Abstract
Intrauterine growth restriction (IUGR) can be described as condition in which fetus fails to reach his potential growth. It is common diagnosis in obstetrics, and carries an increased risk of perinatal mortality and morbidity. Moreover, IUGR has lifelong implications on health, especially on neurological outcome. There is a need for additional neurological assessment during monitoring of fetal well-being, in order to better predict antenatally which fetuses are at risk for adverse neurological outcome. Studies have revealed that the behavior of the fetus reflects the maturational processes of the central nervous system (CNS). Hence, ultrasound investigation of the fetal behavior can give us insight into the integrity and functioning of the fetal CNS. Furthermore, investigations carried out using modern method, four-dimensional (4D) sonography, have produced invaluable details of fetal behavior and its development, opening the door to a better understanding of the prenatal functional development of the CNS. Based on previous observations and several years of investigation, our reaserch group has proposed a new scoring system for the assessment of fetal neurological status by 4D sonography named Kurjak antenatal neurodevelopmental test (KANET). The value of KANET in distinguishing fetal brain and neurodevelopmental alterations due to the early brain impairment in utero is yet to be assessed in large population studies. However, preliminary results are very encouraging.
Keywords: neurological risk, pregnancies complicated with IUGR
1. INTRODUCTION
Fetal growth is a complex developmental process that involves anatomic changes over time. Intrauterine growth restriction (IUGR) can be described as condition in which fetus fails to reach his potential growth (1, 2). Cretan confusion is present in terminology associated with IUGR. By definition, 10% of infants in any population will have birth weights at or below the 10th percentile. IUGR could be manifest at a weight above the population determined at the 10th percentile (eg, an undernourished infant born at the 15th percentile whose genetic makeup would have place it at the 90th percentile). Distinctions between normal and pathologic growth often can not reliably be made in clinical practice. The use of terms IUGR and “small for gestational age” (SGA) has been confusing, and these terms often are used interchangeably (1). SGA and IUGR are not synonymous (3,4,5). SGA, is a different entity than IUGR, but is also associated with poor perinatal outcomes. SGA is defined as a birth weight (BW) below a given (usually) the 10th percentile for gestationalage. The term IUGR should be used only in regard to the fetus, whereas SGA should be used mainly in the newborn (but it can be estimated from sonographic measurements of the fetus) (2). IUGR is ideally detected by a diminished growth velocity of the fetus on serial ultrasonographic scans (6). In this way, the function of growth becomes the object of interest instead of the result (i.e., birth weight).
There are several concepts of IUGR, and information on true IUGR is often missing from retrospective studies. The most common proxy for IUGR is small for gestational age (SGA). However, as it has been already mention, SGA is a heterogeneous category, including not only growth-restricted infants but also infants with chromosomal abnormalities, and small healthy infants as well. Many babies are simply genetically small and are otherwise normal (7). Some women have a tendency to have constitutionally small babies. There are at least three ways to obtain information on true intrauterine growth restriction: 1. by serial ultrasound estimates during pregnancies in which a decreased growth is detected; 2. by anthropometric measures postnatally; and 3. by using individualized or customized growth standards. Kurjak et a (8) illustrated two different patterns of IUGR that may be of significance for the short and long-term prognosis of the fetus using antenatal ultrasonic assessment by measurement of fetal dimensions. They concluded that the late IUGR pattern is frequently associated with conditions that cause reduced placental perfusion, such as hypertension. A typical wasted look and low weight for height is the main characteristic of this group (8). In these fetuses, there is a predisposition to perinatal asphyxia and the Apgar score is low, with an increased brain-to-liver ratio. This type is probably the result of uteroplacental vascular insufficiency (8,9). The symmetric IUGR pattern, which occurs in 20% of SGA fetuses, results from prolonged growth impairment beginning early in the 2nd trimester, even from 18 weeks. There is a proportionate reduction in the fetal head, body length, and body weight, but growth does not generally stop. This type is not typically linked with hypertension or intrapartum asphyxia. Such growth failure has been realized in experimental animals by restriction of the mother’s protein or calorie intake (10). Some of these fetuses have genetic or chromosomal abnormalities and could be examples of reduced growth potential. Long-term follow-up of these fetuses has shown that prolonged IUGR causes stunting of growth in childhood and most likely up to adulthood, and a considerably reduced general development proportion (8).
2. CONSEQUENCES OF IUGR
Fetal growth restriction is one of most common complex problems in modern obstetrics. It is well known that IUGR can lead to significant fetal or neonatal complications. A number of studies have reported a 5–27% incidence of congenital abnormalities associated with IUGR, as compared with a 0.1–4% anomaly rate in control groups of normally grown neonates (11). The incidence of chromosomal abnormalities in IUGR infants is 4–5 times that of appropriate-for-gestational-age (AGA) infants (2% vs 0.4%); and intrauterine infection, especially cytomegalovirus, has been reported in 0.3–3.5% of IUGR infants (11). In addition, growth-restricted infants have up to an 8–10-fold increase in stillbirth and neonatal mortality (11). This, in part, is due to a higher incidence of hypoxia, asphyxia, meconium aspiration, and a generally poorer ability to tolerate labour with IUGR (11). Other developmental problems such as necrotizing enterocolitis, intraventricular hemorrhage (IVH), and neonatal encephalopathy, can also be related to IUGR. Those infants who survive the immediate perinatal period are still at risk for hypothermia, hypoglycemia, polycythemia, and other complications (11, 12). Animal studies have also shown an increased risk for cardiovascular and renal problems later in life (13). IUGR fetuses are connected with high rates of low ponderal indices at birth, hypoglycemia, and admittances to nurseries (7, 14). In infancy, low birthweight is associated with childhood mortality from causes including infectious diseases and congenital anomalies, such as central nervous system and cardiovascular anomalies (15, 16). Numerous adult cardiovascular diseases, including coronary heart disease, hypertension, type II diabetes mellitus, dyslipidemia, and stroke, have been linked with low birthweight;(17) the evidence for the link between risk of coronary heart disease and IUGR comes from the fact that it is independent of gestational age (18). SGA is connected with major psychiatric sequelae in later years. Birthweight less than 3 kg is linked with an increased risk of depression at age 26 years and over, in women but not in men (19). SGA is also connected with an increased risk of suicide and suicide attempts in later life (20). According to some data, severely IUGR fetuses suffer from intellectual impairment in the long term, particularly if neonatal management is less than adequate (14). Furthermore, as described by Jacobsson et al, (21) children with severe IUGR at term have an 8-fold higher risk of CP. These findings highlight the need for close antenatal monitoring of fetal growth (21). Moreover, it is essential to recognize these fetuses - and the earlier in fetal life, the better.
3. FETAL BEHAVIOR AND NEUROBEHAVIORAL ASSESSMENT
As early as possible, neonatologists try to identify neonates at risk of unfavourable neurodevelopmental outcomes. They are fairly reliable in predicting very poor outcomes as well as optimal outcomes. However, within these two extremes, the prediction still remains a challenge (22). Furthermore, there is a growing pool of evidence that many neurological disorders originate from intrauterine rather than perinatal or postnatal period. In addition, clinical and epidemiological studies have shown that even cerebral palsy (CP) most frequently results from prenatal rather than perinatal or postnatal causes (23). As the neuromotor system is the first to mature and cranial expansion passively follows hemispheric growth, neurological assessment should be able to produce early markers to predict later outcomes based on neuromotor and cranial findings. For many years, obstetricians have worked toward the same objective as neonatologists by monitoring fetal well-being during pregnancy. They rely on technical advances, namely ultrasonography (US) which has lead to the following statement: “Fetal behaviour can be defined as fetal activities observed or recorded with ultrasound equipment” (24). The advent of US has led to a kind of revolution. For more than 40 years, ultrasound has been extensively used in medical imaging, providing help for the diagnosis and staging of numerous diseases of different organs and systems of human body. The development of real time two-dimensional (2D) ultrasound has enabled the direct visualization of fetal anatomy and activity in utero. Analysis of the dynamics of fetal behaviour in comparison with morphological studies has led to the conclusion that fetal behavioural patterns directly reflect developmental and maturational processes of fetal central nervous system. Therefore, it was suggested that the assessment of fetal behavior and developmental processes in different periods of gestation may make possible the distinction between normal and abnormal brain development, as well as early diagnosis of various structural or functional abnormalities (25). However, 2D ultrasound was considered somewhat subjective method because information needs observer interpretation. The latest development of three-dimensional (3D) and four-dimensional (4D) sonography that overcame some of the limitations of 2D methods enable precise study of fetal and even embryonic activity and behavior. Contrary to the 3D ultrasound which freezes the image of an object and therefore does not provide information on movements, 4D enablesthe opportunity of simultaneous visualization of the movements of the head, body, and all four limbs and extremities in three dimensions, in a real-time mode. 4D ultrasound or real-time 3D ultrasound makes it straight forward to comprehend some morphological dynamics, such as yawning, sucking, smiling, crying and eye blinking. This offers a practical means for assessment of neurophysiologic development, as well as for detection of anatomical pathology (26, 27). New diagnostic tool additionally provides a possibility of spatial observation of fetal face, which was not provided by 2D sonography (28). 4D therefore allows the appearance and duration most of the each facial movement and expression to be determined and measured (Figure 1, 2).
Figure 1.

A sequence of images of the fetus in the 3rd trimester recorded by 3D/4D sonography, exhibiting smiling movements.
Figure 2.

3D surface rendering mode of the different fetal facial expressions in the third trimester. This ultrasound mode enables the investigation of behavioural fetal facial expression.
In a relatively short period of time, 4D sonography has stimulated multicentric studies on fetal and embryonic behavior with more convincing imaging and data than those obtained by conventional ultrasonic and non-ultrasonic methods (Table 1 (29)).
Table 1.
