Abstract
Peripheral arterial disease (PAD) describes the clinical manifestations of atherosclerosis affecting the circulation in the legs. The severity of PAD is classified according to symptom severity, time course, and anatomical distribution. The signs and symptoms of PAD reflect the degree of circulatory compromise and whether there has been a gradual reduction in the circulation or an abrupt, uncompensated decrease. Accurate clinical assessment underpins decisions on management strategy and should objectively assess the severity of the ischemia and need for revascularization. Clinical history should discriminate symptoms of PAD from other conditions presenting with leg pain, elucidate cardiovascular risk factors and the effect of symptoms on the patient's quality of life. Clinical examination includes signs of general cardiovascular disease and associated conditions before assessing the circulation and viability of the limb. Palpation of peripheral pulses must be augmented by determination of the ankle brachial pressure index using hand held Doppler. A whole patient approach to management is required and must include modification of cardiovascular risk status as well as dealing with the local circulatory manifestation of PAD.
Keywords: intermittent claudication, critical limb ischemia, acute limb ischemia, patient assessment, Doppler, ABPI, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe how to use history and physical examination to identify the different stages of peripheral arterial disease.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Peripheral arterial disease (PAD) is one of the manifestations of generalized atherosclerotic disease, estimated to be present in up to 20% of patients older than 60 years. PAD presents as a spectrum ranging from an asymptomatic reduction in ankle pressures to life- and limb-threatening disease.
In 2012, the National Institute for Health and Care Excellence (NICE) issued guidelines on PAD. NICE highlighted the failure of clinicians to appreciate the link between PAD and cardiovascular disease (CVD).1 Only 1 to 2% of claudicants will ever progress to limb loss, but some 75% will die from a cardiovascular cause.1 PAD is an independent risk factor for CVD and a reduced ankle brachial pressure index (ABPI) (< 0.9) is associated with twice the risk of cardiovascular mortality compared with matched patients with a normal ABPI.2 Furthermore, adding the ABPI into risk calculations based on Framingham score improves the accuracy of cardiovascular risk prediction.3 Critical limb ischemia (CLI) not only represents more severe disease but is also associated with even worse cardiovascular risk. Within 1 year of diagnosis, 30% of patients with CLI will require major amputation and 50% will die, mostly from CVD.4 It is therefore important to keep the high CVD risk of these patients in mind during assessment.5
Correct assessment allows a management strategy to be developed with the objectives of modifying cardiovascular risk, managing symptoms, and preventing major amputation whenever possible. Following a full assessment of the patient, several questions should be considered. First, one must decide if, on the balance of probabilities, the diagnosis of PAD is plausible; second, the severity of the PAD should be quantified. The Fontaine classification (Table 1) and/or TASC II (Trans-Atlantic Inter-Society Consensus document) classification is often used to document this objectively. Third, where the patient should be managed must be determined. In general terms, claudicants should be treated in primary care settings in the first instance, but patients with acute limb ischemia (ALI), worsening disease, or CLI should be treated in secondary care settings. Finally, a decision should be made on the necessity of imaging to guide intervention.
Table 1. The Fontaine classification for PAD4 .
| Fontaine classification | Definition |
|---|---|
| I | Asymptomatic |
| IIa | Compensated intermittent claudication |
| IIb | Decompensated intermittent claudication |
| III | Rest pain |
| IV | Nonhealing ulceration |
Abbreviation: PAD, peripheral arterial disease.
This article presents a strategy for assessing patients presenting with suspected PAD based on the history, clinical examination, and adjunctive tests starting with chronic PAD in asymptomatic patients and progressing through to ALI.
Clinical Assessment of Chronic Limb Ischemia
Chronic limb ischemia refers to a condition characterized by symptoms and signs of vascular disease which have been present for at least 14 days. The history and examination focus on establishing whether symptoms are due to PAD, identification of risk factors, severity of the circulatory compromise, and impact on the patient's life.
Asymptomatic Peripheral Arterial Disease
Signs of PAD may be discovered incidentally, for example, if a pulse is found to be impalpable during clinical examination or a reduced ABPI detected during screening for CVD in primary care. PAD may also be masked in patients who do not exercise sufficiently to produce claudication or whose exercise is limited by other symptoms such as angina or breathlessness. Treatment in these patients is aimed at reducing cardiovascular risk.
