Abstract
Background
The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial provides us an opportunity to describe interval lung cancers not detected by screening chest x-ray (CXR) compared to screen-detected cancers.
Methods
Participants were screened for lung cancer with CXR at baseline and annually for two (never smokers) or three (ever smokers) more years. Screen-detected cancers were those with a positive CXR and diagnosed within 12 months. Putative interval cancers were those with a negative CXR screen but with a diagnosis of lung cancer within 12 months. Potential interval cancers were re-reviewed to determine whether lung cancer was missed and probably present during the initial interpretation or whether the lesion was a “true interval” cancer.
Results
77,445 participants were randomized to the intervention arm with 70,633 screened. Of 5,227 positive screens from any screening round, 299 resulted in screen-detected lung cancers; 151 had potential interval cancers with 127 CXR available for re-review. Cancer was probably present in 45/127 (35.4%) at time of screening; 82 (64.6%) were “true interval” cancers. Compared to screen-detected cancers, true interval cancers were more common among males, persons with <12 years education and those with a history of smoking. True interval lung cancers were more often small cell, 28.1% vs. 7.4%, and less often adenocarcinoma, 25.6% vs. 56.2% (p<0.001), more advanced stage IV (30.5% vs. 16.6%, p<0.02), and less likely to be in the right upper lobe, 17.1% vs. 36.1% (p<0.02).
Conclusion
True interval lung cancers differ from CXR-screen-detected cancers with regard to demographic variables, stage, cell type and location.
Keywords: lung cancer, PLCO Cancer Screening Trial, screening interval lung cancers, chest X-ray screen-detected lung cancers
1. Introduction
Lung cancer is the most common lethal cancer, expected to account for 159,260 deaths in the USA in 2014 [1] and for 1,400,000 deaths in the world in 2008 [2]. Low-dose helical computed tomography (LDCT) was reported in 2011 to reduce lung cancer mortality when it was used to screen high-risk persons [3], but screening with chest radiographs (CXR) has failed to demonstrate reduced mortality compared to historic controls or to usual care in numerous settings [4-9].
The lung component of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial was a comparison of annual screening with CXR to usual care in both never- and ever-smokers. PLCO provided an opportunity to describe characteristics of lung cancers that were not detected by screening CXR and were judged to have developed between screening tests, deemed “interval cancers”. Our objective was to better characterize the nature of interval cancers. We first identified, by re-reviewing the CXR images of putative interval cancers, those that were detectable on the screen but missed (“probably present”) during the original screening review in order to designate the “true interval” cancers. We then analyzed what factors were associated with true interval cancers (and probably present cancers) compared to screen-detected cancers.
We hypothesized that true interval lung cancers are distinct from screen-detected cancers, which would have implications in the development of new screening methodologies. In this report we have performed a detailed comparison of the characteristics of the true interval and screen-detected lung cancers diagnosed during the screening phase in the intervention arm of PLCO.
2. Materials and methods
2.1. Trial design
The design of PLCO has been described previously [9]. Males and females aged 55-74 were recruited between 1993 and 2001 at ten screening centers nationwide. Each institution obtained local Institutional Review Board approval to conduct the study; all participants provided written informed consent. Subjects were randomized to the intervention arm or to usual care within blocks stratified by screening center, sex and age. Exclusion criteria at study entry were history of a PLCO cancer, current cancer treatment and previous removal of one lung. Participants completed a baseline questionnaire at study entry that inquired about socio-demographics, medical history, smoking history, and past screenings.
Intervention arm participants were offered a postero-anterior (PA) CXR at baseline and then annually for three more years; participants who were randomized after April 1995 and who had never smoked were not offered the fourth screen. Subjects and their health care providers were notified of CXR results. A CXR was classified as “abnormal, suspicious for lung cancer” if a nodule, mass, infiltrate or other abnormality suspicious for lung cancer was noted. Those with abnormal suspicious exams were advised to seek diagnostic evaluation. Follow-up was determined by the participants and their physicians, and not by trial protocol. PLCO screening center staff obtained medical records related to diagnostic follow-up of positive screens and certified medical record abstractors recorded information to document lung cancer diagnosis. In addition, an annual questionnaire was completed by all subjects or next-of-kin until 2010, and medical records documenting follow-up also were obtained and abstracted when a lung cancer was reported and was not associated with a positive screen.
