Abstract
The short palate, lung and nasal epithelial clone 1 (SPLUNC1) protein is a member of the palate, lung, and nasal epithelium clone (PLUNC) family, also known as bactericidal/permeability-increasing (BPI) fold-containing protein, family A, member 1 (BPIFA1). SPLUNC1 is an abundant protein in human airways, but its function remains poorly understood. The lipid ligands of SPLUNC1 as well as other PLUNC family members are largely unknown, although some reports provide evidence that lipopolysaccharide (LPS) could be a lipid ligand. Unlike previous hypotheses, we found significant structural differences between SPLUNC1 and BPI. Recombinant SPLUNC1 produced in HEK 293 cells harbored several molecular species of sphingomyelin and phosphatidylcholine as its ligands. Significantly, in vitro lipid-binding studies failed to demonstrate interactions between SPLUNC1 and LPS, lipoteichoic acid, or polymyxin B. Instead, one of the major and most important pulmonary surfactant phospholipids, dipalmitoylphosphatidylcholine (DPPC), bound to SPLUNC1 with high affinity and specificity. We found that SPLUNC1 could be the first protein receptor for DPPC. These discoveries provide insight into the specific determinants governing the interaction between SPLUNC1 and lipids and also shed light on novel functions that SPLUNC1 and other PLUNC family members perform in host defense.—Ning, F., Wang, C., Berry, K. Z., Kandasamy, P., Liu, H., Murphy, R. C., Voelker, D. R., Nho, C. W., Pan, C.-H., Dai, S., Niu, L., Chu, H.-W., Zhang, G. Structural characterization of the pulmonary innate immune protein SPLUNC1 and identification of lipid ligands.
Keywords: lipid ligand, SM, DPPC
More than a decade ago, the palate, lung, and nasal epithelium clone (PLUNC) protein family was identified (1). It belongs to the tubular lipid-binding (TULIP) domain superfamily, which includes bactericidal/permeability-increasing (BPI) protein, lipopolysaccharide (LPS)-binding protein (LBP), cholesteryl ester-transfer protein (CETP), phospholipid-transfer protein (PLTP), PLUNC protein (2), and the house dust mite allergen Der p 7. PLUNC protein family members contain 1 or 2 tandem copies of a fold that usually provides a tubular cavity for the binding of lipids. Gene expression studies show that human PLUNC proteins are produced predominately in the upper airways, nose, and mouth (3). Based on tissue distribution and their structural similarities to the innate immune defense protein BPI, these proteins are probably involved in the innate immune response to pathogens during infection (4). Short palate, lung and nasal epithelial clone 1 [SPLUNC1; also known as BPI fold-containing protein, family A, member 1 (BPIFA1); refs. 5, 6], the most functionally characterized member of the PLUNC protein family, has multiple functions in respiratory infection induced by gram-negative bacteria (7, 8). Its antimicrobial activity was first observed in nasopharyngeal carcinoma epithelial HNE1 cells (9). Recombinant human SPLUNC1 reduces the growth of Pseudomonas aeruginosa (10, 11), and SPLUNC1 from chinchilla shows activity in killing Haemophilus influenzae (12). Additional studies reported that SPLUNC1 and other PLUNC protein family members may act as novel airway surfactants with antibiofilm activity that disrupts the growth of P. aeruginosa in airways (13, 14). Mouse strains overexpressing Clara cell secretory protein (CCSP) and SPLUNC1 protein showed enhanced antimicrobial activity against P. aeruginosa and Klebsiella pneumoniae, as well as the atypical bacterium Mycoplasma pneumoniae (15, 16). Coating of bacterial cells by SPLUNC1 protein inhibits the growth of P. aeruginosa but does not induce bacterial killing as BPI does. SPLUNC1 also acts as a chemoattractant that facilitates migration of macrophages and neutrophils (17). The N terminus of SPLUNC1 (residues G22−A39) inhibits an epithelial sodium channel (ENaC; refs. 18, 19). A recent report suggested that SPLUNC1 may act as a pH-sensitive regulator of ENaC (20). Incubation of recombinant mouse SPLUNC1 protein reduced M. pneumoniae growth significantly in vitro (21). In vivo studies showed that SPLUNC1 is critical for clearing respiratory pathogens such as M. pneumoniae and P. aeruginosa from murine lungs (16, 22). SPLUNC1 deficiency enhances airway eosinophilic inflammation in allergic mice, in part by reducing eotaxin-2 production in alveolar macrophages (23). It is not yet understood how SPLUNC1 carries out these multiple functions, and whether it has a function similar to that of BPI, which kills bacteria by altering outer membrane integrity, or neutralizes LPS to reduce neutrophil recruitment and activation or acts as a chemoattractant (24–26). Notably, based on the high sequence similarity of SPLUNC1 to BPI, it was predicted that SPLUNC1 or other PLUNC family members could bind to LPS (27, 28). Several groups have indeed reported direct binding between SPLUNC1 and LPS (7, 9, 10, 17, 29). However, another report showed that SPLUNC1 protein derived directly from human bronchoalveolar lavage (BAL) fluid does not bind to LPS (30). To address these structural and functional questions regarding SPLUNC1 and its family members, we have solved the structure of the human SPLUNC1 protein. The structure showed a folding pattern similar to that of BPI, although significant differences were revealed, including differing surface charge distribution between SPLUNC1 and BPI. We thus reason that SPLUNC1 may bind preferentially to lipids other than LPS. We found recombinant SPLUNC1 generated in human embryonic kidney (HEK) 293 cells to contain 2 lipid classes: sphingomyelins (SMs; major fraction) and phosphatidylcholines (PCs; minor fraction). Consistent with these observations, direct binding studies demonstrated high-affinity interaction of SPLUNC1 with SM and PC but not LPS. These lipid-protein interactions are likely to play an important role in regulating the innate immune function of SPLUNC1.
MATERIALS AND METHODS
Protein expression, crystallization, and structural determination
Human SPLUNC1 (43–256) was cloned into the EcoRI/SalI site of the modified pMAL-c2X vector (31), which has an AAAEF linker region connecting SPLUNC1 and maltose-binding protein (MBP). To further reduce the flexibility between SPLUNC1 and MBP and improve our chance of obtaining crystals, we shortened the linker region from AAAEF to AAA. The mutant was generated using the Stratagene QuikChange Kit (Stratagene, La Jolla, CA, USA) and verified by sequencing. The mutagenic primers we used were 5′-CTAATGCTGCTGCGAGTCCCACAGGTCTTG-3′ (forward) and 5′-CAAGACCTGTGGGACTCGCAGCAGCATTAG-3′ (reverse). The fusion protein was expressed in Escherichia coli BL21-pLysS(DE3) cells. Large-scale cultures were grown in Luria-Bertani medium with 100 μg/ml ampicillin at 37°C to an OD600 of 0.8. The culture was induced by addition of 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 30°C for 6 h. To express selenomethionine (SeMet)-substituted SPLUNC1, minimal medium was made by addition of 19 aa except Met, 0.2% (w/v) glucose, 2 mM MgSO4, 0.1 mM CaCl2, vitamin B1, and ampicillin into M9 medium. BL21-pLysS(DE3) cells were transferred to minimal medium and grown at 37°C to an OD600 of 1.0. Next, 200 mg of Thr, Lys, Phe, and Cys and 100 mg of Leu, Ile, Val, and Try were added to 2 L of medium, followed by the addition of 200 mg of l-SeMet. SeMet-SPLUNC1 expression was induced by addition of 0.2 mM IPTG and incubated at 16°C for 18 h. Cells were collected by centrifugation at 4000 rpm for 30 min and resuspended in 20 mM Tris-HCl (pH 7.5), 200 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride. The cells were disrupted by French press, and the lysate was clarified by centrifugation at 25,000 rpm for 30 min. The soluble fraction was loaded onto an amylose resin affinity column and MBP-SPLUNC1 fusion was eluted with 10 mM maltose. The MBP-SPLUNC1 fusion was further purified by a HiTrap Q column and a size exclusion Superdex 200 column equilibrated with 5 mM bis-Tris (pH 6.5) and 50 mM NaCl. Purified protein was analyzed by SDS-PAGE and Coomassie Blue staining.