Aditional findings of fetal behavior by 4D ultrasound in published reports. (From 29)

The visualization of fetal activity in utero by 4D ultrasound could allow distinction between normal and abnormal behavioral patterns which might make possible the early recognition of fetal brain impairment (30, 31). As it is not yet possible to assess functional development of the CNS directly, investigators have started to analyze fetal behavior as a measure of neurological maturation including properties of fetal hemodynamics and the muscular system, as well (32).US technique allowed the investigation of spontaneous fetal motoractivity in utero. Since fetal body movements give important information about the condition of the fetus, their quantitative as well as qualitative aspects were analyzed. For many years the interest of obstetricians was focused on the quantity of fetal movements which was considered as an indicator of fetal well-being. Later studies on the subject have shown that movement counts are poor indicator of brain damage, mainly because the great intra- and interindividually differences and the large overlap between normal and abnormal, which makes this method clinically useless. On the other hand, changes in the elegance and fluency, as well as the variability and fluctuation of intensity and speed of motor performances were shown to be prominent in the sick preterm infants. It became evident that the qualitative changes in motor patterns of both the fetus and neonate precede quantitative changes when the integrity of the nervous system is impaired. It must be admitted that it is more difficult to objectify complex qualitative changes than it is simply to count certain events when they occur, but the Gestalt perception is excellent method for dealing with such phenomenon. Important step is video recording of movements of sufficient length to make a selection of several movements from one recording session. The observed general movements are diagnosed as normal if the movements are complex;including neck, trunk and limb movements in a variable sequence, andare fluent and wax and wane in their intensity. GMs are selected for judgement only if they last for 20 seconds or longer. If the GMs are monotonous, have less complexity and are repetitive in pattern, they are judged as abnormal and as being of “poor repertoire”. Other abnormal patterns are the “cramped-synchronised” type, when the movements are occurring en block and generalized muscle contraction and relaxation appear almost simultaneously, or the movements mayoccur in a jerky and exaggerated manner and in chaotic order. If the movements last very shortly and hence, they are difficult to be judged and then we speek of “hypokinesis”. Several studies employing this new assessment have been carried out and they showed that the early normal or abnormal findings of the GM quality are highly predictivefor later outcome (33, 34). Assessment of GMs is based on the concept of ontogenetic adaptation corresponding to the development of human organism, which is during each developmental stage adapted to the internal and external requirements. Prechtl stated that spontaneous motility, as the expression of spontaneous neural activity, is a marker of brain proper or disturbed function (23, 35). The observation of the unstimulated fetus or infant which is the result of spontaneous behavior without sensory stimulation is the best method to assess its central nervous system capacity. Allendogenously generated movement patterns from an unstimulated central nervous system could be observed as early as from the 7 to 8 weeks of postmenstrual age, with a reach repertoire of movements developing within the next two or three weeks, continuing to be present for 5 to 6 months postnatally. The identification of “CNS depression” during fetal life is based on pre-competences (opening of the eyes, variety of facial expressions),primary reflexes (rhythmical bursts in the sucking pattern) and qualityof GMs (34, 36, 37). The addition of cranial signs (such as insufficient head growth and overlapping sutures) to neurological signs could be avaluable complement (34, 36). Moreover, the identification of dynamic andstatic patterns of the symptoms may be as helpful to date the insult as it is postnatally: the more stable the signs, the more precise is the timingof the insult. In the presence of neurological signs in fetuses,the next step is to proceed to the clinical synthesis. In order to do so, all examinees should be followed till the age of two years, when their categorization to disabling or non-disabling CP can be possible, based on clinical neurological findings and presence or absence of the ability to walk. Obstetricians would have a great benefit if it were possible to assess the condition of the fetal nervous system especially due to the fact that in many cases obstetricians are held responsible for brain damage inneonates, regardless of a growing pool of evidence that most of such damages are consequences of prenatal complications. Even after the fetal brain anatomy can be visualized by ultrasound and the development of the fetal brain is well understood, not much is known about the functional development of the fetal CNS. In other words, the fetal CNS is not accessible. It is possible only to ascertain the output ofthe CNS, i.e. ‘fetal behavior’. Observation of fetal behavior provides a direct assessment of the most important human organ. It is possible to look closely at the functioning of the CNS and the brain. Prenatal motilityis considered to reflect the developing nervous system but also involves functional and maturational properties of fetal hemodynamic and the muscular systems (38). Prechtl and his coworkers (39) have explored spontaneous motility during human development. They introduced the concept of ontogenic adaptation, meaning that during each developmental stage, the functional organization has to take into account internal and external requirements. Any fetal brain damage will interfere with endogenous motor activity. Therefore, spontaneous movements, as an expression of neural activity, could be used as a marker for fetal brain status. Consequently, the observation of the unstimulated fetus or infant should contribute significantly to the assessment of central nervous system (CNS) function. Even after delivery, behavioral patterns frequently provide the most useful indicators of brain function in spite of having extending acces to neurological, physiological and pharmacological measures (38). This remarkable continuity of endogenously generated activity from prenatalto postnatal life may allow identifying those fetuses and infants with emerging neurological impairment. During the nine months of gestation, the repertoire of fetal activities constantly expands, correlating precisely with structural developmentof the CNS (40). Major developmental events, such as the establishment of neural connections in the different regions of the brain, are accompanied by the occurrence of new patterns of fetal activity or with the transformation of the existing patterns. The organization of behavioral states during the final weeks of pregnancy shows that the connection between cerebral cortex and periphery is established, and that the cerebral cortex takes control over fetal activity. This also indicates the ability of the fetus to perceive and process external signals. Furthermore, the latest results indicate that even higher brain functions, such as learning, develop in utero during the last weeks of gestation (40). The major problem with the study of fetal behavior is that it is verytime consuming and not enough functional for routine clinical practice. The question of subjectivity should be overcome using recording of information. Nevertheless, there is no other possibility of assessing the function of the CNS in utero, and this is needed for understanding of the hidden information in the neurodevelopmental pathways of the fetal CNS. Only if normal behavior is fairly understood, is it possible toidentify and to perceive abnormal behavior before birth (28, 41). First reports on fetal behavior obviously suggested that these studies should be standardized as much as possible. An objective analysis with strict application techniques and the use of valid reference ranges appropriate for the gestational age are essential (32). Without such standardization, comparisons with former or future measurements of patients and comparable studies cannot be made. In order to achive this goal the Zagreb group published the first study which described the 4D sonographic techniques used for obtaining longitudinal standard parameters of fetal neurological development in all trimesters of anormal pregnancy (42). Measurement of 7 parameters in the 1st trimester and 11 parameters in the 2nd and 3rd trimesters correlated with gestational age. Those parameters have been followed longitudinally through all trimesters and showed increasing frequency of fetalmovements during the first trimester. A tendency towards decreased frequency of facial expressions and movement patterns with increasing gestational age from second to third trimesters has been confirmed (42). Despite the longstanding conclusion that it is possible to make valid conclusion about brain function from observed, no generalized antenatal behavior screening has been developed to identify fetuses that mayhave central nervous system defects. Recent study from Morokumatried to produce screening test that would be less time consuming andin that way cost effective as compared to their previous study (43). They devised a brief ultrasound examination to distinguish fetuses with compromised central nervous system function from the general population and evaluated it with their study (44). The study design compared findings on five behavioral patterns obtained by retrospectively reviewing the ultrasound examinations of 5 fetuses that had abnormal behavior with prospectively obtained findings of 29 normal fetuses. Median time for brief examination criteria was 50minutes (range 30 to 60 minutes) with the only case undetectable by this brief ultrasound examination had an eye-movement period significantly longer than the normal upper limit. Improvements in technology and procedures that provide direct access to the fetus in utero are generating the impetus for prenataldevelopmental research to move beyond the simple documentation that behaviour abnormalities during pregnancy can produce effects that are evident in their offspring after birth. Rather, we see a growing need for developmental researchers to focus attention on how prenatal events affect the fetus, its behavior, and its relationship with environmental conditions in utero. Investigation of behavioral potentials in the fetus will promote understanding of the mechanisms of normal and abnormal development that lead to predictable behavioral outcomes after birth. In other words, behavioral study of the fetus will be necessary to understand the origins of motor and sensory capabilities of infants and the mechanisms of altered developmental outcomes (36). Awareness of changing risk, and the potential for significant neurodevelopmental problem, is an underlying principle of perinatal medicine. Unfortunate neurological outcomes often result from a delay in recognizing or responding to CNS developing risk. This factor may necessitate the timely referral of individuals to an appropriately staffed facility. The timing of referral can be critical but, there are obvious difficulties which may result in the decision to refer being considered unnecessary, or on the other hand, too late.
4. FETAL BEHAVIOR IN PREGNANCIES COMPLICATED BY INTRAUTERINE GROWTH RESTRICTION
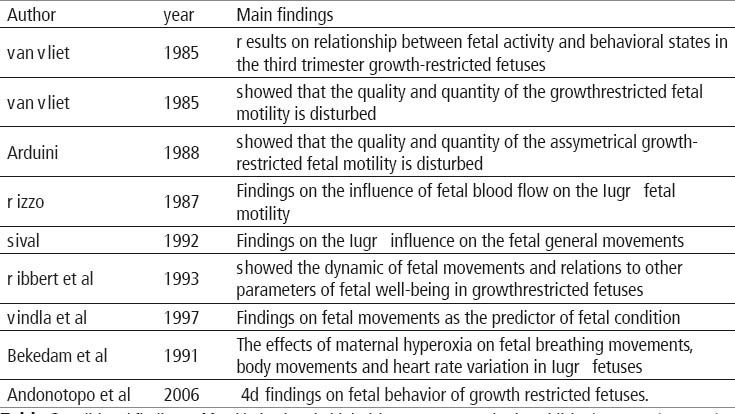
Studies dealing with fetal behavior in IUGR pregnancies are presented in Table 2 (45). One of the first studies on the impact of growth restriction on the fetal behavior focused on fetal breathing and on the course of behavioral state (46). To achieve that goal Van Vliet and his group used real-time ultrasound scanners to detect fetal eye, body, and breathing movements, and the fetal heart rate of 12 growth-retarded fetuses between 36 and 40 weeks of gestation. The mean incidence of fetal breathing was greater during periods of fetal activity (body and eye movements present, greater heart rate variability) than during quiescence (body and eye movements absent, narrowed heart rate variability) at all gestational ages studied in both low-risk and growth retarded fetuses. During periods when one of the state variables was inits active condition while the other two were quiet, or the reverse, the incidence of fetal breathing was intermediate between those found when all three state variables were in agreement. After behavioral states had developed, at 38 and 40 weeks, the mean incidence of fetal breathing in the low-risk fetuses was greater during active states than during the quiet state. There was no apparent increase in the degree of linkage between fetal breathing and other expressions of fetal activity after the emergence of behavioral states (46). In another study by the same group behavioral state observations were carried out on 12 fetuses which subsequently had birth weights below the 10th percentile (47). Their gestational ages at the time of study ranged from 32 to 40 weeks. Real-time ultrasound scanning was used to detect fetal body and eye movements, and the fetal heart rate was continuously recorded using a clinical fetal monitor. The appearance of states seemed to be delayed in the growth-restricted fetuses since states were present in only three of eight growth-restricted fetuses studied at 40 weeks. Also at 40 weeks,the proportion of discordant association of the state variables was increased in the growth-restricted fetuses as compared to the control. There were no consistent differences between the two groups in the occurrence of defined combinations of parameters of the state variables at earlier ages. The results from this study showed that the growth restricted fetuses have impaired quality and quantity of somatic motility in comparison to low risk fetuses of equivalent gestational age. These observations suggest that some aspects of central nervous system function are disturbed in growth-retarded fetuses, even in the absence of fetal distress.19 Since asymmetrical intrauterine growth restriction occurs earlier than symmetrical or combined one it was important to study the fetal behaviour in the group of fetuses that develope growth restriction in earlier gestational age. For that purpose the behavior of 15 asymmetrical intrauterine growth restricted fetuses was compared to that of a control group of healthy fetuses by simultaneous cardiotocographic and ultrasonographic examinations (48). Behavioural states analysis was carried out according to Nijhuis et al. (49) and fetal movements were automatically synchronized with fetal heart rate FHR and grouped for each fetal heart rate pattern FHRP. There were no statistical differences in the distribution of FHRP between healthy and IUGR fetuses. On the other hand quantitative differences were found when the movements investigated were related to FHRP. Moreover IUGR fetuses showed a reduction of state 1F (quiet sleep) and an increase of periods of no coincidence between behavioural state variable when compared to the control group fetuses. These findings, therefore suggest the existence of quantitative differences in fetal behaviour in asymmetrical IUGR fetuses when compared to healthy fetuses (48). To see wether these differences were caused by the compromised vascularization the degree of vascular peripheral resistance was evaluated by means of pulsed doppler ultrasonic equipment in the group of asymmetrical growth restricted fetuses and in control group (50). All fetuses underwent simultaneous cardiotocographicand echographic examinations for two consecutive hours at 36-38 weeks of gestation. The distribution of gross fetal body movements, fetal breathing movements and fetal eye movements was analysed during the different fetal heart rate patterns. Furthermore, the incidence and organization of fetal behavioural states was investigated. Growth restricted fetuses were divided into two groups on the basis of the presence or absence of end diastolic flow in the fetal thoracic descending aorta. The results were in accordance with previous findings that growth restricted fetuses showed a delay in the integration of behavioural patterns and a lower coincidence of behavioural states. These findings are particularly evident in the fetuses with a severe increase of peripheral vascular resistance (absence of end diastolic flowin descending aorta) suggesting that a delay in central nervous system development is present in asymmetrical growth retarded fetuses andthat there is a possible relationship of this delay to the degree of peripheral vascular resistance. Since general movements are considered to be important for prediction of fetal neurobehavior next step was to study the effect ofsevere intrauterine growth restriction on its quality (51). The study was performed longitudinally in 17 human fetuses and fetal movements were recorded by means of weekly 1 h ultrasound and video registrations, following by neurological examinations after