Abstract
Aim
To assess the feasibility and safety of early oral feeding (EOF) after gastrectomy for gastric cancer through a systematic review and meta-analysis based on randomized controlled trials.
Methods
A literature search in PubMed, Embase, Web of Science and Cochrane library databases was performed for eligible studies published between January 1995 and March 2014. Systematic review was carried out to identify randomized controlled trials comparing EOF and traditional postoperative oral feeding after gastric cancer surgery. Meta-analyses were performed by either a fixed effects model or a random effects model according to the heterogeneity using RevMan 5.2 software.
Results
Six studies remained for final analysis. Included studies were published between 2005 and 2013 reporting on a total of 454 patients. No significant differences were observed for postoperative complication (RR = 0.95; 95%CI, 0.70 to 1.29; P = 0.75), the tolerability of oral feeding (RR = 0.98; 95%CI, 0.91 to 1.06; P = 0.61), readmission rate (RR = 1; 95%CI, 0.30 to 3.31; P = 1.00) and incidence of anastomotic leakage (RR = 0.31; 95%CI, 0.01 to 7.30; P = 0.47) between two groups. EOF after gastrectomy for gastric cancer was associated with significant shorter duration of the hospital stay (WMD = −2.36; 95%CI, −3.37 to −1.34; P<0.0001) and time to first flatus (WMD = −19.94; 95%CI, −32.03 to −7.84; P = 0.001). There were no significant differences in postoperative complication, tolerability of oral feeding, readmission rates, duration of hospital stay and time to first flatus among subgroups stratified by the time to start EOF or by partial and total gastrectomy or by laparoscopic and open surgery.
Conclusions
The result of this meta-analysis showed that EOF after gastric cancer surgery seems feasible and safe, even started at the day of surgery irrespective of the extent of the gastric resection and the type of surgery. However, more prospective, well-designed multicenter RCTs with more clinical outcomes are needed for further validation.
Introduction
Recently, the concept of fast-track surgery is drawing increasing attention, which requires multidisciplinary team work to accelerate recovery during perioperative care [1]. Early oral feeding (EOF) is one of the most important parts of fast-track surgery elements. The advantages of early enteral nutrition after colorectal surgery have been demonstrated in several reports, such as a shorter length of hospital stay and less postoperative morbidity and mortality compared with traditional postoperative oral feeding (TOF) [2]–[4].
Gastric cancer is the second most common cause of cancer-related death worldwide, of which the global incidence is declining. However, there remains quite higher morbidity rate in Asia compared to that in western countries [5]. Gastric cancer can be cured successfully with surgical resection and the operative technique and anastomotic type for gastric cancer has been gradually standardized [6]–[8]. Then, surgeons should pay more attention to how to enhance recovery, reduce complications and improve quality of life of patients undergoing gastrectomy for gastric cancer [9]–[11]. To date, the introduction of fast-track surgery following gastrectomy has been demonstrated for nearly one decade [12]–[14]. However, as a key element of fast track surgery pathway, the significance of EOF after gastric cancer surgery is still controversial.
The purpose of this study was to assess the feasibility and safety of EOF in patients after gastric cancer surgery through a systematic review based on randomized controlled trials.
Materials and Methods
Search strategy
The relevant literature was searched from PubMed, Embase, Web of Science and Cochrane library databases published between January 1995 and March 2014. The following search terms was used: gastric cancer, gastrectomy, early oral feeding, early oral intake, enhanced recovery and fast-track surgery. Reference lists within selected studies and abstracts published at major international conferences were also searched for potentially eligible studies.
Study inclusion criteria
The studies were limited to be described as the design type of randomized controlled trials (RCTs) with or without blinding method, comparing EOF with TOF following gastrectomy for gastric cancers. Oral feeding was following a stepwise plan from water to other liquids to semi-fluids to normal food. EOF was defined as oral feeding of water or glucose saline initiated before flatus as tolerated; TOF was defined as oral feeding initiated certainly after flatus. Comparative studies that included patients undergoing EOF after gastric cancer surgery through nasogastric enteral nutrition were excluded. We applied restrictions with respect to language in English.
Quality Assessment
The quality of included RCTs was assessed by two reviewers (XP Liu and D Wang) independently according to the Cochrane Collaboration's tool for assessing risk of bias, which addressed seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, freedom from selective reporting, and freedom from other bias [15]. Disagreement was resolved by consensus and discussion.
Outcomes
The primary outcomes were postoperative complication, tolerability of oral feeding and readmission rates. The secondary outcomes were the duration of hospital stay expressed as hospitalization days after surgery, time of first flatus and incidence of anastomotic leakage. Patients failed to tolerate EOF presented as recurrent nausea, vomiting, abdominal distension without intestinal sound or nasogastric tube was reinserted.
Statistical analysis
Statistical analysis was performed with Cochrane Collaboration's RevMan5.2 software (Cochrane Collaboration, Oxford, UK). For continuous data, results from each study were expressed as a weighted mean difference (WMD) with 95% confidence intervals (CI) and combined for meta-analysis. Data were summarized graphically in forest plots. For dichotomous data, results for each study were performed by using the relative risk (RR). The Mantel–Haenszel method was used to combine the RRs for the outcomes of interest. A funnel plot for postoperative complication was constructed to evaluate publication and other biases [16].