Full-length human SPLUNC1 (20–256) was cloned into the EcoRI/SalI site of the pMAL-c2 vector with a TEV cleavage site between SPLUNC1 and MBP. The full-length MBP-SPLUNC1 (20–256) fusion was expressed and purified using the method mentioned above. To make glutathione transferase (GST)-SPLUNC1 fusion, the full-length human SPLUNC1 (20–256) was cloned into the pGEX-4T2 vector with a TEV cleavage site between SPLUNC1 and GST and was expressed in Rosetta2(DE3) cells. Cultures were induced at OD600 = 1.0 by addition of IPTG to 0.2 mM and incubation at 20°C for 18 h. The fusion proteins were purified using a glutathione agarose resin column followed by a size exclusion Superdex 200 column.
The initial crystallization screening of the MBP-SPLUNC1 (43–256) fusion was conducted at 19°C using the sitting-drop vapor diffusion method. To improve the diffraction quality of crystals, purified MBP-SPLUNC1 (43–256) fusion was incubated with 0.5% n-octyl-β-d-glucoside at 4°C for 12 h and then was concentrated to 13 mg/ml. Crystals appeared in 2 d under conditions of 50 mM HEPES (pH 7.5), 20 mM MgCl2, 25% polyethylene glycol 550 MME.
Crystals were directly transferred to the well solution for a few seconds and then were cryo-cooled in liquid nitrogen for storage and data collection. Single-wavelength anomalous dispersion (SAD) data using 0.9790-Å wavelength X-rays were collected at 100 K on beamline 8.2.2 at the Advanced Light Source (Berkeley, CA, USA) from a single SeMet-SPLUNC1 crystal while using 1.0000-Å wavelength X-rays for native crystals. Data were integrated and scaled with the HKL2000 package (32). Unit cell parameters were used to calculate a Matthews coefficient of 3.07 Å3/Da, 59.96% solvent region, with only 1 molecule/asymmetric unit. With further structure determination, the space group was assigned as P3221. A total of 7 selenium sites were found and verified using HKL2MAP (33). The initial electrodensity map was calculated with Phenix.Autosolve, and the initial model was built with Phenix.AutoBuild. Phenix.Refine was performed for further refinement (34). After several rounds of manual rebuilding in Coot and subsequent cycles of refinement, the model converged to a final Rfactor and Rfree of 20.5 and 25.9%, respectively (Supplemental Tables S1 and S2 and ref. 35).
Sample preparation from HEK 293 cells for mass spectrum experiment
The method for generating human recombinant SPLUNC1 in mammalian cells was described previously (23). The full-length cDNA of human SPLUNC1 with a His tag at the C terminus was cloned into a pRK7-Neo vector, and the protein was expressed in the HEK 293 cell line. The His-SPLUNC1 was secreted by HEK 293 cells; the supernatant containing His-SPLUNC1 was dialyzed 3 times with 20 mM Tris-HCl (pH 7.5) and 200 mM NaCl. The supernatant was loaded onto a nickel-nitrilotriacetic acid (Ni-NTA) column and His-SPLUNC1 was eluted with 250 mM imidazole. The control sample for the mass spectrum experiment was the human IgGγ1 Fc region, which is believed to have no obvious lipid-binding capacity. Fc was secreted by HEK 293 cells and purified using a protein A affinity column. Both His-SPLUNC1 and Fc samples were dissolved in buffer with 20 mM Tris-HCl (pH 7.5) and 200 mM NaCl before further investigation. The lipids bound with His-SPLUNC1 or Fc were extracted using the typical Bligh and Dyer method and phospholipids were detected by phosphorus assay (36, 37). The final purified lipids were subjected to the following mass spectrum analysis.
The extracted phospholipids were injected onto an Ascentis 5-μm Si (2.0 mm×150 mm) column (Supelco, Bellefonte, PA, USA) coupled to a Sciex API 3200 triple quadrupole mass spectrometer (PE Sciex, Toronto, ON, Canada) with an electrospray source. The high-performance liquid chromatograph was operated at a flow rate of 0.2 ml/min with a mobile phase of hexane-isopropanol (30:40; v/v; solvent A) and hexane-isopropanol-water (30:40:7; v/v/v) with a final concentration of 1 mM ammonium acetate (solvent B). The high-performance liquid chromatography (HPLC) gradient was an isocratic hold at 46% solvent B for 3 min, followed by an increase to 70% solvent B at 25 min and another increase to 100% solvent B in 5 min, followed by isocratic elution with 100% solvent B for 20 min. The PC(16:0/18:1) standard eluted at 37 min. The Sciex API 3200 triple quadrupole mass spectrometer was used in the positive ion mode to obtain a full-scan mass spectrum of the phospholipids present in the Bligh and Dyer extract. In addition, the lipids of interest were collisionally activated in the positive ion mode using a collision energy of 35 V. The molecular species of phospholipids were identified at the level of nominal molecular weight and the total number of fatty acyl carbon atoms and double bonds (38).
Lipid-binding assays on solid face
The lipid-binding assays followed established methods (39, 40). In brief, all phospholipids purchased from Avanti Polar Lipids (Alabaster, AL, USA) were dissolved in chloroform, dried, and diluted in pure ethanol. Kdo2-lipid A, rough LPS Ra from E. coli EH100, smooth LPS from E. coli 0111:B4, LPS from P. aeruginosa (PA-LPS), LTA from Bacillus subtilis, and polymyxin B were dissolved in water and diluted in pure ethanol. Lipids (2 μg in 50 μl of ethanol) were pipetted into microtiter wells (Immulon 2 HB; Thermo Scientific, Waltham, MA, USA), and the solvent was evaporated using a warm air blower. After adsorption, the wells were blocked with Tris-buffered saline (TBS) [20 mM Tris-HCl (pH 7.5) and 150 mM NaCl] containing 10% (w/v) nonfat milk (Bio-Rad Laboratories, Hercules, CA, USA) at 37°C for 3 h. After blocking, MBP-SPLUNC1 and GST-SPLUNC1 fusion proteins were added to each well with blocking buffer and incubated at room temperature for 20 min. MBP and GST were used as controls separately. The bound MBP and MBP-SPLUNC1 fusion were detected by enzyme-linked immunosorbent assay (ELISA) using anti-MBP primary antibody (NEB) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Bio-Rad Laboratories). The bound GST and GST-SPLUNC1 fusion were detected by ELISA using HRP-conjugated anti-GST antibody (Abcam, Cambridge, MA, USA). After the wells were washed with TBS, the bound antibodies were detected with o-phenylenediamine as substrate, and absorbance at 490 nm was measured.
To prepare liposomes for the inhibition assay, 18:0 Lyso-PC, 18:1 Lyso-PC, 16:0 Lyso-PC, and dipalmitoylphosphatidylcholine (DPPC) were dissolved in chloroform, dried under nitrogen, and hydrated with TBS at 37°C for 1 h. Liposomes (2 mg/ml) were made by sonication for 5 min. GST-SPLUNC1 and various liposomes were mixed and incubated at 37°C for 1 h before use. To perform the inhibition assay, 1 μg of SM (chicken) in 25 μl of ethanol was pipetted onto half-area microtiter plates (Corning Inc., Corning, NY, USA), and the solvent was evaporated using a warm air blower. After nonspecific binding was blocked with TBS and 5% (w/v) bovine serum albumin (BSA) at 37°C for 2 h, a mixture of GST-SPLUNC1 and liposomes in 40 μl of blocking buffer was added and incubated at 37°C for 1 h. GST-SPLUNC1 was added alone as a control. The wells were then washed with TBS, and HRP-conjugated anti-GST antibody was added and incubated at 37°C for 1 h. After the wells were washed with TBS, the bound antibody was detected with o-phenylenediamine as the substrate, and absorbance at 490 nm was measured. All experiments were repeated ≥3 times in triplicate wells, and data from a representative experiment are shown. All P values and SDs were calculated by Excel (Microsoft, Richmond, WA, USA) and graphs were made by Prism (GraphPad Software, Inc., San Diego, CA, USA).
