Abstract
Purpose of review
Hepatitis B virus (HBV) causes a large proportion of chronic liver disease worldwide. The limited efficiency of current treatments based on the use of nucleotide/nucleoside analogues or interferon-alpha requires the development of new therapeutic tools for the treatment of chronic HBV. We summarize the most recent therapeutic strategies designed to directly target HBV-infected hepatocytes or to restore antiviral immunity during chronic HBV infection.
Recent findings
Novel therapies directly target HBV-infected hepatocytes by inducing covalently closed circular DNA degradation or by inhibiting HBV entry or the expression of viral proteins. In addition, immunotherapeutic approaches may boost HBV-specific T-cell responses or stimulate the intrahepatic innate response.
Summary
These new therapeutic approaches have mainly been tested in animal models. In humans, therapeutic strategies could be tailored to different chronic HBV patients in relation to their clinical and virological disease profile.
Keywords: chronic hepatitis B, toll-like receptor-agonist, vaccine therapy
INTRODUCTION
Hepatitis B virus infection (HBV) is a major public health threat with 350 million people infected worldwide, who are at risk of developing liver cirrhosis and hepatocellular carcinoma (HCC). A prophylactic vaccine for HBV has been available for over 30 years, but the number of infections remains dramatically high, mostly due to perinatal/postnatal mother-to-child transmission of the virus.
HBV is a small, enveloped DNA virus that infects hepatocytes by interacting with the recently identified sodium-taurocholate cotransporting polypeptide present on the surface of these cells [1,2]. Upon hepatocyte infection, the HBV genome is converted to a covalently closed circular DNA (cccDNA) that serves as a template for transcription of all the viral proteins. These are precore [serologically known as HBeAg) (HBV e antigen)], core, polymerase, envelope and X protein. Persistence of the cccDNA in the nucleus of infected cells is believed to be the central mechanism of HBV chronicity. Virus infection results in the production of HBV infectious particles (Dane particles) and of enveloped noninfectious particles devoid of viral DNA [HBV surface antigen (HBsAg)] that are secreted in large excess as compared with the Dane particles [3▪]. HBsAg is believed to be important for immune evasion of HBV through sequestration of HBsAg-specific neutralizing antibody particles and possibly by inducing HBV-specific T-cells tolerance through repetitive stimulation by high-dose antigen [4].
HBV infection during adulthood generally results in an acute self-limited infection, which triggers an effective and broad immune response capable of controlling, but not completely eradicating HBV infection. In contrast, chronic hepatitis B (CHB) patients fail to mount an efficient innate and adaptive immune response to the virus, leading to the onset of chronic liver inflammatory events that lead to cirrhosis and HCC [4]. A strong and broad HBV-specific CD8+T-cell response is essential to clear HBV infection. CD8+T-cell depletion in chimpanzees during acute infection results in persistence of viremia [5]. In contrast, persistence of infection is associated with functional exhaustion of HBV-specific CD8+T cells [6]. As HBV is a noncytopathic virus, HBV-specific T-cell recognition of infected hepatocytes is believed to mediate both virus control and liver damage [7]. However, studies have shown that the number of HBV-specific T cells in the blood and liver compartments is not proportional to liver damage but rather to virus control [8,9]. Instead, inflammation in the liver, which correlates with elevated blood alanine aminotransferase levels, is always associated with liver infiltrates of inflammatory cells, such as granulocytes, monocytes and nonantigen-specific T cells [8,10–12].
In this review, we will describe the recent therapeutic strategies and their potential to be employed as curative strategies for HBV. These therapeutic approaches can be broadly divided into two categories as follows: antiviral therapies that directly target the virus-infected cells and immunotherapeutic strategies that target HBV-infected cells indirectly by boosting the HBV-specific adaptive immune response (vaccination and T-cell engineering) or by directly activating innate intrahepatic immunity (toll-like receptor agonists and cytokine delivery).
Box 1.
no caption available
ANTIVIRAL THERAPIES TARGETING HEPATITIS B VIRUS-INFECTED HEPATOCYTES
Current antiviral therapies for HBV include nucleotide/nucleoside analogues that inhibit the HBV reverse transcriptase. Antiviral treatment can achieve strong inhibition of HBV replication, but it is unable to cure HBV infection due to the persistence of cccDNA in the infected hepatocytes. In contrast, interferon (IFN)-α or pegylated-IFN-α treatment can result in virus clearance, but its efficacy is limited to a proportion of patients and is accompanied by systemic side-effects [13].
