Abstract
Objectives
To address the following focused question: What is the impact of implant–abutment configuration and the positioning of the machined collar/microgap on crestal bone level changes?
Material and methods
Electronic databases of the PubMed and the Web of Knowledge were searched for animal and human studies reporting on histological/radiological crestal bone level changes (CBL) at nonsubmerged one-/two-piece implants (placed in healed ridges) exhibiting different abutment configurations, positioning of the machined collar/microgap (between 1992 and November 2012: n = 318 titles). Quality assessment of selected full-text articles was performed according to the ARRIVE and CONSORT statement guidelines.
Results
A total of 13 publications (risk of bias: high) were eligible for the review. The weighted mean difference (WMD) (95% CI) between machined collars placed either above or below the bone crest amounted to 0.835 mm favoring an epicrestal positioning of the rough/smooth border (P < 0.001) (P-value for heterogeneity: 0.885, I2: 0.000% = no heterogeneity). WMD (95% CI) between microgaps placed either at or below the bone crest amounted to −0.479 mm favoring a subcrestal position of the implant neck (P < 0.001) (P-value for heterogeneity: 0.333, I2: 12.404% = low heterogeneity). Only two studies compared different implant–abutment configurations. Due to a high heterogeneity, a meta-analysis was not feasible.
Conclusions
While the positioning of the machined neck and microgap may limit crestal bone level changes at nonsubmerged implants, the impact of the implant–abutment connection lacks documentation.
Keywords: animal studies, clinical studies, crestal bone level changes, systematic review
Nowadays, there is considerable evidence supporting the view that the insertion of endosseous dental implants is commonly associated with a physiological remodeling process of the alveolar bone (Albrektsson et al. 1986). After 3 years in function, the cumulative interproximal, clinical radiographic bone loss was calculated to be below 0.5 mm (Abrahamsson & Berglundh 2009; Lang & Jepsen 2009). While clinical studies did not reveal a significant influence of any particular implant surface or design on crestal bone preservation (Abrahamsson & Berglundh 2009; Lang & Jepsen 2009), preclinical animal and human studies have pointed to numerous confounding biological, technical, and biomechanical factors.
Of particular importance seems to be the establishment of the biological width (Berglundh et al. 1991), and therefore, the need for a certain soft tissue thickness at implant placement (Linkevicius et al. 2009). A design strategy including the connection of a smaller-diameter abutment relative to the platform diameter of the titanium implant (referred to as platform-switching) was proven to reduce the epithelial component of the biological width, thus resulting in a preservation of crestal bone levels in both animals (Becker et al. 2007, 2009) and humans (Atieh et al. 2010). In addition, the implant–abutment connection (Koo et al. 2012), the size of the machined neck (Schwarz et al. 2008; Hermann et al. 2011), but also the size of the microgap at the implant–abutment interface (Hermann et al. 2001; Broggini et al. 2006), and its insertion relative to the alveolar crest (Jung et al. 1996) may contribute to physiological bone remodeling after implant placement. At the time being, however, these confounding factors have not been systematically evaluated in the available literature.
Therefore, the aim of this systematic review was to address the following focused question: What is the impact of implant–abutment configuration and the positioning of the machined collar/microgap on crestal bone level changes?
Materials and methods
PICO question
The focused question serving for literature search was structured according to the PICO format: P: animals/patients with stable implants at healed ridges; I: Implants exhibiting specific abutment configurations (i.e. internal: flat vs. conical; external vs. internal)/positioning of the machined collar/positioning of the microgap, C: Control implants exhibiting the same macrodesign, but different configurations as I, and O: histological or radiographical crestal bone levels.
Search strategy
The PubMed database of the US National Library of Medicine and the Web of Knowledge of (Thomson Reuters) were used as electronic databases to perform a systematic review of the available literature. Screening was performed independently by two authors (F.S. and A.H.). Disagreement regarding inclusion was resolved by discussion. The level of agreement between both reviewers was determined by free-marginal kappa scores.
The combination of key words (i.e. medical subject headings MeSH) and free text terms included as follows:
“dental implants” (MeSH) OR “implants” OR “titanium implants” OR “titanium dental implants”
AND
“bone remodeling” (MeSH) OR “crestal bone” OR “crestal bone level” OR “crestal bone change” OR “crestal bone loss” OR “crestal bone remodeling”
AND
“implant–abutment connection” (MeSH) OR “implant–abutment configuration” OR “conical” OR “flat” OR “microgap” OR “microbial leakage” OR “machined neck” OR “machined collar” OR “insertion depth”
AND
“animal model” (MeSH) OR “animal study” OR “preclinical study” OR “dog study” OR “canine study” OR “monkey study” OR “swine study” OR “pig study” OR “clinical study” OR “human study” OR “randomized controlled clinical trial” (MeSH) OR “randomized controlled clinical study” OR “comparative study”.
Additionally, the following journals were searched manually between 1990 and November 2012:
Clinical Implant Dentistry and Related Research; Clinical Oral Implants Research; International Journal of Oral and Maxillofacial Implants; Journal of Clinical Periodontology; Journal of Periodontology. Finally, the references of all selected full-text articles and related reviews were scanned.
Study inclusion and exclusion criteria
For electronic search and title management, a commercial software program (Endnote X6; Thomson, London, UK) was used. For the first stage of study selection, the following inclusion criteria were used:
Publication in the international peer-reviewed literature
English language
Animal or clinical (prospective randomized controlled or comparative) studies
Histological (animals) and radiological (animals and humans) assessment of crestal bone levels (CBL) after implant placement and nonsubmerged healing.
At the second stage of selection, all full-text articles identified during the first stage were acquired. During this procedure, the preselected studies were evaluated according to the following exclusion criteria:
Comparison of implants exhibiting a different macrodesign,
Assessment of factors other than implant–abutment configuration/positioning of the machined collar/positioning of the microgap,
Animal studies: inclusion of less than two animals per observation period/group,
Human studies: study design other than prospective randomized controlled or comparative trials,
Locally or systemically compromised sites and/or conditions.
Quality assessment of selected studies
A quality assessment (Berglundh et al. 2012; Tonetti et al. 2012) of all selected full-text articles was performed according to the ARRIVE guidelines for reporting in vivo experiments in animal research (Kilkenny et al. 2010) as well as the revised recommendation of the CONSORT statement for the evaluation of randomized controlled trials (Schulz et al. 2010).