Key Features of History in Asymptomatic Patients
Identifying Risk Factors for Peripheral Arterial Disease
Patient history should include questioning about the standard cardiovascular risk factors including coronary heart disease, cerebrovascular disease (including transient ischemic attacks and stroke), diabetes, hypertension, hypercholesterolemia, family history of PAD, and most importantly, smoking. Smoking history should include both current and past smoking habits with an estimate of lifetime smoking in pack years (pack years = number of years smoked × number of cigarettes smoked per day/20). If diabetes is present, it is important to establish how it is managed, the level of glycemic control, and if there is evidence of other end-organ damage.
Medical History
Previous vascular interventions (surgical and interventional radiological procedures) should be documented along with additional comorbidities. Due to the nature of the risk factors for PAD, concomitant diagnoses of CVD, cancer, and COPD are common. A history of chronic kidney disease should be sought. In patients who are candidates for revascularization, leg or arm veins may be needed as a conduit. A history of varicose veins, venous thromboembolic events (VTE), or coronary artery bypass may therefore be relevant.
Drug History
Patients with PAD require therapy to modify cardiovascular risk and control symptoms. It is vital to establish whether the patient is on any medication to control hypertension, platelet aggregation, and cholesterol. Treatment for diabetes should also be noted in addition to analgesic or vasodilator use.
Examination in Asymptomatic Patients
Clinical examination is an essential component for the assessment of patients with PAD.6 It is important to gain consent for the proposed examination from the patient and ensure the limbs and feet are adequately exposed. Assessment of the shoes may also be helpful, particularly in the context of diabetic foot ulceration. Shoes should be of an appropriate size and shape for the feet so as to be comfortable without damaging the toes.
General Inspection and Systemic Examination
The general peripheral stigmata of CVD should be sought. This includes tar staining on the fingernails as a sign of cigarette smoking, scars from previous vascular or cardiac surgery, amputated limbs or digits, and xanthelasma. The radial and brachial pulses should be palpated to determine rate, rhythm, and volume of the pulse and the heart auscultated for evidence of major valvular pathology (e.g., aortic and mitral stenosis). A systemic examination should be conducted to identify signs of relevant pathology outside the vascular system such as COPD. Abdominal examination includes assessing the aorta for aneurysm and palpating for abdominal masses suggestive of cancer. Focused neurological assessment should be undertaken for signs of prior completed stroke and evidence of peripheral neuropathy in patients with diabetes. Following this, the attention should be focused on the lower limbs.
Inspection of the Limbs
Limb inspection should be focused on scars indicating previous arterial or venous vascular surgery or long saphenous vein harvesting, and the health status of the legs, looking for trophic skin lesions, microemboli (trash), livedo reticularis, ulcers, and areas of necrosis or gangrene (Fig. 1). Hair loss is an unreliable sign of ischemia and is of little clinical value.
Figure 1.
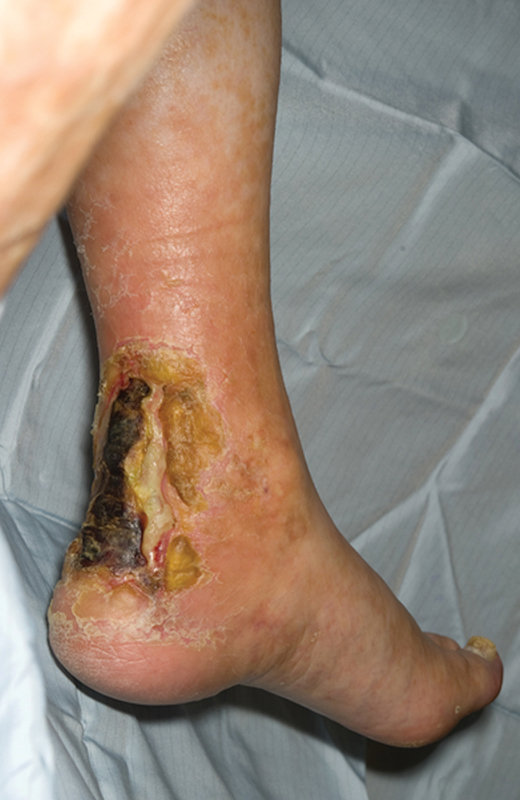
Active ulceration over the Achilles tendon in a patient with critical limb ischemia. Slough can be seen at the base, covered by a layer of necrotic skin. Above the lesion, the typical appearance of the skin in an ischemic leg can be observed. Magnetic resonance angiography confirmed crural vessel disease that responded to angioplasty with long-term healing.