In the last few years, new strategies aimed at improving cccDNA clearance have been developed (Fig. 1a). A recent study proposed lymphotoxin-β-receptor (LTβR) activation of HBV-infected cells as a therapeutic alternative capable of mediating degradation of cccDNA in infected hepatocytes without hepatotoxicity [14▪▪]. Similarly to IFN-α, LTβR (lymphotoxin beta receptor) signalling was shown to induce the upregulation of cytidine deaminases of the APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like) family of proteins that can specifically target the cccDNA for deamination and degradation. These findings highlight the potential use of nuclear deaminases for eradication of cccDNA expression. However, therapeutic LTβR activation needs to be carefully evaluated in patients as LTβR agonists could potentially trigger apoptosis, inflammation and HCC [15].
FIGURE 1.
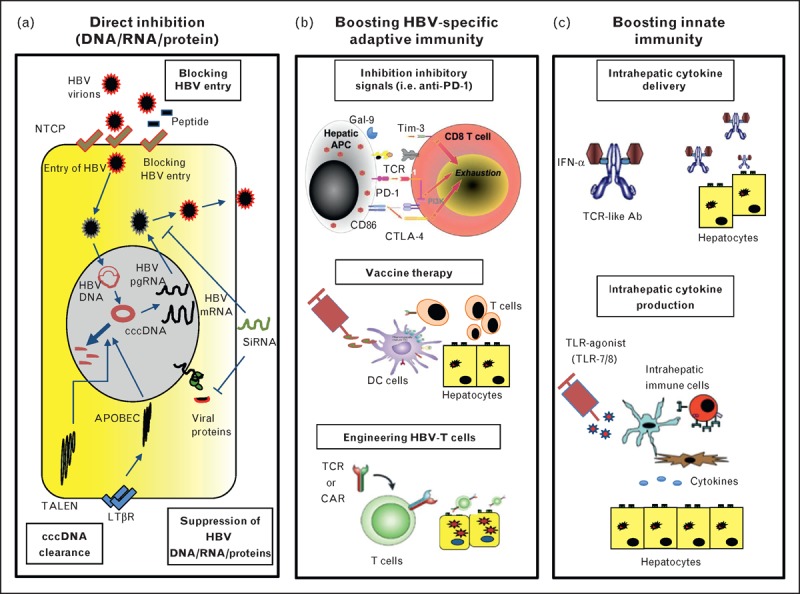
Schematic representation of new hepatitis B virus therapeutic strategies. (a) Direct inhibition (DNA/RNA/Protein). (b) Boosting HBV-specific adaptive immunity. (c) Boosting innate immunity. Ab, antibody; APC, antigen-presenting cell; CAR, chimeric antigen receptors; cccDNA, covalently closed circular DNA; DC, dendritic cell; HBV, hepatitis B virus; IFN, interferon; LTβ, lymphotoxin-β; NTCP, sodium-taurocholate cotransporting polypeptide; TALEN, transcription activator-like effector nucleases; TLR, toll-like receptor.
Degradation of cccDNA can also be achieved by utilizing enzymes termed transcription activator-like effector nucleases (TALENs) that can cleave sequence-specific DNA targets. cccDNA-specific TALENs were shown to significantly reduce cccDNA levels without apparent cytotoxic effect when introduced in HCC cell lines [16▪]. In vivo, in mice hydrodynamically injected with monomeric full length HBV DNA, introduction of TALENs resulted in a significant reduction of serum HBsAg, HBeAg and liver pregenomic RNA and enhanced the antiviral effects induced by IFN-α when used in combination therapy [16▪].
An alternative approach is to modulate the expression of viral proteins, such as HBsAg and HBeAg, which are believed to play a role in induction of T-cell exhaustion [4,17]. This could potentially be achieved by using RNA interference-based therapeutics that target expression of specific viral RNAs. In a transient and transgenic mouse model of HBV infection, it was recently shown that hepatocyte-targeted interference RNA specific for conserved HBV sequences resulted in repression of HBsAg, HBeAg and viral DNA load [18▪].