Heterogeneity was measured through X 2 and I2 test. If between-study heterogeneity existed (I2>50%), random-effect model was used; otherwise, meta-analysis was done with fixed effect model. P<0.05 in two-sided test was considered statistically significant.
Results
Selected studies
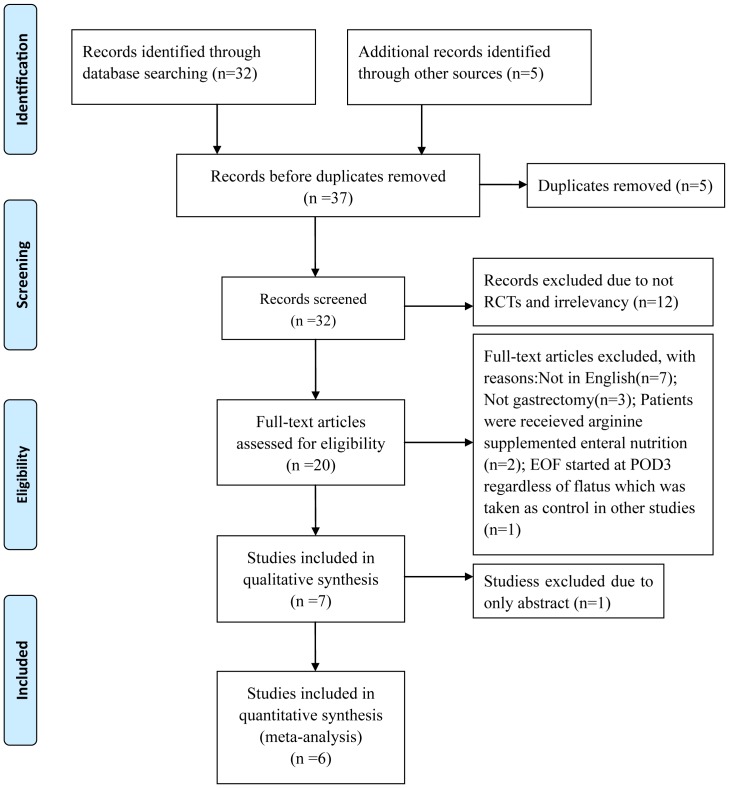
A flow chart detailing the process of study identification and selection following the PRISMA statement (see Checklist S1) is shown in Fig.1. Six studies finally remained for further analysis. Included studies were published between 2005 and 2013 and reported on a total of 454 patients [17]–[22]. In the study by Chen et al., they carried out subgrourp analysis stratified by laparoscopic and open surgery, so L and O were used to tell them apart in the present meta-analysis [17]. The characteristics of these six studies are summarized in Table 1.
Figure 1. Flow chart of studies identified, included and excluded following PRISMA statement.
Table 1. Characteristics of the studies included in meta-analysis.
| Author | Country | Year | Time of oral feeding | Extent of gastrectomy | Group | Number | Age | Gender | BMI | ASA | TNM stage |
| (years) | (m/f) | (kg/m2) | (0/1/2) | (I+II)/(III+IV) | |||||||
| Liu[21] | China | 2010 | day of surgery | TG, PG, DG | EOF | 30 | 60.7±9.7 | 18/15 | 21.84±2.65 | 2c | 8/25 |
| TOF | 33 | 61.9±8.3 | 15/14 | 21.28±2.54 | 2 c | 7/23 | |||||
| Wang[22] | China | 2010 | day of surgery | PG, DG | EOF | 45 | 58.76±9.66 | 32/13 | 23.85±2.40 | NR | NR |
| TOF | 47 | 56.87±9.16 | 29/18 | 23.25±2.79 | |||||||
| Hur[19] | Korea | 2011 | POD1 | TG, SG | EOF | 28 | 24/4 a | 20/8 | 21/7 b | 10/18d | NR |
| TOF | 26 | 21/5a | 13/13 | 19/7b | 4/22d | ||||||
| Chen(L)[17] | China | 2012 | 6–8h after surgery | DG | EOF | 19 | 59(49–71) | 10/9 | 22.94±2.23 | NR | 11/8 |
| TOF | 22 | 62.5(45–72) | 10/12 | 22.99±2.24 | 11/11 | ||||||
| Chen(O)[17] | China | 2012 | 6–8h after surgery | DG | EOF | 21 | 64(40–71) | 9/12 | 23.54±2.59 | NR | 9/12 |
| TOF | 20 | 64.5(49–75) | 12/8 | 23.47±2.62 | 7/13 | ||||||
| Kim[20] | Korea | 2012 | POD2 | DG | EOF | 22 | 52.64±11.57 | 13/9 | 23.40±3.17 | 0/14/8 | 21/1 |
| TOF | 22 | 57.45±14.54 | 15/7 | 23.77±3.54 | 0/14/8 | 22/0 | |||||
| Feng[18] | China | 2013 | day of surgery | TG | EOF | 59 | 54.98±11.35 | 41/18 | 22.44±3.51 | 0/3/56 | 26/33 |
| TOF | 60 | 55.79±10.06 | 44/16 | 21.01±1.78 | 0/1/59 | 39/21 |
BMI: body mass index; ASA: American Society of Anesthesiology; TNM: tumor, node, metastasis; NR: not reported; POD: postoperative day; TG: total gastrectomy; DG: distal gastrectomy; PG: proximal gastrectomy; SG: subtotal gastrectomy;
: Age grouped by <65/≥65;
: BMI grouped by <25 kg/m2/≥25 kg/m2;
: median;
: ASA score grouped by 0/(1 and 2).
