Abstract
Purpose of review
Despite the benefits of rapidly advancing therapeutic and diagnostic possibilities, the perioperative setting still exposes patients to significant risks of adverse events and harm. Anesthesiologists are in midstream of perioperative care and can make significant contributions to patient safety and patient outcomes. This article reviews recent research results outlining the current trends of perioperative patient harm and summarizes the evidence in favor of patient safety practices.
Recent findings
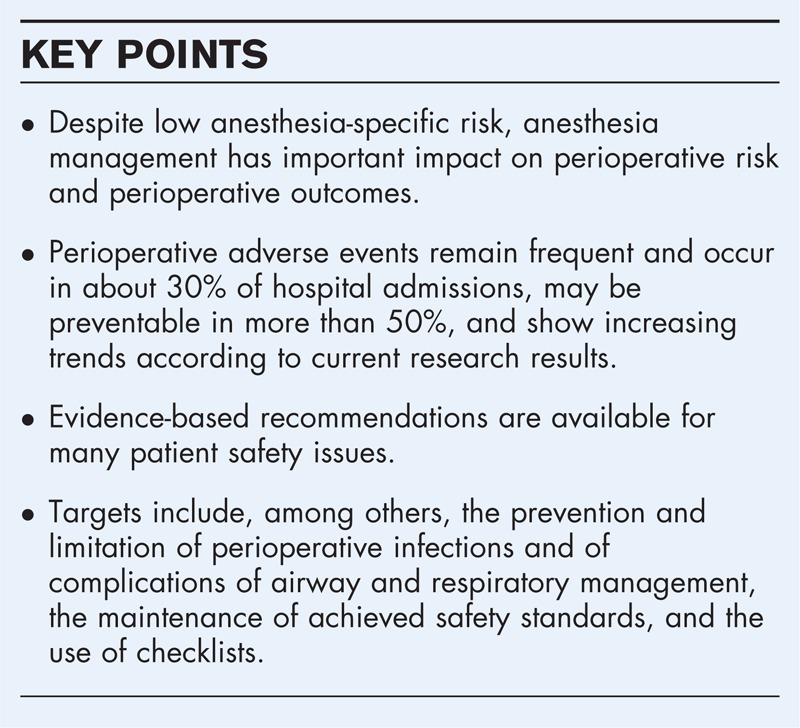
Adverse events and patient harm continue to be frequent in the perioperative period. Adverse events occur in about 30% of hospital admissions, are associated with higher mortality, and may be preventable in more than 50%. Evidence-based recommendations are available for many patient safety issues. No magic bullet practices exist, but promising targets include the prevention and limitation of perioperative infections and of complications of airway and respiratory management, the maintenance of achieved safety standards, the use of checklists, and others.
Summary
Current research provides growing evidence for the effectiveness of several patient safety practices designed to prevent or diminish perioperative adverse events and patient harm. Future investigations will hopefully fill the numerous persisting knowledge gaps.
Keywords: adverse events, anesthesia, patient safety, perioperative management, safety practice
INTRODUCTION
Anesthesiologists have been among the very pioneers of patient safety. According to a definition by Charles Vincent, patient safety is ‘the avoidance, prevention and amelioration of adverse outcomes or injuries stemming from the process of healthcare’ (p. 31) [1], rather than from the patient's underlying medical condition [2]. During the last decades, the risks associated with anesthetic care have been dramatically reduced [3]. Yet anesthesia takes center stage among acute healthcare services, and from a patient's perspective, anesthesia-specific risks cannot be meaningfully isolated from perioperative and peri-interventional risks. In this article, we review the current epidemiological scale of patient safety impairments in adult noncardiac anesthesia and perioperative care, and the evidence supporting interventions to prevent or reduce them. The emphasis of this review is placed on safety issues with increasing occurrence, which can be addressed by effective strategies.
Box 1.
no caption available
TWO DECADES OF STAGGERED PROGRESS IN ELUCIDATING PATIENT HARM
According to the seminal report ‘To err is human’ by the US-based Institute of Medicine in 2000, adverse events occur in 2.9–3.7% of hospital admissions, with 6.6–13.6% of adverse events leading to death. On the basis of these numbers, the report estimated 44 000–98 000 deaths per year due to medical errors in US hospitals, with 50% of these adverse events considered to be preventable [4]. A decade later, however, and using more sensitive research methods such as the ‘Global Trigger Tool (GTT)’, adverse events were estimated to occur 10 times more often, and in 30% of hospital admissions [5]. Adverse events were not declining during the period from 2002 to 2007 [6]. Even worse, more than 400 000 deaths per year were estimated to be due to preventable adverse events according to a review of studies from 2008 to 2011 [7▪▪].
