Abstract
Objectives
The objective of the present study was to evaluate the efficacy of low intensity pulsed ultrasound stimulation (LIPUS) and to determine the optimal frequency for enhancing bone regeneration in sinus augmentation using a rabbit model.
Material and methods
Thirty male rabbits underwent sinus augmentation. Two rectangular nasal bone windows were outlined bilaterally. LIPUS was applied at two different frequencies (1 MHz and 3 MHz) on experimental sites daily for 2, 4 and 8 weeks. Each histological area of the experimental and control sites was divided into upper and lower parts from the parietal region to a depth of 5 mm. Each area of new bone was measured.
Results
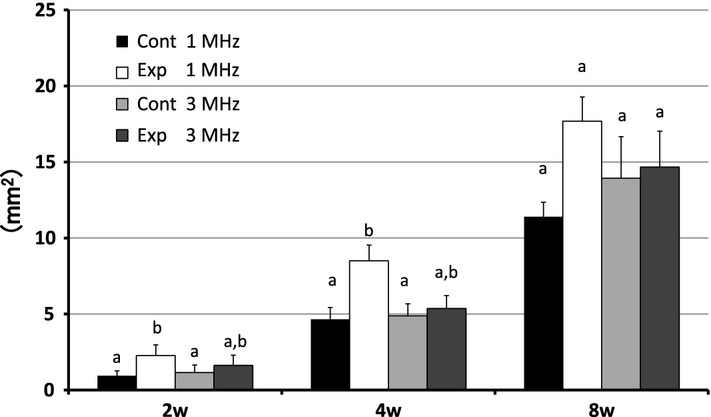
At 2, 4 and 8 weeks, the experimental sites in the 1 MHz group exhibited significantly more new bone growth than the control sites in both the upper and lower parts. When the upper and lower parts of each area were measured in combination there was a statistical difference between the test and control sites in the 1 MHz group at 2, 4 and 8 weeks; however, there were no statistical differences between the test and control sites in the 3 MHz group.
Conclusions
The results suggest that clinical application of LIPUS for sinus augmentation may promote new bone formation, and that the effect of LIPUS for sinus augmentation at a frequency of 1 MHz was greater than at 3 MHz until 8 weeks after sinus augmentation.
Keywords: animal experiments, bone regeneration, low intensity pulsed ultrasound stimulation on sinus augmentation, sinus floor elevation
Oral surgery and especially implantology are among the most valuable procedures in dentistry. Lack of sufficient bone structure is a common problem when the edentulous maxillary bone is used for placement of dental implants. This is because of the poor osseous density typically found in this region, limitation of bone height caused by the existence of sinus pneumatization and atrophy of the bone as a consequence of tooth loss (Yildirim et al. 2000). Sinus augmentation has emerged as a good option for implant site development to ensure long-term biomechanical stability in cases with anatomical limitations (Aparicio et al. 2001). One of the disadvantages of sinus augmentation is that it takes 4–5 months for completion of the repair cycle, and patients are unable to masticate effectively during the healing period (Tatum 1986). Because, patients could not masticate well while they were waiting for the healing period.
Low-intensity pulsed ultrasound stimulation (LIPUS) is a special type of acoustic pulsed energy that is increasingly used in orthopedics as a supplementary therapy to promote healing of pathological and traumatic fractures and wounds. LIPUS has been shown to promote healing and increase the mechanical strength of the fracture callus in clinical studies and animal models without any adverse effects (Duarte 1983; Xavier & Duarte 1983). Recently, several investigators have reported that LIPUS accelerates new bone formation not only in orthopedics but also in oral implantology (Walsh et al. 2008; Kim & Hong 2010; Nakanishi et al. 2011). The healing processes around dental implants are similar to those that occur during the repair of a fractured bone. Because ultrasound stimulation can accelerate the rate of bony healing, LIPUS may be a new way to shorten the duration of osseointegration of dental implants and to improve its quality (Liu et al. 2011). Ultrasound consists of inaudible high-frequency mechanical vibrations created when a generator produces electrical energy that is converted to acoustic energy through mechanical deformation of a piezoelectric crystal located within the transducer. The waves are transmitted by propagation through molecular collisions and vibrations, with a progressive loss of the intensity of the energy for passage through the tissues due to absorption, dispersion or scattering of the waves (Speed 2001). The total amount of energy in an ultrasound beam is its power, expressed in watts. The amount of energy that reaches a specific site is dependent upon parameters of ultrasound such as intensity, frequency, amplitude, focus and beam uniformity. Ultrasound currently is used both operatively and therapeutically; it achieves its biological results by considerably increasing the temperature of the tissue, with intensities ranging from 0.2 to 100 W/cm2. In contrast, safe intensities for diagnostic imaging are much lower (0.5–50 mW/cm2) and are considered non-thermal stimuli. LIPUS used in this study applied 0.5–50 mW/cm2, in the range of diagnostic imaging, to the skin at the site of the fracture (Rubin et al. 2001). Although some researchers have demonstrated that LIPUS enhances fracture healing, the effect of different parameters on the repair process has not been evaluated (Wang et al. 1994; Yang et al. 1996). The depth of penetration of ultrasound depends on the frequency. Ultrasound for healing has a frequency range of 0.75–3 MHz, with most devices set at a frequency of 1 or 3 MHz. Low-frequency ultrasound waves have greater depth of penetration. Ultrasound at a frequency of 1 MHz is absorbed primarily by tissues at a depth of 3–5 cm and is therefore recommended for deeper injuries and in patients with more subcutaneous fat. A frequency of 3 MHz is recommended for more superficial lesions at depths of l–2 cm (Speed 2001). These values are not universally accepted, and ongoing research suggests that in the clinical environment, they may be significantly lower. It is not clear what parameters for LIPUS will achieve optimal therapeutic bone healing in sinus augmentation. Although the application of LIPUS in oral implant treatment has been reported, we found only one clinical report on the effects of ultrasound combined with sinus augmentation; moreover, the mechanism involved is not clear (Kim & Hong 2010).