Methodological quality of the studies
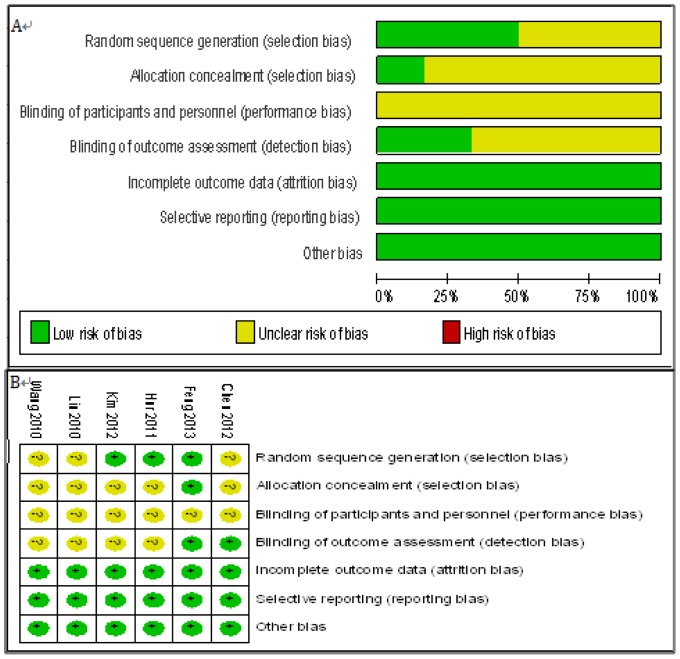
Risk of bias was assessed by the Cochrane Collaboration's tool. As concerns for early oral feeding being compared, blinding was inherent defect for such studies. Generally, the included studies had a moderate risk of bias (Fig.2).
Figure 2. Risk of bias graph.
Judgements about each risk of bias item presented in all include RCTs(A) and for each included RCT(B).
Primary outcome parameters
Postoperative complication
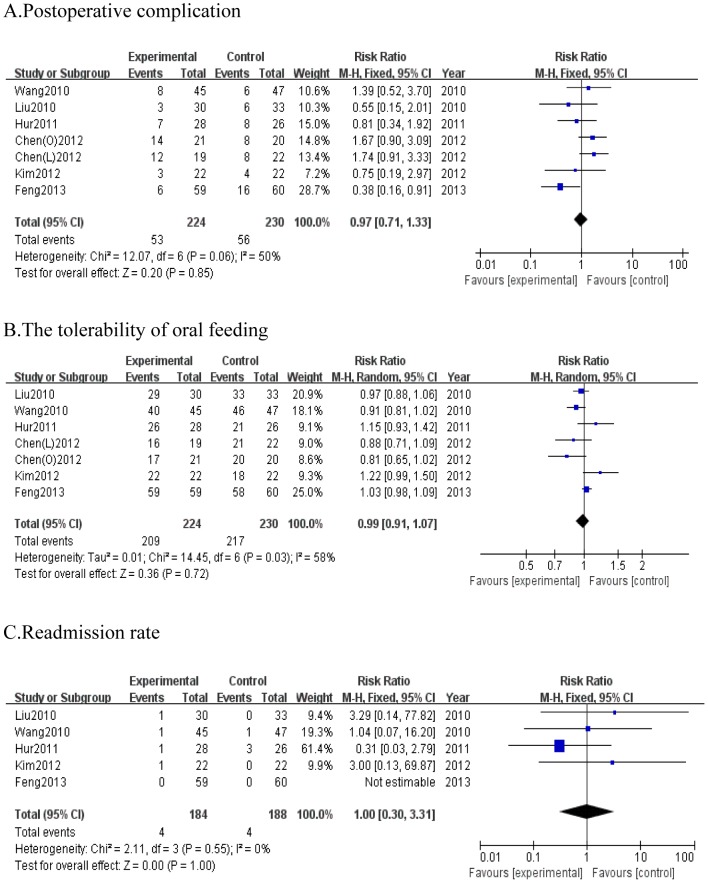
Primary outcome for this systematic review was postoperative complication. All six studies provided information on postoperative complication. The pooled results indicated no evidence of a significant difference in the number of complications between two groups (RR = 0.97; 95% CI, 0.71 to 1.33; P = 0.85), and there was no remarkable heterogeneity among studies (P = 0.06, I2 = 50%) (Fig.3A).
Figure 3. Forest plot displaying the results of the meta-analysis.
A. postoperative complication; B. the tolerability of oral feeding; C. readmission rate. RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
The tolerability of oral feeding
Six studies were all included with 224 and 230 cases in each group respectively. Among them, 209 patients tolerated EOF while 15 failed. Using a random effects model, the pooled results showed that there was no significant difference between two groups about tolerability of oral feeding after gastrectomy (RR = 0.99; 95%CI, 0.91 to 1.07; P = 0.72), with obvious heterogeneity (P = 0.03, I2 = 58%) (Fig.3B).