An attractive therapeutic alternative is represented by the use of acylated peptides derived from the large HBV envelope protein to block HBV entry in hepatocytes [3▪]. These peptides were shown to block virus entry both in vitro and in vivo in mice repopulated with primary human or Tupaia belangeri hepatocytes. These data suggest that inhibition of HBV entry constitutes a therapeutic approach to prevent primary HBV infection, such as after liver transplantation, and it may also control virus infection in chronically infected patients [19].
These novel antiviral strategies represent attractive therapeutic alternatives to the current antiviral therapies. However, the delivery mode of these compounds and safety concerns related to their expression in humans are a complex issue that needs to be carefully evaluated.
IMMUNOTHERAPEUTIC APPROACHES: RESTORATION OF ADAPTIVE IMMUNITY
Elimination of cccDNA from infected cells represents an attractive endpoint for HBV therapy, but it may not be necessary for the cure of CHB. In fact, individuals with resolved acute HBV infection do not achieve complete viral eradication, but they are able to control the virus indefinitely [20] without any signs of liver damage. It may, therefore, be more feasible to restore HBV-specific immunity to mimic the immunological events that occur during acute infection (Fig. 1b).
During CHB infection, HBV-specific T cells are deleted or functionally exhausted most likely due to the repeated exposure of these cells to large quantities of HBsAg and HBeAg. Exhausted virus-specific T cells express inhibitory molecules, such as PD-1 (programmed cell death protein 1), CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), SLAM (signalling lymphocyte activation molecule), TIM-3 (T-cell immunoglobulin domain and mucin domain 3), and acquire a progressive and step-wise loss of their effector functions [21]. Blocking inhibitory receptors was shown to partially recover the exhausted T cells of CHB patients in vitro[6,22,23], but the in-vivo efficacy of this approach is still uncharacterized.
Therapeutic vaccination aimed at eliciting the patient's immune system represents an attractive alternative therapy for HBV [24,25]. The early clinical trials based on the use of the conventional prophylactic HBV vaccine (HBsAg) were unable to show any clinical benefit either when tested alone [26] or in combination with direct antiviral therapy [27]. Failure was believed to be attributable to the use of a single HBV antigen and to the type of adjuvant that was not efficient in generating CD8+T-cell responses [25]. However, new vaccine formulations (HBV envelope-expressing DNA vaccine or peptide-based vaccines) that elicit HBV-specific CD8+T-cell responses in healthy volunteers or in CHB patients [28] were unable to achieve therapeutic effects in patients [29,30▪,31▪].
The clinical failure of these vaccine therapies was attributed to the single targeting of the envelope antigen, produced in high quantity in CHB patients. HBV therapeutic vaccines targeting different HBV proteins with more limited exhaustion potential [32] have been, therefore, developed [33–35]. A therapeutic vaccine comprising particulate HBsAg and HBcAg and a saponin-based adjuvant was able to restore potent multifunctional HBV-specific CD8+T-cell responses in HBV transgenic mice without causing liver disease [34]. Furthermore, a yeast-based vaccine candidate containing X, S and core antigen showed immunogenicity not only in mice but also in peripheral blood of CHB patients [35].
Recently, more structured methods have improved the immunogenicity of different vaccine preparations in animal models of HBV infection. DNA prime-adenovirus boost immunization against woodchuck hepatitis virus (WHV) core antigen (WHcAg) elicited a strong CD8+T-cell response against WHV in mice and naive woodchucks [36]. DNA prime-adenovirus boost immunization using WHV surface antigen (WHsAg) and WHcAg combined with direct antiviral treatment resulted in the induction of a virus-specific CD4+ and CD8+T-cell response and a reduction in WHsAg and viral DNA in chronically WHV-infected woodchucks. Importantly, two of four animals remained WHV-negative after interruption of the antiviral treatment and developed anti-WHV antibodies [37]. The immune therapeutic efficacy of this approach was further improved in a small trial of triple-combination therapy with DNA vaccination encoding the WHcAg and WHsAg, programmed death-ligand 1 blockage and antiviral treatment. This treatment resulted in sustained immunological control of viral infection, generation of specific antibodies and complete viral clearance (with undetectable levels of cccDNA in the liver) in one out of three animals tested [38▪▪].
So far, CHB patients have been refractory to many therapies that were effective in animal models [25]. Therefore, these data obtained in the woodchuck model, which closely mimics human HBV chronic infection, are promising and could provide a valuable approach for the treatment of CHB patients.