Binding of SPLUNC1 to lipids extracted from human and mouse BAL separated by thin-layer chromatography (TLC)
Lipids were extracted from human (healthy, nonsmoker) BAL fluid using the Bligh and Dyer method, and the phospholipids were quantified by phosphorus assay (36, 41). Similarly, lipids were extracted from the pooled samples of BAL fluid from BALB/c mice. Then 20 μg of SM (bovine), SM (chicken), standard DPPC, and lipid extracts from human and mouse BAL were loaded onto a silica gel 60 plate and separated using a solvent system of chloroform-methanol-2-propanol-0.2 M potassium chloride-triethylamine (90:30:75:18:55). After the plate was dried in the air, phospholipid spots were detected by spraying with 0.1% (w/v) anilinonaphthalene sulfonic acid, followed by exposure to UV light. To perform the binding assay, TLC plates were washed with TBS to remove the solvent. After the plates were blocked with TBS containing 5% BSA at room temperature for 1 h, 10 ml of 50 nM MBP/MBP-SPLUNC1 was incubated with the TLC plates for 1 h. After the plates were washed with TBS containing 0.1% BSA, anti-MBP primary antibody was added and incubated for 1 h. Next, the HRP-conjugated goat anti-rabbit IgG secondary antibody was added and incubated for 1 h. Then 1 mg/ml 3,3′-diaminobenzidine tetrahydrochloride dissolved in PBS was used as a substrate to detect the bound antibody.
RESULTS
Structural determination of SPLUNC1
As observed from sequence alignment and analysis, PLUNC family members have significant unique features compared with those for BPI, although all have a similar predicted overall fold (42). High-resolution protein structures are essential to completely understand the relationship between structure and function of SPLUNC1. SPLUNC1 is a lipid-binding protein and thus has a large hydrophobic portion on its surface, which presents numerous challenges for protein expression, crystallization, and determination of structure. To improve solubility, we constructed an MBP at the N terminus of SPLUNC1. To increase the prosperity for crystallization, we truncated the linker region between MBP and SPLUNC1 and removed ∼20 amino acids from the N terminus of SPLUNC1. We verified that the truncated chimera had lipid-binding activity similar to that of full-length SPLUNC1, as described below. We speculate that the N-terminal truncated version of SPLUNC1 maintains the structural integrity of the full-length version, although this region is critical for inhibiting the sodium channel ENaC (18, 19). We were able to crystallize this modified fusion protein, and the crystals diffracted to 2.5 Å with a space group of P3221 and dimensions: a = b = 119.087 Å, c = 98.541 Å, α = β = 90°, γ = 120°. Notably, a traditional molecular replacement (MD) did not yield any solution with significant confidence when the available structure of MBP [RCSB Protein Data Bank (PDB) identification code 4MBP or 1ANF] was used as the starting model. Finally, we produced SeMet-containing proteins and were able to determine the structure using the SAD method. From the final refined model, we found that there are significant structural differences between the MBP structures (RCSB PDB identification code 4MBP or 1ANF; residues 1−360) and the MBP structure in our MBP-SPLUNC1 fusion protein with root mean standard deviation (RMSD) above 3.0 Å (Supplemental Fig. S1). However, the structure of MBP from MBP-SPLUNC1 was found to be similar to that of apo-MBP with RMSD of 0.76 Å (RCSB PDB identification code 1OMP and Supplemental Fig. S1C, residues 1−357; ref. 43). Our final refined model contains a detergent (octyl-β-glucoside) molecule, which was added to speed up crystal growth; this molecule was found to bind to MBP at a site away from the maltose-binding site (Supplemental Fig. S1B).
Overall structure
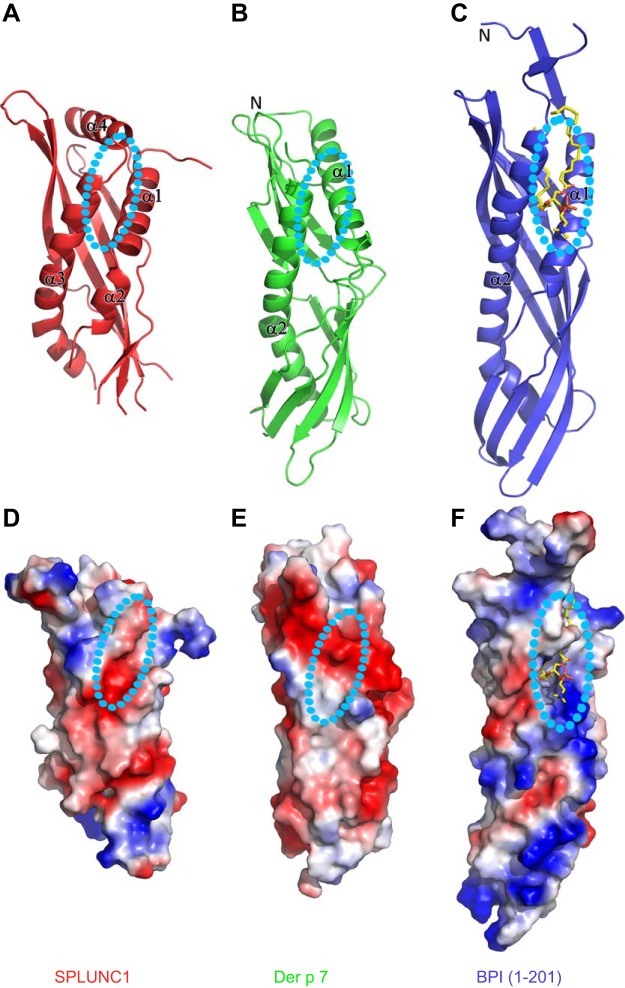
The stereo form of the entire structure of SPLUNC1 is shown in Fig. 1, whereas the structure of the entire fusion protein is shown in Supplemental Fig. S1. The structure of SPLUNC1 begins with a helix (α1, residues 47–61), followed by a short helix (α2, residues 64−70; Fig. 1A). There is a large flexible loop gap between residues 73 and 107 (Fig. 1A), and no continuous density was found to connect this region in either the initial electron density map or the final refined model (data not shown; Fig. 1A). This portion is followed by antiparallel β strands from β1 to β6 (β1, residues 101−111; β2, 119−122; β3,129−141; β4,152−167; β5, 173−182; and β6, 188−191; Fig. 1A). The coil region between β1 and β2 could be a portion of an integrated long β strand, including β1 and β2 in the presence of lipid ligand, since this long potential β strand also comprises a major portion of the potential lipid-binding pocket (Fig. 1A). There are 9 residues missing at the tip of the loop between residues 193 and 203. This is followed by a long bent helix (α3, from residues 206 to 232; Fig. 1A). The structure ends with a unique C-terminal helix (α4, residues 236−248; Fig. 1A). The isolated α4 helix and the extended short coil C terminus (residues 249−253) is stabilized by several hydrogen bonds formed by 2 residue side chains (Fig. 1B), which are absolutely conserved among SPLUNC1 from human, mouse, rat, bovine, and pig (Supplemental Fig. S2). There is one disulfide bridge that links α3 and β5, which is positioned to confer stability of the entire structure. The same version of the SPLUNC1 structure was published from other laboratory (20). Both structures are almost identical except the region between residues 193 and 203, where there is a short helix fragment in the published structure that is completely missing in ours. We believe that the crystal packing within the published structure stabilizes this flexible region.
Figure 1.