birth. No clear effect of uncomplicated intrauterine growth restriction could be detected on the quality of general movements, but the quality was disturbed. General movements became slow and small in amplitude in cases where there was a reduction in the amount of amniotic fluid. Parallel to the onset ofabnormal fetal heart rate patterns, general movements became poor in repertoire, while they were hardly discernible after further deterioration of the fetal condition. With the exception of 3 infants with cerebral haemorrhages, the quality of general movements observed just before and after birth was identical. In these infants, the quality of general movements as well as the results of the standardized neurological examination tended to normalize at 3 months and 1 year, respectively. This study showed that in contrast to prenatal period uncomplicated IUGR had no marked effect on the quality of general movements or on the results of the neurological examination at the age of 1 year (51). Inanother study by the same group 17 fetuses with intrauterine growth restriction (IUGR), the quantity of general movements and fetal breathing movements were studied both cross-sectionally and longitudinally (52). In IUGR fetuses, cross-sectional comparisons were made between the quantity of fetal movements and the fetal clinical condition and the quality of general movements. In addition, the quantity of fetal movements in IUGR was compared with that in uncomplicated pregnancies and in pregnancies complicated by premature rupture of the amniotic membranes. In IUGR, the quantity of general movements declined from 25 weeks gestation onwards, whereas the quantity of fetal breathing movements increased. Longitudinal assessment of these parameters was obtained in four cases and showed a decline of general movements. No relationship between prenatal longitudinal data and neonatal outcome could be observed. The quantity of general movements as well as that of breathing movements was low in IUGR group with abnormal fetal heart rate patterns compared to group with normal parameters. In group with reduced amount of amniotic fluid only thequantity of breathing movements and not of general movements was low. A similar pattern was found in the relation with the quality of general movements observed during fetal deterioration. Cross-sectional analysis of median values (28-31 weeks gestation) did not reveal differences in the quantity of general movements when IUGR, normal pregnancies and premature rupture of the membranes (with or without oligohydramnios) were compared. The quantity of fetal breathing movements was significantly lower in pregnancies complicated by IUGR and by premature rupture of the membranes with oligohydramnios compared to those of normal pregnancies and premature rupture of the membranes without oligohydramnios. In uncomplicated IUGR, the quantity of general movements and breathing movements was in the same range as in normal uncomplicated pregnancies. Similar to the quality of general movements, the quantitative variables were related to the fetal condition. However, in contrast to the quality of general movements, the quantity of general movements and breathing movements showed a high inter- and intraindividual variation. Therefore, the results of this study discouraged the use of quantitative aspects of general movements and breathing movements as reliable indicators of the neurological condition in the individual fetus (52). On theother hand Ribbert and coworkers showed that the assessment of fetal activity may be of help in fetuses with a marginally reduced FHR variation, in which prolongation of pregnancy is considered desirable toallow further maturation in utero (53). In order to determine changes occurring with time they longitudinally studied fetal heart rate variation, general movements, breathing movements and haemodynamics in 19 intrauterine growth restricted fetuses, who eventually were delivered by caesarean section (CS) because of fetal distress. In 14 of 19 fetuses abnormal velocity wave forms were present from the beginning of the study onwards. FHR variation was initially just within or below the norm and fell further during the last 2 days before CS. General movements and breathing movements fell below the normal range later and in alower rate of occurrence than FHR variation. Fetal GM showed a more or less consistent fall in time, whereas fetal breathing movements (FBM) showed a wide range throughout the period of observation. The poorest outcome occurred in fetuses with reversed end-diastolic velocities and rapid fall in FHR variation. It was concluded that with progressive deterioration of the fetal condition abnormal velocity wave form patterns occur first; FHR variation is reduced subsequently while GMs and FBM are the last to become abnormal (53). In another study fetal heart rate (FHR) variation and movements(FA) was investigated in 27 normally grown fetuses and in 18 fetuses with intrauterine growth restriction (54). The results confirmed previously shown decrease of fetal movements in IUGR fetuses ascompared to normally grown fetuses at all gestation times. The investigators reported that IUGR fetuses also spent a significantly lower proportion of time exhibiting high FHR variation at 28-31 weeks. If the fetal movements were compared to FHR one can conclude that more of the IUGR fetuses had abnormalities of movements. Finally, within the IUGR fetuses, those with small head circumferences (less than 3rd centile)had lower movement rates during periods of both low and high FHR variation, though this was only statistically significant for periods of low FHR variation. This published report offered the possibility that objective evaluation of fetal behaviour could be used in a clinical setting and could provide a more sensitive method of fetal assessment than biophysical profile scores (54). A causal relationship with the impairment of fetal oxygenation has been suggested for a reduction in the incidence of fetal movements and in fetal heart rate variation. To test those hypothesis 16 IUGR fetuses and 13 normally grown fetuses were observed during maternal hyperoxygenation that was applied for 40 min in order to increase fetalPO2 levels (55). All IUGR fetuses had abnormal Doppler blood velocity waveforms of the umbilical artery suggesting an impaired uteroplacental exchange. The effect of hyperoxygenation on fetal breathing and body movements and on fetal heart rate was evaluated. In the IUGR fetuses there was a significant increase in fetal breathing and body movements and in heart rate variation during hyperoxygenation as compared to the preceding control period of 40min. No significant changes in fetal breathing and body movements were found in the normally grown control fetuses. A surprising observation was the increase of the number of heart rate decelerations after discontinuation of the maternal hyperoxygenation. It was concluded that in IUGR fetuses the increase in fetal heart rate variation and the increase in the incidence of breathing and body movements during maternal hyperoxygenation substantiates the relationship between these variables and the oxygenation status of the fetus.26 The implementation of 4D sonography was necessary to find out whether the quantity of fetal facial expression and quality of body movements can be used as an additional diagnostic criterion for prenatal brain impairment in fetuses with growth restriction. For that purpose aprospective study was conducted in 50 pregnant women with a growth-restricted fetus and in 50 uncomplicated healthy women in the third trimester of pregnancy (56). 4D ultrasound observation was specially designed to assess whether functional brain impairment and fetal growth restriction had prenatally occurred by the utilization of several behavioral patterns. The results showed that the median value of all movement patterns in the normal fetuses differed from fetuses with intrauterine growth restriction (IUGR). Statistical evaluation revealed significant differences in the distribution of the movements between these groups. A tendency that IUGR fetuses have less behavioral activity than normal fetuses was noted in all observed movement patterns. Correlation reached statistical significance between normal and IUGR fetuses in the third trimester in hand to head, hand to face and head retroflexion. Statistically significant differences could be shown in the distribution of the medianvalues of observation over the five qualitative categories of head and hand movements. Using 4D sonography, this study has opened for the first time the possibility of visualizing the full range of facial expressions in the IUGR condition. The median frequency of all facial expressions in the IUGR groupwas slightly lower compared with the control group. These recent data on IUGR fetuses obtained by 4D sonography are stimulating and might result in a more effective strategy to assess development before birth and may encourage future use of 4D ultrasound for quantitative and qualitative assessment of fetal behavior as possible indicators of the neurological condition in IUGR fetuses (56).
Table 2.
Aditional findings of fetal behaviour in high risk-IUGR pregnancies in published reports. (From 45)
5. IUGR AS AN ANTENATAL RISK FACTOR FOR CEREBRAL PALSY
IUGR entails an increased risk of neonatal morbidity and mortality and also seems to affect brain development (57, 58). Some specific alterations in the brains of IUGR infants, including restriction of the volume of gray matter, a reduced amount of total DNA in glial cells and neurons, and changes in cerebral hemodynamics, have been reported (59). This is also supported by animal studies showing the reduced oxygen delivery to the brain and restricted growth of the forebrain and cerebellum (60). Moreover, it has been established that infants who are somewhat heavier at birth than is average for their gestational age and gender are at the lowest risk of having cerebral palsy and the lowest risk of perinatal death (61, 62). This optimum birth weight for best outcomes seems to be about one standard deviation (SD) heavier than the average birth weight for gestational age among healthy infants. At all gestations, infants who are either smaller or larger than this optimum size have a progressively increased risk of cerebral palsy (63). Furthermore, the frequency of cerebral palsy and the relative severity of the cases also increase away from the same optimum weight for gestational age (62). Other studies have found a dose–response-like relationship between SGA and CP in term infants (64). No such clear association has been found in preterm infants (64) but there are some indications of a similar relationship between SGA and CP in two large preterm studies (65). No data are available for true IUGR, but preliminary data from a Swedish study that used Gardosi’s customized percentiles to the full extent indicate such an association between children born at term with a history of IUGR and CP (21). The gender of the fetus also seems to influence the relationship between cerebral palsy and intrauterine growth (66). Below the 75th weight centile the prevalence of cerebral palsy for males is statistically significantly greater than that for females. At about the 90th centile the difference disappears. Male infants are according to some datas up to a month less mature atterm (and presumably also proportionately less mature at earlier gestations) than their female counterparts (67, 68). This maturity difference is specifically true for cerebral anatomy (lateralization (69) and myelination (70) and can be measured as differences of in utero behavioral adaptation to evoked responses (67). Such immaturity might make male brains more vulnerable to insult at a variety of stages including intrapartum stressors. There is also intriguing possibility that the optimum size at birth for malesis further from their population mean weight than is true for females. The rate of cerebral palsy in males even at the 90th to 97th weight centiles is lower than for males of “normal” birth weight (25th to 75th centiles), whereas for females, the reverse is true. As male infants are significantly heavier than females, being further from optimum birth weight may arise owing to maternal constraint, a limit to intrauterine growth rate created by the limits of maternal resources which are reached earlier for the male infant than for the smaller female infant (71). Recently published review deals with associations and confusions regarding IUGR and CP (62). In these article authors emphasize several problems that arise in the interpretation of the results from reports of the relationship between cerebral palsy and the IUGR. It has been noticed that many studies of the risk for cerebral palsy use birth weight alone unqualified by the gestational age at birth (72, 73), and because of that the observed increase in the risk for cerebral palsy associated with low birth weight has dominated the results and often been attributed to intrauterine growth-retardation (74). Judgment of the relative size of infants at birth must take into account gestational age, because this age has a profound effect on the risk for cerebral palsy (75). Low birth weight infants (< 2500 g) may have elevated risks of cerebral palsy because they are (1) of optimum weight for gestation but are born too early (eg, preterm only), (2) light for gestational age but born at term (small for gestational age [SGA] only), (3) both preterm and light for gestational age, or (4) heavy for gestational age but delivered very early (one fifth of infants at greater than the 90th centile preterm weigh less than 2500 g). Thus, significance of birth weight cannot be properly understood without also considering gestational duration (76). When birth weight and gestational age data are both available, a more sophisticated account of relative size can be made using centile charts. It is important to pay attention that these cetntile charts are not out of date. With the average weight of healthy infants at birth increasing by up to 50 g every 10 years (77, 78), progressively fewer infants are qualifying as SGA as defined by old growth charts. The centile charts may also have insufficient adjustment for nonpathologic determinants of size for gestation, such as gender, parity, and maternal height (79). Furthermore, the size of preterm infants should be compared with that expected of their“healthy” peers. It is now clear that infants born before 37 weeks’ gestation are not healthy in this sense but tend to be lighter (80) and slower growing (81) than fetuses of the same post conceptional age, presumably for reasons related to their preterm birth. Because conventional “neonatal” birth weight standards are based on the observed birth weights of infants born at different gestational ages, comparing the weight of preterm infants with cerebral palsy with these standards compares them with other preterm infants who themselves are more likely to be abnormally grown. To avoid this problem, the relative size of infants born before term should be judged using reference standards based on the intrauterine weight for gestational age (“fetal” standards) rather than birth weights. Such fetal standards are derived from ultrasound based estimates of the weights of healthy infants in utero at known gestational ages (82, 83). The standards can be tailored to allow for other important fetal characteristics, such as sex, ethnicity, and parity, as well as maternal height (84). Authors believe that the use of gestation matched preterm controls can make it impossible to disentangle the risk of cerebral palsy attributable to factors that are themselves associated with poor growth and preterm delivery. Also, in studies investigating the risk of cerebral palsy, neonatal weight standards were used to judge the relative size of cerebral palsy cases, often using gestation-of-delivery matched controls. (85, 86, 87, 88). Typically, these studies reported that the risk for cerebral palsy was not elevated for very preterm SGA infants. Reason for this could be that the neonatal growth standards and the controls used were equally biased by the inclusion of an excess of abnormally light preterm infants. In addition, when fetal growth standards are used, there is a significant elevation of the risk for cerebral palsy for very preterm SGA infants in a similar pattern to that which applies at term. Intriguing question is whether cerebral palsy is a consequence, or a cause of growth deviation, or simply an associated phenomenon (62). Answer on this question can have direct impact on obstetricians. If the brain damage associated with cerebral palsy precedes growth changes, recognition of growth restriction occurs too late for preventative intervention. Furthermore, growth abnormality may be the first, albeit, crude signal that in uteropathology is occurring. This finding may indicate the need for further investigation with a view to potential in utero treatment (eg, of infections) or delivery in optimal circumstances. If the risk for cerebral palsy is elevated as a consequence of growth deviation, underlying causes of growth abnormality may be pursued (placental compromise, gestational diabetes) or early delivery considered before fetal brain damage occurs (62).