Readmission rate
In the five studies [18]–[22] reporting on readmission rate after gastric cancer surgery with 372 patients, the pooled readmission rate was similar between both groups based on fix effects model analysis (RR = 1; 95%CI, 0.30 to 3.31; P = 1.00), without significant heterogeneity (P = 0.55, I2 = 0%) (Fig.3C).
Secondary outcome parameters
The duration of hospital stay
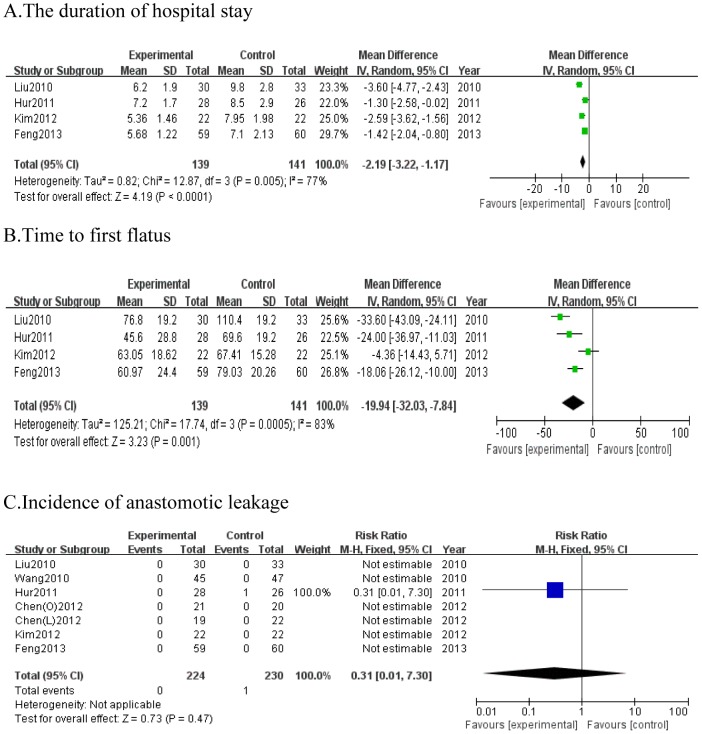
All included studies reported information on the duration of hospital stay, however, two studies, Chen et al. [17] and Wang et al. [22] only described the median postoperative hospital stay. So, we excluded them out of this meta-analysis. The duration of hospital stay was significantly shorter with EOF than TOF in all included studies (WMD = −2.19; 95%CI, −3.22 to −1.17; P<0.001). Pooled analysis of the duration of hospital stay showed obvious heterogeneity between studies (P = 0.005, I2 = 77%) (Fig.4A).
Figure 4. Forest plot displaying the results of the meta-analysis.
A. the duration of hospital stay; B. time of first flatus; C. incidence of anastomotic leakage. RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
Time to first flatus
Four studies [18]–[21] provided the complete information on time to first flatus. Time to first flatus was significantly shorter for EOF group than TOF group after gastric cancer surgery (WMD = −19.94; 95% CI, −32.03 to −7.84; P = 0.001). Because of significant heterogeneity (P = 0.0005, I2 = 83%), a random effects model was used (Fig.4B).
Incidence of anastomotic leakage
Anastomotic leakage is one of the major postoperative complications. We observed that the incidence of anastomotic leakage was comparable for two groups among all patients as similar in all included studies (RR = 0.31; 95%CI, 0.01 to 7.30; P = 0.47) (Fig.4C). There was only one patient suffered anastomotic leakage in Hur et al.'s study [19]. So the heterogeneity was not applicable.
Sensitivity analysis
We reanalyzed the primary outcome parameters by including only studies with total sample size no smaller than 50 in order to perform sensitivity analysis. The results were not substantially influenced by sensitivity analysis as shown in Table 2. The results of sensitivity analysis supported the credibility of the evidence in this meta-analysis.
Table 2. Sensitivity analysis results of primary outcomes by included studies of no less than 50 patients in each group.
| Primary outcomes | Number of studies | Patients | WMD/RR | 95%CI | Analysis model | P | heterogeneity | |
| (EOF/TOF) | I2 | P | ||||||
| postoperative complication | 5 | 202/208 | 0.90 | 0.47, 1.70 | Random | 0.73 | 66% | 0.02 |
| Tolerability of oral feeding | 5 | 202/208 | 0.97 | 0.89, 1.05 | Random | 0.47 | 67% | 0.02 |
| Readmission rates | 4 | 162/166 | 0.78 | 0.20, 3.00 | Fixed | 0.72 | 0% | 0.47 |
RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
Subgroup analysis
Subgroup analysis according to the time of starting EOF, extent of gastrectomy and type of surgery, were also performed to assess potential effect modification of these variables on outcomes. Tables 3–5 show the results of subgroup analysis. The similar outcomes could be observed in the stratified groups irrespective of the time to start EOF, the extent of gastric resection and the type of surgery.