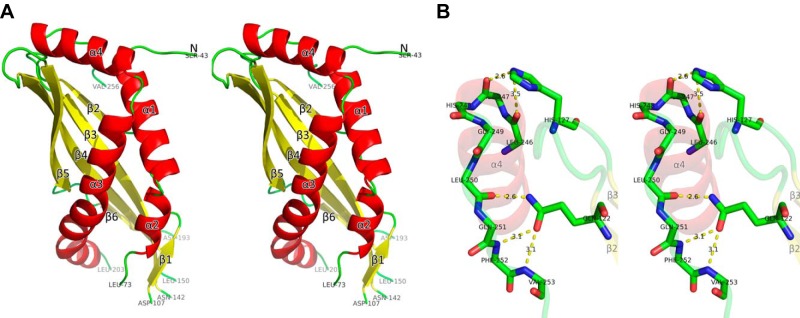
Structure of SPLUNC1. A) The stereo view of the structure of SPLUNC1 with residues from 43 to 256 (colored with secondary structures). There are 3 missing loops: residues from 74 to 106, residues from 143 to 149, and residues from 194 to 202. All structure figures were prepared by the program PyMOL (http://www.pymol.org). B) Detailed interaction between α4 and 2 conserved residues.
Comparison to SPLUNC1 homologs
As expected, the structure of SPLUNC1 has a folding topology similar to those of Der p 7 (RCSB PDB identification code 3H4Z) and BPI (RCSB PDB identification code 1EWF; Fig. 2A–C). Compared with the structure of BPI, the corresponding potential lipid-binding pocket of SPLUNC1 is closed (Fig. 2A). We expect that a significant conformational change is required to open up the hydrophobic pocket to accommodate the hydrophobic tail of lipids. On binding of a lipid ligand, it is expected that some disordered regions within SPLUNC1 become ordered as described above. From the Dali alignment (44), the structure of Der p 7 (RCSB PDB identification code 3H4Z) has a very similar closed form structure at the corresponding lipid-binding site predicted in SPLUNC1, with a RMSD for Cα (total of 119 residues included) of 3.0 Å (Fig. 2B), whereas the RMSD for Cα (total of 129 residues included) between SPLUNC1 and BPI is 3.3 Å (Fig. 2C). Notably, compared with BPI and Der p 7, there is an additional helix (α4) at the C terminus of SPLUNC1 that interacts with an extended loop between β2 and β3 (Figs. 1A and 2A and Supplemental Fig. S2A, B). It will be most informative to determine the role of this helix. As this unique helix locates close to the potential lipid-binding site (Fig. 2A and Supplemental Fig. S2B), we speculate that the helix will move away on lipid binding. The surface properties of SPLUNC1 and Der p 7 differ with BPI containing a highly positively charged region at the substrate binding site (Fig. 2D–F), which functions to neutralize the negative charge of LPS through formation of salt bridges. In contrast, very few positive residues are present in the equivalent region of either SPLUNC1 or Der p 7 (Fig. 2D, E). Based on the similarity in both overall structure and surface charge distribution, it is likely that SPLUNC1 may have a lipid substrate similar to that of Der p 7. However, the dramatic difference in surface charge distribution between SPLUNC1 and BPI indicates that SPLUNC1 may bind to lipids other than LPS.
Figure 2.
Comparison of the SPLUNC1 structure with those of BPI and Der p 7. A) The structure of SPLUNC1. B) The structure of Der p 7. C) The structure of BPI. D) Surface properties of SPLUNC1. E) Surface properties of Der p 7. F) Surface properties of BPI.
Lipid identification of SPLUNC1 protein derived from HEK 293 cells
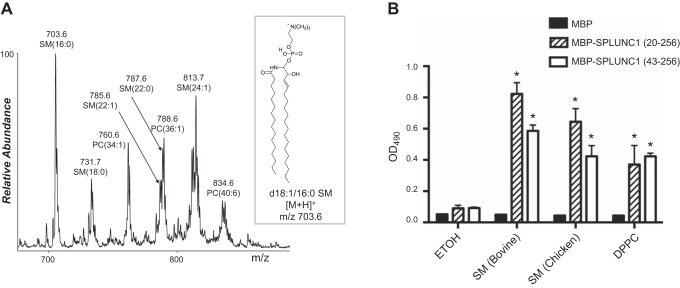
Previous studies have revealed that purified BPI protein expressed in Chinese hamster ovary (CHO) cells contained PC within its lipid-binding pocket (45). We reasoned that SPLUNC1 protein expressed by mammalian cells may also contain a bound lipid molecule. We expressed SPLUNC1 in HEK 293 cell lines and subjected purified SPLUNC1 protein to a simple screening for bound phospholipid constituents. We found that a SPLUNC1 sample of only 60% purity derived from HEK 293 cells was stoichiometrically occupied by phospholipid, based on lipid extraction under denaturing conditions and quantification of lipid phosphorus (36, 41). To identify which lipids were bound, normal-phase HPLC was used to separate the lipids by phospholipid class, and electrospray mass spectrometry was used for detection. It was determined from this experiment that the only lipids present in the Bligh and Dyer extracted sample eluted between 36 and 41 min, which was in the region where the PC(16:0/18:1) standard eluted. Notably, both SM and PC lipids were bound to the SPLUNC1 protein. This identification was verified by the retention time on normal-phase HPLC and by performing collision-induced dissociation (CID) in the positive ion mode. The CID of the lipid species bound to SPLUNC1 resulted in a predominant product ion at m/z 184.1 (phosphocholine) that is characteristic of PC and SM lipids (46). In addition, the number of carbon atoms and double bonds present in these lipids could be established from the nominal molecular weight of the lipids observed in the positive ion mass spectrum (38). Therefore, it was determined that 5 SM molecular species were present in the sample, including SM(16:0), SM(18:0), SM(22:1), SM(22:0), and SM(24:1) (Fig. 3A). In addition, 3 PC molecular species, PC(34:1), PC(36:1), and PC(40:6), were present in the sample. Both lipid classes contain a phosphocholine moiety, which is conjugated to ceramide in SM and conjugated to diacylglycerol in PC.
Figure 3.
Lipids derived from the SPLUNC1 sample from HEK 293 cells and in vitro lipid binding of SPLUNC1 to SM and DPPC. A) Positive ion electrospray mass spectrum over the range of 650−900 Da of the normal-phase high-performance liquid chromatogram from 36 to 41 min of the lipid molecular species bound to SPLUNC1. The most abundant lipid species present was identified by tandem mass spectrometry as an SM lipid due to the presence of a major product ion at m/z 184. In addition, some lipids present were determined to be PC molecular species. The abbreviations of the lipid molecular species of SM or PC observed for the most abundant ions at each m/z have the number of carbon atoms present in the 2 fatty acyl chains and the number of double bonds indicated in parentheses. The inset represents the likely structure of the most abundant SM observed at m/z 703.6. B) SPLUNC1 binds to SM and DPPC. Bars represent means ± sd. Sample size n = 3. All binding assays were repeated >3 times. *P < 0.01 vs. SPLUNC1-ethanol (ETOH) binding.