Assessing skin perfusion by checking temperature and capillary refill is unreliable as an indicator of PAD, as both may be affected by factors such as external temperature. Asymmetric findings may be more useful than absolute findings. Delayed asymmetric capillary refill or very prolonged refill is suggestive, but not diagnostic, of ischaemia.7
Palpation of Pulses and Auscultation of Bruits
Peripheral pulses are compared with the opposite side and the presence of any thrill is noted. Femoral, popliteal, posterior tibial (PT), and dorsalis pedis (DP) pulses are assessed. The femoral pulses are palpated half way between the anterior superior iliac spine and the pubic symphysis on each side. The popliteal artery lies between the heads of gastrocnemius and the pulse is assessed with the knee slightly flexed using the index, second, and third fingers to push the popliteal artery against the tibia. The popliteal pulse is comparatively difficult to identify; a prominent popliteal pulse may indicate popliteal aneurysm and warrants ultrasound imaging. The PT pulse is palpated just behind the medial malleolus and the DP pulse over the navicular bone lateral to the extensor hallucis longus tendon. The peroneal artery cannot be palpated. Pulses should be recorded as present (+), weak (+/−), or absent (−). Listening for arterial bruits is of little value in determining the site and severity of disease.
The Diabetic Foot
In patients with diabetes, it is important to consider the risk of future ulceration. Assessment of the musculoskeletal and neurological status of the foot is essential in addition to the assessment of the vascular supply. Light touch sensation is assessed with standardized monofilaments. A full review on diabetic foot assessment is provided by Boulton et al,8 and management of the diabetic foot is discussed elsewhere in this edition (see Huang et al). All diabetic foot patients should be assessed by vascular and diabetic teams in a multidisciplinary foot clinic.
Bedside Doppler Assessment
Doppler assessment and ABPI should be calculated.9 The Doppler probe (8 MHz) should be angled at 60 degrees in relation to the skin pointing against the direction of blood flow (Fig. 2A). The probe should be manipulated until the clearest signal is heard and should be held just in contact with the skin; excessive pressure can obliterate a weak signal. The other key maneuver is to gently squeeze the foot if one detects a weak signal as well as exclude a venous signal. The Doppler probe can also be used to undertake assessment of the pedal arch (pedal arch patency test).10 11 This involves listening with the Doppler probe in the first metatarsal space (Fig. 2B), then applying pressure to each tibial artery at the malleoli in turn to determine communication with the pedal arch.
Figure 2.
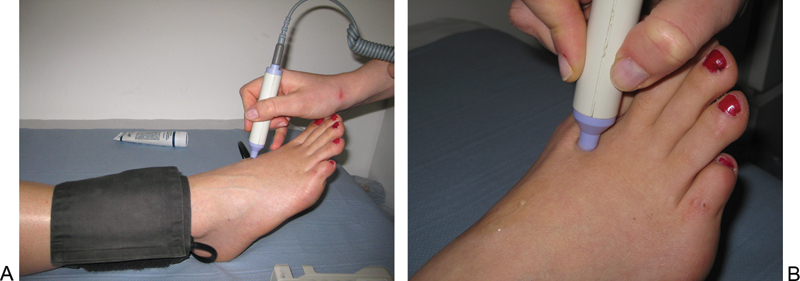
(A) Doppler assessment of the dorsalis pedis pulse in a healthy foot. Note the ∼60 degrees angulation of the probe relative to the skin. (B) Positioning for assessment of pedal arch patency. Pressure is applied to each tibial artery in turn to determine communication with the pedal arch which is assessed by Doppler.
Measuring Ankle Brachial Pressure Index
A manual sphygmomanometer along with an appropriate sized cuff is placed just above the ankle, inflated to occlude the artery, and slowly released. If the vessel cannot be occluded (i.e., a systolic pressure of > 200 mm Hg), this should be recorded as incompressible. The return of signal indicates the systolic pressure by Doppler and should be taken in the PT and DP in each foot. The brachial systolic pressure is recorded in each arm using the Doppler probe and the highest taken as the brachial pressure. The higher of the two pedal pulse pressures is then used for each limb to calculate the ABPI (highest pedal pressure/highest brachial pressure). The nature of the Doppler signal should also be recorded (monophasic, biphasic, triphasic). Toe cuffs are available and can be used to measure systolic pressures at the great toe if the DP and PT are calcified. However, published data suggesting wide variability of the results make its use for clinical decision making limited.12 ABPI of < 0.9 is abnormal. High ABPI (> 1.2) is often associated with arterial calcification in diabetic patients or those patients on long-term renal replacement therapy. Table 2 shows how ABPI correlates with clinical status. It is important to realize that both reduced and elevated ABPI are associated with increased cardiovascular risk.13
Table 2. NICE interpretation of ABPI results1 .
