Abstract
Clinical guidelines recommend concurrent treatment of anemia in end-stage renal disease with erythropoiesis-stimulating agents (ESAs) and iron. However, there are mixed data about optimal iron supplementation. To help address this gap, the relationship between iron markers and hemoglobin (Hb) response to ESA (Epoetin alfa) dose was examined. Electronic medical records of 1902 US chronic hemodialysis patients were analyzed over a 12-month period between June 2009 and June 2010. The analysis included patients who had at least one Hb value during each 4-week interval for four consecutive intervals (k − 2, k − 1, k, and k + 1; k is the index interval), received at least one ESA dose during intervals k − 1 or k, had at least one transferrin saturation (TSAT) value at interval k, and at least one ferritin value during intervals k − 2, k − 1, or k. Effect modification by TSAT and ferritin on Hb response was evaluated using the generalized estimating equations approach. Patients had a mean (standard deviation) age of 62 (15) years; 41% were Caucasian, 34% African American, 65% had hypertension, and 39% diabetes. Transferrin saturation, but not ferritin, had a statistically significant (P < 0.05) modifying effect on Hb response. Maximum Hb response was achieved when TSAT was 34%, with minimal incremental effect beyond these levels. Of the two standard clinical iron markers, TSAT should be used as the primary marker of the modifying effect of iron on Hb response to ESA. Long-term safety of iron use to improve Hb response to ESA warrants further study.
Keywords: Anemia, end-stage renal disease, iron, erythropoiesis-stimulating agent, hemoglobin
Introduction
Iron deficiency and iron-restricted erythropoiesis contribute to anemia in end-stage renal disease (ESRD) and have been identified as factors contributing to resistance to erythropoiesis-stimulating agents (ESAs). Consistent with the Epoetin alfa and Darbepoetin alfa package inserts,1,2 the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative as well as the Kidney Disease: Improving Global Outcomes 2012 guidelines and Best Practice Guidelines of the European Renal Association recommend concurrent iron supplementation with ESA use.3,4
While intravenous (i.v.) iron therapy is an accepted treatment for ESRD-related anemia, optimal dosing and monitoring of iron therapy is not well established. Multiple observational studies have linked various levels of transferrin saturation (TSAT) and ferritin with low hemoglobin (Hb) values, hospitalization, mortality, and compromised Hb response.5–8 Serum iron and TSAT have a significant inverse association with mortality and hospitalization, while increases in serum ferritin have been associated with higher short-term hospitalization and mortality.5,7
A limited number of clinical trials have demonstrated that achieving higher levels of TSAT and ferritin levels result in increased Hb concentrations and decreased ESA requirements.9,10 A randomized clinical trial enrolling 42 maintenance hemodialysis (HD) patients to one of two iron treatment strategies showed that i.v. iron administration to maintain a TSAT >30% reduced ESA requirement by 40%. However, the relationship between iron biomarkers and Hb response to Epoetin alfa was not elucidated in this study.9 In the DRIVE study, administration of 1 g of i.v. iron over eight consecutive dialysis sessions or no i.v. iron was compared in 134 dialysis patients with Hb <11 g/dL, ferritin 500–1200 ng/mL, TSAT <25%, and Epoetin alfa dose >225 IU/kg/wk or >22,500 IU/wk, resulting in higher mean Hb (11.9 vs. 11.3 g/dL) at week 6.10 However, in both trials,9,10 the study period was short, and long-term efficacy or safety could not be established.
These observational studies and clinical trials suggest that there may be considerable Hb response at levels of TSAT and ferritin higher than those recommended by the guidelines, and furthermore, there may be substantial variability in the biological effect of i.v. iron at different levels of TSAT and ferritin. These observations suggest that TSAT and ferritin modify the observed Hb response to a change in ESA dose. To further explore this observation, a nonlinear regression model was recently developed that considered iron markers as effect modifiers of Hb response in a small cohort of maintenance HD patients.11 The model showed that TSAT >30% and ferritin within the range of 350–500 ng/mL were individually associated with the maximum Hb response to ESA dose. Building upon the general principles of this model,11 we performed a retrospective analysis of electronic medical records (EMRs) data obtained from hospital-based dialysis centers (HBDCs) and independent dialysis organizations (IDOs). Specifically, we focused on the interaction between varying levels of TSAT and ferritin and ESA dose in predicting Hb response among US chronic HD patients.