6. NEW DATA ON CEREBRAL PALSY
The traditional concept that brain damage is caused during birth or early neonatal period has been challenged and antenatal and unclassifiable factors are now considered as the most important etiologic factors (23, 89, 90). Cerebral palsy is an “umbrella” term for disorders of development, movements and posture, resulting in limitations of activity due to non-progressive impairment of developing brain (90). This diagnosis describes a group of disorders of development of movement and posture, causing activity limitations that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain. The motor disorders in cerebral palsy are often accompanied by disturbances of sensation, cognition, communication, perception, and/or behavior, and/or by a seizure disorder. “Attributed to” is purposely vague because our understanding of developmental neurobiology is evolving rapidly. “Disturbances” is used as a comprehensive term referring “to events or processes that in some way interrupt damage orotherwise influence the expected pattern of brain maturation” (91). Those events or processes are many, with consequences varying from very conspicuous to very subtle. The worldwide prevalence ranges from 2 to 2.5 per 1000 live-births and the incidence did not change since 1951, respectively. Improvement of obstetrical and neonatal care didnot result in decreasing prevalence rate of CP. On the contrary, the incidence and severity of CP increased due to a better survival rate of very immature and tiny premature infants with significant morbidity and increased number of risk factors. Cerebral palsy is the most common chronic motor disability of childhood. The diagnosis is retrospective and it is rarely made before the age of six months when the infant is severely affected. The specificity of the diagnosis improves as the child ages and the nature of the disability evolves (91). CP does not result from a single event but rather from a sequence of interdependent adverse events. This time frame of evolving adverse events should be taken into account when considering the possibility of CP diagnosis in infants (93). Periventricular white-matter injury is now the most common cause of brain injury in preterm infants and the leading cause of chronic neurological morbidity and CP. Standardized methods of clinical neurological assessment from the neonatal period onwards were developed in order to identify three grades of neurological impairment: severe, moderate and mild. The clinical identification of severely affected patients is less problematic than the identification of moderately and mildly affected infants. Cranial ultrasound, magnetic resonance imaging, magnetic resonance spectroscopy and diffusion weighted imaging are helpful in very low birth weight premature and in terminfants with encephalopathy(90).
From the pediatric experience it is well known that one should wait until the age of 6 months postnatally to be able to diagnose a severe CP, 12 months for a moderate CP and 24 months for a minor non-disabling CP. This delay for the full clinical expression of functional consequences of a brain damage depends on brain maturation. DiPietro was right in saying that a consensus recognizing the fact that fetal neurobehavior reflects the developing nervous system is emerging (93-95). The purposes of early diagnosis of CP could be important from the point of view ofthe infant, the mother, the family, and the gynecologist, who is often accused for clinical negligence. Although randomized studies confirming that the early intervention as an effective strategy for treatment of CP is not available, it should be considered as feasible. Because the etiology of CP is mostly shifted towards the prenatal period,attempts were made to diagnose neurological impairment in the prenatal period (90, 92).
One crucial question often posed to neonatologists is to determine the exact timing of brain damage, prenatal or intrapartum, in the context of neonatal encephalopathy. In this perspective, repeated neurological assessments over the first days of life allow identification of two profiles. The first, a dynamic profile, is associated with signs of CNS depression increasing within the first 3 days and then decreasing gradually with obvious improvement in alertness, motor activity, and sucking (96). This profile is typical of recent insult, most often intrapartum. The second one, a static profile, is disclosed by lack of changes along repeated assessments in the first week of life. This latter profile is typical of a prenatal insult that occurred in utero at least several weeks earlier and therefore, already stable at the time of birth. In addition, the identification of three signs already present at birth offers a precious clue to fetal brain damage, when observed in a cluster:
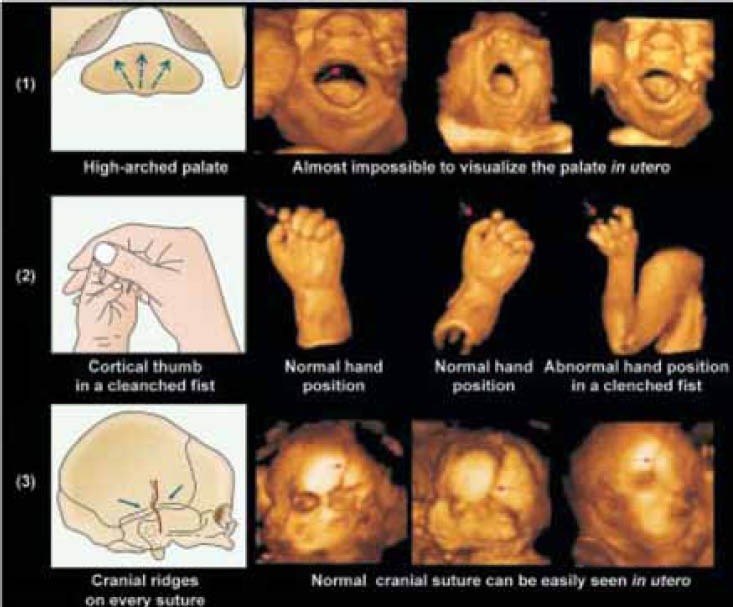
High-arched palate (due to insufficient molding forces of a hypoactive tongue),
Non reducible adduction of the thumb in a clenched fist (due to absence of spontaneous motor activity), and
Cranial ridges over each suture or restricted to the squamous suture(due to severe or moderate impairment of hemispheric growth).
Using 3D US, only two of these three signs can be diagnosed in utero. As for now, it remains impossible to visualize the high arched palate with 3D surface imaging since this technique does not permit visualization of deep structure in the oral cavity. However, detection of the two other signs as a specific expression of brain impairment appears promising (Figure 3).
Figure 3.
Neonatal signs indicating a prenatal insult (sketch pictures) and comparison with 3D-US imagingin utero. (1) High-arched palate (left) and 3D-US imaging of the entire oral cavity(right); (2) Cortical thumb in a clenched fist (left) and 3D-US imaging of the normal and abnormal hand position. (3) Cranial ridges on every suture (left) and 3D-US imaging of the normal cranial suture in utero.
Development of Prechtl’s general movements (GM) for postnatal neurological evaluation encouraged obstetricians to implement this technique for fetal neurological evaluation using 2D ultrasound (23, 35). The fact that the same criteria can be used for the fetus and young infants seemed especially attractive. Development of computer and ultrasound technology enabled evaluation of fetal GM in three dimensions and in real time (97, 98). GM includes the consideration of body movements (arms, legs, neck and trunk) spreading in variable sequences with gradual beginning and end. They wax and wane in intensity, force and speed, being fluent and elegant, revealing the complexity and variability of motor activity already present even in early stage. GM has to be videotaped and then analyzed based on visual “Gestalt perception”,which provides an overall impression of GMs with standardized procedures (37). Subsequently, movement patterns will be described interms of complexity, variability and fluency. GMs will finally be classified as normal-optimal, normal-suboptimal, mildly abnormal and definitely abnormal (96). While the application of GMs in postnatal life is standardized and in spite of encouraging results of fetal GMs in the last 25 years which showed that qualitive assessment of GMs is a goodmarker of brain dysfunction, (32) they did not shift the diagnosis of neurological impairment to the prenatal period.
The Amiel-Tison Neurological Assessment (ATNAT) relyes on responses to specific maneuvers and has specific contribution in the exploration of passive and active tone according to neurological maturation (99). The clinical significance of this type of assessment was more fully understood when Sarnat (100) reviewed anatomical andphysiological correlates of early neurological development. In fact, it became possible to clinically dissociate the development of upper and lower motor systems:
The lower system, consisting of the brainstem and cerebellum, matures early (beginning at 24 GW) in an ascending wave; its essential role is to maintain posture against gravity and flexor tone in the limbs;
The upper system, consisting of the cerebral hemispheres and basalganglia, matures later (beginning at 32 GW) and rapidly for the first 2 years in a descending wave; its essential role is to control the lowersystem, with relaxation of the limbs and control of the antigravity forces, finally allowing erect posture, walking and fine motor skills.
This distinction became even more relevant for clinicians after pathological and radiological data had shown that brain damage is mainly located in cerebral hemispheres, in the full term infant with hypoxic-ischemic encephalopathy or in the preterm infant with periventricular leukomalacia (PVL). The ATNAT may be used to determine the neurological status during the first days of life for fullterm infants or at 40 GWs (corrected age) for premature neonates. Interms of physiological correlates, it is satisfactory to cluster the different items into four subgroups according to their conceptual meaning: adequate hemispheric growth, absence of CNS depression, integrity of the upper motor control, stability of autonomic nervous system (ANS). When result for each item is normal, it seems reasonable to conclude to CNS optimality.
Some criteria are similar in fetal and neonatal assessments: headgrowth parameters including sutures’ status, primary reflexes,(restricted to sucking behavior), fingers’ movements and abduction of thumbs (shaded boxes in Table 3 (29)).
Table 3.
Optimality criteria assessed in the term neonate and comparable optimality criteria observed in the fetus in the second half of pregnancie by 3D/4D sonography. (From 29)
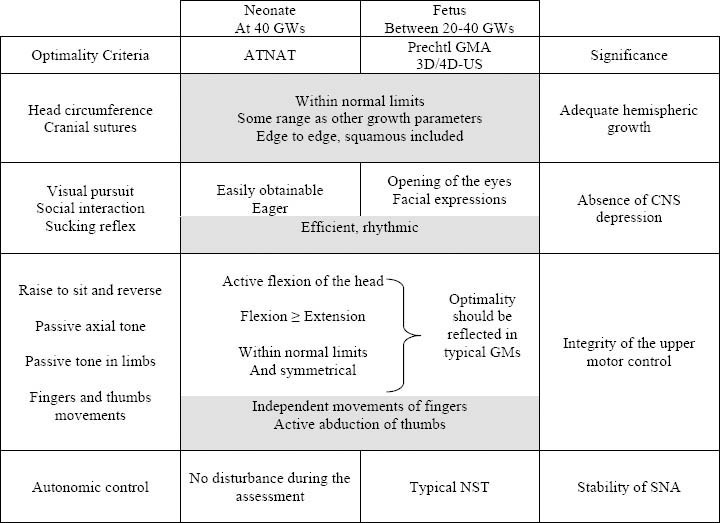
Some criteria observed in the fetus are only prerequisites for ex uterofunctional achievements: opening of the eyes for visual pursuit, facial expressions for social interaction. Their identification by 4D imaging, in addition to efficient and rhythmic sucking supports the absence of CNS depression.