The aim of this study was to evaluate the efficacy of LIPUS and to determine the optimal frequency of LIPUS for enhancing bone regeneration in sinus augmentation in a rabbit model.
Material and methods
Experimental animals
Forty-two adult male Japanese white rabbits (Hokudo, Sapporo, Japan) weighing approximately 3.0 kg were used in this study. The rabbits were kept individually in cages for 1 week. The animal protocol was approved by the Animal Committee of Health Sciences University of Hokkaido. Six groups of seven rabbits were evaluated.
Anatomical and histological examination of the experimental site in a normal rabbit
The rabbit nasal ostium has previously been used as an experimental model (Asai et al. 2002; Marukawa et al. 2010; Lambert et al. 2010). The nasal ostium was found just beneath the nasal bone. The sinus, extending about 2.0 cm anteroposteriorly, was approximately 1.0 cm wide and 1.5 cm high. The sinus cavity was surrounded by mucosa and a thin layer of cortical bone. The sinus mucosa consisted of pseudostratified ciliated epithelium, and numerous serous glands were found in the submucosal tissue. A sagittal view of a dissected rabbit skull showing the location can be seen in Fig.1.
Figure 1.
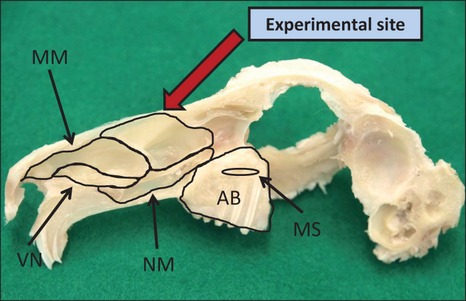
Experimental site (dorsal meatus, red arrow) from the sagittal view AB, alveolar bone; MS, maxillary sinus; VN, ventral nasal meatus; NM, nasopharyngeal meatus; MM, middle meatus.
Sinus augmentation procedure
Sinus augmentation surgery was performed bilaterally, using the right side as the experimental site and the left side as the control site.
The rabbits were anesthetized intravenously with pentobarbital sodium (0.5 mg/kg), and the head was shaved and swabbed with 10% benzethonium chloride solution. At the midline of the nasal dorsum, 0.5 ml of 1% lidocaine with epinephrine (1 : 100,000) was injected subcutaneously. A 50 mm midline incision was made with a #25 scalpel, and the skin and periosteum were elevated sufficiently to expose the nasal bone and the nasoincisal suture line.
Using a round bur, two rectangular nasal bone windows (10 × 5 mm) were outlined bilaterally on the nasal bone. The window was located approximately 10 mm anterior to the nasofrontal suture line and 5 mm lateral to the midline. The windows were completed by osteotomy with continuous cooling with a sterile saline solution (Fig.2).
Figure 2.
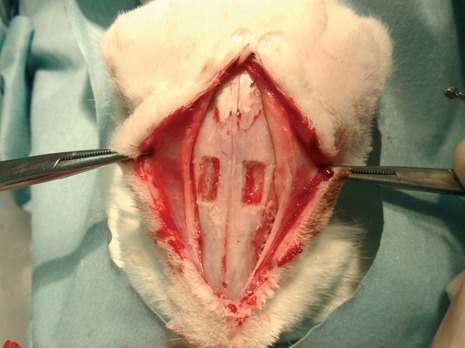
Two bone windows in the nasal region (superior view).
Care was taken to avoid damage of the antral membrane, which moved back and forth with the respiratory rhythm. A Freer elevator was used to gently push the membrane inward. The membrane was then elevated from the bony walls of the antrum to provide a large compartment approximately 5 × 10 × 10 mm, which was filled with 250 mg β-TCP (Osferion® Olympus Terumo Biomaterial Corp., Tokyo, Japan).