NO REASON FOR COMPLACENCY: RECENT DATA ON PERIOPERATIVE PATIENT HARM
Even more recently, perioperative adverse events do not seem to decline. Analyses of large national US databases identified increasing trends of major in-hospital complications despite decreasing in-hospital mortality for patients undergoing total knee and hip arthroplasties (1998–2008) [8], and for major cancer surgery [9,10]. Because of shorter hospital stays, some of the mortality may have shifted to intermediate care facilities [8]. Importantly, most types of these adverse events may be influenced by anesthetic and perioperative care. Further, adverse events were also found in 38.1% of hospital discharges from 2007 to 2011 [11]. Adverse events related to surgery represented the largest category (40.5%) [11], and most were preventable. Moreover, in a recent US study of the records of over 60 000 Medicare patients (2005–2011) [12▪▪], overall trends for 21 different adverse event measures were declining for patients with acute myocardial infarction and congestive heart failure, but not with pneumonia or conditions requiring surgery [12▪▪]. Adverse events in surgical patients were significantly increasing over the study period, occurred in 36.8% of hospitalizations [12▪▪], and were associated with a significantly higher risk of death as compared with patients without adverse events [12▪▪]. Interestingly, postoperative cardiac events and venous thromboembolic events were significantly declining over the study period, potentially due to extensive cardiovascular quality improvement campaigns in the US [12▪▪]. In contrast, rates for events associated with intravenous heparin as well as for postoperative pneumonia increased significantly [12▪▪].
EUROPEAN PERSPECTIVE ON PERIOPERATIVE PATIENT HARM
Recently, a Swedish study using the GTT confirmed these US findings [13▪]. Adverse events, preventable in 71%, were identified in 20.5% of discharges [13▪], and no decline was noted over 4 years [13▪]. Hospital-acquired infections, almost all preventable, represented 47% of adverse events in surgical patients [13▪]. Furthermore, the European Surgical Outcomes Study documented an unexpectedly high mean surgical mortality of 4% before discharge, with pronounced disparities between 28 European countries [14]. In addition, a systematic review found – mostly preventable – surgical and anesthetic adverse events to contribute to 19.3–52.2% of unplanned ICU transfers [15].
ANESTHESIA-SPECIFIC MORTALITY: HIGHER THAN WE THOUGHT?
The anesthesia-specific mortality has been substantially reduced over the last decades [3] and is estimated to be overall about 1/100 000 cases [16]. A recent analysis of a large German national surveillance database identified a risk of 10 per million anesthetics for death or other serious complications from anesthesia in a sample of American Society of Anesthesiologists (ASA) class I and II patients [17▪]. This number approaches the previous estimate of overall anesthesia-specific risk [17▪]. Forty percent of anesthesia-related deaths were due to airway problems [17▪]. If higher ASA classes have higher risks, and the proportion of multimorbid surgical patients with higher ASA class is increasing, the findings of this study suggest a higher overall anesthesia-specific mortality than previously estimated [16,18]. Furthermore, according to the Fourth National Audit Project (NAP4) in the UK, 5.4 deaths per million general anesthetics were estimated to result from airway complications. Because of underreporting, true mortality rate may have been four times higher in this population [19], and also substantially higher in the German study population [17▪]. Anyway, the true airway-related mortality according to NAP4 (about 20 deaths per million general anesthetics) would readily be double of the traditional all-cause anesthesia-specific mortality of 1 : 100 000. Furthermore, future investigation of long-lasting effects of anesthesia-related perioperative hypotension on long-term mortality may question the previous estimate of anesthesia-related risk [20]. From a patient's perspective, however, overall perioperative risk seems more important than speciality-specific risk.
CAN WE TRUST THE DATA?
Data from routine quality or voluntary adverse event reporting should be interpreted cautiously, as underreporting is common and may be quite pronounced (reporting bias). For example, only 5 [11] to 6.3% [13▪] of adverse events identified with the GTT were also reported with voluntary routine reporting instruments. In contrast to systematic routine reporting, voluntary reporting systems for adverse events or critical incidents cannot be used to measure error or event rates, to compare organizations, or to measure changes over time [21]. However, they are important to understand the nature of events and to analyze root causes.
PERIOPERATIVE SAFETY AS A SHARED RESPONSIBILITY OF SURGERY AND ANESTHESIOLOGY
Both surgery and anesthesia contribute to patient harm in the perioperative period. Accordingly, perioperative harm should largely be considered a shared responsibility of surgery and anesthesia. Obviously, patient safety practices should target avoidable patient harm and should be assessed, irrespective of the discipline, as carefully as any other healthcare intervention regarding their effectiveness, potential direct and indirect undesired effects, and cost-effectiveness. A review supported by the US Department of Health and Human Services’ Agency for Healthcare Research and Quality examined 41 patient safety practices. Ten practices were ‘strongly encouraged’, and additional 12 practices were ‘encouraged’ for adoption [22▪▪,23▪]. A selection of practices relevant for increasing safety issues in perioperative care is presented in Table 1[24–32,33▪▪,34,35].