A single, phase III clinical trial of a therapeutic vaccine based on immunogenic complexes composed of HBsAg and antihuman HBsAg antibodies has shown some level of efficacy in vaccine therapy. However, a virological response (HBeAg seroconversion) was not only observed in 20% of individuals treated with the vaccine but also in the control group treated with alum adjuvant alone [39]. The mechanisms causing this clinical response and the immunological perturbations induced by vaccination were not directly investigated in this trial. A possible explanation could be provided by the ability of alum to mediate activation of inflammatory monocyte-derived dendritic cells in a MyD88-dependant mechanism [40]. Recently, a number of reports in different HBV-mouse models have shown that TLR-mediated or anti CD40-mediated stimulation of intrahepatic monocytes or dendritic cells resulted in reversion of immune tolerance and efficient HBV-specific CD8+T-cell expansion [41▪,42▪,43,44]. These data support our recent demonstration that monocytes from CHB patients internalize HBV antigens and after inflammatory activation can activate autologous HBV-specific T cells [45▪▪]. Collectively, these studies suggest that the repetitive injection of adjuvants alone can induce an inflammatory environment that enables the patient's antigen-presenting cells to exploit their endogenous antigen to expand their exhausted HBV-specific T cells. However, this is still a hypothetical scenario, and it will be extremely important to decipher in which CHB patients such a personalized antigenic depot can be effectively used as a therapeutic vaccine.
Currently, the inability to expand an efficient HBV-specific immunity in a large proportion of adult CHB patients (particularly those with high HBV replication levels) [9] might suggest that more radical approaches are necessary to reconstitute a functional efficient HBV-specific T-cell repertoire.
Engineering HBV-specific T cells through transfer of HBV-specific T cell receptor (TCR) or HBV-specific chimeric antigen receptors (CARs) represents a promising alternative strategy to construct an HBV-specific T-cell immunity in many CHB patients [46▪,47]. The two different strategies present different strengths and weaknesses. CARs, which bind HBV antigen in a similar fashion as antibodies [independently of human leukocyte antigen (HLA) restriction], can be easily applied to large CHB patient populations. In contrast, the classical HBV-specific TCR is HLA-restricted and will, thus, need to be tailored to the HLA setup of each patient. On the other hand, circulating HBsAg present in high doses in CHB patients could potentially sequester T cells engineered with CARs and limit their in-vivo therapeutic potential.
Encouraging data for the feasibility of T-cell therapies emerge from studies in CHB patients who were able to control viral replication after receiving bone marrow transplants from HBV-immune donors [48]. Furthermore, HBV-infected livers transplanted in HBV-immune donors resulted in viral control without signs of overt liver disease [49]. Although these data strongly support the use of T cells for gene therapy in CHB patients, restoring HBV-specific T-cell immunity may potentially mediate liver damage through the triggering of an intrahepatic inflammatory response. Ongoing studies in our laboratory are aimed at generating TCR-redirected T cells that transiently express the recombinant TCR and are, thus, safer to control once infused in the patient [50]. We also recently tested HBsAg-specific TCR-redirected T cells in an HCC patient for the treatment of HBsAg+ chemoresistant extrahepatic metastases. This clinical trial demonstrates that TCR-engineered T cells are able to expand in vivo and recognize HCC lesions with a significant reduction in HBsAg levels without exacerbation of liver inflammation and supports the idea that TCR-redirected T cells represent a new therapeutic opportunity for hepatitis B treatment (Wasim et al., in preparation).