The surgical field was covered with a copolymer of cellulose acetate and nitrocellulose membrane (Millipore Co., Billerica, MA, USA) using medical cyanoacrylate adhesive.
The periosteum was then closed using medicinal cyanoacrylate adhesive, and the skin was closed using a 4-0 suture.
Experimental design: effect of LIPUS
LIPUS therapy began on the day after sinus augmentation surgery and was applied to the right (experimental) side. The surgical gingival area was painted with a healing gel, and ultrasound was applied at a frequency of 1 or 3 MHz at an intensity of 240 mW using the BR-Sonic-Pro device (ITO Co., Tokyo, Japan) device (Fig.3). The LIPUS therapy was performed for 15 min every day for 2, 4 and 8 weeks.
Figure 3.

Low-intensity pulsed ultrasound stimulation irradiation equipment.
Observation of new bone formation on non-decalcified specimens
Specimen preparation
The rabbits were anesthetized intravenously with pentobarbital sodium and euthanized at 2, 4 and 8 weeks after surgery. The animals were exsanguinated, and 10% neutral buffered formalin was perfused through the jugular veins. After decapitation and dissection, the entire nose complex, including the nasal ostium, was fixed in 10% neutral buffered formalin for 1 week. Samples were embedded in polyester resin (Rigolac, Ohken, Tokyo, Japan) and sliced into sections of various thickness, perpendicular to the direction of nasal dorsum as much as possible, using an Exakt cutting grinding system (BS 3000, MG 4000; Exakt Co., Hamburg, Germany). Each section was further polished to a thickness of 150 μm.
Histological examination
Specimens were double stained with basic fuchsin and methylene blue. The staining solution consisted of 2% fuchsin and 0.1% methylene blue in sodium hydroxide-alcohol, and the sections were stained as described previously (Murai et al. 2005). The specimens were observed at low magnification under a light microscope (S2H10 BH-2; Olympus, Tokyo, Japan). Using this method, original bone stained pink and new bone stained reddish-violet. The junction between original bone and new bone was clearly visible.
Image analysis
The resulting low magnification images were scanned into a computer and analysed using digital image software (GIMP, GNU General Public Licence: Gnome Foundation Inc, Boston, MA, USA).
Each histological area of the experimental and control sites was divided into upper and lower parts from the parietal region to a depth of 5 mm (Fig.4). The area of new bone was measured in the experimental and control sites using Image J® ver 1.44 software (National Institutes of Health, Bethesda, MD, USA).
Figure 4.
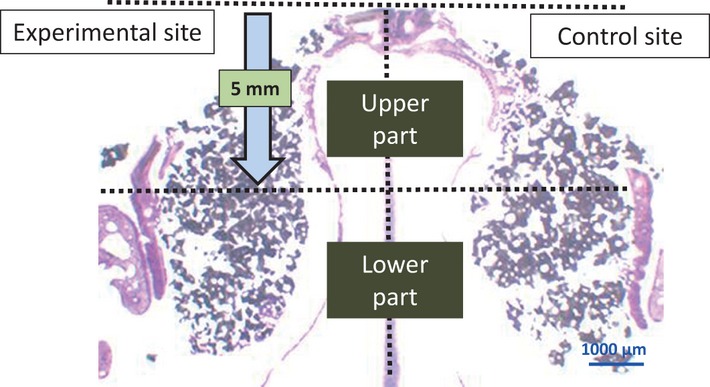
Sagittal section of the rabbit nose.
Statistical analysis
SPSS ver.19.0 (SPSS Inc, Chicago, Il, USA) was used for data analysis. Data were expressed as the mean ± standard deviation and the nonparametric test.
Results
At 2 weeks after implantation, no evidence of inflammation was found in any of the groups. Penetration of connective and vascularized buds into the sub-sinusal space was observed. Newly formed bone was observed along the bone walls and under the lifted antral membrane. Early signs of centripetal new bone formation were observed projecting from the bone walls. In both groups, the bone substitute was almost completely intact. In the 1 MHz group, newly formed bone bridged the particles of β-TCP. In the 3 MHz group of rabbits, new bone formation was only visible at the periphery of the created space on both sides (Fig.5).
Figure 5.