In vitro binding of SPLUNC1 to SMs and PCs
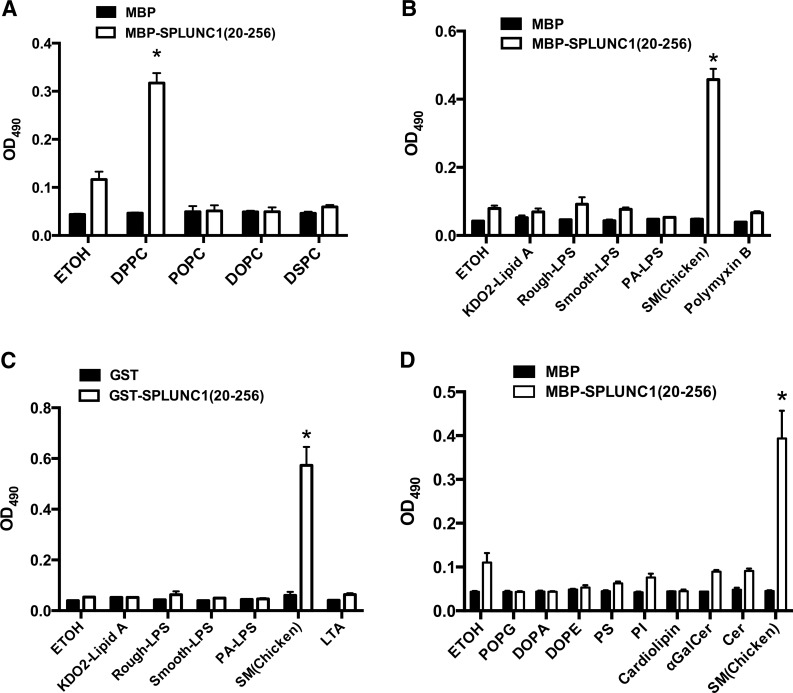
To verify the binding of SPLUNC1 to SMs and PCs, we performed in vitro lipid-binding assays using solid-phase phospholipids according to established methods (39, 40). In accordance with the above mass spectrometry data, we found that several SM samples obtained from bovine (34% of 23:0, 21% of 24:0, 20% of 22:0, and 16% of 16:0) or chicken (>86% of 16:0) bound SPLUNC1 generated from E. coli with high affinity and specificity (Fig. 3B). SPLUNC1 also bound to DPPC (Fig. 3B), which is the major member of the PC class highly enriched in pulmonary surfactant composition (47). However, SPLUNC1 failed to bind to another major member of the PC family, 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC; Fig. 4A). The main difference between DPPC and POPC is that DPPC contains 2 saturated palmitic fatty acids (16:0), whereas POPC contains 1 palmitic fatty acid (16:0) and 1 unsaturated oleic acid (18:1). Notably, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), which contains 2 saturated stearic acids (18:0), did not show any binding affinity to SPLUNC1 (Fig. 4A), demonstrating a high degree of structural specificity of SPLUNC1 in the recognition of PCs.
Figure 4.
In vitro lipid binding of SPLUNC1 screening. A) Binding of SPLUNC1 with DSPC and DOPC. B) Binding of MBP-SPLUNC1 to kdo2-lipid A, smooth LPS, rough LPS, and polymyxin B. C) Binding of GST-SPLUNC1 to kdo2-lipid A, smooth LPS, rough LPS, and LTA. D) Binding of SPLUNC1 to other lipids including POPG, PI, DOPA PS, ceramide (Cer), and galactosylceramide (αGalCer). Bars represent means ± sd. Sample size n = 3. All binding assays were repeated >3 times. *P < 0.01 vs. SPLUNC1-ethanol (ETOH) binding.
In vitro binding of SPLUNC1 to LPS, endotoxins from gram-positive bacteria, and other lipids
Several groups reported the direct binding of SPLUNC1 to LPS (7, 9, 10, 17, 29). However, our preparation of SPLUNC1 protein expressed in E. coli did not contain any lipid based on our solved structure. To specifically test for interactions between SPLUNC1 and LPS, we performed solid-phase binding studies using different versions of LPS. We did not detect any binding activity between SPLUNC1 and LPS using either Kdo2 lipid A, smooth LPS, or rough LPS and MBP-SPLUNC1 fusion protein (Fig. 4B) or GST-SPLUNC1 fusion protein (Fig. 4C). Polymyxin B from gram-positive bacteria binds to Der p 7 with low affinity (42). Based on the strong similarity in both structure and surface charge distribution between Der p 7 and SPLUNC1, we reasoned that polymyxin B could be a potential lipid ligand for SPLUNC1. However, our in vitro assay did not show any binding between SPLUNC1 and polymyxin B (Fig. 4B). Our findings also demonstrated that the gram-positive ligand LTA did not interact with SPLUNC1 (Fig. 4C). To further identify the cognate lipid ligands of SPLUNC1, we systemically screened a panel of lipids that exist widely in mammals, bacteria, and fungi for binding to SPLUNC1, including specific molecular species of 1-palmitoyl-2-oleoyl-sn-glycero-3 (POPG), 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), phosphatidylserine (PS), phosphatidylinositol (PI), cardiolipin, ceramide, and galactosylceramide. Ceramides constitute the hydrophobic portion of simple and complex sphingolipids. However, this expanded screen for lipid ligands failed to identify any additional lipids that interact with SPLUNC1 (Fig. 4D).
In vivo lipid ligands of SPLUNC1
SPLUNC1 protein is mostly found to exist in the upper respiratory airway, whereas DPPC exists mostly in the lung. It will be of great interest to determine whether there are other lipids in the pulmonary system or airways that bind to SPLUNC1. Furthermore, the SPLUNC1 protein was first isolated and identified from human BAL fluid (30). We reason that there may exist lipid candidates in the BAL fluid that bind to SPLUNC1.
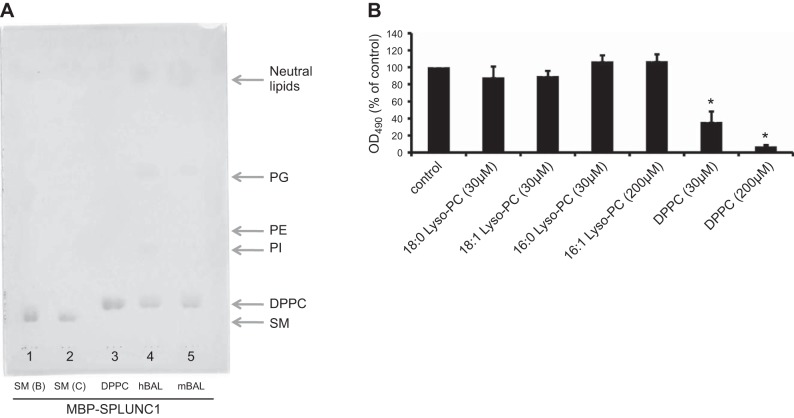
In this regard, lipid extracts of BAL fluids collected from both human and mouse were subjected to TLC analysis. To our surprise, the only lipid found in the BAL fluid to bind SPLUNC1 was DPPC (Fig. 5A). Although SM is found to bind to SPLUNC1, we did not detect any SM in BAL fluids using the TLC approach (Fig. 5A). However, some traces of PI, PE, phosphatidylglycerol (PG), and some neutral lipids were also evident on the gel (Fig. 5A). We interpret these as nonspecific interactions, because individual binding assays of SPLUNC1 to these lipids did not show any binding, as described above. To further confirm the specificity of the binding between DPPC and SPLUNC1, as well as to show that DPPC and SM bind to the same site on SPLUNC1, we challenged the binding of SPLUNC1 to immobilized SM with DPPC and 3 other single-chain lyso-PC lipids (16:0, 18:0, and 18:1). We found that DPPC but not the 3 other lyso-PC lipids could compete for the binding of SPLUNC1 to the plate coated with SM (Fig. 5B). We conclude that DPPC and SM bind to the same site on SPLUNC1 and that the binding is specific.
Figure 5.
Identification of lipid ligands of SPLUNC1 from BAL fluids and competing binding of DPPC and SM to SPLUNC1. A) Samples from both human and mouse BAL fluids show DPPC as the only major lipid bound to SPLUNC1. Lane 1, SM from bovine; lane 2, SM from chicken; lane 3, standard DPPC; lane 4, lipid extracts from human BAL fluid; lane 5, lipid extracts from mouse BAL fluid. Several other major lipid components of BAL fluid also were apparent, including PI, PE, PG, and neutral lipids as labeled, although these had no specific interaction with SPLUNC1. B) DPPC inhibited GST-SPLUNC1 binding to solid-phase SM. However, 18:0 Lyso-PC, 18:1 Lyso-PC, and 16:0 Lyso PC liposomes were not able to inhibit GST-SPLUNC1 binding to solid phase SM. Bars represent means ± sd. Sample size n = 3. All binding assays were repeated >3 times. *P < 0.05 vs. SPLUNC1-SM binding in the absence of phospholipids.