Table 1.
Safety practices encouraged by the Agency for Healthcare Research and Quality for the prevention of increasing substantial perioperative safety issues
| Patient safety issue | Rate (percentage of hospitalizations at risk) | Encouraged safety practice [23▪] | Advantages | Problems (all: varying implementation problems) |
| Clinical issues | ||||
| Ventilator-associated pneumonia | 10.6% [12▪▪] | Bundles: head-of-bed elevation, sedation vacations (holds), oral care with chlorhexidine, and subglottic-suctioning endotracheal tubes (++) [24] | Evidence for effectiveness (evidence): moderate to high (as bundle: synergism)[24] | (Low to) moderate costs [24] |
| Catheter-associated urinary tract infections | 3.7% [12▪▪] | Interventions to reduce urinary catheter use: catheter reminders, stop orders, or nurse-initiated removal protocols (++) [25] | Evidence: moderate to high; low cost [25] | Low risk: premature removal [25] |
| Healthcare-associated infections (HAI) in surgery | Specific fields: 10.5% [10] | Hand hygiene (++) [26]; Barrier precautions, patient isolation, and routine surveillance (++) [27] | Low evidence for harm, low cost [26]; Evidence: moderate [27] | Low strength of evidence for effectiveness [26]; Moderate evidence for harm (contact isolation)[27]; Moderate-to-high cost |
| Central catheter-associated mechanical complications | 3.5% [12▪▪] | Use of real-time ultrasonography for central line placement (++) [28] | Evidence: strong; negligible harm [28] | Moderate cost [28] |
| System issues | ||||
| Adverse events per hospitalization | 36.8% [12▪▪] | Preoperative checklists and anesthesia checklists (++) [29] | Evidence: high, low cost, negligible harm [29] | Multiple implementation issues [29] |
| Rapid-response systems (+) [30] | Evidence: moderate; low harm [30] | Moderate costs [30] | ||
| Use of simulation for patient safety efforts (+) [31] | Evidence: moderate to high [31] | Moderate costs [31] | ||
| Team training (+) [32] | Evidence: moderate, low harm [32,33▪▪] | Impl. moderate to difficult; moderate costs [32] | ||
| Monitoring patient safety problems (e.g., chart reviews; critical incident reporting systems) (+) [34] | Negligible harm [34] | Evidence low; high costs [34] | ||
| Outcome measurements (+) [35] | Evidence: moderate to high, low harm [33▪▪,35] | Moderate costs [35] | ||
This selection presents patient safety issues with the following characteristics: first, they are relevant for anesthesia and perioperative management; second, they have increasing trends of occurrence despite evidence for their partial or extensive preventability; third, safety practices supported by sufficient evidence exist for their prevention. Agency for Healthcare Research and Quality (AHRQ), Department of Health and Human Services, USA. (++) = strongly encouraged; (+) = encouraged practice. Data origin: see references [12▪▪,22▪▪,23▪].
POSTOPERATIVE INFECTIONS: A TARGET FOR ANESTHETIC AND PERIOPERATIVE SAFETY EFFORTS
Postoperative infections remain an area of concern in surgical patients, despite evidence for decreasing mortality from infectious complications [10,12▪▪,13▪]. Increasing trends were noted for postoperative pneumonia, catheter-associated urinary tract infections, ventilator-associated pneumonia [12▪▪], and bloodstream infections and sepsis [8–10]. The rate of postoperative pneumonia has been significantly increasing to 3.3% from 2005 to 2011 [12▪▪]. Evidence for effectiveness to reduce postoperative pneumonia has been found for a multidisciplinary, multiform pulmonary care program [36] and for neuraxial blocks when used instead of general anesthesia or in combination [37]. Surgical site infections (SSIs) have been reported to occur in 13.5% of surgical patients [38▪]. Effective strategies to reduce SSI include timely administration of the correct prophylactic antibiotic, maintenance of normothermia, hand hygiene, and bundles to prevent venous access infections, checklists, and standardization [38▪]. The evidence supporting effectiveness of intraoperative hyperoxia is conflicting [38▪].
Bloodstream infections and sepsis are increasing [8], and may affect more than 2.5% [9] of major cancer surgery patients. General preventive strategies are presented in Table 1. Central line-associated bloodstream infections have been successfully reduced by a number of safety practices, approaches, and technologies [39].