| Clinical status | ABPI |
|---|---|
| Asymptomatic | > 0.9–1.2 |
| Intermittent claudication | 0.9–0.5 |
| Critical ischemia | < 0.5 |
Abbreviations: ABPI, ankle brachial pressure index; NICE, National Institute for Health and Care Excellence.
Additional Tests
All patients with PAD should have an electrocardiogram to identify any evidence of myocardial ischemia, arrhythmia, and/or conduction defects. This is important not only for cardiovascular risk assessment but also for determining interventional risk. Blood should be taken for full blood count (FBC), urea and electrolytes (U&E), and lipid screen. If the presence of diabetes has not been formally excluded, a glucose tolerance test should be requested. If diabetes has been diagnosed, HbA1c is useful as a marker of glycemic control. Imaging is required only if endovascular or surgical therapy is being considered.
Treatment
The aim of treatment in patients with asymptomatic PAD is modification of cardiovascular risk. Acetylcholinesterase inhibitors are considered first-line therapy for hypertension1 and all patients should be commenced on statin and antiplatelet medications.1 Clear communication with colleagues in primary care is vital to ensure these therapies are continued in the long term.
Intermittent Claudication
Patients with PAD commonly present with leg pain on exertion. Intermittent claudication describes a cramp-like pain in the leg(s), commonly in the calf. It generally occurs after a predictable level of exercise, settles with rest, and recurs when exercise is repeated. It is vital to take a careful history of the nature of the symptoms, as other conditions can mimic this presentation.
Key Features of History in Patients with Claudication
History should be obtained as described earlier. In addition, additional information as described here should be obtained.
Establishing Severity of Symptoms
Determining the precise time course of the symptoms and the exacerbating and relieving factors is central to making the correct diagnosis. Questioning should establish the nature and site of the pain within the leg, as well as the precipitating and relieving factors. The location of pain can give a guide to the level of disease but is often inaccurate, so symptoms should be correlated with the state of the peripheral pulses.
Asking patients to quantify the distance they can walk before their pain begins is a helpful guide to assessing severity. Patients use various terminologies to express this: yards, meters, several lampposts or blocks, or to an important landmark such as the shops. Using a distance marker that is accurate from the patient's perspective is helpful, as this will allow more reliable quantification of walking decline or improvement over subsequent clinical assessments by different health care professionals.
Pain comes more rapidly and may be more severe with increasing intensity of exercise, such as walking up hill or walking more quickly. Some patients are able to continue to walk in spite of the pain and this distance should also be assessed. In this case, a record should be made of the reported time and distance to onset of symptoms (claudication distance) and the maximum walking distance before needing to stop. The history should also establish the impact of the symptoms on the patients' daily activities, ability to work, and whether or not they have learned to live with their symptoms. The differential diagnosis of limb pain includes several other conditions, and if symptoms are not typical of intermittent claudication, the history should try to elicit features of other causes of leg pain.
Neurogenic pain from spinal claudication is often exacerbated by walking downhill or sitting, relieved by standing or leaning forward. It is frequently associated with paraesthesia, tingling, or back pain. The onset of symptoms tends to be far less predictable day-to-day in neurogenic compared to intermittent claudication. Lateral femoral cutaneous neuropathy (meralgia paraesthetica) is a rare condition; it is readily discriminated from intermittent claudication by limitation of the pain to the outer aspect of the thigh.
Pain from arthritic joints is not necessarily felt within the joints themselves and can also mimic vascular claudication. Patients with arthritis often experience symptoms immediately on exercise. In some cases, symptoms will be worse in the morning or at the end of the day. A particularly helpful question here can be, “do you have to take the weight off your legs to make the pain disappear?” Arthritic pain often requires the load to be removed from the affected limb, whereas claudication does not. It is important to recognize that atypical symptoms can occur and that conditions may coexist. In this case, it is important to try to establish the relative contribution of each disease.