Analytical criteria of typical passive and active tone in the neonate cannot be elicited in the fetus: head anteflexion versus retroflexion, ventral versus dorsal incurvations in the axis, both being of the utmost importance postnatally to confirm CNS optimality. However, optimality in the fetus should be reflected in typical GMS.
Criteria aiming to check autoregulation are slightly different: typical non stress test (NST) in the fetus and absence of reactions in the neonate.
In the presence of neurological signs, the next step is to proceed to the clinical synthesis. In the full term neonate the final categorization is based on the clustering of signs and symptoms observed within the first week of life; the non-optimal status can be graded into three categories:
minor degree, without CNS depression
moderate degree, with CNS depression
severe degree, with deep CNS depression and repeated seizures
The categorization based on the different findings yielded a good inter-rater reliability with a Kappa coefficient of 0.76. As described above, the identificaion of “CNS depression” during fetal life is based on pre-competences (opening of the eyes, variety of facial expressions), primary reflexes (rhythmical bursts in the sucking pattern) and quality of GMs. The addition of cranial signs (such as insufficient head growth and overlapping sutures) to neurological signs could be a valuable complement. Moreover, the identification of dynamic and static patterns of the symptoms may be as helpful to date the insult as it is postnatally: the more stable the signs, the more precocious the insult. Seizures are known to occur in utero, abnormally rhythmic movements having been occasionally perceived and reported by mothers. However, it is unlikely that such a brief and rare event should be seen with 4D-US.
An atlas on fetal CNS diseases recently reviewed fetal brain imaging; one chapter covers any visible congenital brain anomalies (101) while acquired brain abnormalities in utero including destructive lesions due to hypoxic-ischemic events, intracranial hemorrhage, porencephaliccysts and pseudocysts, are reviewed in another chapter (102). The probable outcome may be estimated according to fetal age at the time of diagnosis, size and location of clastic lesions or structural abnormalities as well as to the degree of severity of functional consequences evaluated by 4D-US. How to use that information still remains litigious in many cases, depending not only on ethical positions (personal or national) but also on expectations concerning the degree of cerebral plasticity at this early stage. We understand morphologists and neurosurgeons that often refer to a few personal cases with a favorable motor outcome despite very destructive brain lesions: they optimistically conclude that morphologydoes not always correlate with neurodevelopmental outcome. On the contrary, pediatricians and neuropsychologists involved in long term follow-up studies certainly are less optimistic. For example, an infant born with a massive destruction of the whole Sylvian artery territory in the left cerebral hemisphere may look fine when he sits independently at 7 months or walks independently at 18 months. However, at age 7 years with a confirmed diagnosis of hemiplegic CP, severe motor disorders are accompanied by disturbances of sensation, cognition, perception, behavior and a seizure disorder. As a conclusion, it is wise to check down the road: for each specific type of fetal brain damage, appropriate decisions for a conservative management have to rely onseries including long term outcome measurements.
Neuropathologists know very well that the best radiological techniques are not microscopes: many changes are below the limits of resolution of neuroimaging. Reviewing fetal and perinatal brain damages in 1998, (103) it was stressed the point that the group of children with normal imaging but non-optimal cerebral function presents an exciting opportunity to hypothesize correlations between neurocognitive disabilities and subtle diffuse brain abnormalities. However, we must refine every method of fetal assessment (fetal neurology included) before providing obstetricians with safe guidelines for the optimal management of fetuses at risk of neurodevelopmental disabilities. The 3D/4D-US gives hope for better future fetal management.
As far as subtle brain lesions are concerned, pathological gliosis hasto be distinguished from PVL as a diffuse lesion of white matter associated with an increase of hypertrophic astrocytes (positive with glial fibrillary acidic protein staining). When using routinely this staining, similar lesions are also detected based on the presence of reactive astrocytes in the germinal matrix; the de-population of this transient structure that can follow a hypoxic-ischemic event may influence the later capacity to produce neuroblasts and glial cells. In The postmigratory phase during the second half of gestation, another transient structure, the subplate, appears to be the site of selective vulnerability (104) with consequences on neocortex formation. The subplate is located between the cortical plate and the intermediate zone, reaching its maximal thickness between 22 and 36 GW. Each neuron will migrate into the subplate which plays several important roles up to term. Programmed cell death, wiring, and synaptogenesis are active processes during the second half of pregnancy, “processes that in some way interrupt, damage or otherwise influence the expected pattern of brain maturation”.71
Those damages can occur in utero but they probably occur as well in postnatal life in many extremely low birth weight (ELBW) infants. It is known that in this risk group the incidence of PVL is not higher than in the LBW group. However, we are aware of the high incidence of learning disabilities in the ELBW group (nearly half of them when tested from 7 to 9 years). It is obviously tempting to correlate those developmental sequellae to those subtle damages, as a result of early cerebral disorganization without macroscopic tissue destruction, and without detectable imaging.
Finally, it appears that when we consider not only CP, the tip of the iceberg, but the full spectrum of motor disorders, the moderate and mild clinical aspects are much more frequent than the severe ones. It is probably the same proportion for the pathologist between the clasticforms of brain damage and the more subtle and diffuses tissue impairment. However, we cannot equate clastic damage-positive imaging and CP on the one hand, and diffuse damage and negative imaging with milder disabilities on the other hand. Those categories overlap, i.e., somecases of the CP are not associated with clastic imaging and some cases with subtle motor disability (non-disabling CP) are associated with, for instance, obvious scars of PVL. In conclusion, clinicopathological correlations are established statistically but have to be applied with caution for each individual case.
The main obstacle to early prediction of CP based on a functional observation of the fetus such as observation by 3D/4D-US is due to the “precompetent” stage of most of the motor abilities observed in utero. In other words, can we predict the presence or absence of hemispheric brain damage, based on the observation of motor and reflex activity under the control of lower structures? The clinician is able to follow the switch in neural circuitry observed for each motor acquisition accordingto a specific chronology. As most of the hypoxic-ischemic damages of minor and moderate degrees are located in the cerebral hemispheres, it seems unwise to expect reassurance about the integrity of upper structures based on precompetent functional stages. Nevertheless, it remains important to test the integrity of the lower system as a prerequisite for a favourable outcome. This dilemma is not specific toneuromotor function. For example, fetal habituation which has attracted a lot of attention as a potential means of assessing fetal neural integrity may just be a prerequisite depending on the lower system (105). More longterm data are necessary to establish its predictive value for later development.
7. New Scoring System For Fetal NeuroBehavior Assessed By 3D And 4D Sonography
One of the most promising improvement in the unknown field of prenatal behavior has been the new 3D/4D-US technology. Its advance has been completed in giving visualizations in almost realtime and production of standards for different movement patterns to appear and develop. The 4D study of fetal behavior provided us with a great possibilityof understanding the hidden function of the developmental pathway of the fetal CNS and the potentialities of originating a neurological investigation in utero. By 4D technology we might be able to visualize an intrauterine neurological condition that would enable to identify which fetus is at risk and which is not. Existence of motoric competence in the newborn, even preterm infants is assumed to have its origins in prenatal life. Behavioral perinatology assessed by 4D sonography should be an interdisciplinary area of research involving concepts and conducting studies of the dynamic interplay between behavioral processes in fetal, neonatal, and infant life. The ultimate clinical application of fetal neurobehavioral assessment will be to identify functional characteristics of the fetus that predict a range of subsequent developmental dysfunction. Establishing this link will require demonstration of positive and negative predictability to outcomes significantly beyond the immediate perinatal period. After standardization of valid reference ranges of movements appropriate for the gestational age, attempts have been made to produce a newscoring system for fetal neurobehavior based on prenatal assessment by 3D/4D sonography (106, 111-114). That preliminary work may help in detecting fetal brain and neurodevelopmental alterations due to in utero brain impairment that is in accessible by any other method.
In the recent study, the Zagreb group published a newscoring system for fetal neurobehavior based on prenatal assessment by 3D/4D sonography (106). That scoring system is a combination of some parameters from fetal GM assessment and parameters from postnatal ATNAT which can be prenatally easily visualized by 4D-US (107, 108). The parameters were chosen basing on developmental approach to the neurological assessment and on the theory of central pattern generators of GM emergence. They were the product of multicentric studies conducted during several years which resulted with the most significant parameters for the assessment of fetal neurological development (Table 4). (42, 109, 110).
Table 4.
Antenatal Neurological Screening Test (KANET)
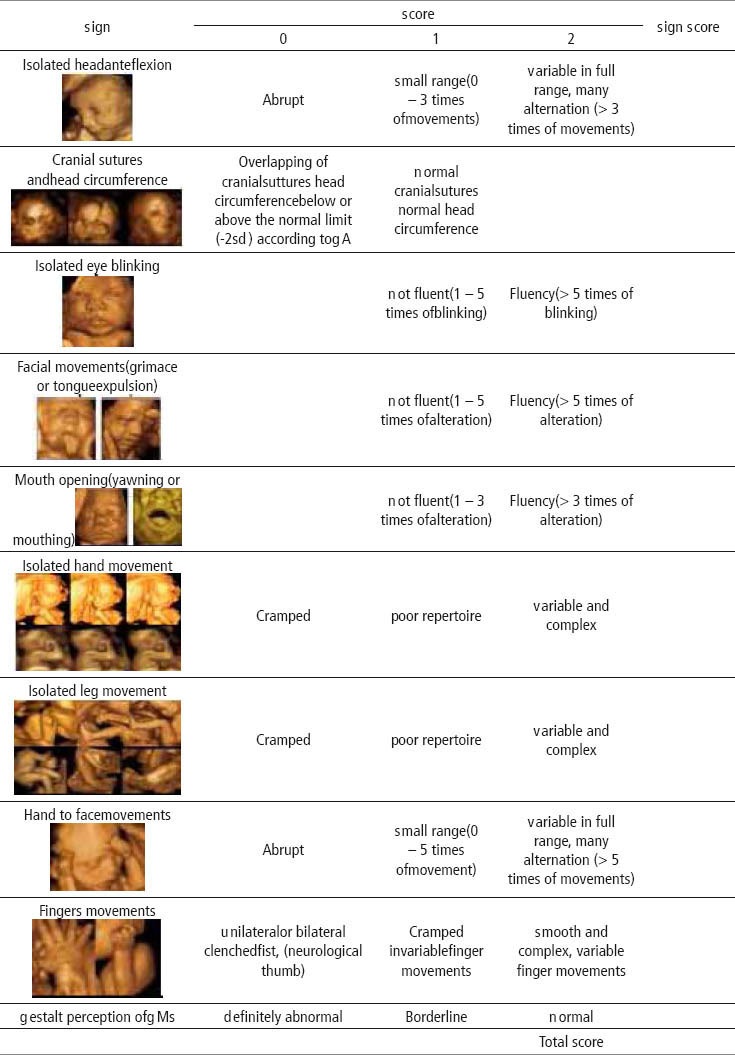
The authors developed a three point scale for isolated head anteflexion, isolated hand, leg, hand to face and finger movements, while for the assessment of cranial sutures, isolated eye blinking, facial alterations and mouth opening two-point scale was applied. The distinction between scores 0 and 2 is evident, whereas uncertainty may exist with regards to the assignation of a score of 1, the latter indicating an abnormal result of moderate degree. The precise description of the moderate abnormal performance is included for each item in the record form. Interpretation of total score is given in Table 5.
Table 5.