2.2. Definition of interval cancers in the PLCO Screening Arm
Among intervention arm subjects, screen-detected cancers were defined as those diagnosed after any positive screen within a window extending twelve months from the positive screen; additionally, a gap of no more than 9 months between screen and first procedure, or between procedures, could be present. Potential interval cancers were defined as those diagnosed within 12 months of a negative or “abnormal but not suspicious for lung cancer” PLCO screening CXR. Cancers diagnosed after the screening phase (more than 12 months after the last scheduled screening CXR) were denoted as “post-screening” and were not included in this analysis. Subjects with carcinoid tumors were also excluded.
After completion of the screening phase of PLCO, CXR that subsequently had been digitized from subjects who had potential interval lung cancers were reinterpreted to determine if there were findings suspicious for lung cancer that were missed at the first interpretation. Two physicians with extensive experience reading CXR (PAK, CJZ)* performed the second interpretations separately, blinded to the location of the cancer, and then compared interpretations with each other. If an abnormality that was suspicious for lung cancer was identified and was in the same lung as the cancer on this second reading by both reviewers, the image was characterized as a missed positive screen and the tumor was characterized as “probably present” at the last PLCO screening. If both reviews agreed with the initial interpretation that the image was not suspicious for cancer, the subsequent cancer was characterized as a “true interval cancer”. Differences of opinions were resolved by discussion between the two reviewers, or with input from a third reviewer (DLS)* if consensus could not be reached.
2.3 Statistical analysis
Descriptive statistics were prepared using contingency table analysis and Fisher's exact test (FET). The 95% confidence intervals (CI) for proportions were estimated using the binomial exact method. Multivariable models were constructed to identify factors or characteristics associated with having a true interval lung cancer vs. screen detected cancer. This was done using two models, one with socio-demographic and exposure predictor variables which preceded diagnosis of lung cancer, and one for the tumor characteristics observed at lung cancer diagnosis. The direction, magnitude and precision of associations were estimated with odds ratios (OR) and their 95% confidence intervals (CI). Logistic regression modeling assumptions were evaluated. Nonlinear effects of continuous variables were evaluated graphically using loess plots and in modeling by using restricted cubic splines. The assumption of additivity of explanatory variables was evaluated by assessing interactions of predictors in final models by including the interaction term along with main effect terms. The added benefit of interactions and categorical variables were assessed by applying the likelihood ratio test (LRT) in the full and nested models with and without the term of interest. Because data were clustered in study centers, all models were adjusted for center as an indicator variable, which is tantamount to fixed effects models. All reported p-values are two-sided. Because the number of true interval cancers is limited, we did not restrict reporting of results to associations with p-values <0.05.
The comparison group of screen-detected lung cancers, which included screen-detected cancers ascertained at the baseline examination, are a mixture of prevalent and incident screen-detected lung cancers. We carried out separate analyses excluding baseline screen-detected (prevalent) cancers from this group to evaluate the use of a more homogeneous comparison group. We found that in all of our models important effect estimates remained the same in direction and magnitude, and our study conclusions remained unchanged. For this reason, we only present the overall results here.
Statistics and figures were prepared using SAS (SAS Institute Inc, SAS 9.1.3, Cary, NC), and Stata 12.1 MP (StataCorp, College Station, Texas) statistical programs.
3. Results
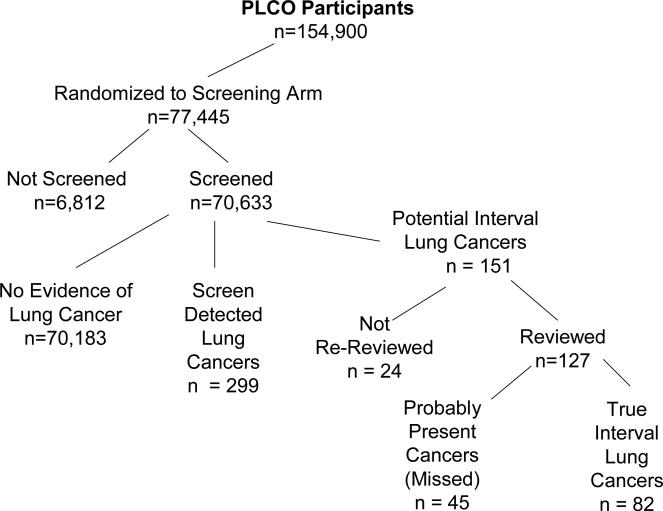
Of the 154,900 participants enrolled in the PLCO trial, 77,445 were randomized to the intervention (screening) arm. In the intervention arm, of the 5,227 subjects with a positive screen, 299 (5.7%) were subsequently classified as having screen-detected lung cancer (Figure 1). There were 151 subjects who were diagnosed with potential interval lung cancers. Digitized images were available for 127 of the 151 subjects.