IMMUNOTHERAPEUTIC APPROACHES: DIRECT STIMULATION OF INNATE INTRAHEPATIC IMMUNITY
Therapeutic strategies aimed at increasing innate immunity (Fig. 1c) exploit the robust antiviral efficacy demonstrated by distinct cytokines (tumour necrosis factor-α, IFN-α, IFN-γ and interleukin-1β) [51–54], mimic the activation of innate immunity during the early phase of acute HBV infection [55] and induce a correct maturation of the adaptive immunity [56▪,57]. Amongst the strategies developed to boost intrahepatic IFN-α levels, we have recently developed TCR-like antibodies conjugated with IFN-α that specifically target HBV-infected hepatocytes, thus increasing the intrahepatic levels of IFN-α [58]. TLR7 agonists have been used to induce IFN-α production in pDCs (plasmacytoid dendritic cells). However, in chronically HBV-infected chimpanzees, TLR7 stimulation triggered only a transient production of IFN-α, and suppression of HBV correlated mostly with IFN-γ production by intrahepatic natural killer cells, natural killer T (NKT) cells, and T cells [59▪▪]. NK and NKT cells, particularly mucosal-associated invariant T cells that constitute a large proportion of T cells-expressing NK markers in human liver [60], can be activated by IL-12 and IL-18. These cytokines are able to mediate not only the robust inhibition of HBV replication [61] but also the partial recovery of exhausted HBV-specific T cells [62▪]. A recent study [63▪] from our group showed that TLR8 stimulation of cells from healthy and HBV or HCV-infected livers can trigger a robust production of IFN-γ by liver-resident NKT mucosal-associated invariant T and CD56bright NK cells, and this was mediated by the production of IL-12 and IL-18 by intrahepatic monocytes. These data suggest that TLR-8 agonists might be ideal candidates to activate intrahepatic immunity in CHB patients.
CONCLUSION
The strategies that we briefly reviewed seek to cure the HBV-related liver disease of CHB patients by controlling HBV infection through direct viral suppression or by restoring antiviral host immunity. Their therapeutic efficiency in animal models supports their translation to clinical practice.
However, as discussed recently [56▪], we propose a radically different perspective according to which CHB is considered an inflammatory rather than a viral disease. This paradigm change has acquired further strength after the demonstration than an anti-inflammatory treatment can suppress the development of HCC in HBV transgenic mice [64].
The balance between a protective antiviral host response and an inflammatory immune reaction is likely to differ within the heterogeneous population of patients with CHB infection that are characterized by different clinical and virological profiles. Thus, therapies designed to control HBV infection or liver inflammation should be tailored to selected population of patients.
We have, for example, recently shown that adolescent and young CHB patients display a less compromised HBV-specific antiviral immune response than their adult counterparts [65]. We hypothesize that the higher proportion of liver inflammatory events detected in adults as compared to adolescent/young CHB patients (Bertoletti and Kennedy, in preparation) might be related to the increased propensity of adults to develop an inflammatory response rather than to the level of their anti-HBV-specific immunity. If further analysis of the inflammatory events, which characterize the different phases of CHB infection, will confirm this hypothesis, it is plausible to think that young CHB patients may be more responsive to immunotherapeutic strategies, whereas adult CHB patients should receive major benefits from therapies aimed at controlling inflammation. The challenge of future HBV research might not only reside in the development of new therapeutic tools but also in the more precise understanding of the population of patients on which these strategies should be applied.
Acknowledgements
This work was supported by a Singapore Translational Research (STaR) Investigator Award (NMRC/STaR/013/2012) (to A.B.).
Conflicts of interest
A.B. declares the following relationship with commercial entities developing immune therapeutics for HBV treatment. A.B. collaborates and receives research support from Gilead and Janseen to test the effect of HBV antigens on immune cell function and TLR-agonists in intrahepatic immune cells. A.B. also participates in Advisory Boards on HBV immune therapy for Gilead, Janseen, Hoffman-La Roche, ISIS, Medimmune.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. ELife 2012; 1:e00049–e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014; 146:1070–1083. [DOI] [PubMed] [Google Scholar]
- 3▪.Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology 2014; 147:48–64. [DOI] [PubMed] [Google Scholar]; An authoritative review of the mechanism of HBV entry and of the strategies to block HBV infection.
- 4.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012; 61:1754–1764. [DOI] [PubMed] [Google Scholar]
- 5.Thimme R, Wieland S, Steiger C, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007; 81:4215–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti LG, Ishikawa T, Hobbs MV, et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996; 4:25–36. [DOI] [PubMed] [Google Scholar]
- 8.Maini MK, Boni C, Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster GJM, Reignat S, Brown D, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol 2004; 78:5707–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando K, Moriyama T, Guidotti LG, et al. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med 1993; 178:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakimi K, Lane TE, Wieland S, et al. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med 2001; 194:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitia G, Isogawa M, Iannacone M, et al. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest 2004; 113:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 2011; 8:275–284. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014; 343:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]; The demonstration that the nuclear transcriptional template of HBV (cccDNA) can be degraded noncytolytically by agents that upregulate APOBEC3A and 3B. This study may lead to the development of new therapies that can achieve HBV clearance.