Allocation of fetuses according to Antenatal Neurological Screening Test

To produce the new scoring test the Zagreb group identified severely brain damaged infants and those with optimal neurological findings by comparing fetal with neonatal findings. In the group of 100 low-risk pregnancies they retrospectively applied new scoring system. After delivery, postnatal neurological assessment (ATNAT) was performed (95) and all neonates assessed as normal reached a score between 14 and 20, which was assumed to be a score of optimal neurological development. New scoring system was applied in the group of 120 high risk pregnancies in which, based on postnatal neurological findings, three subgroups of newborns were found: normal, mildly or moderately abnormal and abnormal. Based on this, a neurological scoring system has been proposed. All normal fetuses reached a score in the range from 14 to 20. Ten fetuses who were postnatally described as mildly or moderately abnormal achieved prenatal score of 5 to 13, while another ten fetuses postnatally assigned as neurologically abnormal had a prenatal score from 0 to 5. Among this group four had alobar holoprosencephally, one had severe hypertensive hydrocephaly, one had tanatophoric dysplasia and four fetuses had multiplemalformations.
Based on several years of research that group of authors has proposed a new test for antenatal application. There is a similarity between neonatal optimality test of Amiel-Tison, and that new scoring system for the assessment of neurological status in fetuses, which is a combination of postnatal ATNAT and GM assessment (100). One of the differences was that the analytical criteria of typical passive and activetone in the neonate cannot be elicited in the fetus: head anteflexionversus retroflexion, ventral versus dorsal incurvations in the axis, both being of the utmost importance postnatally to confirm CNS optimality. However, the status of the fetus should be reflected in the typical GMs.
The potential of the test was investigated at four university departments. The objective of this multricentric study (111) was to apply the new antenatal scoring system, named Kurjak antenatal neurodevelopmental test (KANET) to the fetuses from high risk pregnancies for neurological disorders and to verify the results of the test by two neonatal neurological tests: Amiel-Tison Neurological Assessment at Term (ATNAT) and general movements test by Prechtl. 288 pregnant women meeting the inclusion criteria given in the Table 6 (111) were found eligible to be included in the study.
Table 6.
Inclusion criteria for high risk pregnancies
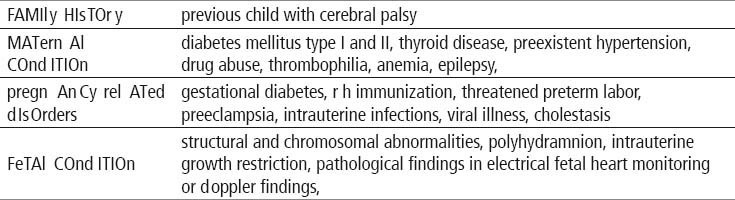
In this study 7 fetuses had abnormal KANET scores, and 25 fetuses were borderline, which gives all together 32 fetuses at neurological risk. Of 7 fetuses with abnormal KANET, postnatal neurological assessment by Amiel Tison’s method (ATNAT) revealed 3 newborns (arthrogryposis, vermis aplasia and neonate of the mother with the previous child with CP) out of 7 fetuses to be abnormal, while 4 were considered normal (ventriculomegaly, preeclampsia, thrombophylia, oligohydramnios). Out of 25 borderline KANET fetuses there were 22 borderline newborns by ATNAT, while 3 were normal (ventriculomegaly, syndrome of intraamniotic infection, mother’s thrombocytopenia). Those who were abnormal prenatally and normal postnatally had following prenatal risk factors: ventriculomegaly, Dandy Walker syndrome, skeletal dysplasia, polihydramnios, hydrocephaly, diabetes in pregnancy, nonimmune hydrops, syndrome of intraamniotic infection, IUGR, trisomy 21, thrombocytopenia, thrombophylia, preeclampsia, achondroplasia, oligohydramnios. Out of 3 abnormal neonates after ATNAT assessment, 2 had definitely abnormal Prechtl’s premature general movements (arthrogryposis and vermis aplasia), and additional 6 were considered abnormal (neonate of the mother with the previous child with CP, Dandy Walker syndrome, hydrocephaly, trisomy 21, ventriculomegaly, non immune hydrops). Rest of 24 children had normal optimal or normal suboptimal GMs.
The three very illustrative cases with abnormal KANET scoring were arthrogryposis, vermis aplasia, and fetus whose previous sibling had verified CP. The fetuses in these three cases had especially reduced facial movements the faces were like mask during repeated scans. Fetuses with vermis aplasia and arthrogryposis had normal cranial sutures but the isolated head flexion was small in range for both cases. Isolated hand movements, hand to face and leg movements were poor in repertoire for all three cases. The finger movements were cramped and invariable in all three cases. The Gestalt perception of General movements was abnormal in all three cases. Results of this study show that the new test might be useful in standardization of neurbehavioural assessments. Furthermore there is a potential for antenatal detection of serious neurological problems. At this stage test easily separates serious structural anomalies associated with brain impairment (artrhoghryposis, vermis aplasia, and anencephaly). Recent study showed a significant difference for 8 out of 10 parameters of KANET: isolated anteflection of the head, eye blinking, facial expressions (grimacing, tong expulsion), mouth movements (mouthing, jawing, swallowing), isolated hand movement, hand to face movement, fist and finger movements, and GMs. Authors have also confirmed statistically significant, moderate correlation of KANET and ATNAT tests. In practical sense, it means that the neuropediatrician who examined the newborns with ATNAT test confirmed the results of KANET.
This is work in progress and four collaborating centers are continuing investigation. In some of the centers (Doha, Zagreb) preliminary results are already obtained after one year of life. The new test might be a promising tool for the assessment of integrity of young central nervous system. However, the test requires further multycentric studies before recommended for wider clinical practice. We believe that the concept of KANET can be simplified and the time of the examination shortened. This could be achieved by grouping several similar parameters in to main categories and by certain changes in the scoring system. In the mean time the potential of antenatal scoring system shouldn’t be neither overestimated nor underestimated.
8. CONCLUSION
Despite medical reports from 100 years ago and 25 years of systematic research initiated by Prechtl and colleagues, the study of prenatal behavior is still in its infancy. One of the most promising advances in the field of ultrasonography has been the new 4D-US technology. Its advance has been completed in giving visualizations in almost real-time. The availability of new diagnostic data has in an extraordinary way raised our knowledge about intrauterine life, substantially modifying some earlier interpretations. The 4D study of fetal behavior provided us with a great possibility of understanding the hidden function of the developmental pathway of the fetal CNS and the potentialities of originating a neurological investigation in utero. Now, by 4D technology, we might be able to visualize an intrauterine neurological condition that would enable to identify which fetus is at risk and which is not. Existence of motoric competence in the newborn, even preterm infants is assumed to have its origins in prenatal life. Behavioral perinatology assessed by 4D sonography should be an interdisciplinary area of research involving concepts and conducting studies of the dynamic interplay between behavioral processes in fetal, neonatal, and infant life. The ultimateclinical application of fetal neurobehavioral assessment will be to identify functional characteristics of the fetus that predict a range of subsequent developmental dysfunction. Establishing this link will require demonstration of positive and negative predictability to outcomes significantly beyond the immediate perinatal period. After standardization of valid reference ranges of movements appropriate for the gestational age, attempts have been made to produce a newscoring system for fetal neurobehavior based on prenatal assessment by 3D/4D sonography. That preliminary work may help in detecting fetal brain and neurodevelopmental alterations due to in utero brain impairment that is inaccessible by any other method. It would be advisable to investigate the usefulness of new KANET test to identify the endangered fetuses from specific high risk pregnancies at neurological risk, like pregnancies complicated with IUGR.
REFERENCES
- 1.ACOG practice bulletin. Intrauterine growth restriction. N.12 January 2000. Int J Gynecol Obstet. 2001;72:85–96. [Google Scholar]
- 2.Mandruzzato G, Antsaklis A, Botet F, Chervenak FA, Figueras F, Grunebaum A, Puerto B, Skupski D, Stanojevic M. WAPM. Intrauterine restriction (IUGR) J Perinat Med. 2008;36(4):277–81. doi: 10.1515/JPM.2008.050. [DOI] [PubMed] [Google Scholar]
- 3.Altman DG, Hytten FE. Intrauterine growth retardation: let's be clear about it. Br J Obstet Gynaecol. 1989;96:1127–8. doi: 10.1111/j.1471-0528.1989.tb03185.x. [DOI] [PubMed] [Google Scholar]
- 4.Ott WJ. Intrauterine growth retardation: refining the definition. J Matern Fetal Invest. 1992;2:101–4. [Google Scholar]
- 5.Soothill PW, Bobrow CS, Holmes R. Small for gestationalage is not a diagnosis. Ultrasound Obstet Gynecol. 1999;13:225. doi: 10.1046/j.1469-0705.1999.13040225.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee PA, Chernausek SD, Hokken-Koelega ACS, Czernichow P. International small for gestational age board consensusdevelopment conference statement: managementof short children born small for gestational age, April24–October 1, 2001. Pediatrics. 2003;111:1253–61. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]
- 7.McCowan LM, Harding JE, Stewart AW. Umbilicalartery Doppler studies in small for gestational agebabies reflect disease severity. Br J Obstet Gynaecol. 2000;107:916–25. doi: 10.1111/j.1471-0528.2000.tb11092.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurjak A, Latin V, Polak J. Ultrasonic recognition of two types of growth retardation by measurement offour fetal dimension. J Perinat Med. 1978;6:102–8. doi: 10.1515/jpme.1978.6.2.102. [DOI] [PubMed] [Google Scholar]
- 9.Ott WJ. Intrauterine growth restriction and Dopplerultrasonography. J Ultrasound Med. 2000;19:661–5. doi: 10.7863/jum.2000.19.10.661. [DOI] [PubMed] [Google Scholar]
- 10.Ergaz Z, Avgil M, Ornoy A. Intrauterine growthrestriction–etiology and consequences: What do weknow about the human situation and experimentalanimal models? Reprod Toxicol. 2005;20:301–22. doi: 10.1016/j.reprotox.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Ott WJ. An update on the ultrasonic diagnosis andevaluation of intrauterine growth restriction. Ultrasound Rev Obstet Gynecol. 2005;5:111–24. [Google Scholar]
- 12.Neligan GA, Kolvin I, Scott D. London: William Heinemann Medical Books; 1978. Born too Soon or Borntoo Small. A Followup Study to Seven Years of Age. Spastic International Medical Publications; p. 66. [Google Scholar]