Predefined gradings (Schwarz et al. 2012) were applied to the following ARRIVE items (Kilkenny et al. 2010): Methods/Ethical statement (5), Methods/Study design (6), Methods/Experimental procedure (7), Methods/Experimental Animals (8), Methods/Housing and keeping (9), Methods/Sample size (10), Methods/Allocation animals to experimental groups (11), Methods/Experimental outcomes (12), and Methods/Statistical methods (13) (Table1). Similar gradings were also used for the quality assessment of finally selected clinical studies (Schulz et al. 2010): Methods/Trial design (3), Methods/Participants (4), Methods/Interventions (5), Methods/Outcomes (6), Methods/Sample size (7), Methods/Randomization (8 and 9), Methods/Blinding (11), and Methods/Statistical methods (12) (Table2).
Table 1.
Categories to assess the quality of finally selected animal studies (Kilkenny et al. 2010; Schwarz et al. 2012)
| Item | Description | Grading |
|---|---|---|
| 5 | METHODS Ethical statement – nature of the review permission, relevant licenses, national and institutional guidelines for the care, and use of animals |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 6 | METHODS Study design – number of experimental and control groups, any steps taken to minimize bias (i.e. allocation concealment, randomization, blinding) |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 7 | METHODS Experimental procedure – precise details (i.e. how, when, where, why) |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 8 | METHODS Experimental animals – species, strain, sex, developmental stage, weight, source of animals |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 9 | METHODS Housing and husbandry – conditions and welfare-related assessments and interventions |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 10 | METHODS Sample size – total number of animals used in each experimental group, details of calculation |
0 = clearly inadequate 1 = possibly adequate 2 = clearly adequate |
| 11 | METHODS Allocation animals to experimental groups – randomization or matching, order in which animals were treated and assessed |
0 = no 1 = yes |
| 12 | METHODS Experimental outcomes – definition of primary and secondary outcomes |
0 = no 1 = unclear/not complete 2 = yes |
| 13 | METHODS Statistical methods – details and unit of analysis |
0 = no 1 = unclear/not complete 2 = yes |
Table 2.
Categories to assess the quality of finally selected clinical studies (Schulz et al. 2010)
| Item | Description | Grading |
|---|---|---|
| 3 | METHODS a. Description of trial design and allocation ratio |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 4 | METHODS a. Eligibility criteria for participants |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 5 | METHODS Interventions for each group – details |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 6 | METHODS a. Definition of primary and secondary outcome measures |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 7 | METHODS a. Sample size calculation |
0 = clearly insufficient 1 = possibly sufficient 2 = clearly sufficient |
| 8 | METHODS a. Method used to generate the random allocation sequence |
0 = no 1 = unclear/not complete 2 = yes |
| 9 | METHODS Mechanism used to implement the allocation sequence |
0 = no 1 = yes |
| 11 | METHODS a. Blinding after assignment to interventions |
0 = no 1 = unclear/not complete 2 = yes |
| 12 | METHODS a. Statistical methods – details and unit of analysis |
0 = no 1 = unclear/not complete 2 = yes |
Quality assessment was performed in two different phases. In particular, during phase I, quality assessment was based on the published full-text article performed independently by both authors. In phase II, disagreements were resolved by discussion.
After forming the scores at the second phase of quality assessment, an overall estimation of plausible risk of bias (low, moderate, or high) was made for each selected study. In brief, a low risk of bias was estimated when all of the criteria were met. A moderate risk was considered when one or more criteria partly met, while a high risk of bias was estimated when one or more criteria not met (Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0., http://www.cochrane.org/resources/handbook).
Data extraction and method of analysis
Median values were calculated for each item (i.e. ARRIVE, CONSORT) and each publication (PASW Statistics 20.0; SPSS Inc., Chicago, IL, USA). For data analysis, the radiographic and/or histological assessment of mean CBL values (i.e. estimated from respective coronal reference points to the first level of bone-to-implant contact) after respective healing periods was extracted and defined as primary outcome. Heterogeneity between studies, subgroup analyses, meta-analyses (fixed-effects model), and forest plots were calculated using a commercially available software program (Comprehensive Meta-Analysis V2; Biostat, Englewood, NJ, USA).
Results
Study selection
A total of 318 potentially relevant titles and abstracts were found during the electronic and manual search. During the first stage of study selection, 293 publications were excluded based on title and abstract (inter-reviewer agreement k = 0.93). For the second phase, the complete full-text articles of the remaining 25 publications were thoroughly evaluated. A total of 12 papers had to be excluded at this stage because they did not fulfill the inclusion criteria of the present review (inter-reviewer agreement k = 1.0) (Tables3 and 4).
Table 3.
Excluded animal studies at the second stage of selection and the reason for exclusion
| Publication | Reason for exclusion |
|---|---|
| Alomrani et al. (2005) | Report on the same (radiographic) data set as Hermann et al. (2011) |
| Jung et al. (2008) | Report on the same (radiographic) data set as Cochran et al. (2009) |
| Pontes et al. (2008b) | Report on the same (radiographic) data set as Pontes et al. (2008a) |
| Novaes et al. (2009) | Report on the same (radiographic) data set as Barros et al. (2010) |
| Welander et al. (2009) | Control implants were lacking a machined collar |
| Weng et al. (2011b) | Report on the same (radiographic) data set as Weng et al. (2010) |
| Heitz-Mayfield et al. (2013) | Different implant designs |
Table 4.
Excluded clinical studies at the second stage of selection and the reason for exclusion
Finally, a total of 13 publications fulfilled the inclusion criteria required for this systematic review (Fig.1).
Figure 1.