Figure 1.
Consort diagram of patients entered into PLCO Cancer Screening Trial, showing those diagnosed with lung cancer during period of this study with classification status (Screen-Detected, Probably Present/Missed, or True Interval).
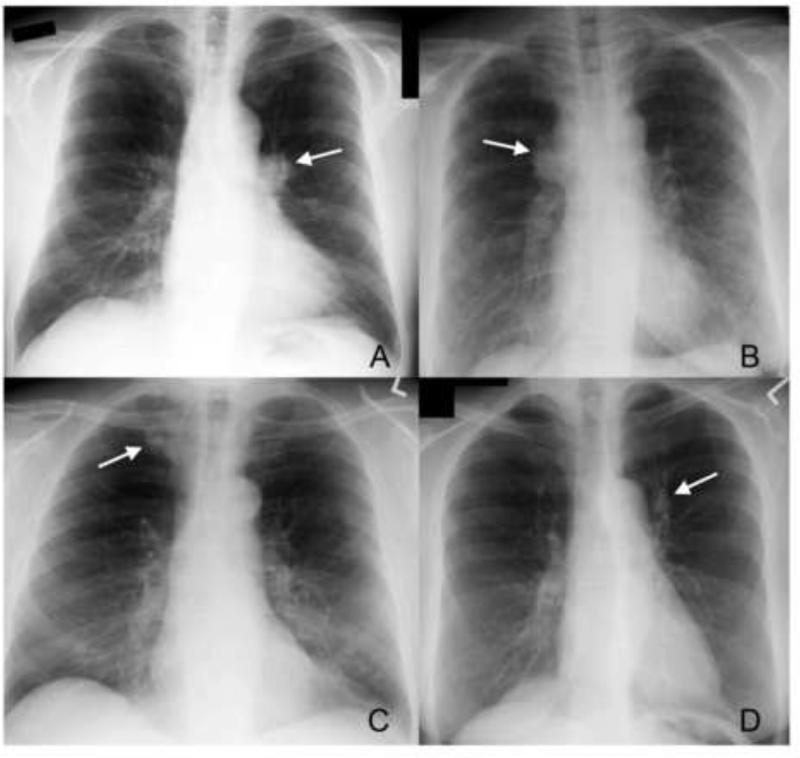
Demographic characteristics of the subjects with screen-detected cancers, true interval cancers, probably present cancers, and the unreviewed interval cancers are presented in Table 1. Missingness of digital images not available for re-review was not significantly associated with any demographic variables evaluated (Table 1); however, there was an association by study center as one center could not provide any CXR for review, accounting for 50% (12/24) of this group. The mean durations between CXR and cancer diagnosis were 246 days (SD 79), 178 days (SD 97) and 93 days (SD 75) for true interval, probably present and screen-detected cancers, respectively. There were 45 of 127 (35.4%) subjects whose images were judged by the second reviews to be missed positive screens and they were classified as “probably present.” Abnormalities on screens classified as probably present included hilar prominence (n=9 on the right side, n=8 on the left side); nodules or masses (n=24), some of which were obscured by overlying bones (n=11) or vascular structures including the heart (n=4); and others with lobar atelectasis, an ill-defined pleural-based mass, or ill-defined air-space opacities. Examples of missed positive screens are shown in Figure 2. The principal difference in characteristics between probably present and screen-detected cases (Table 1) was in stage at diagnosis for non-small cell lung cancers (NSCLC) (P< 0.001), with fewer early stage I & II cancers in the probably present cancers, 32.5% (13/40) versus screen-detected cancers at 59.6% (165/277) (Table 2). The opposite was true for stage IV: 50% (20/40) of the probably present cancers versus 16.6% (46/277) screen-detected cancers.
Table 1.