Materials and Methods
Study design, data, and cohort
In this retrospective observational cohort study, patients were selected from an EMR dataset comprised of approximately 11,000 chronic dialysis patients in 59 HBDCs and 47 IDOs across different geographic regions in the United States. Dataset information was collected by a single EMR provider from dialysis centers using proprietary EMR software to manage dialysis care. The research dataset (acquired by IMS Health U.S., Plymouth Meeting, PA) is Health Insurance Portability and Accountability Act of 1996 compliant and contains de-identified longitudinal patient information such as patient demographics, laboratory results, dialysis sessions, and administered medications.
Hemodialysis patients in the EMR database who had at least one qualifying condition, characterized by stable intra-patient Hb measurements and contemporaneous measurements of TSAT and ferritin, were identified. A qualifying condition was defined by the following criteria (Figure 1) during the period from June 1, 2009 through June 30, 2010:
At least one Hb measurement at each interval for four consecutive 4-week intervals (interval k − 2, k − 1, k, and k + 1), where k is the index period.
At least one TSAT measurement in interval k.
At least one ferritin measurement within intervals k − 2, k − 1, or k.
At least one Epoetin alfa i.v. administration within intervals k − 1 or k.
Figure 1.
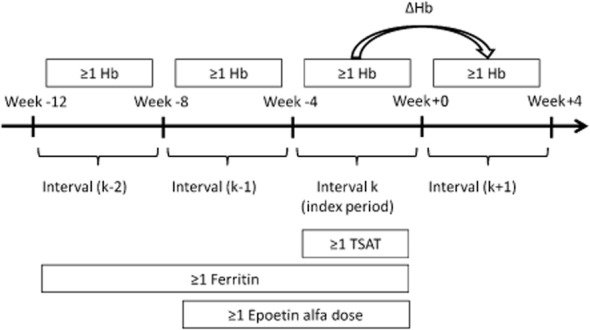
Treatment intervals k − 2 to k + 1 with at least one hemoglobin (Hb) measurement within each interval. Interval k is the index period with at least one transferrin saturation (TSAT) measurement. ΔHb = change of mean Hb from interval k to interval k + 1; ≥1 Hb = at least one Hb measure in each 4-week interval; ≥1 TSAT = at least one TSAT value in interval k; ≥1 ferritin = at least one ferritin value within intervals k − 2, k − 1, or k; ≥1 Epoetin alfa dose = at least one Epoetin alfa i.v. administration within interval k − 1 or k.
Patients could have more than one qualifying condition. Patients were excluded if they were less than 18 years of age as of June 1, 2009; had unknown gender; or had a history of Darbepoetin alfa or Epoetin alfa subcutaneous (s.c.) administration during the period from June 1, 2009 through June 30, 2010.
Model development
The effect of iron on Hb response to Epoetin alfa (ESA) was evaluated using a polynomial regression model with interaction terms (Table 1). The response variable of the model was defined as a change in mean Hb from interval k to k + 1 due to change in ESA dose from the previous time interval of k − 1 to k. The modifying effect of iron on Hb response to ESA was represented by multiplicative interaction between the iron marker and change in ESA dose term in the model equation. From a physiologic standpoint, when ESA dose is increased, the subsequent rise in Hb depends on iron availability. To better understand this interaction, we explored three models:
“TSAT-only” model (TSAT), in which TSAT is used as the single marker of iron status.
“Ferritin-only” model (Ferr), in which ferritin is used as the single marker of iron status.
Joint TSAT and ferritin (TSAT, Ferr) model, including both TSAT and ferritin as markers of iron status.
Table 1.