PERIOPERATIVE RESPIRATORY AND AIRWAY-RELATED COMPLICATIONS
The NAP4 project in the UK provided an occurrence estimate of severe airway complications [19]. Airway management was judged poor in three-quarters of cases, indicating room for improvement [19]. This audit reveals important aspects of patient safety. Despite challenging situations, no ‘plan B’ had been made in most cases. Moreover, well proven technology (e.g., fiberoptic bronchoscopy, capnography) had been disregarded, and well established protocols for airway management had not been followed [19]. Furthermore, difficult intubations as well as aspirations have increasing shares among US closed claim cases. Difficult airway situations occur beyond induction of anesthesia during surgery, during extubation, and during recovery [40]. From closed claims analysis, it is also concluded that a surgical airway should be attempted early in difficult airways, that laryngeal mask airway should not be regarded as a fail safe, and that extubation of the difficult airway remains a significant patient safety issue [40]. Furthermore, residual neuromuscular paralysis occurs in about 30% of patients at risk [41], may be clinically inapparent but associated with increased risk of postoperative adverse events [42], exposes patients to respiratory complications in up to 20%, and can be easily prevented [41]. Careful neuromuscular monitoring [adductor pollicis (thumb), not eyebrow] is more important than indiscriminate use of reversal agents. Sugammadex has been associated with adverse effects, such as allergic reactions, transient increase in prothrombin time, and reparalysis in 2% [41].
INTRAOPERATIVE HYPOTENSION AND ORGAN INJURY
Analysis of a large perioperative database suggested an association between intraoperative hypotension below 55 mmHg mean arterial pressure even for short periods of 1–5 min and the occurrence of postoperative acute kidney injury and myocardial injury [43]. Hypotension has also been associated with the perioperative occurrence of stroke [44▪]. Although no absolute and generalizable lower limit of systemic blood pressure to avoid organ injuries has been defined yet, clinical working definitions are widely used and often represent institutional standards [45]. Some problems interfering with the realization of such standard hemodynamic goals in daily practice may impair patient safety. For example, hemodynamic adverse events are frequent during in-hospital transfers of complex, critically ill patients [46]. Furthermore, unrecognized or inadequately managed drug combinations leading to interactions in the perioperative period may result in severe intraoperative hypotension [47]. In addition, the beach chair (semi-recumbent) position used for shoulder surgery has been associated with risk of perioperative stroke [44▪,48]. Failure to consider the hydrostatic pressure gradient resulting from head elevation in this position when interpreting blood pressure measurements has been proposed as one reason for inadequate intraoperative cerebral perfusion contributing to perioperative stroke [48,49]. Other examples of adverse process-related impact on the clinical management of hemodynamic events include the temporary silencing of audible alarms [50] and distractions or interruptions that may result in delayed or impaired patient care [51–53].
MAINTAINING ESTABLISHED SAFETY STANDARDS
Decreasing trends of some safety issues may be due to successful implementation of effective strategies, or to shifts in their occurrence. For instance, postoperative cardiac events and thromboembolic events have significantly declined from 2005 to 2011 [12▪▪]. Despite decreasing trends, the absolute number and severity of these complications mandate constant vigilance. For clinicians, it will thus be important to retain what has been gained. In the face of limited resources and increasing production pressure [54▪▪], prioritization of emerging safety standards should be accompanied by active upkeep of established, successful safety practices that may otherwise be gradually downgraded [54▪▪,55–57].
SAFER SURGERY CHECKLIST: CHECK, BUT HOW?
Individual studies [29,58,59], reviews [60], and a number of systematic reviews have documented the efficacy of surgical checklists to improve surgical mortality and morbidity [61,62▪,63▪▪,64], teamwork and communication in the operating room [62▪,63▪▪,65,66], and compliance with safety measures [62▪,63▪▪]. Surgical checklists have been widely recommended [18] and adopted [29]. Some inconsistent evidence exists about implementation and effectiveness in routine practice and disparate clinical settings [60]. Interestingly, a number of studies have found no or only minor impact of surgical safety checklists on relevant outcomes in clinical settings with pre-existing comparatively high safety standards [67,68,69▪▪]. One explanation has been that checklist implementation in settings with pre-existing high safety standards and check systems may elicit ‘checklist fatigue’ and promote barriers [60]. Another explanation may be confounding, that is, that the benefits documented with use of the checklist are not resulting from the checklist as such, but from concomitant positive effects of implementation on teamwork, communication, and safety culture [68]. Most studies about the effect of surgical checklists on outcomes then have only moderate methodological quality (pre–post evaluations) [58,69▪▪]. The methodologically best investigation, a stepped wedge cluster randomized controlled trial published a short time ago [70▪], reported significant reductions in length of hospital stay and morbidity, but failed to reproduce the significant reductions in overall mortality identified in previous studies [70▪]. From a clinician's perspective, however, it seems irrelevant if the benefits of this relatively low cost intervention are causally resulting from the checklist, or just by occasion from concomitant effects of its implementation, if these effects are lasting. It is reasonable to take advantage of these benefits, as recommended by many professional organizations [18,23▪,29] and as regulated by law in some countries [69▪▪,70▪]. Given the limited scientific evidence, future research should evaluate if the reported effects prevail over time, and investigate implementation issues in individual settings. In terms of perioperative patient safety, surgical checklists are just one piece of many in a comprehensive safety strategy and should not be regarded as a substitute for other important patient safety activities with comparable evidence for effectiveness [33▪▪].