Distributions of study predictors with outcome categories: Screen-Detected; True Interval (defined as a cancer not present on previous screening x-ray and clinically detected prior to subsequent screen); Probably Present (defined as an interval cancer detected but present on review of preceding screening image); and Not Reviewed Interval (defined as an interval lung cancer whose x-rays were not available for review); N=450.
| Screen-Detected N = 299 | True Interval N=82 | Probably Present N=45 | Not Reviewed Interval N=24 | |
|---|---|---|---|---|
|
Sociodemographic & Exposure Predictors
|
||||
| Sex | ||||
| Female | 120 (40.1%) | 24 (29.3%) | 15 (33.3%) | 13 (54.2%) |
| Male | 179 (59.9%) | 58 (70.7%) | 30 (66.7%) | 11 (45.8%) |
| P-value | 0.09† | 0.42* | 0.20‡ | |
| Race/Ethnicity | ||||
| White | 252 (84.3%) | 70 (85.4%) | 39 (86.7%) | 23 (95.8%) |
| Black | 32 (10.7%) | 7 (8.5%) | 3 (6.7%) | 1 (4.2%) |
| Other (non-white & non-black) | 15 (5.0%) | 5 (6.1%) | 3 (6.7%) | 0 (0.0%) |
| P-value | 1.00† | 1.00* | 1.00‡ | |
| Education | ||||
| Less than high school complete | 39 (13.0%) | 18 (21.9%) | 7 (15.6%) | 4 (16.7%) |
| High school graduate | 88 (29.4%) | 24 (29.3%) | 12 (26.7%) | 6 (25.0%) |
| Some post-high school | 100 (33.4%) | 20 (24.4%) | 14 (31.1%) | 6 (25.0%) |
| College or professional school complete | 70 (23.4%) | 20 (24.4%) | 11 (24.4%) | 8 (33.3%) |
| Missing | 2 (0.66) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) |
| P-value | 0.34† | 1.00* | 1.00‡ | |
| Smoking status | ||||
| Never-smoker | 23 (7.7%) | 2 (2.4%) | 4 (8.9%) | 2 (8.3%) |
| Former-smoker | 164 (54.9%) | 45 (54.9%) | 22 (48.9%) | 13 (54.2%) |
| Current smoker | 111 (37.1%) | 35 (42.7%) | 18 (40.0%) | 9 (37.5%) |
| Missing | 1 (0.3%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) |
| P-value | 0.42† | 1.00* | 1.00‡ | |
|
Tumor-Related Predictors
|
||||
| Histology | ||||
| Adenocarcinoma | 168 (56.2%) | 21 (25.6%) | 24 (53.3%) | 13 (54.2%) |
| Squamous cell | 59 (19.7%) | 15 (18.3%) | 8 (17.8%) | 4 (16.7%) |
| Large cell | 21 (7.0%) | 6 (7.3%) | 2 (4.4%) | 1 (4.2%) |
| Other NSCLC | 29 (9.7%) | 17 (20.7%) | 6 (13.3%) | 3 (12.5%) |
| Small cell | 22 (7.4%) | 23 (28.1%) | 5 (11.1%) | 3 (12.5%) |
| P-value | 0.001† | 1.00* | 1.00‡ | |
| Tumor grade in NSCLC§ | ||||
| Well differentiated | 32 (11.6%) | 2 (3.4%) | 5 (12.5%) | 2 (9.5%) |
| Moderately differentiated | 81 (29.2%) | 12 (20.3%) | 6 (15.0%) | 1 (4.8%) |
| Poorly differentiated | 105 (37.9%) | 16 (27.1%) | 16 (40.0%) | 10 (47.6%) |
| Undifferentiated | 21 (7.6%) | 3 (5.1%) | 0 (0.0%) | 0 (0.0%) |
| Missing | 38 (13.7%) | 26 (44.1%) | 13 (32,5%) | 8 (38.1%) |
| P-value | 0.74† | 0.16* | 0.08‡ | |
| Stage in NSCLC§ | ||||
| I | 139 (50.2%) | 16 (27.1%) | 12 (30.0%) | 3 (14.3%) |
| II | 26 (9.4%) | 6 (10.2%) | 1 (2.5%) | 2 (9.5%) |
| III | 65 (23.5%) | 17 (28.8%) | 7 (17.5%) | 11 (52.4%) |
| IV | 46 (16.6%) | 18 (30.5%) | 20 (50.0%) | 5 (23.8%) |
| Missing | 1 (0.4%) | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) |
| P-value | 0.02† | 0.001* | 0.006‡ | |
| Tumor Lung Location | ||||
| Main stem bronchus | 5 (1.7%) | 4 (4.9%) | 2 (4.4%) | 3 (12.5%) |
| Carina | 1 (0.3%) | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) |
| Left hilum | 7 (2.3%) | 5 (6.1%) | 1 (2.2%) | 0 (0.0%) |
| Lingulum | 4 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Left lower lobe | 33 (11.0%) | 12 (14.6%) | 2 (4.4%) | 5 (20.8%) |