Polynomial regression models of Hb response
| TSAT model | ΔHb(k + 1) = β1ΔESA(k) + β2ΔESA(k)TSAT(k) + β3ΔESA(k)TSAT2(k) + β4ΔESA(k)TSAT3(k) + β5TSAT(k) + β6TSAT2(k) + β7TSAT3(k) + β8ΔIron(k) + β9Cv1(k) + … + β9+m-1Cvm(k) + β0 |
| Ferritin model | ΔHb(k + 1) = β1ΔESA(k) + β2ΔESA(k)Ferr(k) + β3ΔESA(k)Ferr2(k) + β4ΔESA(k)Ferr3(k) + β5Ferr(k) + β6Ferr2(k) + β7Ferr3(k) + β8ΔIron(k) + β9Cv1(k) + … + β9+m-1Cvm(k) + β0 |
| TSAT + ferritin model | ΔHb(k + 1) = β1ΔESA(k) + β2ΔESA(k)TSAT(k) + β3ΔESA(k)TSAT2(k) + β4ΔESA(k)TSAT3(k) + β5ΔESA(k)Ferr(k) + β6ΔESA(k)Ferr2(k) + β7ΔESA(k)Ferr3(k) + β8TSAT(k) + β9TSAT2(k) + β10TSAT3(k) + β11Ferr(k) + β12Ferr2(k) + β13Ferr3(k) + β14ΔIron(k) + β15Cv1(k) + … + β15+m-1Cvm(k) + β0 |
β = unknown regression coefficients; ΔESA = change in weight-adjusted Epoetin alfa dosing from interval k − 1 to k; ΔHb = change in hemoglobin concentration from interval k to k + 1; ΔIron = change in weight-adjusted iron dosing from interval k − 1 to k; Cv1 through Cvm = additional model covariates; Ferr = serum ferritin level at interval k; TSAT = transferrin saturation level at interval k.
Following the approach outlined in Gaweda et al.,11 the effect modification functions can be obtained by factoring the change in ESA dose term out of the interactions:
| (1) |
| (2) |
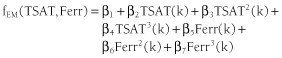 |
(3) |
These equations represent the change in Hb response associated with the iron markers, and the degree of the effect modification is determined by the regression coefficients β1 … β7, which are estimated from the data.
The change in Hb response was normalized to a 0.00–1.00 range, where 1.00 represents maximum change in Hb response and lower values represent proportionally lower relative Hb response at different biomarker levels. For example, a relative Hb response of 0.80 represents 80% of the maximum change in Hb response.
To control for potential confounding, all models included the following additional covariates: (1) the change in cumulative weight-adjusted Epoetin alfa doses from interval k − 1 to k; (2) the change in cumulative weight-adjusted i.v. iron doses from interval k − 1 to k; and (3) the mean Hb at intervals k − 2, k − 1, and k: age, gender, race (i.e., African American, Caucasian, and others), census region (i.e., northeast, midwest, west, and south), dialysis vintage (i.e., <6 months, 6 to <12 months, 1 to <5 years, ≥5 years), dialysis adequacy (Kt/V), dialysis access type (i.e., catheter, fistula, or graft), body mass index, congestive heart failure (i.e., yes or no), and diabetes (i.e., yes or no) at interval k, as well as mean albumin (g/dL) at interval k − 1 and use of i.v. antibiotics between intervals k − 2 and k (i.e., yes or no).
To eliminate possible outliers and erroneous data entries, records meeting the following were included in model estimation: Hb values of 5–20 g/dL, TSAT values of 4–100%, ferritin values of >0 ng/mL, Kt/V values >0 and ≤10, and postdialysis weight of 30–200 kg.
Each model was estimated using the generalized estimating equations (GEE)12 approach to account for intra-subject correlations due to repeated measurements over time in the data. Generalized estimating equation model correlation structures (i.e., auto-regressive, unstructured, independent, and exchangeable) for each of the three models were compared to assess model fit using the Quasi-Akaike Information Criteria (QIC and QIC_u),13 which are fit statistics for likelihood-based models. The exchangeable correlation structure was used for all models based on fit statistics. Statistical significance of parameter estimates was evaluated at the 0.05 level, with no adjustment for multiplicity. All statistical analyses were performed using Stata version 12 (StataCorp LP, College Station, TX, USA).
Results
The analysis included 1902 patients with mean (standard deviation [SD]) age 62 (15) years; 41% were Caucasian and 34% African American; 65% of patients had hypertension and 39% diabetes (Table 2). Across all patients, 7685 qualifying conditions were included in the analysis (Table 3). The mean (SD) of TSAT and ferritin were 30.3% (13.8) and 627.4 (327.8) ng/mL, respectively. The distribution of TSAT was skewed, as 80% of TSAT values were between 10% and <40%, and 15% were between 40% and <60% (Figure 2A). Similarly, 82% of ferritin values were between 200 and <1000 ng/mL, while 8% were between 1000 and <1400 ng/mL (Figure 2B). The joint distribution of TSAT and ferritin was also skewed; 66% of values were between 10% and <40% for TSAT and 200 and <1000 ng/mL for ferritin, and another 19% of values were between 40% and <60% for TSAT and 1000 and <1400 ng/mL for ferritin (Figure 2C).