INTERVENTIONS TO IMPROVE SAFETY CULTURE AND COMMUNICATION
Safety culture, as measured by survey-based ratings of surgeons, nurses, and operating room administrators, has been reported to be associated with rates of serious surgical complications [71▪]. Interventions to improve safety culture may target leadership, teamwork, or behavior change, examples include interdisciplinary rounding, team training, encouraging error reporting, and others [72]. Team training [32] and simulation training [31] also contribute to safety culture. Postoperative handovers are important communications, and incomplete information transfer can be hazardous [73▪▪]. No methodological approach has resulted in improving handover outcomes so far, despite evidence for improved information transfer [73▪▪]. Eventually, promising investments in future safety culture are teaching activities to foster knowledge and attitudes among healthcare trainees and medical students [74]. For this purpose, the WHO has provided teaching materials covering all relevant aspects of patient safety [75].
CONCLUSION
Anesthesia has low specific risk, but has important impact on perioperative risk and outcomes. Adverse events in the perioperative period continue to be frequent, occur in about 30% of hospital admissions, and may be preventable in more than 50%. Evidence-based recommendations are available for many patient safety practices. Important practical targets include perioperative infections, airway and respiratory management, maintenance of safety standards despite production pressure, and others. Future research should provide more high-quality evidence about the effectiveness of patient safety practices, deeper insights into common patterns of preventable events, and into implementation issues of surgical checklists and other practices.
Acknowledgements
The work for this review was funded by resources of the authors and their institutions. The authors received no other external funding or support. Assistance with manuscript formatting was obtained from American Journal Experts (AJE, Durham, North Carolina, USA;http://www.aje.com/en).
Conflicts of interest
Johannes Wacker is member of the Scientific Subcommittee Patient Safety of the European Society of Anaesthesiology (ESA), Brussels, Belgium. Sven Staender is chairman of the Patient Safety Task Force of ESA. No conflicts of interest are declared concerning this publication.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Vincent C. Patient safety. 2nd edOxford: BMJ Books; 2010. [Google Scholar]
- 2.Parry G, Cline A, Goldmann D. Deciphering harm measurement. JAMA 2012; 307:2155–2156. [DOI] [PubMed] [Google Scholar]
- 3.Bainbridge D, Martin J, Arango M, Cheng D. Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet 2012; 380:1075–1081. [DOI] [PubMed] [Google Scholar]
- 4.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 5.Classen DC, Resar R, Griffin F, et al. ’Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 2011; 30:581–589. [DOI] [PubMed] [Google Scholar]
- 6.Landrigan CP, Parry GJ, Bones CB, et al. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med 2010; 363:2124–2134. [DOI] [PubMed] [Google Scholar]
- 7▪▪.James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf 2013; 9:122–128. [DOI] [PubMed] [Google Scholar]; On the basis of four studies from 2008 to 2011 using the GTT, this study updates previous estimates of premature deaths associated with preventable harm in the United States. Such deaths were currently estimated at more than 400 000 per year.
- 8.Kirksey M, Chiu YL, Ma Y, et al. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States. Anesth Analg 2012; 115:321–327. [DOI] [PubMed] [Google Scholar]
- 9.Sukumar S, Roghmann F, Trinh VQ, et al. National trends in hospital-acquired preventable adverse events after major cancer surgery in the USA. BMJ Open 2013; 3:e002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sammon J, Trinh VQ, Ravi P, et al. Healthcare-associated infections after major cancer surgery: temporal trends, patterns of care, and effect on mortality. Cancer 2013; 119:2317–2324. [DOI] [PubMed] [Google Scholar]
- 11.Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large healthcare delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res 2014; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Wang Y, Eldridge N, Metersky ML, et al. National trends in patient safety for four common conditions. N Engl J Med 2014; 370:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important current analysis of a large national United States database indicating declining adverse-event rates among patients hospitalized for acute myocardial infarction or congestive heart failure, but not for pneumonia or conditions requiring surgery.
- 13▪.Rutberg H, Borgstedt Risberg M, Sjodahl R, et al. Characterisations of adverse events detected in a university hospital: a 4-year study using the Global Trigger Tool method. BMJ Open 2014; 4:e004879. [DOI] [PMC free article] [PubMed] [Google Scholar]; This Swedish single-center study using the GTT found adverse event rates higher than reported with voluntary reporting systems, and comparable to United States GTT studies.