DISCUSSION
Currently, the PLUNC family is known to contain ≥8 regular members and 3 pseudo members (3, 6, 49). It can be further divided into 2 subfamilies: the short-form members, which contain 1 BPI fold, and the long-form members, which contain 2 BPI folds (3, 6, 49). However, the functions of these family members in host defense and innate immunity are poorly understood. No protein crystal structures have been reported previously before the recent publication (20), and the lipid ligands for the family members are not well characterized, despite some reports suggesting that SPLUNC1 binds to LPS in a manner similar to that of BPI (7, 9, 10, 17, 29). However, SPLUNC1 is not able to kill gram-negative bacteria, neutralize LPS to suppress inflammation, or act as an opsonin as BPI does. Consistent with our findings, another group also found no direct binding between the SPLUNC1 protein and LPS (30). The lack of structural information as well as knowledge about the lipid substrates for the PLUNC family members impedes our understanding of SPLUNC1 function. Here, we reported the high-resolution structure of the SPLUNC1 protein. We predict that the lipid-binding domains of other family members will produce similar structures. However, differences in surface charge and in structural features in the potential lipid-binding pockets could lead to different lipid specificity for each family member.
The similarity between structures of SPLUNC1 and the house dust mite allergen Der p 7, particularly in the surface charge distributions, raises the possibilities that SPLUNC1 and Der p 7 bind the same lipid substrates and that they could be evolutionarily and/or functionally related. Der p 7 does not bind to LPS but does bind to polymyxin B, a small cationic and hydrophobic peptide from gram-positive bacteria (48). Our binding assays showed no binding of SPLUNC1 to either polymyxin B (Fig. 4B) or LTA (Fig. 4C). It was reported that Der p 2, another allergen, could replace MD2 to mediate the Toll-like receptor (TLR) 4 signal transduction pathways, in which both Der p 2 and MD2 can act as LPS receptor (44). This intriguing result leads us to speculate that Der p 7 and the SPLUNC1 protein may also act as lipid receptors to mediate innate immune signal transduction pathways. One plausible target for SPLUNC1 is TLR2, which acts as a sensor for gram-positive bacteria. However, 2 observations make this unlikely. First, heterodimers of TLR2 and TLR6 directly bind to lipid substrates from gram-positive bacteria without the need for a specific lipid receptor (45). Second, our lipid-binding data showed that SPLUNC1 does not bind to LTA or polymyxin B. Nevertheless, we cannot rule out the possibility that the SPLUNC1 protein may bind to other lipid candidates from gram-positive bacteria. Furthermore, it is also possible that SPLUNC1 may bind to lipids from yeast or fungi so as to trigger other TLR signal pathways. This is an interesting topic needing further exploration.
The inability of SPLUNC1 protein derived from human BAL fluid to bind to LPS could result from the protein being fully occupied by endogenous lipids (30). However, several lines of evidence generated from our experiments demonstrate that LPS does not bind to SPLUNC1 protein. First, LPS did not occupy the lipid-binding site of SPLUNC1 protein derived from gram-negative bacteria (E. coli), which contain LPS within the membrane. Second, SPLUNC1 protein derived from HEK 293 cells was fully occupied by SMs and PCs. In vitro lipid-binding assays showed that only DPPC from the PC family bound to SPLUNC1. Notably, BPI derived from CHO cells is fully occupied only by DSPC (45, 52), which does not show any binding affinity to SPLUNC1 in our assays (Fig. 4A). This finding suggests different binding properties between BPI and SPLUNC1. Third, the negatively charged structural surface of SPLUNC1 suggests that SPLUNC1 should bind to lipids other than LPS. Finally and most importantly, we did not detect any direct binding between SPLUNC1 and lipid A/rough LPS/smooth LPS (Fig. 4B, C), whereas our positive control protein surfactant A protein bound to lipid A and rough LPS but not smooth LPS as previously reported (Supplemental Fig. S3A). To avoid potentially artificial interference introduced by fusion protein MBP and GST in the binding assays, we found that full-length SPLUNC1 without MBP or GST gives us the same binding property as those of fusion proteins (Supplemental Fig. S3B). It is not clear why our data are contradictory to those from other groups, who observed direct binding between SPLUNC1 and LPS. It is possible that the ∼20 residues (residues from A19−L42) at the N terminus, which is highly hydrophobic, could be the main cause of the discrepancy. We found that the full-length SPLUNC1 protein has strong nonspecific binding to ELISA plates. A His-tagged version of the full-length SPLUNC1 was easily attached tightly to the plate surface. Our MBP-SPLUNC1 fusion proteins, with or without the truncation of the N terminus, resolved this nonspecific binding problem (Fig. 4B). Our parallel experiments using GST-SPLUNC1 fusion proteins showed binding properties of SPLUNC1 exactly the as same as all lipids tested in our experiments (Fig. 4C), further supporting our conclusions.
The strong binding affinity of the SPLUNC1 protein to SMs and DPPC but not to LPS or other PC members raises the question of what other lipids SPLUNC1 could bind to and what the cognate lipid ligands for the protein is. To this end, we performed a systemic screening of lipid ligands for SPLUNC1 and found that SPLUNC1 protein did not bind to endotoxin lipid candidates from either gram-negative (LPS) or gram-positive bacteria (LTA). SPLUNC1 protein did not bind to other mammalian lipids including DOPE, DOPA, POPG, PI, PS, cardiolipin, ceramide, and galactosylceramide. On analysis of these lipids, we found some interesting features that may be determinants of the specificity to SPLUNC1. We found that a phosphocholine headgroup on the lipid is essential for specificity. For example, all SM species have a ceramide core, but galactosylceramide and ceramide did not bind to SPLUNC1. We reason that the positive charge from the choline group could interact with the negatively charged patch on the surface of SPLUNC1. Besides the headgroup, the fatty acid chains also appear to play critical roles in determining the binding of lipids to SPLUNC1. This conclusion is based on the fact that POPC, which contains the phosphocholine group, 1 saturated fatty acid chain, and 1 nonsaturated fatty acid chain, does not bind to SPLUNC1. In contrast, DPPC, which contains 2 saturated and short fatty acid chains, binds to SPLUNC1. The question is how to explain the unsaturated PC components [(PC(34:1), PC(36:1), and PC(40:4)] derived from the 293 cell samples of SPLUNC1. As mentioned earlier, full-length SPLUNC1 contains a large portion of hydrophobic residues, and it is hard to obtain pure full-length SPLUNC1 protein from different resources. The sample we obtained from the HEK 293 cell lines was only ∼60−80% pure (Supplemental Fig. S3C). We surmise that all nonsaturated PC components are nonspecific contamination. Notably, all species of SMs identified from the HEK 293 cell samples contained saturated fatty acid chains including SM(16:0), SM(18:0), and SM(22:0) except SM(22:1) and SM(24:1). We reason that SM(22:1) and SM(24:1) were a result of contamination, similar to most PC components derived from the HEK 293 cell sample. To further determine whether any PC that contains saturated fatty acid chains will bind to SPLUNC1, we introduced DSPC (Fig. 4A), which is the main component within BPI samples derived from CHO cell lines (52). Notably, DSPC, which contains 2 saturated fatty acid chains (18:0), bound to BPI but not to SPLUNC1. This suggests that the length of the 2 fatty acid chains is also a determinant for SPLUNC1 specificity (only 16 carbons for DPPC), although bovine SM (rich with 23:0, which has one 23-carbon fatty acid chain) could bind SPLUNC1. It may suggest that the length of 1 of 2 fatty acid chains is specific, whereas the other is not. This would be a logical scenario, because of the limited depth of the pocket to accommodate one of the fatty chains, whereas there could be more room for the other within SPLUNC1.
The identity of the cognate lipid ligands for SPLUNC1 is an obvious remaining question. Based on our in vivo data and our in vitro binding assays, we expect that SPLUNC1 may have multiple lipid ligands, including those from the SM family, DPPC, and other PC family members. It has been found that SPLUNC1 is enriched in the upper airways, but it also exists in the trachea, bronchi, and lung (4). PC comprises almost 80% of the total lipids of pulmonary surfactant, almost half of which are DPPC (47). The distributions of SPLUNC1 and DPPC appear not to overlap exactly. This raises the question of whether there are other lipids in the airway that act as cognate lipid ligands for SPLUNC1. However, our TLC experiment showed that BAL fluids from both human and mouse contained only DPPC that bound specifically to SPLUNC1 (Fig. 5A). Lipid competing binding experiments further showed that DPPC inhibited the binding of SPLUNC1 to immobilized SM, suggesting that both SM and DPPC have the same binding site on SPLUNC1 (Fig. 5B). One explanation is that although DPPC is highly concentrated in the alveolar compartment, the mucociliary escalator can carry surfactant constituents up into the airways, and this may be an adequate source of DPPC to provide a ligand for SPLUNC1. The amount of DPPC in the airway may be low compared with the content of DPPC in the lung, but it may still be enough to saturate SPLUNC1. In this regard, we speculate that SPLUNC1 could be occupied in vivo mainly by DPPC and minorly by SMs, in contrast to samples obtained from HEK 293 cells.
