Abstract
Background
Keloid and hypertrophic scar (HS) are two pathological forms of excessive dermal fibrosis, which are due to aberrant wound-healing responses. Accumulating evidence suggests that aberrant activity of growth factors and increased numbers of growth factor receptors play an important role in the formation of pathological scar.
Aim
We examined the expression level of insulin-like growth factor-1 receptor (IGF-IR) in keloid, HS and normal skin.
Methods
IGF-IR expression was analyzed by immunohistochemistry, real-time PCR and western blotting on tissues and fibroblasts from 30 patients, comprising 10 patients with keloid and 20 with HS (10 with immature and 10 with mature HS), and from 10 age-matched and sex-matched healthy controls.
Results
Immunoreactivity to IGF-IR was found in dermal fibroblasts of keloid (90%), immature HS, (80%) and mature HS (30%), but not in normal skin. There was no statistically significant difference in immunoreactivity scores between keloid and immature HS, but there was a significant difference (P < 0.01) between mature and immature HS. Real-time PCR and western blot analysis confirmed that there was high expression of IGF-IR in keloid and immature HS fibroblasts, but not in mature HS or normal skin fibroblasts. IGF-IR was expressed in the overlying epidermis, and there was no significant difference between the groups.
Conclusions
IGF-IR may be involved in the pathogenesis of keloid and HS. Given that IGF-IR are predominantly expressed on dermal fibroblasts, targeting of IGF-IR in fibroblasts may be of benefit to prevent scarring.
Introduction
Keloid and hypertrophic scar (HS) are two pathological forms of excessive dermal fibrosis, which are due to aberrant wound-healing responses. Both produce similar uncomfortable signs and symptoms, and both are characterized by excessive proliferation of fibroblasts and overproduction of extracellular matrix (ECM). However, unlike HS, keloid is characterized by invasion, immortality and a high rate of recurrence after surgery. Histopathologically, the presence of distinct collagenized nodules in the dermis is a characteristic feature of HS, whereas the absence of collagenized nodules and the presence of thick collagen bundles characterize keloid.1 In addition, the major components of HS are α-smooth muscle actin (SMA)-expressing myofibroblasts, which are generally absent in keloid.2,3
Despite extensive research, the exact pathogenesis of dermal fibrosis remains unclear. Accumulating evidence suggests that aberrant activity of growth factors, such as platelet-derived growth factor,4 transforming growth factor-β5 and connective tissue growth factor,6 contribute to the pathogenesis of keloid and HS. In addition, increased numbers of growth factor receptors, which result in faster response to their relevant growth factors, also play an important role in the formation of pathological scar.
Insulin-like growth factors (IGF)-1 and IGF-2 are potent mitogens and inhibitors of apoptosis. Activation of the insulin-like growth factor-I receptor (IGF-IR) by binding of IGF-I and IGF-2 plays important roles in cell proliferation, survival and inhibition of apoptosis.7,8 The IGF-1/IGF-IR pathway has been implicated in a number of fibrotic diseases, such as renal fibrosis in IgA nephropathy, fibroproliferative acute respiratory distress syndrome and rat liver fibrogenesis.9–11 Moreover, previous studies have demonstrated that IGF and IGF-IR are involved in keloid and HS.12–16
Recently, several studies reported that IGF-IR was overexpressed in keloid fibroblasts, which was shown to enhance the invasive activity of fibroblasts and to make fibroblasts resistant to ceramide-induced apoptosis.12–14 However, the expression and role of IGF-IR in HS remains unclear.
To explore the role of IGF-IR in the pathogenesis of HS, we investigated expression levels of IGF-IR in both epidermis and dermis in immature HS, mature HS, keloid and normal skin using immunohistochemistry, real-time PCR and western blotting.
Methods
The study was approved by the Medical and Ethics Committees of the First Affiliated Hospital of Sun Yat-Sen University, and informed consent was obtained from each patient.