- 13.Briscoe TA, Rehn AE, Dieni S, et al. Cardiovascularand renal disease in the adolescent guinea pig afterchronic placental insufficiency. Am J Obstet Gynecol. 2004;191:847–55. doi: 10.1016/j.ajog.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 14.Tan TY, Yeo GS. Intrauterine growth restriction. CurrOpin Obstet Gynecol. 2005;17:135–42. doi: 10.1097/01.gco.0000162181.61102.d7. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert WM, Danielsen B. Pregnancy outcomes associatedwith intrauterine growth restriction. Am J ObstetGynecol. 2003;188:1596–9. doi: 10.1067/mob.2003.384. [DOI] [PubMed] [Google Scholar]
- 16.Li CI, Daling JR, Emanuel I. Birthweight and risk ofoverall and causespecific childhood mortality. PaediatrPerinat Epidemiol. 2003;17:164–70. doi: 10.1046/j.1365-3016.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–23. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ, Osmond C, Simmonds SJ, et al. Therelation of small head circumference and thinness atbirth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–6. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale CR, Martyn CN. Birthweight and later risk ofdepression in a national birth cohort. Br J Psychiatry. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- 20.Mittendorfer-Rutz E, Rasmussen F, Wasserman D. Restricted fetal growth and adverse maternal psychosocialand socioeconomic conditions as risk factors forsuicidal behaviour of offspring: a cohort study. Lancet. 2004;364:1135–40. doi: 10.1016/S0140-6736(04)17099-2. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson B, Francis A, Hagberg G, et al. Cerebralpalsy is strongly associated with severe intrauterinegrowth restriction in term but not in preterm cases. AmJ Obstet Gynecol. 2003;189:S74. [Google Scholar]
- 22.Amiel-Tison C, Gosselin J. From Neonatal to Fetal Neurology: Some Clues For Interpreting Fetal Findings. In: Pooh RK, Kurjak A, editors. Fetal neurology. New Delhi: Jaypee Brothers; 2009. [Google Scholar]
- 23.Back SA. Perinatal white matter injury: the changing spectrum of pathologyand emerginginsights into pathogenetic mechanisms. Ment Retard Dev DisabilRes Rev. 2006;12:129–40. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 24.Salihagic-Kadic A, Medic M, Kurjak A. Recent advances in neurophysiology. In: Kurjak A, Azumendi G, editors. The Fetus in Three Dimensions: Imaging, Embryology, And Fetoscopy. UK: Informa Health Care; 2007. [Google Scholar]
- 25.Prechtl HFR. Qualitative changes of spontaneous movements in fetus andpreterm infant are a marker of neurological dysfunction. Early Hum Dev. 1990;23:151–8. doi: 10.1016/0378-3782(90)90011-7. [DOI] [PubMed] [Google Scholar]
- 26.Kurjak A, Miskovic B, Andonotopo W, Stanojevic M, Azumendi G, Vrcic H. How useful is 3D and 4D ultrasound in perinatal medicine? J Perinat Med. 2007;3:10–27. doi: 10.1515/JPM.2007.002. [DOI] [PubMed] [Google Scholar]
- 27.Kurjak A, Vecek N, Hafner T, Bozek T, Funduk-Kurjak B, Ujevic B. Prenataldiagnosis: what does four-dimensional ultrasound add? J Perinat Med. 2002;30:57–62. doi: 10.1515/JPM.2002.008. [DOI] [PubMed] [Google Scholar]
- 28.Azumendi G, Kurjak A. Three-dimensional and fourdimensional sonographyin the study of the fetal face. Ultrasound Rev Obstet Gynecol. 2003;3:1–10. [Google Scholar]
- 29.Kurjak A, Pooh RK, Tikvica A, Stanojevic M, Miškovic B, Ahmed B, Azumendi G. Assessment of Fetal Neurobehavior By 3D/4D Ultrasound. In: Pooh RK, Kurjak A, editors. Fetal neurology. New Delhi: Jaypee Brothers; 2009. [Google Scholar]
- 30.Kurjak A, Vecek N, Kupesic S. Four dimensional ultrasound: how much doesit improve perinatal practice? In: Carrera JM, Chervenak FA, Kurjak A, editors. Controversies in Perinatal Medicine, Studies on the Fetus as a Patient. NewYork: Parthenon Publishing; 2003. pp. 222–34. [Google Scholar]
- 31.Kurjak A, Carrera J, Medic M, Azumendi G, Andonotopo W, Stanojevic M. The antenatal development of fetal behavioral patterns assessed by fourdimensionalsonography. J Matern Fetal Neonatal Med. 2005;17:401–16. doi: 10.1080/14767050400029657. [DOI] [PubMed] [Google Scholar]
- 32.Nijhuis JG, editor. Oxford: Oxford University Press; 1992. Fetal Behaviour: Developmental and Perinatal Aspects. [Google Scholar]
- 33.Prechtl HFR. How can we assess the integrity of the fetal nervous system? [Google Scholar]
- 34.Einspieler C, HFR Prechtl, Bos AF, Ferrari F, Cioni G. London: Mac Keith Press; 2004. Prechtl's method on thequalitative assessment of general movements in preterm, term and younginfants. [Google Scholar]
- 35.de Vries JIP, Visser GH, Prechtl HF. Early Hum Dev. Vol. 7. Oxford: Oxford University Press; 1982. The emergence of fetal behavior, I. Qualitative aspect; pp. 301–22. [DOI] [PubMed] [Google Scholar]
- 36.Amiel-Tison C. Clinical assessment of the infant nervous system. In: Levene MI, Chervenak FA, Whittle M, editors. Fetal and Neonatal Neurology and Neurosurgery. 3rd ed. London: Churchill Livingstone; 2001. [Google Scholar]
- 37.Sarnat HB. Functions of the corticospinal and corticobulbar tracts in the humannewborn. J Pediatr Neurol. 2003;1:3. [Google Scholar]
- 38.Shawker TH, Schuette WH, Whitehouse W, Rifka SM. Early fetal movement: a real time ultrasound study. Obstet Gynecol. 1980;55:194–8. [PubMed] [Google Scholar]
- 39.Prechtl HFR. How can we assess the integrity of the fetal nervous system? [Google Scholar]
- 40.Kurjak A, Carrera JM, Stanojevic M, Andonotopo W, Azumendi G, Scazzocchio E, Medic M, Salihagic-Kadic A. The role of 4D sonography in the neurologicalassessment of early human development. Ultrasound Rev Obstet Gynecol. 2004;4:148–59. [Google Scholar]
- 41.Kurjak A, Vecek N, Hafner T, Bozek T, Funduk-Kurjak B, Ujevic B. Prenataldiagnosis: what does four-dimensional ultrasound add? J Perinat Med. 2002;30:57–62. doi: 10.1515/JPM.2002.008. [DOI] [PubMed] [Google Scholar]
- 42.Kurjak A, Andonotopo W, Hafner T, Salihagic Kadic A, Stanojevic M, Azumendi G, Ahmed B, Carrera JM, Troyano JM. Normal standards for fetalneurobehavioral developments —longitudinal quantification by fourdimensionalsonography. J Perinat Med. 2006;34:56–65. doi: 10.1515/JPM.2006.007. [DOI] [PubMed] [Google Scholar]
- 43.Horimoto N, Koyanagi T, Maeda H, Satoh S, Takashima T, Minami T, Nakano H. Can brain impairment be detected by in utero behavioural patterns? ArchDis Child. 1993;69:3–8. doi: 10.1136/adc.69.1_spec_no.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morokuma S, Fukushima K, Yumoto Y, Uchimura M, Fujiwara A, Matsumoto M, Satoh S, Nakano H. Simplified ultrasound screening for fetal brain functionbased on behavioral pattern. Early Hum Dev. 2007;83:177–81. doi: 10.1016/j.earlhumdev.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Kurjak A, Pooh RK, Tikvica A, Miškovic B, Stanojevic M, Ahmed B. Fetal Behavior in High-risk Pregnancies Assessed by Different forms of Ultrasound Techniques. In: Pooh RK, Kurjak A, editors. Fetal neurology. New Delhi: Jaypee Brothers; 2009. [Google Scholar]
- 46.Van Vliet MA, Martin CB, Jr, Nijhuis JG, Prechtl HF. The relationship between fetal activity and behavioral states and fetal breathing movements in normaland growth-retarded fetuses. Am J Obstet Gynecol. 1985 Nov 1;153(5):582–8. doi: 10.1016/0002-9378(85)90483-1. [DOI] [PubMed] [Google Scholar]
- 47.Van Vliet MA, Martin CB, Jr, Nijhaus JG, Prechtl HF. Behavioural states in growth-retarded human fetuses. Early Hum Dev. 1985;12:183–97. doi: 10.1016/0378-3782(85)90181-1. [DOI] [PubMed] [Google Scholar]
- 48.Arduini D, Rizzo G, Romanini C, Mancuso S. Computerized analysis of behavioural states in asymmetrical growth retarded fetuses. J Perinat Med. 1988;16(4):357–63. doi: 10.1515/jpme.1988.16.4.357. [DOI] [PubMed] [Google Scholar]
- 49.Nijhuis JG, Prechtl HF, Martin CB, Jr, Bots RS. Are there behavioural states in the human fetuses? Early Hum Dev. 1982;6(2):177–95. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- 50.Rizzo G, Arduini D, Pennestri F, Romanini C, Mancuso S. Fetal behavior in growth retardation: its relationship to fetal blood flow. Prenat Diagn. 1987;7(4):229–38. doi: 10.1002/pd.1970070402. [DOI] [PubMed] [Google Scholar]
- 51.Sival DA, Visser GH, Prechtl HF. The effect of intrauterine growth retardation on the quality of general movements in the human fetus. Early Hum Dev. 1992 Feb;28(2):119–32. doi: 10.1016/0378-3782(92)90107-r. [DOI] [PubMed] [Google Scholar]
- 52.Sival DA, Visser GH, Prechtl HF. The relationship between the quantity and quality of prenatal movements in pregnancies complicated by intra-uterine growth retardation and premature rupture of the membranes. Early Hum Dev. 1992 Oct;30(3):193–209. doi: 10.1016/0378-3782(92)90069-s. [DOI] [PubMed] [Google Scholar]
- 53.Ribbert LS, Visser GH, Mulder EJ, Zonneveld MF, Morssink LP. Changes with time in fetal heart rate variation, movement incidences and haemodynamics in intrauterine growth retarded fetuses: a longitudinal approach to the assessment of fetal well being. Early Hum Dev. 1993;31(3):195–208. doi: 10.1016/0378-3782(93)90195-z. [DOI] [PubMed] [Google Scholar]
- 54.Vindla S, James DK, Sahota DS, Coppens M. Computerised analysis of behavior in normal and growth-retarded fetuses. Eur J Obstet Gynecol Reprod Biol. 1997 Dec;75(2):169–75. doi: 10.1016/s0301-2115(97)00131-0. [DOI] [PubMed] [Google Scholar]
- 55.Bekedam DJ, Mulder EJ, Snijders RJ, Visser GH. The effects of maternal hyperoxia on fetal breathing movements, body movements and heart rate variation in growth retarded fetuses. Early Hum Dev. 1991;27(3):223–32. doi: 10.1016/0378-3782(91)90196-a. [DOI] [PubMed] [Google Scholar]
- 56.Andonotopo W, Kurjak A. The assessment of fetal behavior of growth restricted fetuses by 4D sonography. J Perinat Med. 2006;34:471–8. doi: 10.1515/JPM.2006.092. [DOI] [PubMed] [Google Scholar]
- 57.Baschat AA. Doppler application in the delivery timingof the preterm growth restricted fetus: another step inthe right direction. Ultrasound Obstet Gynecol. 2004;23:111–9. doi: 10.1002/uog.989. [DOI] [PubMed] [Google Scholar]
- 58.Jacobsson B, Hagberg G. Antenatal risk factors forcerebral palsy. Best Pract Res Clin Obstet Gynaecol. 2004;18:425–36. doi: 10.1016/j.bpobgyn.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Toft PB, Leth H, Ring PB, et al. Volumetric analysis ofthe normal infant brain and in intrauterine growthretardation. Early Hum Dev. 1995;43:15–29. doi: 10.1016/0378-3782(95)01657-o. [DOI] [PubMed] [Google Scholar]
- 60.Rees S, Mallard C, Breen S, et al. Fetal brain injuryfollowing prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol A Mol Integr Physiol. 1998;19:653–60. doi: 10.1016/s1095-6433(98)01001-0. [DOI] [PubMed] [Google Scholar]
- 61.Wilcox AJ, Russell I. Birthweight and perinatal mortality, II. On weight specific mortality. Int JEpidemiol. 1983;12(3):319–25. doi: 10.1093/ije/12.3.319. [DOI] [PubMed] [Google Scholar]
- 62.Jarvis S, Glinianaia SV, Blair E. Cerebral palsy and intrauterine growth. Clin Perinatol. 2006;33(2):285–300. doi: 10.1016/j.clp.2006.03.009. Review. [DOI] [PubMed] [Google Scholar]