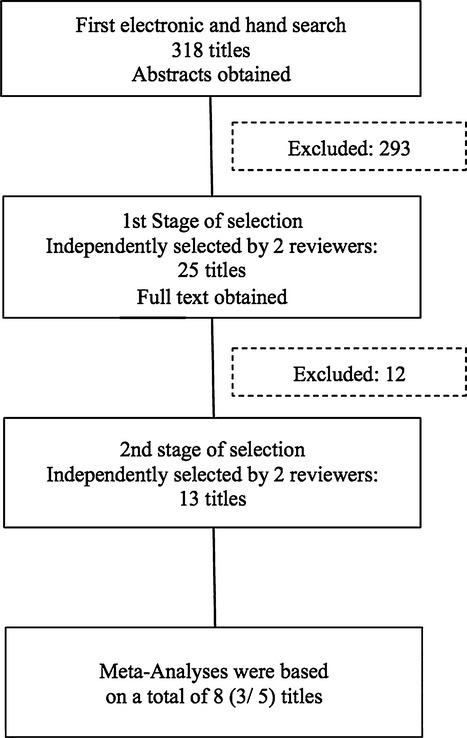
Search strategy.
Quality assessment of selected publications
Quality assessment of selected studies per checklist item (i.e. ARRIVE, CONSORT) is summarized in Tables7.
Table 7.
Quality assessment of finally selected (a) animal and (b) human studies: implant–abutment connection
| Publication | Animals (n) | Methods | Abutment connection | Follow-up | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||||||||
| Becker et al. (2007) | 9 Dogs | Histology (v-o) | Internal: flat vs. conical | 4 Weeks | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | High |
| Publication | Implants (n) | Methods | Abutment connection | Follow-up | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (b) | ||||||||||||||
| Koo et al. (2012) | 40 | Radiology (m-d) | External vs. internal | 1 Year | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 2 | High |
m-d, mesio-distal; m-v, vestibulo-oral.
In particular, the majority of publications reporting on animal studies were associated with minimum gradings when evaluating checklist items 9 (i.e. Housing and husbandry), 10 (i.e. Sample size), and 11 (i.e. Allocation animals to experimental groups). For items 6 (i.e. Study design) and 8 (i.e. Experimental animals), the majority of publications were graded with medium scores, while maximum gradings were commonly assigned to the remaining checklist items 5 (Ethical Statement) and 6 (Study design) (Tables5a, 6a, and 7a).
Table 5.
Quality assessment of finally selected (a) animal and (b) human studies: machined collar and insertion depth
| Publication | Animals (n) | Methods | Implant type | Depth (mm) | Follow-up | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||||||
| Hermann et al. (2000) | 5 Dogs | Histology (m-d) | Exp. (one-piece) | (0/−1) | 6 Months | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | High |
| Schwarz et al. (2008) | 4 Dogs | Histology (m-d) | Camlog (two-piece) | (0/−1.1) | 12 Weeks | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | High |
| Hermann et al. (2011) | 5 Dogs | Histology (m-d) | Exp. (one-piece) | (0/−1) | 6 Months | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 2 | High |
| Median | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | ||||||
| Publication | Patients (n) | Methods | Implant type | Depth (mm) | Follow-up | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (b) | |||||||||||||||
| Hammerle et al. (1996) | 11 | Radiology (m-d) | ITI (one-piece) | (0/−1) | 1 Year | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 2 | High |
| Hartman & Cochran (2004) | 27 | Radiology (m-d) | ITI (one-piece) | (0/−1.1) | 5 Years | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | High |
| Median | 1 | 0.5 | 2 | 1 | 0 | 0.5 | 0 | 0 | 2 | ||||||
Exp., experimental; negative values: subcrestal positioning of the machined neck; m-d: mesio-distal.
Table 6.
Quality assessment of finally selected (a) animal and (b) human studies: microgap and insertion depth
| Publication | Animals (n) | Methods | Implant type | Depth (mm) | Follow-up | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||||||
| Pontes et al. (2008a) | 6 Dogs | Histology (m-d)* | connect (two-part) | (0/−1/−2) | 3 Months | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | High |
| Cochran et al. (2009) | 5 Dogs | Histology (m-d)* | ITI (two-part) | (+1/0/−1) | 8 Months | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 2 | 2 | High |
| Barros et al. (2010) | 6 Dogs | Histology (m-d) | Neodent (two-part) | (0/−1.5) | 2 Months | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 2 | 2 | High |
| Weng et al. (2010) | 6 Dogs | Histology (m-d) | Dentsply, NobelBiocare (two-part) | (0/−1.5) | 3 Months | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | High |
| Huang et al. (2012) | 6 Dogs | Radiology | Astra, Bicon (two-part) | (0/−1.5) | 4 Months | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | High |
| Median | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | ||||||
| Publication | Implants (n) | Methods | Implant type | Depth (mm) | Follow-up | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (b) | |||||||||||||||
| Veis et al. (2010) | 282 | Radiology (m-d) | Biomet 3i (two-piece) | (+1–2/0/−1−2) | 2 Years | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | High |
Negative values: subcrestal positioning of the microgap; m-d, mesio-distal; *not indicated but estimated based on histological views.
The majority of publications reporting on human studies were associated with minimum gradings when evaluating checklist items 4 (i.e. Eligibility criteria), 7 (i.e. Sample size calculation), 8 (i.e. Random allocation sequence), 9 (i.e. Allocation concealment), and 11 (i.e. Blinding of examiner). While the evaluation of items 5 (i.e. Interventions) and 6 (i.e. Outcome measures) was heterogeneous among publications, medium scores were commonly assigned to checklist item 3 (i.e. Study design). Maximum gradings were only assigned to checklist item 12 (i.e. Statistical method) (Tables5b, 6b, and 7b).
According to the given definition, in all publications evaluated, the estimated risk of bias was considered as high (Tables7).
Subdivision of selected publications
All selected publications were subdivided according to differences in the implant–abutment configuration, machined collar size, and insertion depth, respectively:
Three animal and two human studies assessed the impact of different locations of the machined collar on crestal bone level changes (Table5a and b),
Five animal and one human studies assessed the impact of the positioning of the microgap relative to the alveolar crest on bone remodeling (Table6a and b), and
One animal and one human study compared crestal bone level changes at different implant–abutment configurations (i.e. internal: flat vs. conical or external vs. internal) (Table7a and b).
Machined collar and insertion depth
All three animal studies used the canine model for research on the impact of the positioning of the machined collar on crestal bone level changes (Hermann et al. 2000, 2011; Schwarz et al. 2008) (Table5a).