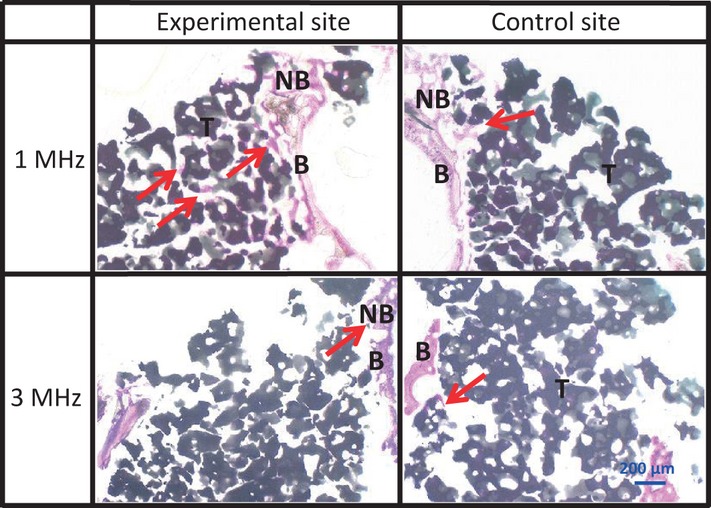
Sagittal section of the upper part at 2 weeks Newly formed bone was observed along the antral membrane at all sites (arrows). NB, newly formed bone; T, β-TCP; B, bone wall.
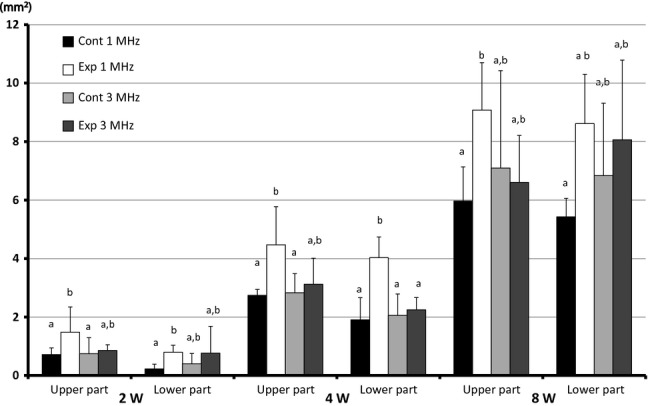
In both the 1 and 3 MHz experimental sites, newly formed bone was observed along the bony walls in the upper part, but not the lower part. There was no difference in the histological findings between the upper and lower parts of the 3 MHz experimental sites (Figs5 and 6). The differences in the area of newly formed bone were not significant between the experimental sites and the control sites of the 3 MHz group, but significantly more new bone formed at the experimental sites than at the control sites of the 1 MHz group. When the upper and lower parts of each area were measured in combination, there was a statistical difference between the experimental and control sites in the 1 MHz group at 2 weeks; however, there was no statistical difference between the experimental and control sites in the 3 MHz group (Fig.7).
Figure 6.
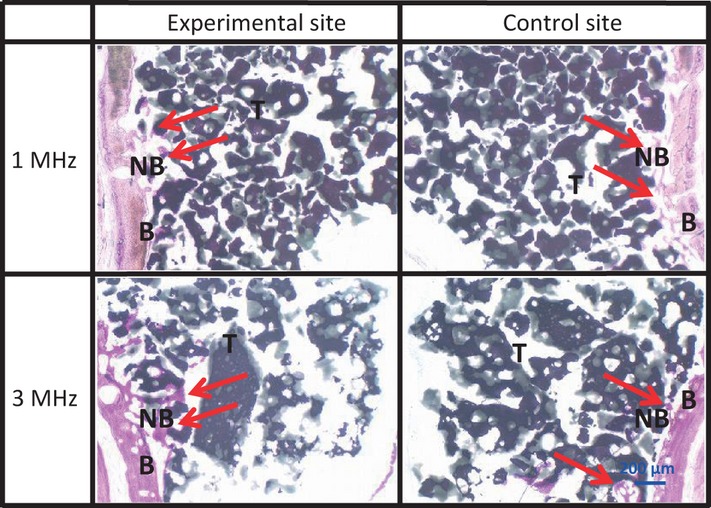
Sagittal section of the lower part at 2 weeks There were no histological differences between any of the sites (arrows).
Figure 7.
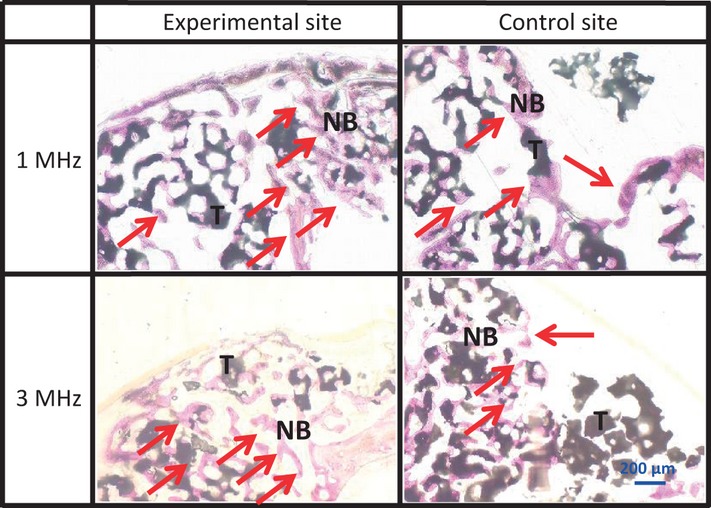
Total area of upper and lower parts of newly formed bone Area of newly formed bone in the upper and lower parts at 2, 4 and 8 weeks. a,b Values with different letters within a group are significantly different (Nonparametric test).