- 14.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlayen A, Verelst S, Bekkering GE, et al. Incidence and preventability of adverse events requiring intensive care admission: a systematic review. J Eval Clin Pract 2012; 18:485–497. [DOI] [PubMed] [Google Scholar]
- 16.Staender SE, Mahajan RP. Anesthesia and patient safety: have we reached our limits? Curr Opin Anaesthesiol 2011; 24:349–353. [DOI] [PubMed] [Google Scholar]
- 17▪.Schiff JH, Welker A, Fohr B, et al. Major incidents and complications in otherwise healthy patients undergoing elective procedures: results based on 1.37 million anaesthetic procedures. Br J Anaesth 2014; 113:109–121. [DOI] [PubMed] [Google Scholar]; This analysis of a large German surveillance database found that for ASA class I and II patients, the risk of death or other serious complication from anaesthesia was about 10 per million anaesthetics.
- 18.Mellin-Olsen J, Staender S, Whitaker DK, Smith AF. The Helsinki declaration on patient safety in anaesthesiology. Eur J Anaesthesiol 2010; 27:592–597. [DOI] [PubMed] [Google Scholar]
- 19.Cook TM, Woodall N, Frerk C. Fourth National Audit Project Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 2011; 106:617–631. [DOI] [PubMed] [Google Scholar]
- 20.Avidan MS, Kheterpal S. Perioperative mortality in developed and developing countries. Lancet 2012; 380:1038–1039. [DOI] [PubMed] [Google Scholar]
- 21.Pham JC, Girard T, Pronovost PJ. What to do with healthcare Incident Reporting Systems. J Public Health Res 2013; 2:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.Shekelle PG, Wachter RM, Pronovost PJ, et al. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. 2013; Rockville, MD: Agency for Healthcare Research and Quality, [Google Scholar]; Comprehensive review and critical analysis of the evidence for the effectiveness of a broad range of patient safety practices. Open online access: http://www.ahrq.gov/research/findings/evidence-based-reports/ptsafetyuptp.html; all individual sections (see references) can be downloaded as single pdf files.
- 23▪.Shekelle PG, Pronovost PJ, Wachter RM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med 2013; 158:365–368. [DOI] [PubMed] [Google Scholar]; Focus article highlighting 10 patient safety strategies ‘strongly encouraged’ and 12 patient safety strategies ‘encouraged’ for adoption into clinical practice.
- 24.Winters BD, Berenholtz SM. Shekelle PG, Wachter RM, Pronovost PJ. Ventilator-associated pneumonia: brief update review. Making healthcare safer II: An updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 110–116. [Google Scholar]
- 25.Meddings J, Krein SL, Fakih MG. Shekelle PG, Wachter RM, Pronovost PJ, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infections: brief update review. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 73–87. [Google Scholar]
- 26.Pfoh E, Dy S, Engineer C. Shekelle PG, Wachter RM, Pronovost PJ. Interventions to improve hand hygiene compliance: brief update review. Making healthcare safer II: An updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 67–72. [Google Scholar]
- 27.Schweizer M. Shekelle PG, Wachter RM, Pronovost PJ. Barrier precautions, patient isolation, and routine surveillance for prevention of healthcare-associated infections: brief update review. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 55–66. [Google Scholar]
- 28.Shekelle PG, Dallas P. Shekelle PG, Wachter RM, Pronovost PJ. Use of real-time ultrasound guidance during central line insertion: brief update review. Making healthcare safer II: an updated critical analysis of the 17 evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 172–177. [Google Scholar]
- 29.Treadwell JR, Lucas S. Shekelle PG, Wachter RM, Pronovost PJ. Preoperative checklists and anesthesia checklists. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 122–139. [Google Scholar]
- 30.Winters BD, Weaver S, Dy S. Shekelle PG, Wachter RM, Pronovost PJ. Rapid-response systems (NEW). Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 257–269. [Google Scholar]
- 31.Schmidt EM, Goldhaber-Fiebert SN, Ho LA, McDonald KM. Shekelle PG, Wachter RM, Pronovost PJ. Use of simulation exercises in patient safety efforts. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 472–479. [Google Scholar]
- 32.Weaver SJ, Rosen MA. Shekelle PG, Wachter RM, Pronovost PJ. Team-training in healthcare: brief update review. (Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 472–479. [Google Scholar]
- 33▪▪.Howell AM, Panesar SS, Burns EM, et al. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg 2014; 259:630–641. [DOI] [PubMed] [Google Scholar]; Interesting systematic review of interventions to reduce adverse events in surgery. A number of interventions producing a significant decrease in surgical morbidity and mortality are identified.