Table 2.
Patient demographics (N = 1902)
| Variables |
Mean (SD) |
Median (25%, 75%) |
|---|---|---|
| Age, years | 62.0 (14.9) | 63.0 (52.0, 75.0) |
| n | % | |
| Age group, years | ||
| 18–44 | 247 | 13.0 |
| 45–64 | 792 | 41.6 |
| 65–74 | 368 | 19.4 |
| 75+ | 495 | 26.0 |
| Gender | ||
| Female | 886 | 46.6 |
| Male | 1016 | 53.4 |
| Census region | ||
| Northeast | 380 | 20.0 |
| Midwest | 968 | 50.9 |
| South | 394 | 20.7 |
| West | 160 | 8.4 |
| Race | ||
| African American | 647 | 34.0 |
| Caucasian | 782 | 41.1 |
| Other | 473 | 24.9 |
| Comorbid conditions | ||
| Congestive heart failure | 466 | 24.5 |
| Diabetes | 750 | 39.4 |
| Hypertension | 1244 | 65.4 |
Table 3.
Mean (standard deviation) and median (25%, 75%) laboratory values at interval k by number of qualifying conditions (N = 7685)
| Variable | Mean (SD) | Median (25%, 75%) | Min, Max |
|---|---|---|---|
| Hb (g/dL) | 11.4 (1.1) | 11.5 (10.8, 12.1) | 6.7, 17.5 |
| TSAT (%) | 30.3 (13.8) | 28.0 (21.0, 37.0) | 4.0, 99.0 |
| Ferritin (ng/mL) | 627.4 (327.8) | 602.0 (394.0, 830.0) | 8.0, 3433.0 |
| Epoetin alfa (1000 U/4 wks) | 56.4 (63.8) | 35.0 (15.0, 72.0) | 0.0, 440.0 |
Ferritin values were averaged over observed values within intervals k − 2, k − 1, and k, where 82% of values were observed during interval k.
Hb = hemoglobin, TSAT = transferrin saturation, U = units, wks = weeks.
Figure 2.
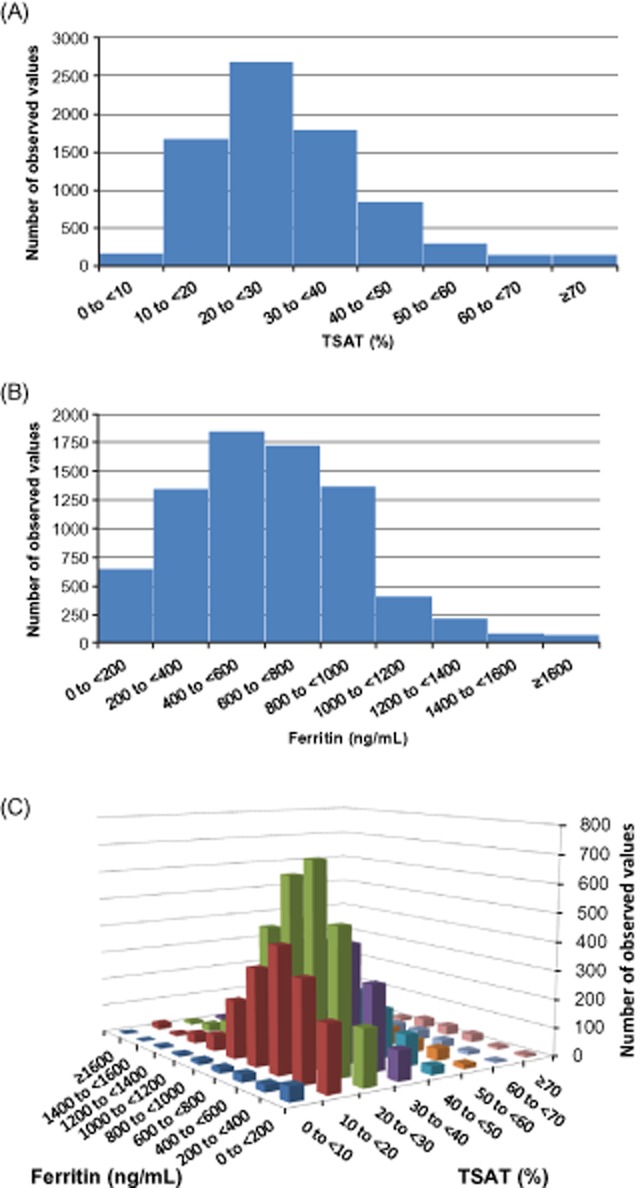
(A–C) Distribution of observed transferrin saturation (TSAT) and ferritin values during interval k, which are used in polynomial regression models.
