10.1093/eurheartj/ehs060 [DOI] [PubMed] [Google Scholar]
- 58. Li R. C., Tao J., Guo Y. B., Wu H. D., Liu R. F., Bai Y., Lv Z. Z., Luo G. Z., Li L. L., Wang M., Yang H. Q., Gao W., Han Q. D., Zhang Y. Y., Wang X. J., Xu M., Wang S. Q. (2013) In vivo suppression of microRNA-24 prevents the transition toward decompensated hypertrophy in aortic-constricted mice. Circ. Res. 112, 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Care A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., Bang M. L., Segnalini P., Gu Y., Dalton N. D., Elia L., Latronico M. V., Hoydal M., Autore C., Russo M. A., Dorn G. W., 2nd, Ellingsen O., Ruiz-Lozano P., Peterson K. L., Croce C. M., Peschle C., Condorelli G. (2007) MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 13, 613–618 [DOI] [PubMed] [Google Scholar]
- 60. Krutzfeldt J., Rajewsky N., Braich R., Rajeev K. G., Tuschl T., Manoharan M., Stoffel M. (2005) Silencing of microRNAs in vivo with “antagomirs.” Nature 438, 685–689 [DOI] [PubMed] [Google Scholar]