Table 3. Results of subgroup analysis comparing the time of EOF after gastrectomy.
| Outcomes | Subgroup | Number of studies | Patients | WMD/RR | 95%CI | P | Heterogeneity | |
| (EOF/TOF) | I2 | P | ||||||
| Postoperative complication | Day of surgery | 5 | 174/182 | 1.02 | 0.72, 1.45 | 0.91 | 64% | 0.02 |
| Day after surgery | 2 | 50/48 | 0.79 | 0.38, 1.65 | 0.53 | 0% | 0.92 | |
| Tolerability of oral feeding | Day of surgery | 5 | 174/182 | 0.94 | 0.86, 1.03 | 0.21 | 67% | 0.02 |
| Day after surgery | 2 | 50/48 | 1.18 | 1.02, 1.37 | 0.03 | 0% | 0.71 | |
| Readmission rates | Day of surgery | 3 | 134/140 | 1.78 | 0.24, 13.09 | 0.57 | 0% | 0.59 |
| Day after surgery | 2 | 50/48 | 0.68 | 0.14, 3.28 | 0.63 | 26% | 0.25 | |
| Duration of hospital stay | Day of surgery | 2 | 89/93 | −2.45 | −4.58,−0.32 | 0.02 | 90% | 0.001 |
| Day after surgery | 2 | 50/48 | −2.00 | −3.26,−0.74 | 0.002 | 58% | 0.12 | |
| Time to first flatus | Day of surgery | 2 | 89/93 | −25.62 | −40.84,−10.40 | 0.001 | 83% | 0.01 |
| Day after surgery | 2 | 50/48 | −13.74 | −32.96, 5.49 | 0.16 | 82% | 0.02 | |
RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
Day of surgery: 6 h≤ time to start EOF≤ 24 h after gastrectomy;
Day after surgery: time to start EOF>24 h after gastrectomy.
Table 5. Results of subgroup analysis comparing EOF in Laparoscopy and open surgery for gastric cancer.
| Outcomes | Subgroup | Number of studies | Patients | WMD/RR | 95%CI | P | Heterogeneity | |
| (EOF/TOF) | I2 | P | ||||||
| Postoperative complication | Laparoscopy | 2 | 41/44 | 1.39 | 0.77, 2.51 | 0.27 | 18% | 0.27 |
| Open | 5 | 183/186 | 0.86 | 0.59, 1.25 | 0.43 | 57% | 0.06 | |
| Tolerability of oral feeding | Laparoscopy | 2 | 41/44 | 1.04 | 0.76, 1.42 | 0.82 | 77% | 77% |
| Open | 5 | 183/186 | 0.97 | 0.90, 1.06 | 0.54 | 61% | 0.04 | |
| Readmission rates | Laparoscopy | 1 | 22/22 | 3.00 | 0.13, 69.87 | 0.49 | Not applicable | |
| Open | 4 | 162/166 | 0.78 | 0.20, 3.00 | 0.72 | 0% | 0.47 | |
| Duration of hospital stay | Laparoscopy | 1 | 22/22 | −2.59 | −3.62,−1.56 | <0.001 | Not applicable | |
| Open | 3 | 117/119 | −2.07 | −3.45, −0.70 | 0.003 | 82% | 0.004 | |
| Time of first flatus | Laparoscopy | 1 | 22/22 | −4.36 | −14.43, 5.71 | 0.40 | Not applicable | |
| Open | 3 | 117/119 | −25.07 | −35.05, −15.09 | <0.001 | 67% | 0.05 | |
RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
Table 4. Results of subgroup analysis comparing EOF in TG and SG for gastric cancer.
| Outcomes | Subgroup | Number of studies | Patients | WMD/RR | 95%CI | P | Heterogeneity | |
| (EOF/TOF) | I2 | P | ||||||
| Postoperative complication | TG | 1 | 59/60 | 0.38 | 0.16, 0.91 | 0.03 | Not applicable | |
| SG | 4 | 107/111 | 1.54 | 1.04, 2.28 | 0.03 | 0% | 0.72 | |
| Tolerability of oral feeding | TG | 1 | 59/60 | 1.03 | 0.98, 1.09 | 0.25 | Not applicable | |
| SG | 4 | 107/111 | 0.94 | 0.81, 1.09 | 0.44 | 63% | 0.05 | |
| Duration of hospital stay | TG | 1 | 59/60 | −1.42 | −2.04,−0.80 | <0.00001 | Not applicable | |
| SG | 1 | 22/22 | −2.59 | −3.62,−1.56 | <0.00001 | Not applicable | ||
| Time of first flatus | TG | 11 | 59/60 | −18.06 | −26.12,−10.00 | <0.0001 | Not applicable | |
| SG | 1 | 22/22 | −4.36 | −14.43, 5.71 | 0.40 | Not applicable | ||
TG: total gastrectomy; SG: subtotal gastrectomy; RR: Risk ratio; WMD: Weighted mean difference. CI: confidence intervals.
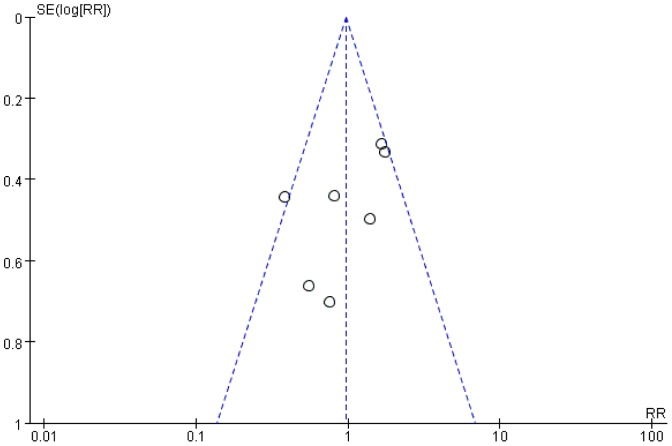
Publication bias
Publication bias was evaluated by performing a funnel plot of postoperative complication (Fig.5). All included studies reported on this outcome, which were equally distributed on the vertical axis, the result showed no evidence of obvious publication bias.