It is well accepted that DPPC is the strongest in vivo surfactant molecule in the airway surfactant mixture and plays critical roles in preventing fluid accumulation and keeping lung airways dry. We are inclined to speculate that one of the main functional roles of SPLUNC1 is to recruit DPPC, SM, and other lipids in the airway or that SPLUNC1 is recruited or constrained by these surfactant lipids. It is also possible that DPPC may act as a host ligand that serves this function until it is displaced by a microbial ligand. There is clear evidence in the literature that SPLUNC1 can exert antimicrobial properties, which could be due to SPLUNC1 releasing DPPC and binding to microbial lipids. Alternatively, the host lipid ligand may enable SPLUNC1 to assume a conformation that is required for its antimicrobial activity. Notably, Gakhar and colleagues (13, 14) reported that the SPLUNC1 protein interferes with biofilm formation in bacteria. Recently, it has been reported that 2 salt bridges within the SPLUNC1 act as pH sensors to regulate the function of ENaC (20). However, residues involved in the formation of the 2 salt bridges are the least conserved within the members of the PLUNC family. A possible scenario is that the total surfactant mixture, including SPLUNC1, other PLUNC family members, all lipids, and other surfactant proteins in the airways, work in concert to prevent fluid accumulation and bacterial biofilm formation and to provide other innate immune defense functions for the host. The specific interactions among these surfactant components are probably essential for maintaining the precise makeup of the surfactant mixture.
Supplementary Material
Acknowledgments
The authors thank the Howard Hughes Medical Institute, the Zuckerman/Canyon Ranch, and Alan Lapporte for supporting their X-ray and computing facilities; Dr. Philippa C. Marrack, Dr. John Kappler, Dr. Lei Yin, Dr. Laurel Lenz, and other researchers at National Jewish for their kind support; and Dr. Jennifer Kemp for editing. All datasets were collected from Howard Hughes Medical Institute beamlines 8.2.1 and 8.2.2 at the Advanced Light Source (Berkeley, CA, USA).
This work was supported by the U.S. National Institutes of Health (grants HL088264 to H.W.C., AI109219 to G.Z, and GM80719 and AI22295 to P.M.) and by an intramural grant (2Z03300) from the Korea Institute of Science and Technology.
The structure factors and coordinates have been deposited in PDB (identification codes 4KEG and 4N4X).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BAL
- bronchiolar lavage
- BPI
- bactericidal/permeability-increasing protein
- BSA
- bovine serum albumin
- CHO
- Chinese hamster ovary
- CID
- collision-induced dissociation
- DOPA
- 1,2-dioleoyl-sn-glycero-3-phosphate
- DOPE
- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DPPC
- dipalmitoylphosphatidylcholine
- DSPC
- 1,2-distearoyl-sn-glycero-3-phosphocholine
- ELISA
- enzyme-linked immunosorbent assay
- ENaC
- epithelial sodium channel
- GST
- glutathione transferase
- HEK
- human embryonic kidney
- HPLC
- high-performance liquid chromatography
- HRP
- horseradish peroxidase
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- LPS
- lipolysaccharide
- LTA
- lipoteichoic acid
- MBP
- maltose-binding protein
- PC
- phosphatidylcholine
- PDB
- Protein Data Bank
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PLUNC
- palate, lung, and nasal epithelium clone
- POPC
- 1-palmitoyl-2-oleoyl-sn-phosphatidylcholine
- POPG
- 1-palmitoyl-2-oleoyl-sn-glycero-3
- PS
- phosphatidylserine
- RMSD
- root mean standard deviation
- SAD
- single-wavelength anomalous dispersion
- SeMet
- selenomethionine
- SM
- sphingomyelin
- SPLUNC1
- short palate, lung and nasal epithelial clone 1
- TBS
- Tris-buffered saline
- TLC
- thin-layer chromatography
- TLR
- Toll-like receptor
REFERENCES
- 1. Bingle C. D., Bingle L. (2000) Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim. Biophys. Acta 1493, 363–367 [DOI] [PubMed] [Google Scholar]
- 2. Kopec K. O., Alva V., Lupas A. N. (2011) Bioinformatics of the TULIP domain superfamily. Biochem. Soc. Trans. 39, 1033–1038 [DOI] [PubMed] [Google Scholar]
- 3. Bingle L., Bingle C. D. (2011) Distribution of human PLUNC/BPI fold-containing (BPIF) proteins. Biochem. Soc. Trans. 39, 1023–1027 [DOI] [PubMed] [Google Scholar]
- 4. Zhou H. D., Fan S. Q., Zhao J., Huang D. H., Zhou M., Liu H. Y., Zeng Z. Y., Yang Y. X., Huang H., Li X. L., Shen S. R., Li G. Y. (2006) Tissue distribution of the secretory protein, SPLUNC1, in the human fetus. Histochem. Cell Biol. 125, 315–324 [DOI] [PubMed] [Google Scholar]
- 5. Weston W. M., LeClair E. E., Trzyna W., McHugh K. M., Nugent P., Lafferty C. M., Ma L., Tuan R. S., Greene R. M. (1999) Differential display identification of plunc, a novel gene expressed in embryonic palate, nasal epithelium, and adult lung. J. Biol. Chem. 274, 13698–13703 [DOI] [PubMed] [Google Scholar]
- 6. Bingle C. D., Bingle L., Craven C. J. (2011) Distant cousins: genomic and sequence diversity within the BPI fold-containing (BPIF)/PLUNC protein family. Biochem. Soc. Trans. 39, 961–965 [DOI] [PubMed] [Google Scholar]
- 7. Di Y. P. (2011) Functional roles of SPLUNC1 in the innate immune response against gram-negative bacteria. Biochem. Soc. Trans. 39, 1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartlett J. A., Gakhar L., Penterman J., Singh P. K., Mallampalli R. K., Porter E., McCray P. B., Jr. (2011) PLUNC: a multifunctional surfactant of the airways. Biochem. Soc. Trans. 39, 1012–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou H. D., Li G. Y., Yang Y. X., Li X. L., Sheng S. R., Zhang W. L., Zhao J. (2006) Intracellular co-localization of SPLUNC1 protein with nanobacteria in nasopharyngeal carcinoma epithelia HNE1 cells depended on the bactericidal permeability increasing protein domain. Mol. Immunol. 43, 1864–1871 [DOI] [PubMed] [Google Scholar]
- 10. Zhou H. D., Wu M. H., Shi L., Zhou M., Yang Y. X., Zhao J., Deng T., Li X. L., Sheng S. R., Li G. Y. (2006) Effect of growth inhibition of the secretory protein SPLUNC1 on Pseudomonas aeruginosa. Zhong Nan Da Xue Xue Bao Yi Xue Ban 31, 464–469 [PubMed] [Google Scholar]
- 11. Zhou H. D., Li X. L., Li G. Y., Zhou M., Liu H. Y., Yang Y. X., Deng T., Ma J., Sheng S. R. (2008) Effect of SPLUNC1 protein on the Pseudomonas aeruginosa and Epstein-Barr virus. Mol. Cell. Biochem. 309, 191–197 [DOI] [PubMed] [Google Scholar]