All three GEE models had similar QIC and QIC_u fit statistics. Change in iron dose (P <0.001), Hb at interval k and k − 1 (P < 0.001), dialysis adequacy (P < 0.05), the northeast and south regions (P < 0.05), as well as for the African American race (P < 0.05) covariates were statistically significant across the three models (data not shown), and are interpreted at the population level. All interaction terms between TSAT and ESA dose change were statistically significant (Table 4). However, all interaction terms between ferritin and ESA dose change were not statistically significant.
Table 4.
Parameter estimates of effect modification covariates in hemoglobin change models
| Covariate | Model |
|||||
|---|---|---|---|---|---|---|
| TSAT |
Ferritin |
TSAT + Ferritin |
||||
| Estimate | P | Estimate | P | Estimate | P | |
| ΔESA | −0.03 | 0.84 | 0.36 | <0.001 | −0.11 | 0.48 |
| ΔESA*TSAT | 0.04 | <0.001 | – | – | 0.04 | <0.01 |
| ΔESA*TSAT2 | −9.2E–04 | <0.01 | – | – | −9.0E–04 | <0.01 |
| ΔESA*TSAT3 | 5.9E–06 | <0.01 | – | – | 5.8E–06 | <0.01 |
| ΔESA*Ferr | – | – | 5.0E–04 | 0.07 | 3.8E–04 | 0.15 |
| ΔESA*Ferr2 | – | – | −2.6E–07 | 0.34 | −2.3E–07 | 0.39 |
| ΔESA*Ferr3 | – | – | 4.2E–11 | 0.54 | 4.25E–11 | 0.54 |
ΔESA = weight-adjusted dose change of Epoetin alfa from interval k to k + 1; ΔESA*Ferr = interaction between adjusted Epoetin alfa dose change and ferritin; ΔESA*Ferr2 = interaction between adjusted Epoetin alfa dose change and ferritin2; ΔESA*Ferr3 = interaction between adjusted Epoetin alfa dose change and ferritin3; ΔESA*TSAT = interaction between adjusted Epoetin alfa dose change and TSAT; ΔESA*TSAT2 = interaction between adjusted Epoetin alfa dose change and TSAT2; ΔESA*TSAT3 = interaction between adjusted Epoetin alfa dose change and TSAT3.
Effect modification by TSAT
Figure 3 depicts the change in Hb response as a function of TSAT (Equation (1)). Maximum Hb response to ESA was reached at TSAT of 35%. As indicated by the increasing slope of the curve, increasing TSAT from 0% to 10% leads to a strong improvement in Hb response. Each subsequent increase in TSAT, from 10% to 20% and again from 20% to 30%, results in diminished improvement in Hb response by approximately one-half. Finally, when TSAT changes between 30% and 40%, there is no apparent improvement in Hb response. Hemoglobin response to ESA appeared to decrease at TSAT levels above 40%, but caution should be used when interpreting this result because of data sparseness at higher TSAT values.
Figure 3.
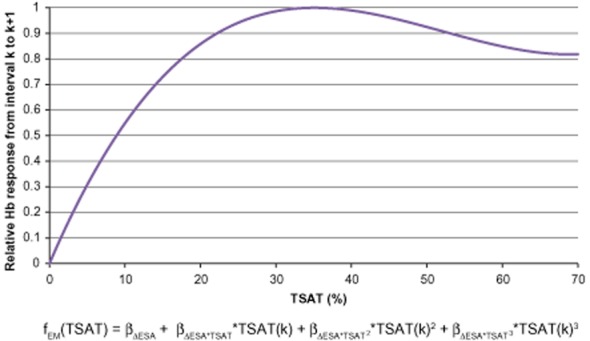
Relative hemoglobin (Hb) response from interval k to k + 1 to a fixed-unit Epoetin alfa dose change from interval k − 1 to k across transferrin saturation (TSAT) values, derived from the TSAT model. ESA = erythropoiesis-stimulating agent.