Effect of PAD on Quality of Life
It is important to establish the activities the patient can complete despite the pain (e.g., putting on shoes, climbing stairs, shopping, working, etc.) and restrictions in activity due to the pain. These should be considered in absolute terms and in relation to the premorbid status of the individual patient.
Considering the effect that symptoms are having on a patient's quality of life (QoL) is important to enable treatment decisions to be made appropriately and to assess the impact of any intervention. While generic QoL questionnaires are useful from a research perspective,14 PAD-specific tools may be more sensitive to subtle changes in disease progression.15 Until the gold standard assessment tool for PAD is determined, questions such as, “what would you like to be able to do if you did not have the pain?” are useful along with “what changes have you made in order to live with the pain?.” The impact of PAD on social functioning and emotional health of a patient is more difficult to quantify.16 What is clear, however, is that changes in QoL measures do not necessarily correlate with changes in clinical parameters, such as ABPI.17 From a patient perspective, the impact of an intervention on QoL is much more important than arbitrary measures of vessel patency.
Examination in Claudication
Examination is performed as for the asymptomatic patient. The aim is to use the pulse to try to localize the level of disease to the aortoiliac, femoropopliteal, or crural segment.
Ankle Brachial Pressure Index
In patients where the ABPI appears normal at rest and there is a good history to support the diagnosis of PAD, the ABPI is repeated after exercise. In the clinical environment, this can be achieved by asking the patient to do 20 heel–toe raises; a fall in ABPI after exercise indicates PAD.18 A more formal assessment can be made by combining ABPI measurement with a treadmill exercise test to establish claudication distance. Segmental pressure measurements can also help with localization.
Imaging in Claudication
The NICE guidelines propose a pragmatic approach to the use of imaging based on cost and safety.1 Imaging is reserved for patients being considered for endovascular or surgical therapy. Ultrasound is the first-line investigation; it is the cheapest and safest modality. Magnetic resonance angiography (MRA) is the second-line investigation; it is more expensive but safer than computed tomography angiography. It is reasonable to adopt an MRA-first approach in patients in whom ultrasound is unlikely to be successful or will be very time consuming. This includes obese patients and those with extensive dressings on the legs. Patients with known or suspected multilevel disease are also likely to require additional imaging, particularly if endovascular treatment is being considered, as this greatly assists planning.
Treatment of Claudication
Treatment should be the same as described earlier for the asymptomatic patient. In addition, NICE recommends initial treatment with a supervised exercise program.1 If this fails, endovascular therapy should be considered with surgery reserved for patients unsuitable for or who fail endovascular treatment.
Critical Limb Ischemia
The NICE1 and TASC II4 documents define CLI as rest pain and/or tissue loss lasting more than 2 weeks. Patients with CLI are at risk of limb loss and have a very high mortality from CVD. The identification of symptoms or signs of CLI should set the patient on the path of urgent assessment for potential limb salvaging intervention.
Key History of Critical Limb Ischemia
Rest Pain and Tissue Loss
Classically rest pain is unremitting and is exacerbated when the leg is elevated. Often pain wakes up the patient after a few hours of sleep and is alleviated by hanging the foot out of the bed or by getting up, walking around, and (in England) making a cup of tea. Invariably, over time the patient finds that it is easier to sleep in a chair rather than going to bed. These patients often present late and are frequently in a poor nutritional state due to the inability to undertake their normal activities of daily living. Patients may also present with skin changes, nonhealing ulcers, or even gangrene. Ulcers commonly occur at pressure points in the foot after minimal trauma.
Examination in Critical Limb Ischemia
Examination should be performed as described earlier for the asymptomatic patient. In addition, additional examination should be performed as follows.
Documentation of Ulceration and Skin Changes
Inspection for ulceration should pay particular attention to the pressure areas (behind the malleoli, the metatarsal heads, heel, and plantar aspect of the foot) and between the toes. A blister in an ischemic foot is the precursor of an ulcer.
The size and distribution of ulceration and the health of the surrounding skin should be recorded. Mapping the size and shape of the ulcer using clinical photographs or clear acetate helps assess changes objectively. The ulcer base should be inspected for granulation tissue (sign of ulcer healing) or slough (possible development of infective processes). Bacterial culture is only needed to guide antimicrobial therapy in cases of superadded infection. Musculoskeletal deformity such as hallux valgus, hammer toe deformity (with displacement/loss of the submetatarsal head fat pads) or Charcot's foot, as well as any calluses or corns of the foot should be noted. The state of the nails, particularly in diabetic patients, should be examined, as poor foot care can increase the risk of ulceration.