- 15.Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther 2013; 22:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first description of a new potential strategy to treat HBV infection using enzymes that can cleave sequence specific DNA.
- 17.Milich DR, Jones JE, Hughes JL, et al. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A 1990; 87:6599–6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Wooddell CI, Rozema DB, Hossbach M, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther 2013; 21:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]; Targeted delivery of HBV-specific small interfering RNA in hepatocytes lead to suppression of viral RNA, DNA and protein in a mouse model of HBV.
- 19.Petersen J, Dandri M, Mier W, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol 2008; 26:335–341. [DOI] [PubMed] [Google Scholar]
- 20.Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest 1994; 94:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maini MK, Schurich A. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol 2010; 52:616–619. [DOI] [PubMed] [Google Scholar]
- 22.Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long term effective treatment with nucleos(t)ide analogues. Gastroenterology 2012; 143:963.e9–973.e9. [DOI] [PubMed] [Google Scholar]
- 23.Schurich A, Khanna P, Lopes AR, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011; 53:1494–1503. [DOI] [PubMed] [Google Scholar]
- 24.Michel ML, Pol S, Brechot C, Tiollais P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: from present to future. Vaccine 2001; 19:2395–2399. [DOI] [PubMed] [Google Scholar]
- 25.Bertoletti A, Gehring A. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev Gastroenterol Hepatol 2009; 3:561–569. [DOI] [PubMed] [Google Scholar]
- 26.Pol S, Nalpas B, Driss F, et al. Multicenter study group: efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol 2001; 34:917–921. [DOI] [PubMed] [Google Scholar]
- 27.Vandepapelière P, Lau GKK, Leroux-Roels G, et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007; 25:8585–8597. [DOI] [PubMed] [Google Scholar]
- 28.Mancini-Bourgine M, Fontaine HLN, Scott-Algara D, et al. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 2004; 40:874–882. [DOI] [PubMed] [Google Scholar]
- 29.Heathcote J, McHutchison J, Lee S, et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology 1999; 30:531–536. [DOI] [PubMed] [Google Scholar]
- 30▪.Godon O, Fontaine H, Kahi S, et al. Immunological and antiviral responses after therapeutic DNA immunization in chronic hepatitis B patients efficiently treated by analogues. Mol Ther 2013; 22:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported the clinical and virological efficacy of therapeutic vaccination in CHB patients.
- 31▪.Fontaine H, Kahi S, Chazallon C, et al. ANRS HB02 study group. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial–ANRS HB02 VAC-ADN. Gut 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This study reported the clinical and virological efficacy of therapeutic vaccination in CHB patients.
- 32.Kakimi K, Isogawa M, Chung J, et al. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol 2002; 76:8609–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depla E, Van der Aa A, Livingston BD, et al. Rational design of a multiepitope vaccine encoding T-lymphocyte epitopes for treatment of chronic hepatitis B virus infections. J Virol 2007; 82:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchmann P, Dembek C, Kuklick L, et al. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice. Vaccine 2013; 31:1197–1203. [DOI] [PubMed] [Google Scholar]
- 35.King TH, Kemmler CB, Guo Z, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One 2014; 9:e101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosinska AD, Johrden L, Zhang E, et al. DNA prime-adenovirus boost immunization induces a vigorous and multifunctional T-cell response against hepadnaviral proteins in the mouse and woodchuck model. J Virol 2012; 86:9297–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosinska AD, Zhang E, Johrden L, et al. Combination of DNA prime–adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog 2013; 9:e1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪▪.Liu J, Zhang E, Ma Z, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014; 10:e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed analysis of different strategies to improve therapeutic vaccination in the woodchuck HBV model.
- 39.Xu DZ, Wang XY, Shen XL, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 2013; 59:450–456. [DOI] [PubMed] [Google Scholar]
- 40.Kool M, Soullié T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med 2008; 205:869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Huang LR, Wohlleber D, Reisinger F, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8+ T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013; 14:574–583. [DOI] [PubMed] [Google Scholar]; TLR-mediated activation of intrahepatic myeloid cells triggers expansion of virus-specific cytotoxic T lymphocytes in a mouse model of chronic infection. A possible new strategy to overcome HBV-specific CD8+ T-cell exhaustion.