Participants
Thirty patients (12 men, 18 women; mean ± SD age 29.10 ± 6.34 years, range 18–44) who had not received any treatment prior to surgical excision were enrolled into the study from March 2010 to June 2012. These 30 cases comprised 10 keloid and 20 HS (10 immature stage and 10 mature stage). Normal skin tissues were obtained from 10 healthy volunteers (5 men, 5 women; mean ± SD 27.30 ± 5.27 years, range 18–36). The patient and control groups had similar distributions with respect to sex, age, ethnicity, and the type, site and cause of scar) (Table S1).
Tissue sections stained with haematoxylin and eosin were used for histopathological confirmation of the clinical diagnosis of keloid or HS, according to the histological criteria described by Lee et al.2 HS was characterized by presence of collagen fibres arranged in nodules (Fig.1b,c), while keloid had less cellularity and contained thick collagen bundles arranged parallel to epidermis (Fig.1d). Additionally, we divided the HS into mature and immature stages according to the criteria described by Abdou et al.17 Immature HS shows numerous capillaries, fibroblasts, inflammatory cells and collagen fibres, and the epidermis may be flattened, ulcerated, or normal (Fig.1c). Mature HS shows more markedly thickened and hyperesinophilic collagen bundles arranged in whorls or nodular patterns, with minimal inflammation and less vascularity (Fig.1b).
Fig 1.
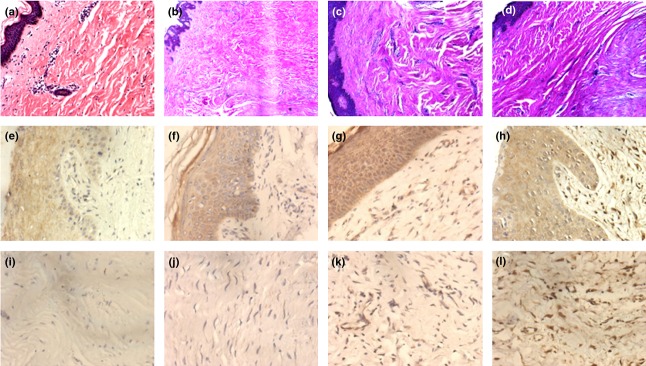
Histological staining of (a) normal skin, (b) mature hypertrophic scar (HS), (c) immature HS and (d) keloid. Haematoxylin and eosin, original magnification (a–d) × 100. Immunohistochemical staining of insulin-like growth factor receptor (IGF-IR) was similar in the epidermis of (e) normal skin, (f) mature HS, (g) immature HS and (h) keloid. (i–l) Immunohistochemical staining of IGF-IR in the dermal fibroblasts: (i) no IGF-IR staining in the normal skin fibroblasts, (j) weak IGF-IR staining in mature HS fibroblasts, (k) moderate IGF-IR staining in immature HS fibroblasts and (l) strong IGF-IR staining in keloid fibroblasts. Original magnification (a–d) × 100; (e–l) × 400.
Cell culture
Primary fibroblast cultures were established as previously described.18 The specimens were digested in Dulbecco modified Eagle medium (DMEM) with 0.5% dispase overnight at 4 °C, then cultured in DMEM with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 U/mL penicillin and 0.1 g/mL streptomycin at 37 °C in a humidified incubator with 5% CO2. Fibroblasts at passages 3–6 were used in this study.