In particular, Hermann et al. (Hermann et al. 2000) used an experimental, one-piece full-body screw-type implant design (length: 9 mm, inner diameter: 3.5 mm, outer diameter 4.1 mm) exhibiting different lengths of the machined collar (the present data set considered types A: 3 mm and B: 4 mm). While type A implants were placed with the rough (sand-blasted and acid-etched surface – SLA)/smooth border at the alveolar bone crest, this border was located 1.0 mm below the crest at type B implants. All implants were connected with healing abutments and left to heal in a nonsubmerged position for 6 months. Mean histological CBL values were 2.98 ± 0.27 mm and +3.88 ± 0.42 mm in the A and B groups, respectively. Accordingly, at 6 months, the alveolar crest was commonly located close to the rough/smooth border in both groups.
In a similar study design, Schwarz et al. (2008) used commercially available SLA-surfaced two-piece implants (flat-to-flat internal connection, length: 11 mm, outer diameter 3.8 mm) with machined neck sizes of 1.6 mm (CAM) and 0.4 mm (CAM+). According to the suggestions given by the manufacturer, both CAM and CAM+ implants were inserted at 0.4 mm above the alveolar bone. While the rough/smooth border was located in an epicrestal position at CAM+ implants, a machined neck part of 1.2 mm was positioned subcrestally in the CAM group. Healing abutments were connected, and the animals were killed after 2 and 12 weeks of healing (the present data set considered 12 week data of 2 animals). At 12 weeks, mean CBL values were significantly higher at CAM (2.4 ± 0.3 mm) when compared with CAM+ (1.6 ± 0.1 mm) implants (Schwarz et al. 2008).
In a most recent animal study (Hermann et al. 2011), one-piece implants (length: 9 mm, inner diameter: 3.5 mm, outer diameter 4.1 mm) exhibiting a machined collar of 1.8 mm (the present data set considered types A and C) were inserted either with the rough/smooth border located at (control) or 1 mm below the bone crest (test). After a nonsubmerged healing period of 6 months, mean bone loss was 0.52 ± 0.4 mm at control and 1.28 ± 0.21 mm at test sites.
A comparable implant type and design (hollow screw and hollow cylinder, machined collar of 2.8 mm) was also used in a prospective clinical study (Hammerle et al. 1996) and inserted according to the same surgical procedures (i.e. rough/smooth border located at, or 1 mm below the bone crest). After 12 months, mean CBL values were 2.5 ± 0.7 mm and 2.6 ± 0.8 mm at test and control implants, respectively. An identical relationship between the amount of crestal bone remodeling and the location of the rough/smooth border at one-piece implants (hollow cylinder and solid-screw implants, titanium plasma-sprayed surface, machined collar of 2.8 mm) was also reported by Hartman and Cochran (Hartman & Cochran 2004). In particular, at 6 months, test implants (1.72 mm) exhibited a more pronounced bone remodeling than control implants (0.68 mm). In both groups, these values remained almost unchanged over an observation period of up to 60 months (Table5b). Unfortunately, SD values were not reported in this publication, and therefore, these data were could not be considered in the meta-analysis (Hartman & Cochran 2004).
Based on these three animal (Hermann et al. 2000, 2011; Schwarz et al. 2008) studies, the weighted mean difference (95% CI) between machined collars placed either above or below the bone crest amounted to 0.835 mm (standard error: 0.130; variance: 0.017; lower limit: 0.580; upper limit: 1.090; Z-value: 6.420) favoring an epicrestal positioning of the rough/smooth border (P < 0.001) (P-value for heterogeneity: 0.885, I2: 0.000% = no heterogeneity) (Fig.2).
Figure 2.
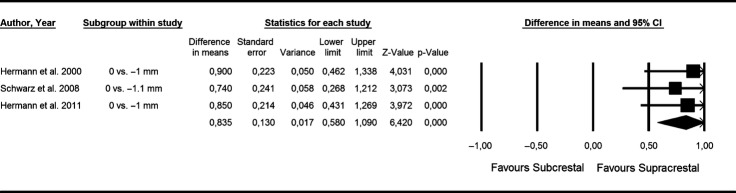
Forest plot indicating weighted mean difference (95% CI) between machined collars placed either above (i.e. supracrestal) or below (i.e. subcrestal) the bone crest. P-value for heterogeneity: 0.885, I2: 0.000% (= no heterogeneity).
Microgap and insertion depth
All animal studies (n = 5) also used the canine for research on the impact of the positioning of the microgap relative to the alveolar crest (i.e. epicrestal vs. subcrestal) on bone remodeling (Pontes et al. 2008a; Cochran et al. 2009; Barros et al. 2010; Weng et al. 2010; Huang et al. 2012).