Buerger Test
Buerger test is performed by raising the legs to 45 degrees (from the couch) and observing the foot perfusion. A clear reduction in foot perfusion along with venous guttering is termed a positive Buerger test. Holding the legs in this position for 2 minutes followed by hanging the leg over the edge of the bed and watching for a reactive hyperemia (dependent rubor or a sunset foot) is also a positive Buerger sign. These tests are, however, unlikely to alter management in contemporary vascular practice.
Ankle Brachial Pressure Index
ABPI should be performed as previously described.
Imaging in Critical Limb Ischemia
Imaging is required in most patients, as some form of revascularization will be needed. The strategy proposed earlier should be considered with a relatively low threshold for adopting an MRA-first approach.
Treatment of Critical Limb Ischemia
Cardiovascular risk control should be undertaken as stated previously. Pain control starts with simple analgesia but may progress to opiate drugs (in combination with stool softeners) if necessary. Revascularization using endovascular techniques or bypass grafting depends on individual patient factors such as distribution of disease and the availability of vein. If revascularization is not possible or has failed, palliative procedures such as elective amputation or chemical sympathectomy should be considered.
Clinical Assessment of Acute Limb Ischemia
ALI occurs when there is a sudden reduction in arterial flow in a limb. ALI is arbitrarily defined as being less than 14 days duration. ALI encompasses a clinical spectrum ranging from sudden onset or worsening of intermittent claudication to limb and life-threatening ischemia.
Acute ischemia can occur in patients with normal arteries in the context of embolus, dissection, and arterial injury. In the presence of preexisting PAD, this is an acute-on-chronic event most commonly due to thrombotic occlusion of a diseased artery. Bypass graft thrombosis is another important cause of severe ALI. The severity of ischemia is variable depending on the level of the vascular occlusion and magnitude of flow reduction. Acute-on-chronic ALI is usually better tolerated than an embolic occlusion or bypass graft thrombosis due to preexisting reduction in circulation and the presence of established collateral flow.
ALI typically has sudden onset with severe pain associated with worsening paraesthesia, paralysis, and ultimately irreversible ischemic damage. Nerves and muscle tissue are particularly vulnerable to ischemia and will be irreversibly damaged if severely ischemic for 4 to 6 hours. Assessment of patients with ALI aims to establish (i) the viability of the limb, (ii) the urgency of intervention, and (iii) determination of the cause (embolic vs. thrombotic), as these factors will decide the management.
History in Acute Limb Ischemia
History should be obtained as described earlier for asymptomatic and claudicant patients. In addition, additional information as described here should be obtained. The time of onset and duration of symptoms should be noted. Additional questions will help establish whether this is an embolic event or thrombosis of a diseased vessel.
Medical History/Risk Factors
Questioning should include conditions which are associated with an increased risk of ALI (Table 3).
Table 3. Conditions associated with increased risk of ALI.
| Factor | Likely thrombotic/embolic cause |
|---|---|
| Preexisting PAD | Both |
| Aortic or popliteal aneurysm | Both |
| Cardiac condition: recent MI, atrial fibrillation, mitral or aortic valve disease, right-to-left shunt | Embolus |
| Thrombophilia/thrombocytosis | Thrombus |
| Past arterial/venous thrombosis | Thrombus |
| Malignancy | Thrombus |
| Severe dehydration | Thrombus |
Abbreviation: PAD, peripheral arterial disease.
Examination in Acute Limb Ischemia
Examination should be performed as described earlier for asymptomatic and claudicant patients. In addition, additional examination as described here should be performed. The extremity must be checked for sensory deficit and loss of motor function and can be classified using the Rutherford classification19 (Table 4). The classical presentation is the “six Ps” (pale, painful, pulseless, paralyzed, paraesthetic, and perishing cold); a white leg without evidence of skin perfusion (Fig. 3) is akin to Rutherford IIb. Over the next few hours, the skin will develop a mottled blue appearance representing stagnant deoxygenated blood. If the discoloration blanches on pressure, the limb remains salvageable. Signs of irreversible ischemia include tender muscle compartments, fixed skin mottling, and myoglobinuria. Fixed skin mottling (Rutherford III) represents coagulation in the cutaneous capillaries. The extent of nonviable tissue should be documented (Fig. 4).