Effect modification by ferritin
Figure 4 shows the change in Hb response as a function of ferritin level (Equation (2)). As indicated by the change in the slope of the curve, increasing ferritin from 0 to 200 ng/mL leads to the strongest improvement in Hb response. Increases in ferritin levels from 200 to 500 ng/mL and from 500 to 800 ng/mL result in constantly diminishing improvements in Hb response. Although the maximum Hb response to ESA appears to be above 1300 ng/mL, the apparent improvement in Hb response as ferritin increases beyond 800 ng/mL is clinically negligible.
Figure 4.
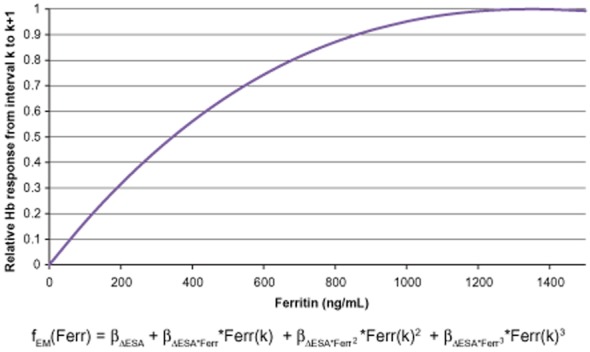
Relative hemoglobin (Hb) response from interval k to k + 1 to a fixed-unit Epoetin alfa dose change from interval k − 1 to k across ferritin values, derived from the ferritin model. ESA = erythropoiesis-stimulating agent.
Effect modification by TSAT and ferritin
The joint effect of both TSAT and ferritin on change in Hb response after ESA dose increase is shown in Figure 5. The maximum Hb response was achieved when TSAT was 34% and ferritin was 1300 ng/mL. When TSAT was 34% and ferritin was 500 ng/mL, relative Hb response was 92% of its maximum value. The shape of the surface plot in Figure 5 shows that increases in TSAT over the range from 0% to 35% result in a steeper increase in Hb response than changes in ferritin over the entire range of physiologically plausible values.
Figure 5.
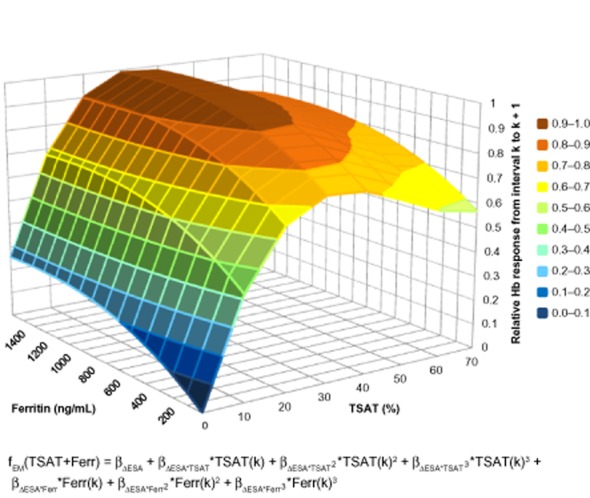
Relative hemoglobin (Hb) response from interval k to k + 1 to a fixed-unit Epoetin alfa dose change from interval k − 1 to k across transferrin saturation (TSAT) and ferritin values, derived from the TSAT + ferritin model. Relative Hb response is 92% of its maximum value when TSAT is 34% and ferritin is 500 ng/mL. ESA = erythropoiesis-stimulating agent.
Discussion
In order to understand the relationship between levels of TSAT and ferritin, ESA dose change and Hb response, we developed polynomial regression models relating Hb response to ESA dose as a function of iron marker levels. Treatment of iron markers as effect measure modifiers is plausible on the basis of both previous observational evidence6,8,11 and known biology because in normal iron homeostasis, varying levels of ferritin and TSAT reflect the differences in iron stores and iron available for incorporation into heme, respectively.