Figure 5. Funnel plot of the studies reporting on postoperative complication.
RR: risk ratio; SE: standard error.
Discussion
The present meta-analysis showed that early oral feeding (EOF) did not increase postoperative complication, readmission rate and the incidence of anastomotic leakage, either. We found no significant difference in tolerability of oral feeding after gastrectomy between both groups. Furthermore, EOF following gastric surgery was associated with a significant reduction in duration of postoperative hospital stay and time to first flatus compared with TOF.
Though emerging evidence for the advantages of EOF has been demonstrated, many surgeons are still reluctant to administer EOF to patients after gastrectomy. One reason is fearing an increase of postoperative complications, such as gastric retention and anastomosis dehiscence[23], [24]. Regarding the safety of EOF, previous evidence from a systematic review in patients undergoing colonic surgery has shown that EOF is safe without any significant increase in complications [25], [26]. What's more, in a meta-analysis of studies comparing early enteral feeding versus “nil by mouth”, those patients receiving enteral nutrition had a lower incidence of infection complications undergoing gastrointestinal surgery [27]. The major factor used to justify the traditional practice of oral intake restriction and EOF after gastrectomy is concern for anastomosis leakage [28]. However, restricting EOF is not evidence-based. On the contrary, EOF after upper gastrointestinal surgery was found to promote anastomotic healing and anastomotic strength in intestines and somatic tissues of a rat model [29]. Early postoperative oral diet is easily absorbed, which may accelerate recovery of peristalsis, protect gut mucosal barrier function, and strengthen immune response [30]. Traditional nutritional routes still adhere to the first flatus with postoperative fasting, decompression of nasogastric tube and the supply of a large number of intravenous fluids for several days after gastrectomy. Thus, some pulmonary complications such as atelectasis, pneumonia, gastroesophageal reflux in patients may be induced [31]. In the present meta-analysis, we observed no significant differences between two groups concerning postoperative complications, including the presence of anastomotic leakage.
Inducing gastrointestinal symptoms is another concern to justify EOF restriction after gastrectomy [32], [33]. It was supposed that EOF might result in the risk of increasing postoperative nausea and vomiting [4], [34], [35]. Thus, the patients would not only suffer from intolerance of EOF, but also encounter severe adverse events. Traditionally, the time to resume diet depends on the passage of flatus. However, such an approach was considered extremely conservative through the physiology research of postoperative ileus [36]. Difronzo et al. [37] showed that over 80% of patients tolerated EOF after colonic surgery by analyzed 200 patients during a five-year period. From all included studies of this meta-analysis, in spite that nausea, vomitting or abdominal distension occurred in some patients receiving EOF, the symptoms mostly happened in the initial stage of oral diet and did not develop into severe complications. The pooled data showed that there was no significant difference between two groups about tolerability of oral feeding after gastrectomy, and EOF didn't increase the risk of postoperative morbidity and mortality. Therefore, removing a nasogastric tube and early oral intake after gastrectomy as soon as possible is a considerable strategy for postoperative patients [38], [39]. Moreover, on the basis of safety and tolerability of EOF, we also see from this meta-analysis, EOF might further shorten the hospital stays, lessen first flatus time and didn't increase the patient's readmission to the hospital. It was considered that EOF following gastrectomy could benefit patients.
From the studies included in this meta-analysis, various time of EOF after surgery between studies was found such an 6 to 8 hours after surgery, the first postoperative day and the third postoperative day et al. Jeong et al. [40] reported EOF was safe and feasible on the first postoperative day after gastrectomy, however, an old age (≥70 years) required careful monitoring when applying EOF after surgery. Lewis et al. [41] compared early enteral nutrition within 24 h of gastrointestinal surgery versus later commencement of feeding, it was found that mortality was reduced with early postoperative feeding even though increased vomiting. Thus, the given time of EOF after surgery is still controversial. In our included studies, time of EOF after gastrectomy was mostly based on an accelerated rehabilitation protocol designed for colorectal resection surgery. According to the time of EOF, we divided the included studies into two subgroup, day of surgery subgroup [17], [18], [21], [22] and day after surgery subgroup [19], [20]. Most outcomes in EOF were found similar with TOF in the stratified subgroups consistent with the pooled analysis, which somewhat suggested that to start EOF at 6 or 8 hours after surgery might be safe, in spite of small sample studies contributing to it.