At 4 weeks after implantation, newly formed bone was observed surrounded by β-TCP particles in the 1 and 3 MHz groups. The 1 MHz group manifested advanced bone formation and calcification. Although in the 3 MHz group newly formed bone was found in both the experimental sites and the control sites, there were no significant differences between them (Fig.8). Newly formed bone was present underneath both the upper and lower parts in the 1 and 3 MHz groups. There was no difference in histological findings between the upper and lower parts in the 1 and 3 MHz groups. There was more newly formed bone in the experimental sites than in the control sites in the 1 and 3 MHz groups (Figs8 and 9). The experimental sites exhibited significantly more new bone growth than the control sites in both the upper and lower parts of the 1 MHz group, but there was no significant difference between the experimental sites and the control sites in both the upper and lower parts of the 3 MHz group. The experimental sites of the 1 MHz group exhibited significantly more new bone growth than the experimental sites of the 3 MHz group in the lower part. (Fig.10). When the upper and lower parts of each area were measured in combination, there was a statistical difference between the experimental and control sites in the 1 MHz group at 4 weeks; however, there were no statistical differences between the experimental and control sites in the 3 MHz group (Fig.7).
Figure 8.
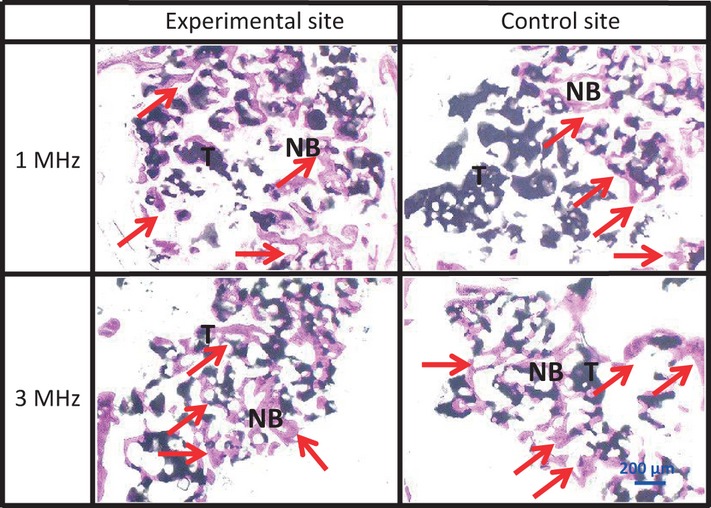
Sagittal section of the upper part at 4 weeks The 1 MHz group manifested advanced bone formation and calcification. Although in the 3 MHz group newly formed bone was found in both the experimental and control sites, there was no significant difference in the newly formed bone (arrows).
Figure 9.
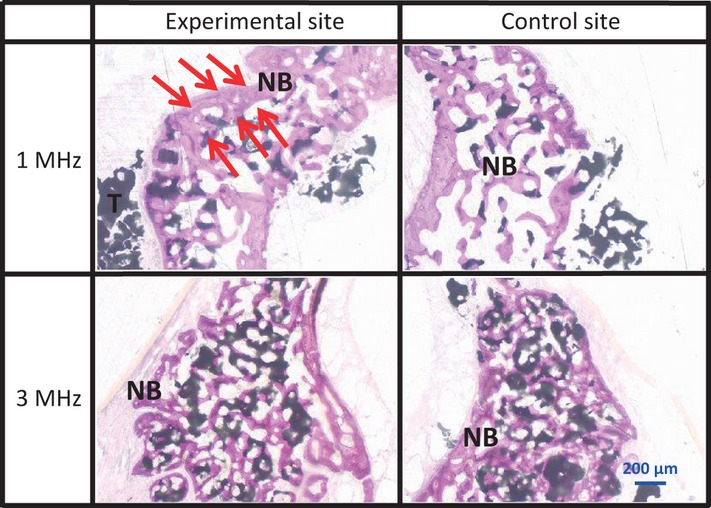
Sagittal section of the lower part at 4 weeks There was no difference in the newly formed bone between any of sites (arrows).
Figure 10.
Area of newly formed bone Area of newly formed bone in the upper and lower parts at 2, 4 and 8 weeks. a,b Values with different letters within a group are significantly different (Nonparametric test).