Immunohistochemistry
Tissue sections on slides were incubated with 3% H2O2 to block endogenous peroxidase, then the slides were incubated with antibodies against IGF-IR (ab39398; Abcam Inc. Boston, MA, USA) overnight at 4 °C. Negative controls were incubated with phosphate-buffered saline. A DAB kit (AR1022; Boster, Wuhang, China) was then used for visualization. The immunostaining results were then evaluated. If the cellular membrane or cytoplasm stained dark-brown, it was considered as positive and evaluated as a percentage19 (0 was ≤ 5%; 1 was 6–25%; 2 was 26–50%; 3 was 51–75%; and 4 was ≤ 76%). Evaluation of IGF-IR expression was calculated by a double scoring system (multiplying the immunoreactive percentage score by the intensity score).20 Immunoreactive intensity was graded as 0 = negative, 1 = weakly positive, 2 = moderately positive, and 3 = strongly positive. The immunohistochemistry results were evaluated and scored by two independent observers who were blinded to the clinicopathological information of the patients. The average value from the two observers was used as the final score.
Real-time PCR
Real-time PCR analyses were performed using a real-time PCR system (LightCycler; Roche Molecular Biochemicals, Mannheim, Germany) in accordance with the manufacturer's instructions. Total RNA was extracted from fibroblasts using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized from 2 μg of mRNA using reverse transcriptase (Superscript™; Invitrogen) with oligo(dT) as primers. Real-time quantitative (q)PCR was performed using a SYBR Green I Core Kit (Eurogentec, Southampton, UK) and a qPCR thermal cycler (Opticon; Bio-Rad, Hertfordshire, UK). Relative IGF-IR transcript levels were corrected by normalization based on glyceraldehyde 3-phosphate (GAPDH) levels. The sequences of the IGF-IR primers are shown in Table1.
Table 1.
Primers used for real-time quantitative PCR.
| Primer | Direction | Sequence 5′→3′ |
|---|---|---|
| IGF-IR | Forward | CTCCTGTTTCTCTCCGCCG |
| Reverse | ATAGTCGTTGCGGATGTCGAT | |
| GADPH | Forward | CCATGGAGAAGGCTGGGG |
| Reverse | CAAAGTTGTCATGGATGACC |
GADPH, glyceraldehyde 3-phosphate dehydrogenase; IGF-IR, insulin-like growth factor-1 receptor.
Western blot analysis
Western blotting was performed as described previously.18 Briefly, the membrane was subjected to immunoblotting with primary antibodies for IGF-IR and GAPDH (#5174, Cell Signaling Technology Inc., Danvers, MA, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized using the ECL chemiluminescence system (Amersham, Piscataway, NJ, USA). The intensity of the bands was quantified using imaging software (ImageQuant; Molecular Dynamics, Sunnyvale, CA, USA).
Statistical analysis
The results of real-time PCR are shown as bar figures. The western blot bands were quantified by densitometry. Results were analysed using SPSS software (version 13.0; SPSS Inc. Chicago, IL, USA). ANOVA and independent t-tests with Bonferroni correction were used to compare groups of continuous, normally distributed variables, while χ² or Fisher exact test with Bonferroni correction were used to determine the significant difference between groups for qualitative variables. The IGF-1R intensity and immunoreactivity scores were assessed by Kruskal–Wallis analysis, and Bonferroni-corrected Mann–Whitney U-test was used when comparing two groups. Statistical significance was set at P < 0.05 (P < 0.0083 for Bonferroni correction).
Results
Immunohistochemistry staining of insulin-like growth factor-1 receptor in keloid and hypertrophic scar
Fibroblasts in the dermis showed immunoreactivity to IGF-IR in 9 of the 10 (90%) keloid cases (Fig.1l), 8 of the 10 (80%) immature HS cases (Fig.1k), and 3 of the 10 (30%) mature HS cases (Fig.1j), whereas no immunoreactivity was seen in normal skin specimens (Fig.1i). Of the 10 keloid cases, 8 (80%) had strong staining intensity for IGF-IR, 1 (10%) had moderate staining and 1 (10%) had no staining. Of the 10 immature HS cases, 3/10 (30%) had strong staining, 4/10 (40%) had moderate staining, 1/10 (10%) had weak staining and 2/10 (20%) had no staining. Of the 10 mature HS cases, 3 (30%) had weak staining, while the other 7 (70%) had no staining. There was no significant difference in immunoreactivity scores for the dermis between keloid and immature HS, but there was a significant difference between immature and mature HS (P < 0.01) for IGF-IR expression (Table2). IGF-IR was also found to be expressed in the overlying epidermis of all samples, with no significant difference between them (Fig.1e–h; Table1).