- 34.Sun F. Shekelle PG, Wachter RM, Pronovost PJ. Monitoring patient safety problems (NEW). Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 405–424. [Google Scholar]
- 35.Maggard-Gibbons M. Shekelle PG, Wachter RM, Pronovost PJ. Use of report cards and outcome measurements to improve safety of surgical care: American College of Surgeons National Quality Improvement Program (NEW). Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 140–157. [Google Scholar]
- 36.Cassidy MR, Rosenkranz P, McCabe K, et al. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg 2013; 148:740–745. [DOI] [PubMed] [Google Scholar]
- 37.Guay J, Choi P, Suresh S, et al. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014; 1:CD010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Forbes SS, McLean RF. Review article: the anesthesiologist's role in the prevention of surgical site infections. Can J Anaesth 2013; 60:176–183. [DOI] [PubMed] [Google Scholar]; Review of anesthesia practices to prevent SSIs, particularly preoperative administration of antibiotics, perioperative normothermia, and perioperative hyperoxia.
- 39.Chopra V, Krein SL, Olmsted RN. Shekelle PG, Wachter RM, Pronovost PJ, et al. Prevention of central line-associated bloodstream infections: brief update review. Making healthcare safer II: an updated critical analysis 19 of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 88–109. [Google Scholar]
- 40.Metzner J, Posner KL, Lam MS, Domino KB. Closed claims’ analysis. Best Pract Res Clin Anaesthesiol 2011; 25:263–276. [DOI] [PubMed] [Google Scholar]
- 41.Donati F. Residual paralysis: a real problem or did we invent a new disease? Can J Anaesth 2013; 60:714–729. [DOI] [PubMed] [Google Scholar]
- 42.Cedborg AI, Sundman E, Boden K, et al. Pharyngeal function and breathing pattern during partial neuromuscular block in the elderly: effects on airway protection. Anesthesiology 2014; 120:312–325. [DOI] [PubMed] [Google Scholar]
- 43.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119:507–515. [DOI] [PubMed] [Google Scholar]
- 44▪.Bijker JB, Gelb AW. Review article: the role of hypotension in perioperative stroke. Can J Anaesth 2013; 60:159–167. [DOI] [PubMed] [Google Scholar]; This article reviews the role of hypotension as a possible primary or secondary factor in the develpment of perioperative stroke.
- 45.Brady K, Hogue CW. Intraoperative hypotension and patient outcome: does ‘one size fit all?’. Anesthesiology 2013; 119:495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmentier-Decrucq E, Poissy J, Favory R, et al. Adverse events during intrahospital transport of critically ill patients: incidence and risk factors. Ann Intensive Care 2013; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orser BA, Hyland S, U D, et al. Review article: improving drug safety for patients undergoing anesthesia and surgery. Can J Anaesth 2013; 60:127–135. [DOI] [PubMed] [Google Scholar]
- 48.Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth 2005; 17:463–469. [DOI] [PubMed] [Google Scholar]
- 49.Villevieille T, Delaunay L, Gentili M, Benhamou D. [Arthroscopic shoulder surgery and ischemic cerebral complications]. Ann Fr Anesth Reanim 2012; 31:914–918. [DOI] [PubMed] [Google Scholar]
- 50.Beatty PC, Beatty SF. Anaesthetists’ intentions to violate safety guidelines. Anaesthesia 2004; 59:528–540. [DOI] [PubMed] [Google Scholar]
- 51.Savoldelli GL, Thieblemont J, Clergue F, et al. Incidence and impact of distracting events during induction of general anaesthesia for urgent surgical cases. Eur J Anaesthesiol 2010; 27:683–689. [DOI] [PubMed] [Google Scholar]
- 52.Jothiraj H, Howland-Harris J, Evley R, Moppett IK. Distractions and the anaesthetist: a qualitative study of context and direction of distraction. Br J Anaesth 2013; 111:477–482. [DOI] [PubMed] [Google Scholar]
- 53.Campbell G, Arfanis K, Smith AF. Distraction and interruption in anaesthetic practice. Br J Anaesth 2012; 109:707–715. [DOI] [PubMed] [Google Scholar]
- 54▪▪.Eichhorn JH. Review article: practical current issues in perioperative patient safety. Can J Anaesth 2013; 60:111–118. [DOI] [PubMed] [Google Scholar]; Compelling review article summarizing contemporary problems of perioperative patient care. In particular, the article points to the threat of loosing safety gains achieved over decades because of complacency.