In particular, Pontes et al. (2008a) used root-form two-piece SLA-surfaced implants (internal hexagon, length: 10 mm, outer diameter 4.3 mm) with the microgap located at (BoneLevel), or 1 (minus 1) or 2 (minus 2) mm below the bone crest. These implants were allocated to either a conventional (i.e. 120 days after submerged healing) or immediate (i.e. 24 h) restoration. At 90 days, the histological analysis revealed no significant differences in mean CBL values within groups (Conventional – BoneLevel: 1.46 ±0.31 mm; minus 1: 1.26 ± 0.43 mm; Minus 2: 1.0 ± 0.32 mm; Immediate – BoneLevel: 1.54 ± 0.57 mm; minus 1: 1.07 ± 0.73 mm; minus 2: 0.82 ± 0.51 mm (the present data set considered the immediate restoration protocol). However, between group comparisons have pointed to a significantly improved outcome at immediately restored sites (Pontes et al. 2008a). In contrast, Cochran et al. (2009) reported on increased mean CBL values, when two-piece SLA (hydrophilic)-surfaced implants (internal conical abutment connection, platform-switched configuration, length: 8 mm, outer diameter 4.1 mm) were placed at 1 mm below the bone crest (1.13 mm vs. 0.38 mm) (as respective standard deviations have not been reported, the data could not be considered in the meta-analysis). Interestingly, a slight bone gain of 0.19 mm was noted for implants placed with the neck 1 mm above the crest (Cochran et al. 2009). The influence of insertion depth and interimplant distances (i.e. 2 and 3 mm) at two-piece SLA-surfaced implants (internal Morse cone connection, platform-switched configuration, length: 9 mm, outer diameter 4.5 mm) was investigated by Barros et al. (2010). After 8 weeks of healing, mean CBL values in the interimplant area were significantly lower at implants placed in a subcrestal (i.e. 1.5 mm), when compared with implants placed in an epicrestal position (2 mm – epicrestal: 0.92 ± 0.61 mm; subcrestal: 0.49 ± 0.38 mm; 3 mm – epicrestal: 0.68 ± 0.57 mm; subcrestal: 0.37 ± 0.29 mm). These differences, however, were less pronounced when the free end areas were considered (2 mm – Epicrestal: 0.91 ± 0.60 mm; subcrestal: 0.79 ± 0.31 mm; 3 mm – epicrestal: 0.92 ± 0.56 mm; subcrestal: 0.79 ±0.46 mm) (the present data set considered the free ends area) (Barros et al. 2010). Weng et al. (2010) evaluated the location of the microgap (epicrestal vs. 1.5 mm subcrestal) at two different screw-type implants exhibiting either an external-hex (smooth collar: 0.75 mm, length: 8.5 mm, outer diameter 3.75 mm) or an internal morse-taper connection (smooth collar: 1. 5 mm, length: 8 mm, outer diameter 3.5 mm). After 3 months of nonsubmerged healing, mean CBL values were comparable within groups (hexed group – epicrestal: 0.98 ± 0.41 mm; subcrestal: 0.76 ± 0.41 mm; Morse group – epicrestal: 2.08 ± 1.20 mm; subcrestal: 1.26 ± 1.48mm) (Weng et al. 2010). In a radiographic study, Huang et al. (2012) compared two different implant types (screw-design) exhibiting either a tapped-in (TI) (length: 8 mm, outer diameter 3.5 mm), or screwed-in (SI) (length: 8 mm, outer diameter 3.5 mm) tapered internal abutment connection. After 16 weeks of healing, mean CBL values were significantly lower in the subcrestal (TI: 0.78 ± 0.42 mm; SI: 0.46 ± 0.26 mm), when compared with the epicrestal (TI: 1.36 ± 0.31 mm; SI: 1.27 ± 0.42 mm) groups, respectively (Huang et al. 2012) (Table6). In a prospective clinical study, Veis et al. (2010) evaluated radiographic bone levels at two-piece implants with either matching or platform-switched abutment connections. After 2 years, mean CBL values were significantly lower at implants placed in either a subcrestal (i.e. 1–2 mm), or a supracrestal (i.e. 1–2 mm) position (matching – supracrestal: 0.60 ±0.50 mm; epicrestal: 1.23 ± 0.96 mm; subcrestal: 0.81 ± 0.79 mm; platform-switch – supracrestal: 0.69 ± 0.47 mm; epicrestal: 1.13 ± 0.42 mm; subcrestal: 0.39 ± 0.52 mm) (Veis et al. 2010) (Table6b).
Based on four animal (Pontes et al. 2008a; Barros et al. 2010; Weng et al. 2011a; Huang et al. 2012) studies, the weighted mean difference (95% CI) between microgaps placed either at or below the bone crest amounted to −0.479 mm (standard error: 0.096; variance: 0.009; lower limit: -0.668; upper limit: −0.290; Z-value: −4.977) favoring a subcrestal position of the implant neck (P < 0.001) (P-value for heterogeneity: 0.333, I2: 12.404% = low heterogeneity) (Fig.3).
Figure 3.
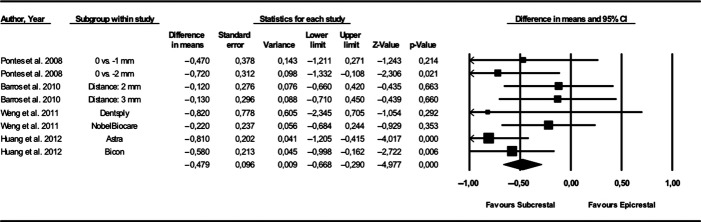
Forest plot indicating weighted mean difference (95% CI) between microgaps placed either at (i.e. epicrestal) or below (i.e. subcrestal) the bone crest. P-value for heterogeneity: 0.333, I2: 12.404% (low heterogeneity).
Due to the low number and high heterogeneity among the selected studies, a meta-analysis was not feasible for microgaps placed either at or above/above or below the bone crest.
Implant–abutment connection
In the canine model, Becker et al. (2007) histologically assessed crestal bone changes at screw-typed wide-diameter two-piece implants (SLA, machined neck: 0.4 mm, length: 11 mm, outer diameter 5.0 mm) exhibiting either a conical (CPS) or tube-in-tube (i.e. flat-to-flat) internal connection (CAM). Wide-body healing abutments were connected and left to heal in a nonsubmerged position. Between group comparisons at 28 days revealed slightly reduced mean CBL values at CPS (vestibular aspect: 1.3 ± 0.4 mm; lingual aspects: 1.2 ± 0.5 mm) when compared with CAM (vestibular aspect: 1.9 ± 0.3 mm; lingual aspects: 1.8 ± 0.6 mm) implants. Despite an identical macrodesign, however, both groups also differed with regard to the outer implant–abutment configuration (CAM: matching abutments; CPS: platform-switched abutments) (Becker et al. 2007) (Table7a).
In a prospective clinical study, Koo et al. (2012) compared epicrestally inserted root-form implants (acid-etched surface, microthreads in the neck area, length: 8.5–13 mm, outer diameter 4.3 mm) exhibiting either an external or internal implant–abutment connection. Radiographic evaluation after 1 year revealed significantly higher mean CBL values for the external (1.14 ± 0.54 mm), when compared with the internal (0.24 ± 0.29 mm)–abutment connection (Table7b).
Due to the limited number and high heterogeneity among the selected studies (i.e. differences in implant–abutment configurations – external vs. internal; conical vs. flat), a meta-analysis was not feasible.
Discussion
The present systematic review was conducted to address the following focused question: What is the impact of implant–abutment configuration and the positioning of the machined collar/microgap on crestal bone level changes?
In general, literature search revealed that the impact of these confounding factors has only been addressed in a total of thirteen publications. Most of these studies, among others, had a focus on the positioning of the machined collar and the implant neck relative to the alveolar bone and were mainly conducted in animals using the canine model. Basically, quality assessment of the selected animal and few human studies, performed according to the ARRIVE guidelines and CONSORT statement (Berglundh & Stavropoulos 2012; Tonetti & Palmer 2012), revealed a high risk of bias, which should be taken into account when evaluating the overall outcome of this systematic review. In this context, it must also be emphasized that systematic reviews of research involving animals are feasible and commonly used to “maximize the use of animal evidence to inform likely health effects in humans” (Peters et al. 2006).