- 42▪.Isogawa M, Chung J, Murata Y, et al. CD40 activation rescues antiviral CD8+ t cells from PD-1-mediated exhaustion. PLoS Pathog 2013; 9:e1003490. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article, rescue of intrahepatic exhausted CD8+ T cells is achieved through CD40-mediated activation of intrahepatic dendritic cells.
- 43.Lan P, Zhang C, Han Q, et al. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology 2013; 58:73–85. [DOI] [PubMed] [Google Scholar]
- 44.Lv S, Wang J, Dou S, et al. Nanoparticles encapsulating hepatitis B virus cytosine-phosphate-guanosine induce therapeutic immunity against HBV infection. Hepatology 2014; 59:385–394. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Gehring AJ, Haniffa M, Kennedy PT, et al. Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest 2013; 123:3766–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]; A demonstration that circulating monocytes of chronic HBV patients can be activated to cross-present viral antigen that is already present in the circulation of infected patients. Vaccination therapy without a vaccine?
- 46▪.Krebs K, Böttinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 2013; 145:456–465. [DOI] [PubMed] [Google Scholar]; The demonstration of the therapeutic efficacy of CAR-modified T cells in a mouse model of HBV infection.
- 47.Gehring AJ, Xue S-A, Ho ZZ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011; 55:103–110. [DOI] [PubMed] [Google Scholar]
- 48.Lau GK, Lok AS, Liang RH, et al. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology 1997; 25:1497–1501. [DOI] [PubMed] [Google Scholar]
- 49.Loggi E, Bihl F, Chisholm JV, et al. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J Hepatol 2009; 50:625–630. [DOI] [PubMed] [Google Scholar]
- 50.Koh S, Shimasaki N, Suwanarusk R, et al. A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol Ther Nucleic Acids 2013; 2:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol 2000; 74:4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol 2000; 74:2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puro R, Schneider RJ. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J Virol 2007; 81:7351–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watashi K, Liang G, Iwamoto M, et al. Interleukin-1 and tumor necrosis factor-α trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J Biol Chem 2013; 288:31715–31727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisicaro P, Valdatta C, Boni C, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009; 58:974–982. [DOI] [PubMed] [Google Scholar]
- 56▪.Bertoletti A, Gehring AJ. Immune therapeutic strategies in chronic hepatitis B virus infection: virus or inflammation control? PLoS Pathog 2013; 9:e1003784. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent review that discusses in detail the possible immune-based therapies in HBV infection.
- 57.Zoulim F, Luangsay S, Durantel D. Targeting innate immunity: a new step in the development of combination therapy for chronic hepatitis B. Gastroenterology 2013; 144:1342–1344. [DOI] [PubMed] [Google Scholar]
- 58.Ji C, Sastry KSR, Tiefenthaler G, et al. Targeted delivery of interferon-α to hepatitis B virus-infected cells using T-cell receptor-like antibodies. Hepatology 2012; 56:2027–2038. [DOI] [PubMed] [Google Scholar]
- 59▪▪.Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis b virus in chronically infected chimpanzees. Gastroenterology 2013; 144:1508.e10–1517.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]; The demonstration of the potential clinical and virological efficacy of TLR-agonists in the treatment of chronic HBV infection.
- 60.Tang XZ, Jo J, Tan AT, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 2013; 190:3142–3152. [DOI] [PubMed] [Google Scholar]
- 61.Kimura K, Kakimi K, Wieland S, et al. Interleukin-18 inhibits hepatitis b virus replication in the livers of transgenic mice. J Virol 2002; 76:10702–10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Schurich A, Pallett LJ, Lubowiecki M, et al. The third signal cytokine IL-12 rescues the antiviral function of exhausted HBV-specific CD8 T cells. PLoS Pathog 2013; 9:e1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]; An insight into the impact of different cytokines in the functional rescue of human exhausted CD8 T cells.
- 63▪.Jo J, Tan AT, Ussher JE, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014; 10:e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]; A direct characterization of the hierarchy of TLR-mediated activation of intrahepatic human immune cells.
- 64.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012; 109:E2165–E2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy PTF, Sandalova E, Jo J, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012; 143:637–645. [DOI] [PubMed] [Google Scholar]