| Left upper lobe | 73 (24.4%) | 16 (19.5%) | 14 (31.1%) | 3 (12.5%) |
| Right hilum | 7 (2.3%) | 4 (4.9%) | 1 (2.2%) | 0 (0.0%) |
| Right lower lobe | 47 (15.7%) | 13 (15.9%) | 9 (20.0%) | 2 (8.3%) |
| Right middle lobe | 11 (3.7%) | 5 (6.1%) | 3 (6.7%) | 1 (4.2%) |
| Right upper lobe | 108 (36.1%) | 14 (17.1%) | 12 (26.7%) | 7 (29.2%) |
| Missing | 3 (1.0%) | 8 (9.8%) | 1 (2.2%) | 3 (12.5%) |
| P-value | 0.02† | 1.00* | 0.24‡ |
Abbreviations: N, number; NSCLC; non-small cell lung cancer.
This is the two-sided Fisher's exact test p-value comparing the variable in those with true interval cancers to those with screen-detected lung cancers. Missing values were excluded from the computation.
This is the two-sided Fisher's exact test p-value comparing the variable in those with interval cancers missed in screening to those with screen-detected lung cancers. Missing values were excluded from the computation.
This is the two-sided Fisher's exact test p-value comparing the variable in those with un-reviewed interval cancers to those with screen-detected lung cancers. Missing values were excluded from the computation.
Small cell lung cancers were excluded from these analyses because they were not staged or graded in the same manner as the NSCLC (n=397).
Figure 2.
Examples of findings on second review of screening chest x-rays. A. Prominent left hilum (arrow). B. Prominent right hilum (arrow). C. 1 cm nodule under medial end of right clavicle (arrow). D. 1.5 cm nodule lateral to aortic arch (arrow).
Table 2.
Odds ratios for the association between true interval lung cancer and lung location, adjusted for tumor stage, histology and study centers
| Lung location | OR (95% CI) |
|---|---|
| Right upper lobe | Reference |
| Right hilum | 2.66 (.57-12.45) |
| Right lower lobe | 1.51 (.58-3.91) |
| Right middle lobe | 3.55 (.94-13.38) |
| Left hilum | 2.45 (.57-10.47) |
| Left lower lobe | 3.90 1.46-10.41) |
| Left upper lobe | 1.68 (.71-3.95) |
| Main stem bronchus | 4.99 (.98-25.46) |
| Other/Unknown | 15.71 (2.94-83.89) |
There were 82 subjects with, upon re-review, a negative CXR, implying that their subsequent cancer was a true interval cancer. Although there were no statistically significant associations between sociodemographic and exposure variables at the nominal significance cut-point of 0.05, there were associations that were of sizeable magnitude which approached significance that are noteworthy (Table 1). The univariate analysis suggested that males were more likely to have a true interval cancer (p=0.09). In the multivariable logistic regression model, the following associations suggested increased risk: males versus females (OR = 1.44; 95% CI 0.83 to 2.52; p = 0.197), those with less than a high school education vs. high school completed or above (OR = 1.91; 95% CI 0.98 to 3.73; p = 0.057); and ever-smokers versus never-smokers (OR 3.07; 95% CI 0.67-14.07; p = 0.149).
Compared to screen-detected cancers, true interval NSCLC were significantly more advanced stage IV (30.5% vs 16.6%) and were significantly less early stage I disease (27.1% vs 50.2%) (p = 0.02) (Table 1). There was little difference in the groups by grade for NSCLC, but grade was missing for many cancers. For small cell lung cancers (SCLC), 87% (20/23) of the true interval cancers were classified as extensive versus 42.9% (9/22) of the screen-detected SCLC (p = 0.0018).