Simultaneously, we also analyzed the outcomes stratified into total gastrectomy (TG) subgroup [18] and subtotal gastrectomy (SG) subgroup [17], [20], [22]. Similar findings were found in both TG and SG group with regard to tolerability of oral feeding, duration of hospital stay and time of first flatus except postoperative complication. For TG, the incidence of postoperative complications seemed lower in EOF group than that in TOF group, which turned out to be opposite for SG. The possible reasons underlying the distinctions between TG and SG might be that the extent of the gastric resection decided types of digestive tract reconstruction, which led to different effects on postoperative physiological functions, like gut motility and metabolism. Thus, some minor gastrointestinal symptoms such as abdominal cramps, colic, nausea and vomiting would be induced, especially in SG which preserved the function of gastric acid secretion, resulting in increased overall postoperative complications. However, no major complications like anastomotic leakage were observed in most studies that had reported on this issue. Besides, it was too difficult to reach a conclusive outcome based on pooled analysis including only two studies with quite small sample sizes. On the whole, no obvious change was observed regarding the primary outcomes of the present meta-analysis. It might still be feasible under careful assessment for both TG and SG. And similar findings were also observed in the subgroup analyses stratified by laparoscopic and open surgery. It is believed that postoperative recovery of bowel motion could be affected by abdominal incision size. Patients in laparoscopy group are supposed to have a better recovery for the intrinsic advantages of minimally invasive surgery over conventional one. Unfortunately, due to limited sample size, such conclusions could not be draw out of the given data. So a large-scale well-designed RCT is warranted to clarify this difference more conclusively. However, in this meta-analysis EOF seemed acceptable for patients in both laparoscopic and open surgery group.
Several limitations were associated with included randomized studies deserving consideration in the interpretation of this meta-analysis. First, small sample size, single-center experience and moderate quality of included studies might decrease the reliability of the results. Second, insufficient background of clinical information, differences in operating technique, perioperative nursing system and outcomes examined were discovered in our included studies. Third, obvious bias in population was found. Most of the studies were done in East Asian countries. However, the studies of white and black population were lacked. As widely known, factors such as dietary history, preoperative obesity could also influence the EOF on patients after gastric cancer surgery. Thus, given the above defects, different strategies were used to eliminate bias. Then, subgroup analysis was performed to detect potential bias sources, stratifying the time to start EOF, the extent of the gastric resection and the type of surgery to acquire robust evidence for the conclusions. All these attempts supported the credibility of the evidence in this meta-analysis.
In conclusion, this meta-analysis supported that EOF after gastric cancer surgery seemed feasible and safe, even started at the day of surgery irrespective of the extent of the gastric resection and the type of surgery. However, more prospective, well-designed multicenter RCTs with more clinical outcomes are needed for further validation.
Supporting Information
PRISMA Checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by The National Key Technology R&D Program (No. 2013BAI05B00), the Major Program of Science and Technology Program of Guangzhou (No. 201300000087), Research Fund of Public welfare in Health Industry of Health (No. 201402015), Ministry of Health of PR China, and The program of the national key clinical medical specialty. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 248: 189–198. [DOI] [PubMed] [Google Scholar]
- 2. Feo CV, Romanini B, Sortini D, Ragazzi R, Zamboni P, et al. (2004) Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg 74: 298–301. [DOI] [PubMed] [Google Scholar]
- 3. Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC, et al. (2013) Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg 30: 225–232. [DOI] [PubMed] [Google Scholar]
- 4. Gianotti L, Nespoli L, Torselli L, Panelli M, Nespoli A (2011) Safety, feasibility, and tolerance of early oral feeding after colorectal resection outside an enhanced recovery after surgery (ERAS) program. Int J Colorectal Dis 26: 747–753. [DOI] [PubMed] [Google Scholar]
- 5. de Martel C, Forman D, Plummer M (2013) Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am 42: 219–240. [DOI] [PubMed] [Google Scholar]
- 6. Hanna GB, Amygdalos I, Ni M, Boshier PR, Mikhail S, et al. (2013) Improving the standard of lymph node retrieval after gastric cancer surgery. Histopathology 63: 316–324. [DOI] [PubMed] [Google Scholar]
- 7. Lee HJ, Shiraishi N, Kim HH, Hiki N, Uyama I, et al. (2012) Standard of practice on laparoscopic gastric cancer surgery in Korea and Japan: experts' survey. Asian J Endosc Surg 5: 5–11. [DOI] [PubMed] [Google Scholar]
- 8. Koeda K, Nishizuka S, Wakabayashi G (2011) Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg 35: 1469–1477. [DOI] [PubMed] [Google Scholar]
- 9. Chen ZX, Liu AH, Cen Y (2014) Fast-track program vs traditional care in surgery for gastric cancer. World J Gastroenterol 20: 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu Z, Zhuang CL, Ye XZ, Zhang CJ, Dong QT, et al. (2014) Fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Langenbecks Arch Surg 399: 85–92. [DOI] [PubMed] [Google Scholar]
- 11. Zhao H, Zhao H, Wang Y, Jing H, Ding Q, et al. (2013) Randomized clinical trial of arginine-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing gastric cancer surgery. J Cancer Res Clin Oncol 139: 1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, et al. (2012) Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer 15: 34–41. [DOI] [PubMed] [Google Scholar]