Table 2.
Differences in insulin-like growth factor-1 receptor (IGF-IR) immunohistochemistry expression between normal skin, mature and immature hypertrophic scar (HS), and keloid.
| IGF-1R | Normal skin (n = 10) | HS | Keloid (n = 10) | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|---|
| Mature (n = 10) | Immature (n = 10) | ||||||
| Dermal expression | |||||||
| Positive | 0 (0) | 3 (30) | 8 (80) | 9 (90) | χ² = 21.6, P < 0.001 | P = 0.07† | P = 0.10† |
| Negative | 10 (100) | 7 (70) | 2 (20) | 1 (10) | |||
| Intensity | |||||||
| Weak | 0 (0) | 3 (30) | 1 (10) | 0 (0) | χ² = 11.20; P < 0.01 | U = 1.50‡; P < 0.1 | U = 17.00‡; P = 0.03 |
| Moderate | 0 (0) | 0 (0) | 4 (40) | 1 (10) | |||
| Strong | 0 (0) | 0 (0) | 3 (30) | 8 (80) | |||
| Scoring | 0 (0, 0)§ | 0 (0, 1) | 5 (1.5, 12) | 12 (11.25, 12) | U = 25.9‡ P < 0.001 | U = 13.5‡ P < 0.01 | U = 20‡ P = 0.02 |
| Epidermal expression | |||||||
| Positive | 9 (90) | 8 (80) | 9 (90) | 9 (90) | χ² = 0.97; P = 1.0 | ||
| Negative | 1 (10) | 2 (20) | 1 (10) | 1 (10) | |||
comparison between the four groups
comparison between immature and mature HS
comparison between immature HS and keloid. P < 0.0083 was considered significant for Bonferroni correction. Data are n (%) unless otherwise stated.
Fisher exact test;
Mann–Whitney U-test;
interquartile range.
High expression of insulin-like growth factor-1 receptor by fibroblasts in keloid and immature hypertrophic scar
To confirm IGF-IR expression in keloid and immature HS, we performed western blotting studies using anti-IGF-IR antibody in cultured fibroblasts. No clear staining bands were present for cultured fibroblasts from normal skin or mature HS, and there was no significant difference between them. However, positive IGF-IR bands were evident for cultured fibroblasts from keloid and immature HS, and both were significantly different from normal skin fibroblasts (P < 0.001 and P = 0.001, respectively). The IGF-IR band was also significantly stronger (P < 0.001) for cultured fibroblasts from keloid than for those from immature HS (Fig.2).
Fig 2.
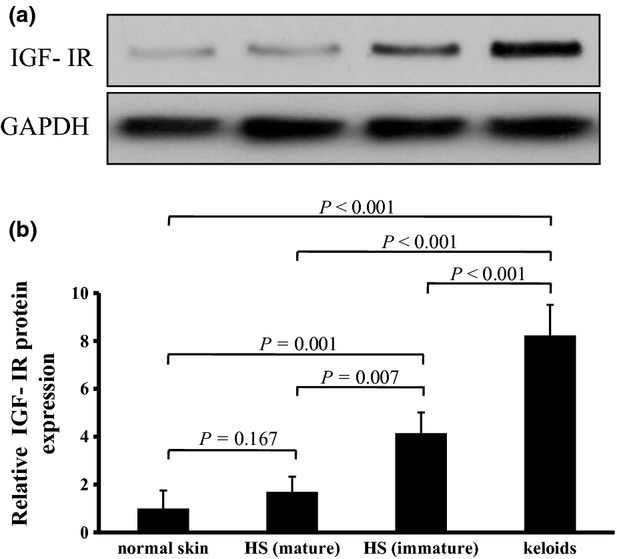
(a,b) Western blotting analysis of insulin-like growth factor-I receptor (IGF-IR) protein expression in cultured fibroblasts of keloid, immature hypertrophic scar (HS), mature HS and normal skin. (a) Cell lysates from fibroblasts were prepared and subjected to Western blotting with antibodies against IGF-IR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (b) Mean densitometric data showing the level of IGF-IR protein normalized to that of GAPDH protein. Data are expressed as mean ± SD (n = 3). Statistical analysis was performed by ANOVA followed by Bonferroni-corrected independent t-tests. ANOVA = 0.000. P < 0.0083 was considered statistically significant.