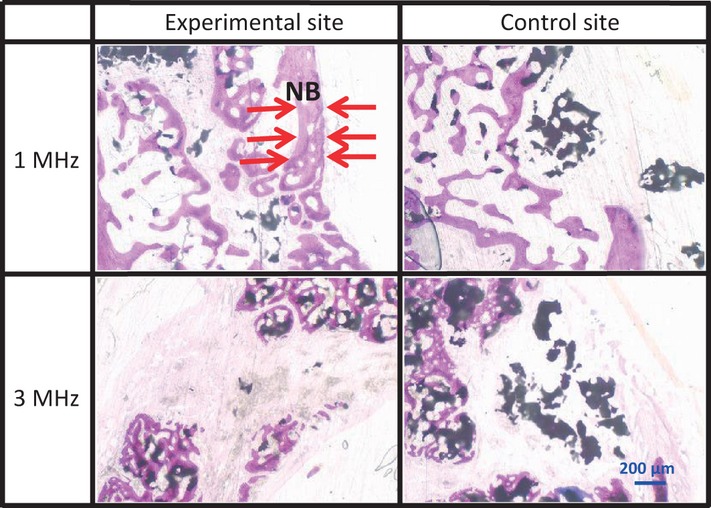
At 8 weeks after implantation, both the experimental and the control sites in the 1 and 3 MHz groups contained fewer β-TCP particles than at 4 weeks (Fig.11). In the experimental sites of the 1 MHz group, the newly formed bone was thicker than in the control sites. We did not find any differences between the upper and lower parts in the 1 and 3 MHz groups (Figs11 and 12). The experimental sites in the 1 MHz group exhibited significantly more new bone growth than the control sites in the upper parts. There were no significant differences between the experimental sites and the control sites in both the upper and the lower parts of the 3 MHz group, and also no significant differences between the experimental sites of the 1 and 3 MHz groups (Fig.10). When the upper and lower parts of each area were measured in combination, there was no statistical difference between the experimental and control sites in the 1 and 3 MHz group at 8 weeks.
Figure 11.
Sagittal section of the upper part at 8 weeks In the 1 MHz group, the area of newly formed bone at the experimental site was greater than at the control site (arrows).
Figure 12.
Sagittal section of the lower part at 8 weeks In the 1 MHz group the area of newly formed bone at the experimental site was greater than at the control site as well as at both sites in the 3 MHz group (arrows).
Discussion
Sinus augmentation has emerged as a good option for implant site development to ensure long-term biomechanical stability in cases with anatomical limitations (Aparicio et al. 2001). However, a disadvantage of this technique is that it takes 4–5 months for completion of the repair cycle (Tatum 1986), during which time the patient is unable to masticate.
LIPUS has been shown to accelerate new bone formation in animals not only in orthopedics but also in oral implantology (Walsh et al. 2008; Kim & Hong 2010; Nakanishi et al. 2011). The healing processes around dental implants are similar to those that occur during repair of a fractured bone. Because ultrasound stimulation can accelerate the rate of bony healing, LIPUS may offer a new technique to shorten the duration of osseointegration of dental implants and to improve outcomes (Liu et al. 2011).
Ultrasound, a form of mechanical energy that is transmitted through and into biological tissues as an acoustic pressure wave at frequencies above the limit of human hearing, is used widely in medicine as a therapeutic, operative and diagnostic tool (Rubin et al. 2001). It has been hypothesized that the micromechanical strains produced in biological tissues may result in biochemical events that could stimulate fracture healing (Pilla et al. 1990; Gleizal et al. 2006). The intensity of LIPUS used for the treatment of fractures is 30–40 mW/cm2, which is considerably lower than the intensity of 1000–3000 mW/cm2 used in physiotherapy. Moreover, the increase in temperature of the deep tissue is assumed not to be caused by pulse waves (Saito et al. 2004; Lavandier et al. 2009).
Although therapeutic ultrasound has been used for over 40 years, its current use in the clinical environment has changed significantly over this period. Whereas in the past, its use was primarily for its thermal effect, it is now more widely employed for its non-thermal effects, especially in relation to bone fracture healing (Sapareto & Dewey 1984; Uchiyama et al. 2007; Watson & Young 2008; Fu et al. 2010).
Only one clinical study to date has suggested that LIPUS has a significant effect on new bone formation in sinus augmentation (Kim & Hong 2010). We therefore aimed to evaluate the efficacy of LIPUS and to determine the optimal frequency for enhancing bone regeneration in sinus augmentation using a rabbit model. Our results showed that LIPUS has the potential to accelerate bone regeneration in sinus augmentation within certain parameters. Studies investigating the optimal LIPUS setting for enhancing fracture healing have examined several different low intensities.
Bone injury has been shown to occur if the intensity level is more than 1.5 W/cm2. The device used in our study was applied at approximately 27 mW/cm2 because this study designed to have a fixed parameter. (Pilla et al. 1990; Heckman et al. 1994).