Data synthesis has identified that a supracrestal positioning of the machined collar at both one- and two-piece implants may be favored over a subcrestal positioning (weighted mean difference [WMD]: 0.835 mm) (Hammerle et al. 1996; Hermann et al. 2000, 2011; Schwarz et al. 2008). However, when evaluating the outcomes reported in these studies, it must be emphasized that mean CBL values assessed after respective healing periods commonly did not consider the initial positioning of the implant neck relative to the alveolar crest (Hammerle et al. 1996; Hermann et al. 2000, 2011). Accordingly, the net bone loss at implants exhibiting a subcrestal insertion of the machined neck was even more pronounced than implied by mean CBL values, thus endorsing an epicrestal positioning of the smooth/rough border. Basically, this outcome is also supported by the results of a retrospective follow-up examination of 127 patients treated with CAM tube-in-tube implants. Implants that had been placed with the machined neck in a supracrestal position revealed a significantly lower radiographic bone remodeling (1.11 ± 0.87 mm) than implants that were placed according to the original protocol (i.e. 0.4 mm above the crest) (1.71 ± 1.14 mm) (Al-Nawas et al. 2011). As microbial leakage apparently did not contribute to the marginal bone resorption at either CAM or CAM+ implants (Schwarz et al. 2008; Steinebrunner et al. 2008), the pronounced bone remodeling at subcrestally inserted machined necks was most likely due to their reduced osteoconductive surface properties (Wennerberg & Albrektsson 2009). However, further controlled clinical studies also considering different neck designs are needed to clarify these issues.
When evaluating the positioning of the microgap at two-piece implants relative to the alveolar crest, data synthesis has identified that a subcrestal positioning may be favored over an epicrestal insertion of the implant neck (WMD: −0.479 mm) (Pontes et al. 2008a; Barros et al. 2010; Weng et al. 2011a; Huang et al. 2012). The evaluated histological data were commonly in agreement with the respective radiographic assessments (Pontes et al. 2008b; Novaes et al. 2009; Weng et al. 2011b), but also supported by the clinical analysis of Veis et al. (2010). Accordingly, despite a more pronounced bone remodeling, a subcrestal positioning of the microgap may help to retain the bony coverage of the rough surface. However, as this aspect has mainly been addressed for implants placed in animals with different surface characteristics, diameters, and follow-up times, it remains unclear whether a subcrestal positioning of two-piece implants should be advocated in patients.
The observation that a subcrestal positioning of the neck may limit crestal bone changes is also supported by a retrospective histological analysis of retrieved human implants. While the subcrestal insertion was commonly associated with the establishment of a close bone-to-implant contact on top of the implant shoulder, a crestal bone resorption of 0.5–1.5 mm was present at implants placed in an epicrestal position (Degidi et al. 2011). In this context, however, it must also be emphasized that, at least adjacent to experimental implants, the location and size of the microgap at or below the alveolar crest had a major impact on bone remodeling. These detrimental effects were less pronounced when the interface was initially positioned in a supracrestal position (Hermann et al. 2000, 2001). The present systematic review revealed that a direct comparison of either epi- vs. subcrestal or sub- vs. supracrestal positions of the implant neck has, so far, only been addressed in two studies (Cochran et al. 2009; Veis et al. 2010). The available data suggest that a supracrestal position may be favored over an epicrestal position; however, this trend could not be confirmed when comparing implants placed in supra- and subcrestal positions. Similarly, only two studies (1 animal; 1 human) addressed the influence of different implant–abutment configurations on crestal bone level changes. While an internal connection has been proven to be superior to an external connection (Koo et al. 2012), the beneficial aspect of either internal conical or internal flat-to-flat implant–abutment configurations cannot be estimated (Becker et al. 2007). However, a historical comparison of nonsubmerged conical and internal flat-to-flat (tube-in-tube) implants exhibiting the same macrodesign did not reveal any relevant differences in mean CBL values (1.11–1.14 mm vs. 1.0 mm) after 8 weeks of healing in the canine model (Becker et al. 2009, 2012). Finally, it must be noted that the consensus of the eighth European workshop on periodontology has acknowledged that “animal models may not completely recreate the anatomical, physiological, biomechanical/functional, or pathological environment of the clinical conditions in humans” (Berglundh & Stavropoulos 2012). Nevertheless, the present data synthesis has pointed to a close correlation of mean CBL values assessed in the canine and humans and may therefore underline the applicability of preclinical in vivo research in implant dentistry.
Within its limitations, the present systematic review has identified the importance of a correct positioning of the machined neck and microgap to limit crestal bone level changes at nonsubmerged implants. At the time being, however, the impact of the implant–abutment connection lacks documentation and may not allow for any conclusions.
Conflict of interests
The authors declare that they have no conflict of interests related to this systematic review.
Source of funding
This systematic review was supported by an unrestricted grant of the Camlog Foundation, Basel, Switzerland.