- 13. Grantcharov TP, Kehlet H (2010) Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg 97: 1547–1551. [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa T, Kaneko H, Konagaya T, Shinozaki K, Kasahara A, et al. (2003) Enhanced somatostatin secretion into the gastric juice with recovery of basal acid output after Helicobacter pylori eradication in gastric ulcers. J Gastroenterol Hepatol 18: 505–511. [DOI] [PubMed] [Google Scholar]
- 15. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG (2012) Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 18: 12–18. [DOI] [PubMed] [Google Scholar]
- 16. Liao G, Chen J, Ren C, Li R, Du S, et al. (2013) Robotic versus open gastrectomy for gastric cancer: a meta-analysis. PLoS One 8: e81946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, et al. (2012) Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg 16: 1830–1839. [DOI] [PubMed] [Google Scholar]
- 18. Feng F, Ji G, Li JP, Li XH, Shi H, et al. (2013) Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 19: 3642–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hur H, Kim SG, Shim JH, Song KY, Kim W, et al. (2011) Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery 149: 561–568. [DOI] [PubMed] [Google Scholar]
- 20. Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, et al. (2012) Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg 36: 2879–2887. [DOI] [PubMed] [Google Scholar]
- 21. Liu XX, Jiang ZW, Wang ZM, Li JS (2010) Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr 34: 313–321. [DOI] [PubMed] [Google Scholar]
- 22. Wang D, Kong Y, Zhong B, Zhou X, Zhou Y (2010) Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg 14: 620–627. [DOI] [PubMed] [Google Scholar]
- 23. Bisgaard T, Kehlet H (2002) Early oral feeding after elective abdominal surgery—what are the issues? Nutrition 18: 944–948. [DOI] [PubMed] [Google Scholar]
- 24. Csendes A, Diaz JC, Burdiles P, Braghetto I, Maluenda F, et al. (1990) Classification and treatment of anastomotic leakage after extended total gastrectomy in gastric carcinoma. Hepatogastroenterology 37 Suppl 2: 174–177. [PubMed] [Google Scholar]
- 25. Vlug MS, Wind J, van der Zaag E, Ubbink DT, Cense HA, et al. (2009) Systematic review of laparoscopic vs open colonic surgery within an enhanced recovery programme. Colorectal Dis 11: 335–343. [DOI] [PubMed] [Google Scholar]
- 26. Minig L, Biffi R, Zanagnolo V, Attanasio A, Beltrami C, et al. (2009) Reduction of postoperative complication rate with the use of early oral feeding in gynecologic oncologic patients undergoing a major surgery: a randomized controlled trial. Ann Surg Oncol 16: 3101–3110. [DOI] [PubMed] [Google Scholar]
- 27. Lewis SJ, Egger M, Sylvester PA, Thomas S (2001) Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 323: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sierzega M, Kolodziejczyk P, Kulig J, Polish Gastric Cancer Study G (2010) Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg 97: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 29. Fukuzawa J, Terashima H, Ohkohchi N (2007) Early postoperative oral feeding accelerates upper gastrointestinal anastomotic healing in the rat model. World J Surg 31: 1234–1239. [DOI] [PubMed] [Google Scholar]
- 30. Minig L, Biffi R, Zanagnolo V, Attanasio A, Beltrami C, et al. (2009) Early oral versus “traditional” postoperative feeding in gynecologic oncology patients undergoing intestinal resection: a randomized controlled trial. Ann Surg Oncol 16: 1660–1668. [DOI] [PubMed] [Google Scholar]
- 31. Schuchert MJ, Pettiford BL, Landreneau JP, Waxman J, Kilic A, et al. (2008) Transcervical gastric tube drainage facilitates patient mobility and reduces the risk of pulmonary complications after esophagectomy. J Gastrointest Surg 12: 1479–1484. [DOI] [PubMed] [Google Scholar]
- 32. Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S (2011) A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo) 66: 2001–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Nakeeb A, Fikry A, El Metwally T, Fouda E, Youssef M, et al. (2009) Early oral feeding in patients undergoing elective colonic anastomosis. Int J Surg 7: 206–209. [DOI] [PubMed] [Google Scholar]
- 34. Palmer DJ, Prescott SL (2012) Does early feeding promote development of oral tolerance? Curr Allergy Asthma Rep 12: 321–331. [DOI] [PubMed] [Google Scholar]
- 35. Prescott SL, Smith P, Tang M, Palmer DJ, Sinn J, et al. (2008) The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatr Allergy Immunol 19: 375–380. [DOI] [PubMed] [Google Scholar]
- 36. Klappenbach RF, Yazyi FJ, Alonso Quintas F, Horna ME, Alvarez Rodriguez J, et al. (2013) Early oral feeding versus traditional postoperative care after abdominal emergency surgery: a randomized controlled trial. World J Surg 37: 2293–2299. [DOI] [PubMed] [Google Scholar]
- 37. Di Fronzo LA, Cymerman J, O'Connell TX (1999) Factors affecting early postoperative feeding following elective open colon resection. Arch Surg 134: 941–946. [DOI] [PubMed] [Google Scholar]
- 38. Wu B, Chen XZ, Wen L, Chen XL, Yang K, et al. (2013) The feasibility and safety of early removal of nasogastric tube after total gastrectomy for gastric cancer. Hepatogastroenterology 60: 387–389. [DOI] [PubMed] [Google Scholar]
- 39. Zhou T, Wu XT, Zhou YJ, Huang X, Fan W, et al. (2006) Early removing gastrointestinal decompression and early oral feeding improve patients' rehabilitation after colorectostomy. World J Gastroenterol 12: 2459–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeong O, Ryu SY, Jung MR, Choi WW, Park YK (2014) The safety and feasibility of early postoperative oral nutrition on the first postoperative day after gastrectomy for gastric carcinoma. Gastric Cancer 17: 324–331. [DOI] [PubMed] [Google Scholar]
- 41. Lewis SJ, Andersen HK, Thomas S (2009) Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg 13: 569–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.