- 55.de Saint Maurice G, Auroy Y, Vincent C, Amalberti R. The natural lifespan of a safety policy: violations and system migration in anaesthesia. Qual Saf Healthcare 2010; 19:327–331. [DOI] [PubMed] [Google Scholar]
- 56.Haut ER, Lau BD. Shekelle PG, Wachter RM, Pronovost PJ. Prevention of venous thromboembolism: brief update review. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 303–309. [Google Scholar]
- 57.Amalberti R, Vincent C, Auroy Y, de Saint Maurice G. Violations and migrations in healthcare: a framework for understanding and management. Qual Saf Healthcare 2006; 15 Suppl 1:i66–i71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009; 360:491–499. [DOI] [PubMed] [Google Scholar]
- 59.Trimmel H, Fitzka R, Kreutziger J, von Goedecke A. [Anesthetist's briefing check. Tool to improve patient safety in the operating room]. Anaesthesist 2013; 62:53–60. [DOI] [PubMed] [Google Scholar]
- 60.Tang R, Ranmuthugala G, Cunningham F. Surgical safety checklists: a review. ANZ J Surg 2014; 84:148–154. [DOI] [PubMed] [Google Scholar]
- 61.Borchard A, Schwappach DL, Barbir A, Bezzola P. A systematic review of the effectiveness, compliance, and critical factors for implementation of safety checklists in surgery. Ann Surg 2012; 256:925–933. [DOI] [PubMed] [Google Scholar]
- 62▪.Thomassen O, Storesund A, Softeland E, Brattebo G. The effects of safety checklists in medicine: a systematic review. Acta Anaesthesiol Scand 2014; 58:5–18. [DOI] [PubMed] [Google Scholar]; This systematic review found checklists to be effective tools for improving patient safety in various clinical settings.
- 63▪▪.Lyons VE, Popejoy LL. Meta-analysis of surgical safety checklist effects on teamwork, communication, morbidity, mortality, and safety. West J Nurs Res 2014; 36:245–261. [DOI] [PubMed] [Google Scholar]; This meta-analysis of 19 studies about surgical safety checklists found significant effects on morbidity and mortality, teamwork and communication, and compliance with safety measures.
- 64.Bergs J, Zurel O, Cleemput I, et al. The implementation of a perioperative checklist increases patients’ perioperative safety and staff satisfaction. Acta Anaesthesiol Scand 2013; 57:675. [DOI] [PubMed] [Google Scholar]
- 65.Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf 2014; 23:299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russ S, Rout S, Sevdalis N, et al. Do safety checklists improve teamwork and communication in the operating room? A systematic review. Ann Surg 2013; 258:856–871. [DOI] [PubMed] [Google Scholar]
- 67.Lubbeke A, Hovaguimian F, Wickboldt N, et al. Effectiveness of the surgical safety checklist in a high standard care environment. Med Care 2013; 51:425–429. [DOI] [PubMed] [Google Scholar]
- 68.Sewell M, Adebibe M, Jayakumar P, et al. Use of the WHO surgical safety checklist in trauma and orthopaedic patients. Int Orthop 2011; 35:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪▪.Urbach DR, Govindarajan A, Saskin R, et al. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014; 370:1029–1038. [DOI] [PubMed] [Google Scholar]; This eminent study surprisingly found no significant reductions in operative mortality or complications associated with the implementation of surgical safety checklist in Ontario, Canada. This study provides important information about possible implementation problems and/or weaknesses of surgical safety checklists.
- 70▪.Haugen AS, Softeland E, Almeland SK, et al. Effect of the World Health Organization checklist on patient outcomes: a stepped wedge cluster randomized controlled trial. Ann Surg 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; Stepped wedge cluster randomized controlled trial of surgical safety checklist. This is methodologically probably the best study investigating the effectiveness of surgical safety checklist.
- 71▪.Birkmeyer NJ, Finks JF, Greenberg CK, et al. Safety culture and complications after bariatric surgery. Ann Surg 2013; 257:260–265. [DOI] [PubMed] [Google Scholar]; By linking survey data about safety culture to clinical registry outcome data in bariatric surgery, this study found correlation of better safety ratings with lower rates of serious complications.
- 72.Weaver SJ, Dy S, Lubomski LH, Wilson R. Shekelle PG, Wachter RM, Pronovost PJ. Promoting a culture of safety. Making healthcare safer II: an updated critical analysis of the evidence for patient safety practices. Comparative effectiveness review no. 211. Rockville, MD: Agency for Healthcare Research and Quality; 2013. 362–371. [Google Scholar]
- 73▪▪.Robertson ER, Morgan L, Bird S, et al. Interventions employed to improve intrahospital handover: a systematic review. BMJ Qual Saf 2014; 23:600–607. [DOI] [PubMed] [Google Scholar]; Systematic review on intrahospital patient handover improvement interventions. Despite improved information transfer, no methodology reliably improved the outcomes of clinical handover.
- 74.Aboumatar HJ, Thompson D, Wu A, et al. Development and evaluation of a 3-day patient safety curriculum to advance knowledge, self-efficacy and system thinking among medical students. BMJ Qual Saf 2012; 21:416–422. [DOI] [PubMed] [Google Scholar]
- 75.Walton M, Woodward H, Van Staalduinen S, et al. The WHO patient safety curriculum guide for medical schools. Qual Saf Healthcare 2010; 19:542–546. [DOI] [PubMed] [Google Scholar]