The effect of LIPUS on the repair process at different frequencies has not been clarified; however, many studies have demonstrated that LIPUS enhances fracture healing (Speed 2001). The depth of penetration of LIPUS depends on the frequency regardless of intensity. Ultrasound at a frequency of 1 MHz penetrates 3–5 cm into the tissue and is therefore recommended for deeper injuries and in patients with more subcutaneous fat. A frequency of 3 MHz is recommended for more superficial injuries at depths of 1–2 cm (Speed 2001). There are no reports comparing LIPUS at frequencies of 1 and 3 MHz in sinus augmentation in rabbit models. Consequently, in this study, each histological area of the experimental and control sites was divided into upper and lower parts from the parietal region to a depth of 5 mm to detect specific differences between the frequencies of 1 and 3 MHz.
Our results showed that LIPUS at a frequency of 3 MHz did not even penetrate all of the upper parts at 2, 4 and 8 weeks, because the energy was absorbed in the superficial tissue and did not reach the deep tissue. It is possible that the penetration was affected by the position and angle of the probe at the experimental site, which varied because it was held in the researcher’s hand and not fixed in place, by movement of the experimental animals, which were also not fixed in place. However, LIPUS at a frequency of 1 MHz penetrated all upper and lower sites, despite the existence of factors that may have affected LIPUS penetration. The characteristics of certain tissues have been shown to affect the depth of penetration (Duarte 1983). For example, tissues such as blood and fat, which have a high water content and a low protein content, absorb little of the ultrasound energy, but those with a lower water content and a higher protein content absorb far more ultrasound energy. It is difficult to predict the thickness of each of these layers in individual patients. There was no significant difference in maximum torque or torsional stiffness in rat femora with LIPUS treatment (30 mW/cm2) using a frequency of 0.5 MHz compared with 1.5 MHz. After 21 days of healing, both frequencies led to increased maximum torque and torsional stiffness compared with untreated controls (Wang et al. 1994). Another researcher observed significantly greater mineral apposition rates at 2 and 3 weeks post-fracture in rabbit fibulae that had been treated with 1.5 MHz compared with 3 MHz frequencies, both using a LIPUS intensity of 500 mW/cm2 (Tsai et al. 1992).
In this study, we showed that there were significant differences between the experimental sites and control sites of the 1 MHz group in the upper and lower part at 2 or 4 weeks. These findings are in contrast with another study which demonstrated that new bone growth around an implant was significantly greater at 3 MHz than at 1 MHz in dogs’ jaws (Fujii et al. 2004). The reason for this discrepancy may be that LIPUS at frequency of 3 MHz was effective over the short distance between the probe and dental implants because LIPUS was applied to the side of the implants rather than to the long axis. It was hypothesized that the high-frequency ultrasound waves were reflected by the oral implants, thus reducing the acceleration of bone regeneration. Further research is needed to determine the optimal parameters for applying LIPUS in sinus augmentation, relating not only to frequency but also to intensity, pulse width, pulse cycle and exposure time.
This study suggests that LIPUS stimulates bone regeneration in sinus augmentation although it is thought that the areas of new bone formation at the control sites in the 1 and 3 MHz groups were potentially subject to bias by administering LIPUS at the experimental site although all the control sites in the 1 and 3 MHz group were involved negligible LIPUS exposure as well. Challenges for the future of this study need to be modified so that the control sites are not subject to bias. In conclusion, our study shows that LIPUS stimulates bone regeneration in sinus augmentation and has a significant effect on the development of newly formed bone at a frequency of 1 MHz.