Table 4. The Rutherford classification of ALI19 .
| Class | Definition | Sensory loss | Muscle weakness | Doppler signal |
|---|---|---|---|---|
| I | Viable | None | None | Strong |
| IIA | Threatened: marginal | Minimal/None | None | Weak |
| IIB | Threatened: immediate | Moderate | Mild/Moderate | Weak/None |
| III | Irreversible | Profound | Profound | None |
Abbreviation: ALI, acute limb ischemia.
Figure 3.
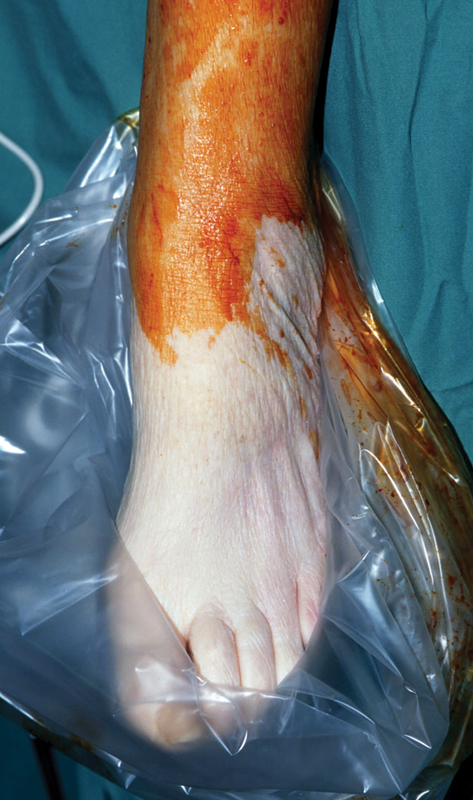
Acute limb ischemia of the left foot on the operating table just before the emergency thromboembolectomy; all “six Ps” were present.
Figure 4.
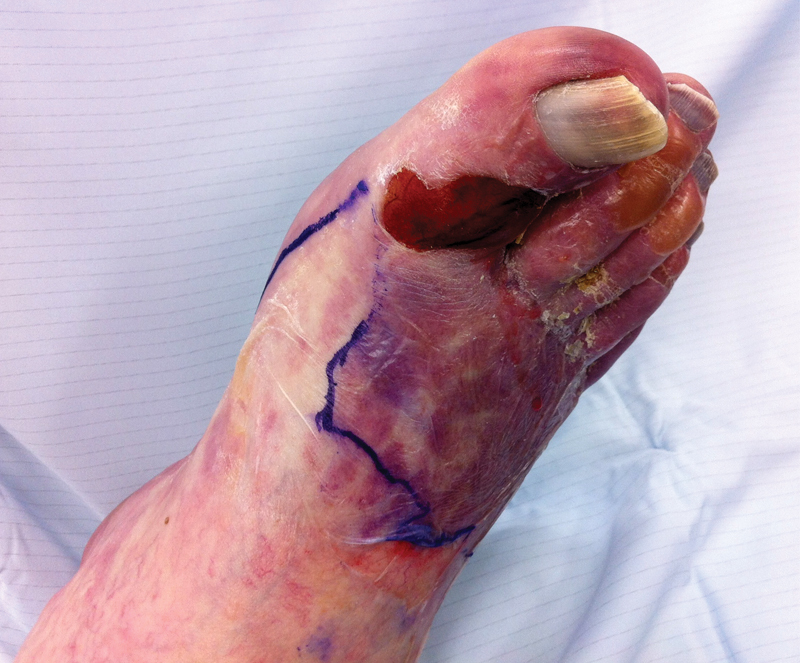
Fixed mottling of the forefoot in a patient with acute-on-chronic ischemia. A line has been drawn at the interface of the viable and nonviable tissues.
Additional Tests
Blood samples should be taken for FBC, U&E, viscosity, clotting screen, creatinine kinase, thrombophilia screen (before intravenous heparin or therapeutic subcutaneous low-molecular-weight heparin) and fasting homocysteine.
Imaging in Acute Limb Ischemia
The physical state of the limb will determine the urgency of the investigation, and treatment will be dictated by local factors and access to the appropriate imaging and resources. Multidetector CT (MDCT) scanning from the heart to the feet can be completed in a few minutes and may be better tolerated than MRI or ultrasound. MDCT will establish the site and nature of the occlusion and may identify cardiac and aortic sources of emboli and occult malignancy.