Real-time PCR also showed that IGF-IR mRNA was overexpressed in primary culture fibroblasts of keloid and immature HS, and was significantly different from that of normal skin and mature HS (keloid vs. normal skin P < 0.001; keloid vs. mature HS P < 0.001; immature HS vs. normal skin P = 0.002; immature HS vs. mature HS P = 0.003). The level of IGF-IR mRNA in keloid fibroblasts was higher than that in immature HS fibroblasts (P < 0.01). In addition, there was no significant difference in IGF-IR mRNA level between mature HS and normal skin fibroblasts (Fig.3).
Fig 3.
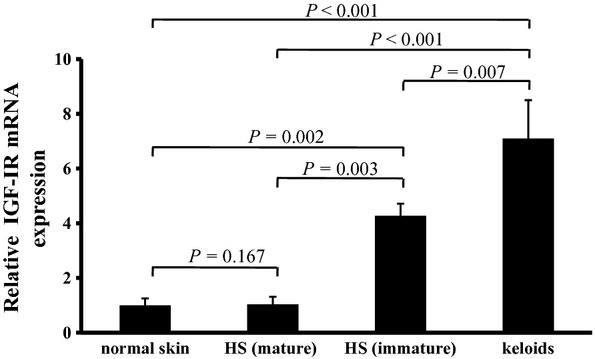
Real-time quantitative PCR analysis of insulin-like growth factor-I receptor (IGF-IR) mRNA expression in cultured fibroblasts of keloid, immature hypertrophic scar (HS), mature HS and normal skin. Data are expressed as mean ± SD (n = 4). Statistical analysis was performed by ANOVA followed by Bonferroni-corrected independent t-tests. ANOVA = 0.000. P < 0.0083 was considered statistically significant.
Discussion
In the current study, we found significant upregulation of IGF-IR protein and mRNA in dermal fibroblasts from keloid and immature HS, but only weak expression of both in mature HS dermal fibroblasts, which was almost similar to that of normal skin fibroblasts. In addition, expression of IGF-IR protein and mRNA in keloid fibroblasts was greater than in immature HS fibroblasts. Both Ohtsuru et al.12 and Yoshimoto et al.13 reported increased expression of IGF-IR in dermal fibroblasts from keloid, but not in those from normal skin. However, in our study, we found overexpression of IGF-IR in dermal fibroblasts from immature HS, but only weak expression in mature HS. Moreover, our study also found expression of IGF-IR in the overlying epidermis of keloid, HS (both stages) and normal skin, with no significant difference between them.