- 63.Jarvis SN, Glinianaia SV, Torrioli M-G, et al. Cerebral palsy and intrauterine growth in singlebirths: a European collaborative study. Lancet. 2003;362:1106–11. doi: 10.1016/S0140-6736(03)14466-2. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsson B, Hagberg G, Hagberg B, et al. Cerebralpalsy in preterm infants: a population-basedcase–control study of antenatal and intrapartal riskfactors. Acta Paediatr. 2002;91:946–51. doi: 10.1080/080352502760148685. [DOI] [PubMed] [Google Scholar]
- 65.Thorngren-Jerneck K. Lund: Lund University; 2002. Cerebral Injury in PerinatalAsphyxia. [Google Scholar]
- 66.Jarvis SN, Glinianaia SV, Arnaud C, et al. Case gender and severity in cerebral palsy varies withintrauterine growth. Arch Dis Child. 2005;90:474–9. doi: 10.1136/adc.2004.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leader LR, Baille P, Martin B, et al. The assessment and significance of habituation to a repeatedstimulus by the human fetus. Early Hum Dev. 1982;7(3):211–9. doi: 10.1016/0378-3782(82)90084-6. [DOI] [PubMed] [Google Scholar]
- 68.Birkbeck JA. Metrical growth and skeletal development of the human fetus. In: Roberts DF, Thomson AM, editors. The biology of human fetal growth. Symposia of the Society for theStudy of Human Biology. Vol. 15. London: 7 Taylor and Francis; 1976. pp. 39–68. [Google Scholar]
- 69.Taylor DC. Differential rates of cerebral maturation between sexes and between hemispheres: evidence from epilepsy. Lancet. 1969;2(7612):140–2. doi: 10.1016/s0140-6736(69)92445-3. [DOI] [PubMed] [Google Scholar]
- 70.Geschwind N, Galaburda A. Cerebral lateralization: biological mechanisms, associations andpathology. Arch Neurol. 1985;42:428–59. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 71.Ounsted M, Ounsted C. Maternal regulation of intrauterine growth. Nature. 1966;212:995–7. doi: 10.1038/212995a0. [DOI] [PubMed] [Google Scholar]
- 72.Pharoah POD, Cooke T, Johnson MA, et al. Epidemiology of cerebral palsy in England andScotland 1984–9. Arch Dis Child Fetal Neonatal Ed. 1998;79:F21–5. doi: 10.1136/fn.79.1.f21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colver A, Gibson M, Hey EN, et al. Increasing rates of cerebral palsy across the severityspectrum in North East England 1964–1993. Arch Dis Child Fetal Neonatal Ed. 2000;83(1):F7–13. doi: 10.1136/fn.83.1.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stanley F, Blair E, Alberman E. Cambridge: 7 Cambridge University Press; 2000. Cerebral palsies: epidemiology and causal pathways. [Google Scholar]
- 75.Drummond PM, Colver AF. Analysis by gestational age of cerebral palsy in singleton births innortheast England 1970–1994. Paediatr Perinat Epidemiol. 2002;16:172–80. doi: 10.1046/j.1365-3016.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 76.Blair E. The undesirable consequences of controlling for birthweight in perinatal epidemiologicstudies. J Epidemiol Comm Health. 1996;50:559–63. doi: 10.1136/jech.50.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonellie SR, Raab GM. Why are babies getting heavier? Comparison of Scottish births from 1980 to 1992. BMJ. 1997;315:1205. doi: 10.1136/bmj.315.7117.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. ActaObstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 79.Gardosi J, Chang A, Kalyan B, et al. Customised antenatal growth charts. Lancet. 1992;339:283–7. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 80.Gardosi J. Customized fetal growth standards: rationale and clinical application. Semin Perinatol. 2004;28(1):33–40. doi: 10.1053/j.semperi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Hediger ML, Scholl TO, Schall JI, et al. Fetal growth and the etiology of preterm delivery. Obstet Gynecol. 1995;85(2):175–82. doi: 10.1016/0029-7844(94)00365-K. [DOI] [PubMed] [Google Scholar]
- 82.Hadlock F, Harrist R, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weightstandard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 83.Marsal K, Persson P-H, Larsen T, et al. Intrauterine growth curves based on ultrasonicallyestimated fetal weights. Acta Paediatr. 1996;85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 84.Gardosi J, Mongelli M, Wilcox M, et al. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–74. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- 85.Blair E, Stanley F. Intrauterine growth and spastic cerebral palsy. I. Association with birthweightfor gestational age. Am J Obstet Gynecol. 1990;162(1):229–37. doi: 10.1016/0002-9378(90)90856-3. [DOI] [PubMed] [Google Scholar]
- 86.Uvebrant P, Hagberg G. Intrauterine growth in children with cerebral palsy. Acta Paediatr. 1992;81:407–12. doi: 10.1111/j.1651-2227.1992.tb12259.x. [DOI] [PubMed] [Google Scholar]
- 87.Foley J. Birth-weight ratio and cerebral palsy. Early Hum Dev. 1995;40(2):145–56. doi: 10.1016/0378-3782(94)01602-L. [DOI] [PubMed] [Google Scholar]
- 88.Kyllerman M, Bager B, Bensch J, et al. Dyskinetic cerebral palsy. I. Clinical categories,associated neurological abnormalities and incidences. Acta Paediatr Scand. 1982;71:543–50. doi: 10.1111/j.1651-2227.1982.tb09472.x. [DOI] [PubMed] [Google Scholar]
- 89.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability ofperinatal white matter in premature birth. Stroke. 2007;38:724–30. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 90.Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D. Executive Committe for the Definition of Cerebral Palsy et al. Proposed definition and classification of cerebral palsy, April 2005. Dev MedChild Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 91.Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev MedChild Neurol. 2005;47:571. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 92.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy. DevMed Child Neurol. 2007;109:8–14. [PubMed] [Google Scholar]
- 93.DiPietro JA, Bronstein MH, Costigan KA, et al. What does fetal movementpredict about behavior during the first two years of life? Dev Psychobiol. 2002;40:358–71. doi: 10.1002/dev.10025. [DOI] [PubMed] [Google Scholar]
- 94.DiPietro JA, Hodgson DM, Costigan KA, Johnson TR, et al. Fetal antecedentsof infant temperament. Child Dev. 1996;67:2568–83. [PubMed] [Google Scholar]
- 95.DiPietro JA, Costigan KA, Pressman EK. Fetal state concordance predicts infantstate regulation. Early Hum Dev. 2002;68:1–13. doi: 10.1016/s0378-3782(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 96.Palmer FB. Strategies for the early diagnosis of cerebral palsy. J Pediatr. 2004;145:8–11. doi: 10.1016/j.jpeds.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 97.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of grossfetal body movements over 24-hours observation intervals during the last 10weeks of pregnancy. Am J Obstet Gynecol. 1982;142:363–71. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- 98.Kurjak A, Stanojevic M, Azumendi G, Carrera JM. The potential of fourdimensional (4D) ultrasonography in the assessment of fetal awareness. JPerinat Med. 2005;33:46–53. doi: 10.1515/JPM.2005.008. [DOI] [PubMed] [Google Scholar]
- 99.Gosselin J, Amiel-Tison C, Infante-Rivard C, Fouron C, Palmer FB. Strategiesfor the early diagnosis of cerebral palsy. J Pediatr. 2004;145:8–11. [Google Scholar]
- 100.Pooh RK, Maeda K, Pooh KH. Acquired brain abnormalities in utero. In: Pooh RK, Maeda K, Pooh KH, editors. An Atlas of Fetal Central Nervous System Disease – Diagnosis and Management. Parthenon, New York: 2003. [Google Scholar]
- 101.Pooh RK, Pooh KH. Congenital brain anomalies. In: Pooh RK, Maeda K, Pooh KH, editors. An Atlas of Fetal Central Nervous System Disease – Diagnosis and Management. Parthenon, New York: 2003. [Google Scholar]
- 102.Amiel-Tison C, Laurini RN. Fetal and perinatal brain damage: aclinicopathological correlation. In: Kurjak A, editor. Textbook of Perinatal Medicine. Parthenon, London: 1998. [Google Scholar]
- 103.Kostovic I, Judas M, Rados M, Hrabac Laminar organization of the humanfetal cerebrum revealed by histochemical markers and MRI. Cereb Cortex. 2002;12:536. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 104.Hepper PG. Fetal habituation: another Pandora's box? Dev Med Child Neurol. 1997;39:274. doi: 10.1111/j.1469-8749.1997.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 105.Strijbis EMM, Oudman I, van Essen P, MacLennan AH. Cerebral palsy andthe application of the international criteria for acute intrapartum hypoxia. Obstet Gynecol. 2006;107:1357–65. doi: 10.1097/01.AOG.0000220544.21316.80. [DOI] [PubMed] [Google Scholar]
- 106.Kurjak A, Miskovic B, Stanojevic M, Amiel-Tison C, Ahmed B, Azumendi G, Vasilj O, Andonotopo W, Turudic T, Salihagic-Kadic A. New scoring systemfor fetal neurobehavior assessed by three-and four-dimensional sonography. J Perinat Med. 2008;36(1):73–81. doi: 10.1515/JPM.2008.007. [DOI] [PubMed] [Google Scholar]
- 107.Amiel-Tison C. Neurological assessment of the neonate revisited: a personalview. Dev Med Child Neurol. 1990;32:1105–13. doi: 10.1111/j.1469-8749.1990.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 108.Amiel-Tison C, Gosselin J, Kurjak A. Neurosonography in the second half offetal life: a neonatologist's point of view. J Perinat Med. 2006;34:437–46. doi: 10.1515/JPM.2006.088. [DOI] [PubMed] [Google Scholar]
- 109.Kurjak A, Stanojevic M, Andonotopo W, Scazzocchio-Duenas E, Azumendi G, Carrera JM. Fetal behavior assessed in all three trimesters of normalpregnancy by four-dimensional ultrasonography. Croat Med J. 2005;46:772–80. [PubMed] [Google Scholar]
- 110.Kurjak A, Stanojevic M, Andonotopo W, Salihagic-Kadic A, Carrera JM, Azumendi G. Behavioral pattern continuity from prenatal to postnatal life - a study by four-dimensional (4D) ultrasonography. J Perinat Med. 2004;32:346–53. doi: 10.1515/JPM.2004.065. [DOI] [PubMed] [Google Scholar]
- 111.Kurjak A, Abo-Yaqoub S, Stanojevic M, Yigiter AB, Vasilj O, Lebit D, Shaddad AN, Ahmed B, Nese Kavak Z, Miskovi B, Vladareanu R, Spalldi L, Guillermo Azumendi G, Younis M, Pooh RK, Salihagic Kadic A. The potential of 4D sonography in the assessment of fetal neurobehavior–multicentric study in high risk pregnancies. J Perinat Med. 2010;38(1):77–82. doi: 10.1515/jpm.2010.012. [DOI] [PubMed] [Google Scholar]
- 112.Miškovic B, Vasilj O, Stanojevic M, Ivankovic D, Kerner M, Tikvica A. The comparison of fetal behavior in high risk and normal pregnancies assessed by four dimensional ultrasound. J Matern Fetal Neonatal Med. 2010 doi: 10.3109/14767051003678200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 113.Kurjak A, Ahmed B, Abo-Yaguab S, Younis M, Saleh H, Shaddad AN, Vasilj O, Al Bahar AJ, Miškovic B. An Attempt to Introduce Neurological Test for Fetus-based on 3D and 4D Sonography. Donald School Journal of Ultrasound in Obstetrics and Gynecology. 2008;2(4):29–44. [Google Scholar]
- 114.Kurjak A, Tikvica Luetic A, Stanojevic M, Talic A, Zalud I, Al-Noobi M, Perva S, Abushama M, Tomasovic S, Zaputovic S, Honemeyer U. Further Experience in the Clinical Assessment of Fetal NeurobehaviorDonald School Journal of Ultrasound in Obstetrics and Gynecology. 2010;4(1):59–71. [Google Scholar]