Cell types and locations of lung cancers were different for true interval cancers (Table 1). Adenocarcinoma was less common (21/82, 25.6%) compared to screen-detected lung cancers (168/299, 56.2%) and more true interval cancers were SCLC (23/82, 28.1%), as compared with screen-detected cancers (22/299, 7.4%) (p=0.001). True interval cancers were less likely to be located in the right upper lobe (14/82, 17.1%) than screen-detected cancers (108/299, 36.1%) (p<0.02).
Considering tumor characteristic variables together, using multivariable logistic regression, stage, histology and tumor location were significantly associated with true interval cancers: respective likelihood ratio test p-values were 0.009, <0.0001 and 0.012. Compared to stage I, the odds ratio for being a true interval cancer for stages II, III and IV were 1.33 (95% CI 0.46-3.88), 1.38 (95% CI 0.61-3.15) and 3.58 (1.63-7.88), respectively. Compared to adenocarcinoma, the odds of a true interval cancer being a non-adenocarcinoma NSCLC were 3.68 fold greater (95% CI 1.83-7.42) and of being a SCLC were 5.52 fold greater (95% CI 2.24-13.62). The odds ratios associated with lung locations are presented in Table 2, indicating that true interval tumors were consistently more likely to be located elsewhere than the right upper lobe.
4. Discussion
Lung cancers that are not seen on a screening CXR may either not exist at the time the CXR is taken, be radiographically occult, or simply be missed when the CXR is interpreted. There may be characteristics peculiar to some lung cancers that will defeat the efficacy of any screening tool. We have analyzed subjects from the PLCO in an effort to compare the true interval cancers that were not detected by CXR screening to those that were detected by CXR screening.
Our data suggested that several characteristics distinguished true interval cancers as being different from screen-detected cancers. True interval cancers were more likely to occur in males and those with less than a high school education, although there were no notable differences by race/ethnicity. Smoking (current and previous) was associated with interval cancers compared to no history of smoking; however, this study did not have the power to definitively demonstrate these associations. True interval cancers were more often advanced stage (III – IV) and were different histologically. There were more SCLC. This was not unexpected since SCLC are known to be more aggressive and grow rapidly. However, we speculate that some NSCLC that are true interval cancers may be biologically different than most NSCLC and have more aggressive behavior.[10] These aggressive tumors (both SCLC and NSCLC) may simply not have existed or have been radiographically occult when the screening CXR was taken, and they appeared and progressed rapidly sometime during the next 12 months.
Upon re-reviewing the CXR, 17 of the 45 probably present cancers were manifest on re-review as an enlarged hilum and another four were obscured by the heart or the great vessels of the mediastinum. The cancers we classified as true interval cancers were more often central in the hilum or adjacent to the mediastinum, which suggests that some of these may also have been present but that the screening CXR was not sufficiently sensitive to identify them and they were therefore misclassified.
The CXR of 35% of the persons initially classified as negative but with cancers diagnosed within 12 months were characterized upon re-review as suspicious for lung cancer. As the second interpretations were done with knowledge that each of the subjects had a lung cancer that was diagnosed within twelve months from the time that the CXR was taken, there was a clear bias to find an abnormality that could be characterized as suspicious for lung cancer, although the readers were blinded as to the location of the diagnosed cancer. In the literature, missed NSCLC that presented as peripheral nodules on chest radiographs has varied between 19% and 90% [11-14]. The explanation given for missing abnormalities is usually that other superimposed structures obscure the abnormality in as many as 71% of the cases [12]. However, fully 65% of the second reviews led to agreement with the first interpretation that the CXR was normal.
More advanced stage and a disproportionate number of SCLC for interval cancers (as well as the presence of symptoms) are the only characteristics that have been described as distinguishing features of interval cancers in most earlier publications of cancer-screening trials [5;8;12], which our study confirms. Lung cancer arises more frequently in the right upper lobe [11;15;16], but the true interval cancers in the PLCO were less commonly located in the right upper lobe than were screen-detected cancers. There are probably some lung cancers that are so fast-growing and aggressive that perhaps no screening test will ever prove to be useful.