We observe that when ESA dose is increased, the associated rise in Hb depends on the level of TSAT and ferritin. Increases in TSAT led to incrementally greater increases in Hb response at lower TSAT ranges, but the relative impact on Hb response plateaus at TSAT levels between 30% and 40%. This finding is consistent with the observation that in normal iron homeostasis, about one-third of transferrin iron-binding sites are occupied by iron molecules.14 This translates to a TSAT level of about 33.3%, which is very close to our reported optimal TSAT levels. Furthermore, Hb response to ESA improves markedly over the range of TSAT from 0% to 20%, consistent with the known relationship between iron deficiency and ESA resistance. There appeared to be little additional effect at TSAT levels above 34%. The somewhat counterintuitive decrease in Hb response at TSAT levels above 40% may be an artifact due to sparse data in that TSAT range. However, this phenomenon could potentially be attributed to decrease in total iron-binding capacity due to protein energy wasting,15 a common comorbidity in renal disease that is associated with poor response to ESA.16
Similarly for ferritin, incrementally greater increases in Hb response were observed at lower levels of ferritin with a plateau in Hb response at higher ferritin values. With regard to ferritin, Gaweda et al.'s original study11 showed a bell-shaped effect modification function, with a steep increase in ESA response at very low levels of ferritin, consistent with the known effect of iron deficiency. Maximum Hb response to ESA was observed at ferritin levels between 350 and 500 ng/mL, with declining effect at higher levels of ferritin. Our present study shows quite a different trend, with Hb response to ESA increasing monotonically over the range of ferritin values. In the combined TSAT and ferritin model, maximum Hb response to ESA is achieved at ferritin levels of 1300 ng/mL when TSAT is held constant, but the relationship between ferritin and Hb response is nonlinear, with 92% of maximum Hb response achieved at ferritin levels of 500 ng/mL. Our result is surprising, as no effect of absolute iron deficiency is observed nor is there any decline at high ferritin that could be conceptualized as the effect of inflammatory blockade of reticuloendothelial stores of iron.
The differences between Gaweda et al.'s previous finding11 and our current study are likely to be due to differences in the populations being observed. The original study used a dataset from a single dialysis center collected over the 5-year period between 1996 and 2001. Our present study uses data from multiple providers collected over a much more recent time frame. Differences may result from secular trends in anemia management. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Dialysis Practice Monitor reports trends in dialysis care in the United States during and after the transition to a new prospective payment system.17 Reports from DOPPS show a progressive rise in i.v. iron use during calendar year 2010, a moderate reduction in use of Epoetin alfa, slight downward trends in achieved Hb concentrations, and increases in average TSAT and ferritin.18 It is important to note that high ferritin levels can indicate either increased stores of iron or acute inflammatory response. As inflammation is known to compromise Hb response to ESA, the decrease in Hb response as ferritin increases above 500 ng/mL reported in the original study11 may have been predominantly due to ferritin acting as a marker of inflammation. The change in the observed effect modification function of ferritin might be due to changes in patterns of i.v. iron use. Perhaps in the current cohort, higher levels of ferritin are less likely to reflect inflammatory blockade and are more likely to be a marker of increased demand for iron storage resulting from i.v. iron doses. Measurements of clinical markers of inflammation, such as C-reactive protein, were unavailable in our current dataset.
While we observe increasing Hb response to ESA over the range of high ferritin values, it is important to note that on the whole, parameter estimates for the model using ferritin as the single iron marker, as well as the combined TSAT and ferritin model, indicate that ferritin is not a statistically significant (P < 0.05) predictor of Hb response to ESA. Therefore, our findings are inconclusive with regard to the use of ferritin as the laboratory marker to guide i.v. iron dosing. Additionally, there are safety concerns related to the use of i.v. iron to target higher levels of iron markers in pursuit of a minimum ESA dose. Free iron levels increase acutely after administration of i.v. iron. Because bacteria require free iron to replicate, there is theoretical concern that i.v. iron use can promote the risk of infection in chronic dialysis patients, although this effect has not been observed in the largest observational study to date.19 Levels of oxidative stress are also increased in response to i.v. iron, an effect that may contribute to the excess burden of cardiovascular disease in ESRD.20 In addition, a recent study of adverse events (AEs) reported to the US Food and Drug Administration indicated significant differences among i.v. iron products in the rates of death, serious nonfatal AEs, and all AE classifications combined.21 Of note, in a recent prospective study of 119 stable HD patients receiving i.v. iron and ESA, iron overload was observed in 84% of the patients, with levels of hepatic iron approaching those seen in hemochromatosis in some patients.22 The longer term consequences of high cumulative doses of i.v. iron and high-cellular iron levels resulting from i.v. iron therapy in ESRD remain unknown and are not addressed by our current study. Long-term prospective studies examining the safety of various types of chronic i.v. iron therapy and high levels of cellular iron stores are needed. Dialysis providers should balance these safety concerns when administering i.v. iron to manage anemia and consider individualizing care when targeting higher iron marker levels to achieve optimal Hb response to ESA.