IGF-I and IGF–II are mitogens and differentiation factors, which have been shown to facilitate wound healing by stimulating fibroblast proliferation and enhancing collagen synthesis.21 Both receptors exert their effects via IGF-IR, which in turn phosphorylates phosphoinositide 3-kinase and Ras/Raf/mitogen-activated protein kinase (MAPK),22 two kinases that play important roles in IGF-IR-induced cellular proliferation and apoptosis inhibition.23 Overexpression of IGF-IR was shown to make keloid fibroblasts resistant to ceramide-induced apoptosis,14 and targeted disruption of IGF-IR resulted in growth inhibition.24 Both keloid and HS occur as the result of a pathological wound-healing process, characterized by excess collagen deposition and hyperproliferation of fibroblasts. Keloid and HS fibroblasts have been shown to possess greater proliferative capacity and to be more resistant to apoptosis than normal dermal fibroblasts.25 In the current study, we found that IGF-IR protein and mRNA were overexpressed not only in keloid fibroblasts but also in immature HS fibroblasts. In addition, positive IGF-IR staining and expression intensity in dermal fibroblasts were associated with scar in the immature stages. As the HS matured, expression of IGF-IR nearly disappeared, reaching a level comparable with that of normal skin. These findings demonstrate that the main role of IGF-IR occurs during scar formation rather than the mature stages. Therefore, we speculate that IGF-IR might be involved in the pathogenesis of keloid and HS by inhibiting fibroblast apoptosis and stimulating collagen synthesis by fibroblasts. However, the precise role of IGF-IR in scar formation needs to be explored further.
Epidermal and mesenchymal homeostasis, growth and differentiation are regulated by epidermal–mesenchymal interactions. During wound healing, epidermal keratinocytes play an important regulatory role in the proliferation and apoptosis of the underlying fibroblasts and the production of ECM by cytokines from keratinocytes. Our study found that IGF-IR is expressed in the overlying epidermis of all samples, without any difference between them, indicating that IGF-IR may help in epithelialization and healing of wound by inhibiting keratinocyte apoptosis and promoting keratinocyte renewal. However, the specific role of IGF-I/IGF-IR in epithelial–mesenchymal interactions is unclear, and further studies are needed to answer this question.
Conclusion
We analyzed the expression profile of IGF-IR in keloid, HS and normal skin, and identified significant upregulation of IGF-IR in dermal fibroblasts from keloid and immature HS. Our results indicate that IGF-IR might be is involved in the pathogenesis of keloid and HS by inhibiting fibroblast apoptosis and stimulating collagen synthesis, although the precise mechanism remains to be clarified.
What's already known about this topic?
IGF-IR is a potent inhibitor of apoptosis.
IGF-IR has been detected in keloid, in which it was shown to enhance the invasive activity of fibroblasts and cause them to become resistant to ceramide-induced apoptosis.
What does this study add?
Overexpression of IGF-IR was found in dermal fibroblasts from keloid and immature HS, but not in those from mature HS and normal skin.
IGF-IR is similarly expressed in the overlying epidermis of normal skin, keloid and HS (both stages).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81272096 and 30973128) and the Research Fund for the Doctoral Program of Higher Education of China (PhD supervisor) (grant number 20110171110064).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplementary
Table S1. Patient demographics and clinical characteristics (Keloids and HS).
References
- 1.Köse O, Waseem A. Keloids and hypertrophic scars: are they two different sides of the same coin? Dermatol Surg. 2008;34:336–46. doi: 10.1111/j.1524-4725.2007.34067.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Yang CC, Chao SC, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26:379–84. doi: 10.1097/00000372-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Sarrazy V, Billet F, Micallef L, et al. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl):s10–15. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 4.Haisa M, Okochi H, Grotendorst GR. Elevated levels of PDGF alpha receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol. 1994;103:560–3. doi: 10.1111/1523-1747.ep12396856. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara M, Muragaki Y, Ooshima A. Upregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activity. Arch Dermatol Res. 2005;297:161–9. doi: 10.1007/s00403-005-0596-2. [DOI] [PubMed] [Google Scholar]