Decision Making Based on the Assessment
In patients with a viable limb, restoration of arterial inflow is the priority. Patients with Rutherford IIa ischemia are candidates for thrombolysis, and patients with Rutherford IIb ischemia generally require surgical revascularization by emergency thromboembolectomy or bypass grafting. True class III ischemia is beyond salvage.
Conclusion
In summary, a whole patient approach is required in patients presenting with PAD. For claudicants, the emphasis should be on cardiovascular risk assessment and risk factor control; in patients with critical ischemia, the emphasis is on limb salvage in selected patients, and in ALI, determination of limb viability is key. Detailed clinical assessment in conjunction with ABPI measurement will allow decisions regarding treatment to be made. If endovascular or surgical treatment is appropriate then imaging assessment will also be required.
Footnotes
Funding M. A. B. and K. J. G. are funded by the British Heart Foundation.
References
- 1.National Institute for Health and Care Excellence . London: National Institute for Health and Care Excellence; 2012. Lower Limb Peripheral Arterial Disease: Diagnosis and Management CG147. [Google Scholar]
- 2.Heald C L Fowkes F G Murray G D Price J F; Ankle Brachial Index Collaboration. Risk of mortality and CVD associated with the ankle-brachial index: systematic review Atherosclerosis 200618961–69. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes F G, Murray G D, Butcher I. et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norgren L Hiatt W R Dormandy J A Nehler M R Harris K A Fowkes F G; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg 200745(Suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 5.Welten G M, Schouten O, Chonchol M. et al. Prognosis of patients with peripheral arterial disease. J Cardiovasc Surg (Torino) 2009;50(1):109–121. [PubMed] [Google Scholar]
- 6.Khan N A, Rahim S A, Anand S S, Simel D L, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536–546. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]
- 7.Anderson B, Kelly A M, Kerr D, Clooney M, Jolley D. Impact of patient and environmental factors on capillary refill time in adults. Am J Emerg Med. 2008;26(1):62–65. doi: 10.1016/j.ajem.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Boulton A J, Armstrong D G, Albert S F. et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange S F, Trampisch H J, Pittrow D. et al. Profound influence of different methods for determination of the ankle brachial index on the prevalence estimate of peripheral arterial disease. BMC Public Health. 2007;7:147. doi: 10.1186/1471-2458-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roedersheimer L R, Feins R, Green R M. Doppler evaluation of the pedal arch. Am J Surg. 1981;142(5):601–604. doi: 10.1016/0002-9610(81)90435-9. [DOI] [PubMed] [Google Scholar]
- 11.Attinger C E, Meyr A J, Fitzgerald S, Steinberg J S. Preoperative Doppler assessment for transmetatarsal amputation. J Foot Ankle Surg. 2010;49(1):101–105. doi: 10.1053/j.jfas.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Romanos M T, Raspovic A, Perrin B M. The reliability of toe systolic pressure and the toe brachial index in patients with diabetes. J Foot Ankle Res. 2010;3:31. doi: 10.1186/1757-1146-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinola-Klein C, Rupprecht H J, Bickel C. et al. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118(9):961–967. doi: 10.1161/CIRCULATIONAHA.107.763227. [DOI] [PubMed] [Google Scholar]
- 14.Gulati S, Coughlin P A, Hatfield J, Chetter I C. Quality of life in patients with lower limb ischemia; revised suggestions for analysis. J Vasc Surg. 2009;49(1):122–126. doi: 10.1016/j.jvs.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Disease-specific quality of life assessment in intermittent claudication: review [Review] Eur J Vasc Endovasc Surg. 2003;25(3):202–208. doi: 10.1053/ejvs.2002.1837. [DOI] [PubMed] [Google Scholar]
- 16.Liles D R, Kallen M A, Petersen L A, Bush R L. Quality of life and peripheral arterial disease. J Surg Res. 2006;136(2):294–301. doi: 10.1016/j.jss.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Mazari F AK, Carradice D, Rahman M N. et al. An analysis of relationship between quality of life indices and clinical improvement following intervention in patients with intermittent claudication due to femoropopliteal disease. J Vasc Surg. 2010;52(1):77–84. doi: 10.1016/j.jvs.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 18.Diehm C, Darius H, Pittrow D. et al. Prognostic value of a low post-exercise ankle brachial index as assessed by primary care physicians. Atherosclerosis. 2011;214(2):364–372. doi: 10.1016/j.atherosclerosis.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford R B, Baker J D, Ernst C. et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]