5.0 Conclusion
CXR screening in PLCO detected two-thirds of lung cancers that occurred in the screening arm of the Trial during the screening period, but one-third of the lung cancers that occurred in this arm were outside of the screening process. Approximately 35% of the non-screen-detected cancers were probably detectable at the time of the screening CXR, but were not recognized by the radiologist during the initial interpretation. Those defined as true interval cancers were more often stage III – IV NSCLC and small cell type classified as extensive, less often in the right upper lobe and more common among males, lower educated individuals, and those with a history of smoking.
Acknowledgments
Funding
This work was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, to the 10 screening centers and to the coordinating center (N01-CN-25476, to Westat; N01-CN-25512, to Henry Ford Health System; N01-CN-25518, to Marshfield Clinic Research Foundation; N01-CN-25513, to the University of Minnesota School of Public Health; N01-CN-25511, to the University of Pittsburgh Cancer Institute; N01-CN-25514, to the University of Colorado; N01-CN-25515, to Pacific Health Research and Education Institute; N01-CN-25516, to Washington University; N01-CN-25522, to Georgetown University Medical Center; N01-CN-25524, to the University of Utah; and N01-CN-75022, to the University of Alabama at Birmingham).
Abbreviations list
- CXR
chest x-ray
- LDCT
low dose helical computed tomography
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- NIOSH
National Institute of Occupational Safety and Health
- FET
Fisher's exact test
- CI
confidence interval
- OR
odds ratio
- LRT
likelihood ratio test
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- RUL
right upper lobe
- RML
right middle lobe
- RLL
right lower lobe
- LUL
left upper lobe
- LLL
left lower lobe
- L
left
- R
right
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov number, NCT00002540
PAK is a board-certified pulmonologist who has been certified for 28 years as a “B” reader by the National Institute of Occupational Safety and Health (NIOSH), and CJZ and DLS are board-certified radiologists with added proficiency in thoracic radiology.
Conflict of interest statement
None of the authors has any conflict of interest.
References
- 1.Siegel R, Ma J, Zhaohui Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–65. doi: 10.1164/arrd.1984.130.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Fontana RS. Screening for lung cancer: recent experience in the United States. In: Hansen HH, editor. Lung Cancer: Basic and Clinical Aspects. Martinus Nijhoff Publishers; Boston: 1986. pp. 91–111. [Google Scholar]
- 6.Frost JK, Ball WC, Jr., Levin ML, Tockman MS, Baker RR, Carter D, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–54. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 7.Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis. 1984;130:555–60. doi: 10.1164/arrd.1984.130.4.555. [DOI] [PubMed] [Google Scholar]
- 8.Kubik A, Parkin DM, Khlat M, Erban J, Polak J, Adamec M. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer. 1990;45:26–33. doi: 10.1002/ijc.2910450107. [DOI] [PubMed] [Google Scholar]
- 9.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–73. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 10.Tammemagi CM, Freedman MT, Church TR, Oken MM, Hocking WG, Kvale PA, et al. Factors associated with human small aggressive non small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2082–89. doi: 10.1158/1055-9965.EPI-07-0251. [DOI] [PubMed] [Google Scholar]
- 11.Muhm JR, Miller WE, Fontana RS, Sanderson DR, Uhlenhopp MA. Lung cancer detected during a screening program using four-month chest radiographs. Radiology. 1983;148:609–15. doi: 10.1148/radiology.148.3.6308709. [DOI] [PubMed] [Google Scholar]
- 12.Quekel LG, Kessels AG, Goei R, van Engelshoven JM. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest. 1999;115:720–24. doi: 10.1378/chest.115.3.720. [DOI] [PubMed] [Google Scholar]
- 13.Forrest JV, Friedman PJ. Radiologic errors in patients with lung cancer. West J Med. 1981;134:485–90. [PMC free article] [PubMed] [Google Scholar]
- 14.Giger ML, Doi K, MacMahon H, Metz CE, Yin FF. Pulmonary nodules: computer-aided detection in digital chest images. Radiographics. 1990;10:41–51. doi: 10.1148/radiographics.10.1.2296696. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey EW, Smart CR, Winchester DP, Steele GD, Jr., Yarbro JW, Chu KC, et al. National survey of the pattern of care for carcinoma of the lung. J Thorac Cardiovasc Surg. 1990;100:837–43. [PubMed] [Google Scholar]
- 16.Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest. 1984;86:44–53. doi: 10.1378/chest.86.1.44. [DOI] [PubMed] [Google Scholar]