- 6.Khoo YT, Ong CT, Mukhopadhyay A, et al. Upregulation of secretory connective tissue growth factor (CTGF) in keratinocyte-fibroblast coculture contributes to keloid pathogenesis. J Cell Physiol. 2006;208:336–43. doi: 10.1002/jcp.20668. [DOI] [PubMed] [Google Scholar]
- 7.Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 8.Khandwala HM, McCutcheon IE, Flyvbjerg A, et al. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 9.Hahn WH, Suh JS, Cho BS. Polymorphisms of insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) contribute to pathologic progression in childhood IgA nephropathy. Growth Factors. 2011;29:8–13. doi: 10.3109/08977194.2010.532126. [DOI] [PubMed] [Google Scholar]
- 10.Krein PM, Sabatini PJ, Tinmouth W, et al. Localization of insulin-like growth factor-I in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:83–90. doi: 10.1164/rccm.2201012. [DOI] [PubMed] [Google Scholar]
- 11.Novosyadlyy R, Dudas J, Pannem R, et al. Crosstalk between PDGF and IGF-I receptors in rat liver myofibroblasts: implication for liver fibrogenesis. Lab Invest. 2006;86:710–23. doi: 10.1038/labinvest.3700426. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuru A, Yoshimoto H, Ishihara H, et al. Insulin-like growth factor-I (IGF-I)/IGF-I receptor axis and increased invasion activity of fibroblasts in keloid. Endocr J. 2000;47(Suppl):S41–4. doi: 10.1507/endocrj.47.supplmarch_s41. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto H, Ishihara H, Ohtsuru A, et al. Overexpression of insulin-like growth factor-1 (IGF-I) receptor and the invasiveness of cultured keloid fibroblasts. Am J Pathol. 1999;154:883–9. doi: 10.1016/S0002-9440(10)65335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara H, Yoshimoto H, Fujioka M, et al. Keloid fibroblasts resist ceramide-induced apoptosis by overexpression of insulinlike growth factor I receptor. J Invest Dermatol. 2000;115:1065–71. doi: 10.1046/j.1523-1747.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghahary A, Shen YJ, Nedelec B, et al. Enhanced expression of mRNA for insulin-like growth factor-1 in post-burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mol Cell Biochem. 1995;148:25–32. doi: 10.1007/BF00929499. [DOI] [PubMed] [Google Scholar]
- 16.Ghahary A, Shen YJ, Nedelec B, et al. Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol. 1996;106:476–81. doi: 10.1111/1523-1747.ep12343658. [DOI] [PubMed] [Google Scholar]
- 17.Abdou AG, Maraee AH, Al-Bara AM, et al. Immunohistochemical expression of TGF-β1 in keloids and hypertrophic scars. Am J Dermatopathol. 2011;33:84–91. doi: 10.1097/DAD.0b013e3181d0c3ad. [DOI] [PubMed] [Google Scholar]
- 18.Tang B, Zhu B, Liang Y, et al. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res. 2011;303:563–72. doi: 10.1007/s00403-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 19.Jackson D, Rowlinson RA, Eaton CK, et al. Prostatic tissue protein alterations due to delayed time to freezing. Proteomics. 2006;6:3901–8. doi: 10.1002/pmic.200500794. [DOI] [PubMed] [Google Scholar]
- 20.Cai MB, Han HQ, Bei JX, et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int J Biol Sci. 2012;8:891–900. doi: 10.7150/ijbs.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Yan GC, Gishizky ML. Identification of structural characteristics that contribute to a difference in antiapoptotic function between human insulin and insulin-like growth factor I receptors. Cell Growth Differ. 1998;9:939–47. [PubMed] [Google Scholar]
- 23.LeRoith D. Insulin-like growth factor I receptor signaling—overlapping or redundant pathways? Endocrinology. 2000;141:1287–8. doi: 10.1210/endo.141.4.7475. [DOI] [PubMed] [Google Scholar]
- 24.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog. 1997;8:71–92. doi: 10.1615/critrevoncog.v8.i1.40. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A, Hatoko M, Tada H, et al. Expression of p53 family in scars. J Dermatol Sci. 2004;34:17–24. doi: 10.1016/j.jdermsci.2003.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary
Table S1. Patient demographics and clinical characteristics (Keloids and HS).