References
- Aparicio C, Perales P, Rangert B. Tilted implants as an alternative to maxillary sinus grafting: a clinical, radiologic, and periotest study. Clinical Implant Dentistry and Related Research. 2001;3:39–49. doi: 10.1111/j.1708-8208.2001.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clinical Oral Implants Research. 2002;13:405–409. doi: 10.1034/j.1600-0501.2002.130409.x. [DOI] [PubMed] [Google Scholar]
- Duarte LR. The stimulation of bone growth by ultrasound. Archives of Orthopaedic and Trauma Surgery. 1983;101:153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- Fu SC, Hung LK, Shum WT, Lee YW, Chan LS, Ho G, Chan KM. In vivo low-intensity pulsed ultrasound (LIPUS) following tendon injury promotes repair during granulation but suppresses decorin and biglycan expression during remodeling. The Journal of Orthopaedic and Sports Physical Therapy. 2010;40:422–429. doi: 10.2519/jospt.2010.3254. [DOI] [PubMed] [Google Scholar]
- Fujii S, Kajimoto T, Nagahara K, Yamamoto K. Usefulness of low-intensity pulsed ultrasound in reducing the healing period of dental implant therapy. Journal of Japanese Society of Oral Implantology. 2004;17:183–195. [Google Scholar]
- Gleizal A, Li S, Pialat JB, Beziat JL. Transcriptional expression of calvarial bone after treatment with low-intensity ultrasound: an in vitro study. Ultrasound in Medicine and Biology. 2006;32:1569–1574. doi: 10.1016/j.ultrasmedbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. Journal of Bone and Joint Surgery. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hong KS. Histologic evaluation of low-intensity pulsed ultrasound effects on bone regeneration in sinus lift. Journal of Periodontal and Implant Science. 2010;40:271–275. doi: 10.5051/jpis.2010.40.6.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert F, Léonard A, Drion P, Sourice S, Layrolle P, Rompen E. Influence of space-filling materials in subantral bone augmentation: blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clinical Oral Implants Research. 2010;22:538–545. doi: 10.1111/j.1600-0501.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- Lavandier B, Gleizal A, Bera JC. Experimental assessment of calvarial bone defect re-ossification stimulation using low-intensity pulsed ultrasound. Ultrasound in Medicine and Biology. 2009;35:585–594. doi: 10.1016/j.ultrasmedbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu X, Liu B, Hu K, Zhou X, Ding Y. The effect of low-intensity pulsed ultrasound on the osseointegration of titanium dental implants. British Journal of Oral and Maxillofacial Surgery. 2011;50:244–250. doi: 10.1016/j.bjoms.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Marukawa K, Ueki K, Okabe K, Nakagawa K, Yamamoto E. Use of self-setting α-tricalcium phosphate for maxillary sinus augmentation in rabbit. Clinical Oral Implants Research. 2010;22:606–612. doi: 10.1111/j.1600-0501.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- Murai M, Sato S, Koshi R, Yokoyama K, Ikeda K, Narukawa M, Takayama T, Yoshinuma N, Ito K. Effects of the enamel matrix derivative and β-tricalcium phosphate on bone augmentation within a titanium cap in rabbit calvarium. Journal of Oral Science. 2005;47:209–217. doi: 10.2334/josnusd.47.209. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Wang PL, Ochi M, Nakanishi K, Matsubara H. Low-intensity pulsed ultrasound stimulation significantly enhances the promotion of bone formation around dental implants. Journal of Hard Tissue Biology. 2011;20:139–146. [Google Scholar]
- Pilla AA, Mont M, Nasser P, Khan S, Figueiredo M, Kaufman J, Siffert R. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. Journal of Orthopaedic Trauma. 1990;4:246–253. doi: 10.1097/00005131-199004030-00002. [DOI] [PubMed] [Google Scholar]
- Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. The Journal of Bone and Joint Surgery. 2001;83:259–270. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- Saito M, Soshi S, Tanaka T, Fujii K. Intensity-related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low- and high-intensity pulsed ultrasound. Bone. 2004;35:644–655. doi: 10.1016/j.bone.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. International Journal of Radiation Oncology, Biology, Physics. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology. 2001;40:1331–1336. doi: 10.1093/rheumatology/40.12.1331. [DOI] [PubMed] [Google Scholar]
- Tatum HJ. Maxillary and sinus implant reconstructions. Dental Clinics of North America. 1986;30:207–209. [PubMed] [Google Scholar]
- Tsai CL, Chang WH, Liu TK. Preliminary studies of duration and intensity of ultrasonic treatments on fracture repair. The Chinese Journal of Physiology. 1992;35:21. [PubMed] [Google Scholar]
- Uchiyama Y, Nakamura Y, Mochida J, Tamaki T. Effect of low-intensity pulsed ultrasound treatment for delayed and non-union stress fractures of the anterior mid-tibia in five athletes. Tokai Journal of Experimental and Clinical Medicine. 2007;32:121–125. [PubMed] [Google Scholar]
- Walsh WR, Langdown AJ, Auld JW, Stephens P, Yu Y, Vizesi F, Bruce WJ, Pounder N. Effect of low intensity pulsed ultrasound on healing of an ulna defect filled with a bone graft substitute. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;86:74–81. doi: 10.1002/jbm.b.30989. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Lewallen DG, Bolander ME, Chao E, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. Journal of Orthopaedic Research. 1994;12:40–47. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- Watson T, Watson T, Young SR, editors. Electrotherapy: Evidence-Based Practice. 12th edition. New York: Elsevier; 2008. Therapeutic ultrasound; pp. 180–181. [Google Scholar]
- Xavier C, Duarte L. Ultrasonic stimulation on bone callus: clinical application. Revista Brasileira de Ortopedia. 1983;18:73–80. [Google Scholar]
- Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, Bolander ME. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. Journal of Orthopaedic Research. 1996;14:802–809. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clinical Oral Implants Research. 2000;11:217–229. doi: 10.1034/j.1600-0501.2000.011003217.x. [DOI] [PubMed] [Google Scholar]