In summary, the current analysis makes use of a large contemporary dataset that reflects current trends in the management of HD patients in the United States to evaluate the effect of varying levels of TSAT and ferritin in predicting Hb response to ESA. We found that TSAT, but not ferritin, had a statistically significant modifying effect on Hb response to changes in ESA dose. Given the possible risks of excessive i.v. iron dosing, including infection and iron overload, our data support the use of TSAT as a better marker than ferritin of i.v. iron therapy.
Acknowledgments
The authors wish to thank Holly Tomlin (employee and stockholder, Amgen Inc.) and Linda Rice (employee and stockholder, Amgen Inc.) for their manuscript editing and formatting assistance. All authors contributed to the writing of this manuscript according to the International Committee of Medical Journal Editors criteria.
References
- EPOGEN®. Thousand Oaks, CA: Amgen Inc; 2012. (Epoetin Alfa) Package Insert. [Google Scholar]
- Aranesp®. Thousand Oaks, CA: Amgen Inc; 2012. (Darbepoetin Alfa) Package Insert. [Google Scholar]
- Locatelli F, Nissenson AR, Barrett BJ, et al. Clinical practice guidelines for anemia in chronic kidney disease: Problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2008;74:1237–1240. doi: 10.1038/ki.2008.299. [DOI] [PubMed] [Google Scholar]
- NKF. National Kidney Foundation Dialysis Outcomes Quality Initiative: Clinical practice guidelines: Anemia of chronic renal failure. Am J Kidney Dis. 2001;37:S182–S238. [Google Scholar]
- Kalantar-Zadeh K, Don BR, Rodriguez RA, et al. Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis. 2001;37:564–572. [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Lee GH, Miller JE, et al. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53:823–834. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, McAllister CJ, Lehn RS, et al. A low serum iron level is a predictor of poor outcome in hemodialysis patients. Am J Kidney Dis. 2004;43:671–684. doi: 10.1053/j.ajkd.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Regidor DL, McAllister CJ, et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- Besarab A, Amin N, Ahsan M, et al. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol. 2000;11:530–538. doi: 10.1681/ASN.V113530. [DOI] [PubMed] [Google Scholar]
- Coyne DW, Kapoian T, Suki W, et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- Gaweda AE, Goldsmith LJ, Brier ME, et al. Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol. 2010;5:576–581. doi: 10.2215/CJN.04710709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7:209–220. [Google Scholar]
- Tsagalis G. Renal anemia: A nephrologist's view. Hippokratia. 2011;15:39–43. [PMC free article] [PubMed] [Google Scholar]
- Bross R, Zitterkoph J, Pithia J, et al. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am J Nephrol. 2009;29:571–581. doi: 10.1159/000191470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, McAllister CJ, Lehn RS, et al. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- Robinson B, Fuller D, Zinsser D, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: Rationale and methods for an initiative to monitor the new US bundled dialysis payment system. Am J Kidney Dis. 2011;57:822–831. doi: 10.1053/j.ajkd.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOPPS Practice Monitor. 2013. Available from: http://www.dopps.org (accessed date: February 21)
- Hoen B, Paul-Dauphin A, Hestin D, et al. EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869–876. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- Drueke T, Witko-Sarsat V, Massy Z, et al. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation. 2002;106:2212–2217. doi: 10.1161/01.cir.0000035250.66458.67. [DOI] [PubMed] [Google Scholar]
- Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm. 2012;69:310–320. doi: 10.2146/ajhp110262. [DOI] [PubMed] [Google Scholar]
- Rostoker G, Griuncelli M, Loridon C, et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: A MRI study. Am J Med. 2012;125:991.e1–999.e1. doi: 10.1016/j.amjmed.2012.01.015. [DOI] [PubMed] [Google Scholar]