- 12. McGillivary G., Bakaletz L. O. (2010) The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One 5, e13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartlett J. A., Gakhar L., Penterman J., Singh P. K., Mallampalli R. K., Porter E., McCray P. B., Jr. (2011) PLUNC: a multifunctional surfactant of the airways. Biochem. Soc. Trans. 39, 1012–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gakhar L., Bartlett J. A., Penterman J., Mizrachi D., Singh P. K., Mallampalli R. K., Ramaswamy S., McCray P. B., Jr. (2010) PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS One 5, e9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukinskiene L., Liu Y., Reynolds S. D., Steele C., Stripp B. R., Leikauf G. D., Kolls J. K., Di Y. P. (2011) Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J. Immunol. 187, 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gally F., Di Y. P., Smith S. K., Minor M. N., Liu Y., Bratton D. L., Frasch S. C., Michels N. M., Case S. R., Chu H. W. (2011) SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am. J. Pathol. 178, 2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sayeed S., Nistico L., St Croix C., Di Y. P. (2013) Multifunctional role of human SPLUNC1 in Pseudomonas aeruginosa infection. Infect. Immun. 81, 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaillard E. A., Kota P., Gentzsch M., Dokholyan N. V., Stutts M. J., Tarran R. (2010) Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflügers Arch. 460, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hobbs C. A., Blanchard M. G., Kellenberger S., Bencharit S., Cao R., Kesimer M., Walton W. G., Redinbo M. R., Stutts M. J., Tarran R. (2012) Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways. FASEB J. 26, 4348–4359 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Garland A. L., Walton W. G., Coakley R. D., Tan C. D., Gilmore R. C., Hobbs C. A., Tripathy A., Clunes L. A., Bencharit S., Stutts M. J., Betts L., Redinbo M. R., Tarran R. (2013) Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 110, 15973–15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chu H. W., Thaikoottathil J., Rino J. G., Zhang G., Wu Q., Moss T., Refaeli Y., Bowler R., Wenzel S. E., Chen Z., Zdunek J., Breed R., Young R., Allaire E., Martin R. J. (2007) Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J. Immunol. 179, 3995–4002 [DOI] [PubMed] [Google Scholar]
- 22. Chu H. W., Gally F., Thaikoottathil J., Janssen-Heininger Y. M., Wu Q., Zhang G., Reisdorph N., Case S., Minor M., Smith S., Jiang D., Michels N., Simon G., Martin R. J. (2010) SPLUNC1 regulation in airway epithelial cells: role of Toll-like receptor 2 signaling. Respir. Res. 11, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thaikoottathil J. V., Martin R. J., Di P. Y., Minor M., Case S., Zhang B., Zhang G., Huang H., Chu H. W. (2012) SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice. Am. J. Respir. Cell Mol. Biol. 47, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiss J. (2003) Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against gram-negative bacteria. Biochem. Soc. Trans. 31, 785–790 [DOI] [PubMed] [Google Scholar]
- 25. Balakrishnan A., Marathe S. A., Joglekar M., Chakravortty D. (2013) Bactericidal/permeability increasing protein: A multifaceted protein with functions beyond LPS neutralization. Innate Immun. 19, 339–347 [DOI] [PubMed] [Google Scholar]
- 26. Elsbach P., Weiss J. (1998) Role of the bactericidal/permeability-increasing protein in host defence. Curr. Opin. Immunol. 10, 45–49 [DOI] [PubMed] [Google Scholar]
- 27. LeClair E. E. (2003) Four reasons to consider a novel class of innate immune molecules in the oral epithelium. J. Dent. Res. 82, 944–950 [DOI] [PubMed] [Google Scholar]
- 28. Schumann R. R. (2011) Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem. Soc. Trans. 39, 989–993 [DOI] [PubMed] [Google Scholar]
- 29. Ghafouri B., Kihlstrom E., Tagesson C., Lindahl M. (2004) PLUNC in human nasal lavage fluid: multiple isoforms that bind to lipopolysaccharide. Biochim. Biophys. Acta 1699, 57–63 [DOI] [PubMed] [Google Scholar]
- 30. Campos M. A., Abreu A. R., Nlend M. C., Cobas M. A., Conner G. E., Whitney P. L. (2004) Purification and characterization of PLUNC from human tracheobronchial secretions. Am. J. Respir. Cell Mol. Biol. 30, 184–192 [DOI] [PubMed] [Google Scholar]
- 31. Liu Y., Manna A., Li R., Martin W. E., Murphy R. C., Cheung A. L., Zhang G. (2001) Crystal structure of the SarR protein from Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 98, 6877–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otwinowski Z., Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 33. Pape T., Schneider T. R. (2004) HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Cryst. 37, 843–844 [Google Scholar]
- 34. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 36. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 37. Chen P., Toribara T. Y., Warner H. (1956) Microdetermination of phosphorus. Anal. Chem. 28, 1756–1758 [Google Scholar]
- 38. Liebisch G., Vizcaino J. A., Kofeler H., Trotzmuller M., Griffiths W. J., Schmitz G., Spener F., Wakelam M. J. (2013) Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 54, 1523–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiba H., Pattanajitvilai S., Evans A. J., Harbeck R. J., Voelker D. R. (2002) Human surfactant protein D (SP-D) binds Mycoplasma pneumoniae by high affinity interactions with lipids. J. Biol. Chem. 277, 20379–20385 [DOI] [PubMed] [Google Scholar]
- 40. Piboonpocanun S., Chiba H., Mitsuzawa H., Martin W., Murphy R. C., Harbeck R. J., Voelker D. R. (2005) Surfactant protein A binds Mycoplasma pneumoniae with high affinity and attenuates its growth by recognition of disaturated phosphatidylglycerols. J. Biol. Chem. 280, 9–17 [DOI] [PubMed] [Google Scholar]
- 41. Rouser G., Siakotos A. N., Fleischer S. (1966) Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids 1, 85–86 [DOI] [PubMed] [Google Scholar]
- 42. Bingle C. D., Seal R. L., Craven C. J. (2011) Systematic nomenclature for the PLUNC/PSP/BSP30/SMGB proteins as a subfamily of the BPI fold-containing superfamily. Biochem. Soc. Trans. 39, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharff A. J., Rodseth L. E., Spurlino J. C., Quiocho F. A. (1992) Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis. Biochemistry 31, 10657–10663 [DOI] [PubMed] [Google Scholar]
- 44. Hasegawa H., Holm L. (2009) Advances and pitfalls of protein structural alignment. Curr. Opin. Struct. Biol. 19, 341–348 [DOI] [PubMed] [Google Scholar]
- 45. Beamer L. J., Carroll S. F., Eisenberg D. (1997) Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 276, 1861–1864 [DOI] [PubMed] [Google Scholar]
- 46. Pulfer M., Murphy R. C. (2003) Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 22, 332–364 [DOI] [PubMed] [Google Scholar]
- 47. Willson D. F., Notter R. H. (2011) The future of exogenous surfactant therapy. Respir. Care 56, 1369–1386; discussion 1386–1368 [DOI] [PubMed] [Google Scholar]
- 48. Mueller G. A., Edwards L. L., Aloor J. J., Fessler M. B., Glesner J., Pomes A., Chapman M. D., London R. E., Pedersen L. C. (2010) The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J. Allergy Clin. Immunol. 125, 909–917.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bingle C. D., Seal R. L., Craven C. J. Systematic nomenclature for the PLUNC/PSP/BSP30/SMGB proteins as a subfamily of the BPI fold-containing superfamily. Biochem. Soc. Trans. 39, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trompette A., Divanovic S., Visintin A., Blanchard C., Hegde R. S., Madan R., Thorne P. S., Wills-Karp M., Gioannini T. L., Weiss J. P., Karp C. L. (2009) Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kang J. Y., Nan X., Jin M. S., Youn S. J., Ryu Y. H., Mah S., Han S. H., Lee H., Paik S. G., Lee J. O. (2009) Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31, 873–884 [DOI] [PubMed] [Google Scholar]
- 52. Kleiger G., Beamer L. J., Grothe R., Mallick P., Eisenberg D. (2000) The 1.7 A crystal structure of BPI: a study of how two dissimilar amino acid sequences can adopt the same fold. J. Mol. Biol. 299, 1019–1034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.