References
- Abrahamsson I, Berglundh T. Effects of different implant surfaces and designs on marginal bone-level alterations: a review. Clinical Oral Implants Research. 2009;20(Suppl 4):207–215. doi: 10.1111/j.1600-0501.2009.01783.x. & . [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. International Journal of Oral and Maxillofacial Implants. 1986;1:11–25. & . [PubMed] [Google Scholar]
- Al-Nawas B, Kammerer PW, Morbach T, Ophoven F, Wagner W. Retrospective clinical evaluation of an internal tube-in-tube dental implant after 4 years, with special emphasis on peri-implant bone resorption. International Journal of Oral and Maxillofacial Implants. 2011;26:1309–1316. & . [PubMed] [Google Scholar]
- Alomrani AN, Hermann JS, Jones AA, Buser D, Schoolfield J, Cochran DL. The effect of a machined collar on coronal hard tissue around titanium implants: a radiographic study in the canine mandible. International Journal of Oral and Maxillofacial Implants. 2005;20:677–686. & . [PubMed] [Google Scholar]
- Atieh MA, Ibrahim HM, Atieh AH. Platform switching for marginal bone preservation around dental implants: a systematic review and meta-analysis. Journal of Periodontology. 2010;81:1350–1366. doi: 10.1902/jop.2010.100232. & . [DOI] [PubMed] [Google Scholar]
- Barros RR, Novaes AB, Jr, Muglia VA, Iezzi G, Piattelli A. Influence of interimplant distances and placement depth on peri-implant bone remodeling of adjacent and immediately loaded Morse cone connection implants: a histomorphometric study in dogs. Clinical Oral Implants Research. 2010;21:371–378. doi: 10.1111/j.1600-0501.2009.01860.x. & . [DOI] [PubMed] [Google Scholar]
- Becker J, Ferrari D, Herten M, Kirsch A, Schaer A, Schwarz F. Influence of platform switching on crestal bone changes at non-submerged titanium implants: a histomorphometrical study in dogs. Journal of Clinical Periodontology. 2007;34:1089–1096. doi: 10.1111/j.1600-051X.2007.01155.x. & . [DOI] [PubMed] [Google Scholar]
- Becker J, Ferrari D, Mihatovic I, Sahm N, Schaer A, Schwarz F. Stability of crestal bone level at platform-switched non-submerged titanium implants: a histomorphometrical study in dogs. Journal of Clinical Periodontology. 2009;36:532–539. doi: 10.1111/j.1600-051X.2009.01413.x. & . [DOI] [PubMed] [Google Scholar]
- Becker K, Mihatovic I, Golubovic V, Schwarz F. Impact of abutment material and dis-/re-connection on soft and hard tissue changes at implants with platform-switching. Journal of Clinical Periodontology. 2012;39:774–780. doi: 10.1111/j.1600-051X.2012.01911.x. & . [DOI] [PubMed] [Google Scholar]
- Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clinical Oral Implants Research. 1991;2:81–90. doi: 10.1034/j.1600-0501.1991.020206.x. & . [DOI] [PubMed] [Google Scholar]
- Berglundh T, Stavropoulos A Working Group 1 of the, V. E. W. o. P. Preclinical in vivo research in implant dentistry. Consensus of the eighth European workshop on periodontology. Journal of Clinical Periodontology. 2012;39(Suppl. 12):1–5. doi: 10.1111/j.1600-051X.2011.01827.x. & & . [DOI] [PubMed] [Google Scholar]
- Boynuegri AD, Yalim M, Nemli SK, Erguder BI, Gokalp P. Effect of different localizations of microgap on clinical parameters and inflammatory cytokines in peri-implant crevicular fluid: a prospective comparative study. Clinical Oral Investigations. 2012;16:353–361. doi: 10.1007/s00784-010-0497-4. & . [DOI] [PubMed] [Google Scholar]
- Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D, Cochran DL. Peri-implant inflammation defined by the implant-abutment interface. Journal of Dental Research. 2006;85:473–478. doi: 10.1177/154405910608500515. & . [DOI] [PubMed] [Google Scholar]
- Cochran DL, Bosshardt DD, Grize L, Higginbottom FL, Jones AA, Jung RE, Wieland M, Dard M. Bone response to loaded implants with non-matching implant-abutment diameters in the canine mandible. Journal of Periodontology. 2009;80:609–617. doi: 10.1902/jop.2009.080323. & . [DOI] [PubMed] [Google Scholar]
- Degidi M, Perrotti V, Shibli JA, Novaes AB, Piattelli A, Iezzi G. Equicrestal and subcrestal dental implants: a histologic and histomorphometric evaluation of nine retrieved human implants. Journal of Periodontology. 2011;82:708–715. doi: 10.1902/jop.2010.100450. & . [DOI] [PubMed] [Google Scholar]
- Hammerle CH, Bragger U, Burgin W, Lang NP. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clinical Oral Implants Research. 1996;7:111–119. doi: 10.1034/j.1600-0501.1996.070204.x. & . [DOI] [PubMed] [Google Scholar]
- Hartman GA, Cochran DL. Initial implant position determines the magnitude of crestal bone remodeling. Journal of Periodontology. 2004;75:572–577. doi: 10.1902/jop.2004.75.4.572. & . [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ, Darby I, Heitz F, Chen S. Preservation of crestal bone by implant design. A comparative study in minipigs. Clinical Oral Implants Research. 2013;24:243–249. doi: 10.1111/j.1600-0501.2012.02513.x. & . [DOI] [PubMed] [Google Scholar]
- Hermann JS, Buser D, Schenk RK, Cochran DL. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. Journal of Periodontology. 2000;71:1412–1424. doi: 10.1902/jop.2000.71.9.1412. & . [DOI] [PubMed] [Google Scholar]
- Hermann JS, Jones AA, Bakaeen LG, Buser D, Schoolfield JD, Cochran DL. Influence of a machined collar on crestal bone changes around titanium implants: a histometric study in the canine mandible. Journal of Periodontology. 2011;82:1329–1338. doi: 10.1902/jop.2011.090728. & . [DOI] [PubMed] [Google Scholar]
- Hermann JS, Schoolfield JD, Schenk RK, Buser D, Cochran DL. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. Journal of Periodontology. 2001;72:1372–1383. doi: 10.1902/jop.2001.72.10.1372. & . [DOI] [PubMed] [Google Scholar]
- Huang B, Meng H, Piao M, Xu L, Zhang L, Zhu W. Influence of placement depth on bone remodeling around tapered internal connection implant: a clinical and radiographic study in dogs. Journal of Periodontology. 2012;83:1164–1171. doi: 10.1902/jop.2012.110617. & . [DOI] [PubMed] [Google Scholar]
- Jung YC, Han CH, Lee KW. A 1-year radiographic evaluation of marginal bone around dental implants. International Journal of Oral and Maxillofacial Implants. 1996;11:811–818. & . [PubMed] [Google Scholar]
- Jung RE, Jones AA, Higginbottom FL, Wilson TG, Schoolfield J, Buser D, Hammerle CH, Cochran DL. The influence of non-matching implant and abutment diameters on radiographic crestal bone levels in dogs. Journal of Periodontology. 2008;79:260–270. doi: 10.1902/jop.2008.070132. & . [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. & . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo KT, Lee EJ, Kim JY, Seol YJ, Han JS, Kim TI, Lee YM, Ku Y, Wikesjo UM, Rhyu IC. The effect of internal versus external abutment connection modes on crestal bone changes around dental implants: a radiographic analysis. Journal of Periodontology. 2012;83:1104–1109. doi: 10.1902/jop.2011.110456. & . [DOI] [PubMed] [Google Scholar]
- Lang NP, Jepsen S. Implant surfaces and design (Working Group 4) Clinical Oral Implants Research. 2009;20(Suppl 4):228–231. doi: 10.1111/j.1600-0501.2009.01771.x. & . [DOI] [PubMed] [Google Scholar]
- Linkevicius T, Apse P, Grybauskas S, Puisys A. The influence of soft tissue thickness on crestal bone changes around implants: a 1-year prospective controlled clinical trial. International Journal of Oral and Maxillofacial Implants. 2009;24:712–719. & . [PubMed] [Google Scholar]
- Novaes AB, Jr, Barros RR, Muglia VA, Borges GJ. Influence of interimplant distances and placement depth on papilla formation and crestal resorption: a clinical and radiographic study in dogs. Journal of Oral Implantology. 2009;35:18–27. doi: 10.1563/1548-1336-35.1.18. & . [DOI] [PubMed] [Google Scholar]
- Penarrocha-Diago MA, Flichy-Fernandez AJ, Alonso-Gonzalez R, Penarrocha-Oltra D, Balaguer-Martinez J, Penarrocha-Diago M. Influence of implant neck design and implant-abutment connection type on peri-implant health. Radiological study. Clinical Oral Implants Research. 2012 doi: 10.1111/j.1600-0501.2012.02562.x. & doi:10.1111/j.1600-0501.2012.02562.x. [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR. A systematic review of systematic reviews and meta- analyses of animal experiments with guidelines for reporting. Journal of Environmental Science and Health. Part. B. 2006;41:1245–1258. doi: 10.1080/03601230600857130. & . [DOI] [PubMed] [Google Scholar]
- Pontes AE, Ribeiro FS, da Silva VC, Margonar R, Piattelli A, Cirelli JA, Marcantonio E., Jr Clinical and radiographic changes around dental implants inserted in different levels in relation to the crestal bone, under different restoration protocols, in the dog model. Journal of Periodontology. 2008b;79:486–494. doi: 10.1902/jop.2008.070145. & . [DOI] [PubMed] [Google Scholar]
- Pontes AE, Ribeiro FS, Iezzi G, Piattelli A, Cirelli JA, Marcantonio E., Jr Biologic width changes around loaded implants inserted in different levels in relation to crestal bone: histometric evaluation in canine mandible. Clinical Oral Implants Research. 2008a;19:483–490. doi: 10.1111/j.1600-0501.2007.01506.x. & . [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. British Medical Journal. 2010;340:c332. doi: 10.1136/bmj.c332. & . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Herten M, Bieling K, Becker J. Crestal bone changes at nonsubmerged implants (Camlog) with different machined collar lengths: a histomorphometric pilot study in dogs. International Journal of Oral and Maxillofacial Implants. 2008;23:335–342. & . [PubMed] [Google Scholar]
- Schwarz F, Iglhaut G, Becker J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. Journal of Clinical Periodontology. 2012;39(Suppl. 12):63–72. doi: 10.1111/j.1600-051X.2011.01838.x. & . [DOI] [PubMed] [Google Scholar]
- Shin YK, Han CH, Heo SJ, Kim S, Chun HJ. Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. International Journal of Oral and Maxillofacial Implants. 2006;21:789–794. & . [PubMed] [Google Scholar]
- Stein AE, McGlmphy EA, Johnston WM, Larsen PE. Effects of implant design and surface roughness on crestal bone and soft tissue levels in the esthetic zone. International Journal of Oral and Maxillofacial Implants. 2009;24:910–919. & . [PubMed] [Google Scholar]
- Steinebrunner L, Wolfart S, Ludwig K, Kern M. Implant-abutment interface design affects fatigue and fracture strength of implants. Clinical Oral Implants Research. 2008;19:1276–1284. doi: 10.1111/j.1600-0501.2008.01581.x. & . . [DOI] [PubMed] [Google Scholar]
- Tonetti M, Palmer R Working Group 2 of the, V. E. W. o. P. Clinical research in implant dentistry: study design, reporting and outcome measurements: consensus report of Working Group 2 of the VIII European Workshop on Periodontology. Journal of Clinical Periodontology. 2012;39(Suppl. 12):73–80. doi: 10.1111/j.1600-051X.2011.01843.x. & & . [DOI] [PubMed] [Google Scholar]
- Veis A, Parissis N, Tsirlis A, Papadeli C, Marinis G, Zogakis A. Evaluation of peri-implant marginal bone loss using modified abutment connections at various crestal level placements. International Journal of Periodontics and Restorative Dentistry. 2010;30:609–617. & . [PubMed] [Google Scholar]
- Welander M, Abrahamsson I, Berglundh T. Subcrestal placement of two-part implants. Clinical Oral Implants Research. 2009;20:226–231. doi: 10.1111/j.1600-0501.2008.01637.x. & . [DOI] [PubMed] [Google Scholar]
- Weng D, Nagata MJ, Bell M, de Melo LG, Bosco AF. Influence of microgap location and configuration on peri-implant bone morphology in nonsubmerged implants: an experimental study in dogs. International Journal of Oral and Maxillofacial Implants. 2010;25:540–547. & . [PubMed] [Google Scholar]
- Weng D, Nagata MJ, Bosco AF, de Melo LG. Influence of microgap location and configuration on radiographic bone loss around submerged implants: an experimental study in dogs. International Journal of Oral and Maxillofacial Implants. 2011a;26:941–946. & . [PubMed] [Google Scholar]
- Weng D, Nagata MJ, Leite CM, de Melo LG, Bosco AF. Influence of microgap location and configuration on radiographic bone loss in nonsubmerged implants: an experimental study in dogs. International Journal of Prosthodontics. 2011b;24:445–452. & . [PubMed] [Google Scholar]
- Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clinical Oral Implants Research. 2009;20(Suppl 4):172–184. doi: 10.1111/j.1600-0501.2009.01775.x. & . [DOI] [PubMed] [Google Scholar]


