Abstract
Peripheral arterial disease (PAD) is a progressive disease with significant morbidity and mortality. Risk factor control, using diet and lifestyle modification, exercise, and pharmacological methods, improves symptoms and reduces associated cardiovascular events in these patients. Antiplatelet agents and anticoagulants may be used to reduce the incidence of acute events related to thrombosis. The armamentarium available for symptom relief and disease modification is discussed. Novel treatments such as therapeutic angiogenesis are in their evolutionary phase with promising preclinical data.
Keywords: peripheral arterial disease, pharmacotherapy, antiplatelet therapy, symptom relief, gene therapy, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the pharmacological options for risk factor modification and symptom control in patients with PAD.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The primary aim of medical therapy in patients with peripheral arterial disease (PAD) is to reduce the incidence of cardiovascular morbidity and mortality, improve function, limb salvage, and quality of life. PAD patients irrespective of their symptoms are at increased risk of vascular events and mortality.1 Hence, atherosclerotic risk factors should be identified and aggressively managed in all PAD patients.2 3 4 5 6
Risk Factor Management
Smoking Cessation
Smoking is an independent risk factor for PAD, and the magnitude of this association is greater than with coronary heart disease.7 All types of smoking including cannabis, cigar, pipe, smokeless tobacco, and cigarettes predispose to PAD.8 There is a dose-dependent relationship and even passive smoking is associated with increased risk of developing PAD.9 10 The postangioplasty/surgical revascularization patency rates are lower in patients who continue to smoke,4 11 and smoking cessation reduces postoperative complications with both short-term and long-term benefits.12
The effect of smoking, and the importance of smoking cessation, should be explained to all PAD patients. Simple advice is rarely effective and hence patients are usually referred to their primary care physician for active interventions.13 These include behavioral therapy, nicotine receptor partial agonists, antidepressants, and nicotine replacement therapy (NRT). A detailed review of the current pharmacotherapy for smoking cessation is available elsewhere.14 Varenicline, bupropion, and NRT are first line medications, which achieve high-smoking cessation rates and have good safety profiles. There is no convincing evidence to suggest that these drugs increase the risk of cardiovascular events during the cessation period.15
Lipid Control
There is a strong association between elevated low-density lipoprotein (LDL) cholesterol levels and PAD.16 An aggressive reduction in LDL cholesterol with statins improves walking distance and reduces all-cause mortality, cardiac death, progression to renal failure, and increases amputation-free survival in patients with PAD.17 18 19 20 It also reduces perioperative mortality and improves long-term outcomes in patients undergoing both endovascular and surgical revascularization.21 22 23 24 25 In spite of these known benefits, effective lipid management remains poor in PVD patients compared with patients with coronary artery disease.26
Lipid control can be achieved by dietary and lifestyle changes, exercise, and drugs such as 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors (statins), fibrates, bile acid sequestrants, nicotinic acids, and Ezetimibe (cholesterol absorption blocker).27 28 Along with diet modification, the authors initiate all patients on simvastatin 40 mg, which may be increased up to 80 mg depending upon the response. The goal is to decrease LDL to below 2 mmol/L (77 mg/dL).28 29 The Heart Protection study has shown that benefits from statin therapy can be obtained in patients with PAD and total cholesterol level greater than 3.5 mmol/L.30 Ezetimibe is used as an alternative in patients who have contraindications, intolerance or do not respond to statins.28 31 Aiming for a lower LDL level below 1.8 mmol/L (70 mg/dL) is desirable in higher risk patients with disease in multiple vascular beds, but it can be difficult in practice because of patient compliance.6
Hypertension
Hypertension is an independent risk factor for PAD.16 32 In contrast to coronary artery disease, raised systolic blood pressure (SBP) and a lower pulse pressure are associated with the greatest risk of PAD.33 In diabetic patients with PAD, reduction of SBP by 10 mm Hg confers a 16% decrease in either lower limb amputation rate or death from PAD over 1,000 person years of follow-up.34
The actual choice of antihypertensive drug35 36 is less relevant as long as the blood pressure should be maintained below 140/90 mm Hg (below 130/80 mm Hg in the presence of diabetes or renal failure).6 37 38 In the authors practice, thiazide diuretics and angiotensin-converting enzyme (ACE) inhibitors are the initial drugs of choice. In addition to their effect on hypertension, ACE inhibitors reduce cardiovascular events39 and improve walking distance.40 Renal function should be checked before and monitored closely following initiation of ACE inhibitors. The results from a nationwide study from Denmark demonstrating that ACE inhibitors may be associated with an increased long-term risk of recurrence after vascular reconstruction need to be confirmed.41 Similarly, there is no convincing evidence to suggest that β-blockers have a detrimental effect in PAD patients.42
Diabetes Control
Diabetes is a major risk factor for PAD. The pattern of PAD in diabetic patients is usually multilevel and predominantly affects the infrapopliteal segments with relative sparing of the pedal vessels.43 Diabetes affects both the micro- and macro-vessels and increases cardiovascular morbidity and mortality. Diabetes increases perioperative cardiovascular morbidity and mortality, and is an independent risk factor for poor outcome after surgical or endovascular revascularization.44 45 46 There is no evidence to suggest that tight glycemic control reduces the progression of PVD or affects the amputation rate.47 It may however reduce the incidence of myocardial infarction (MI), stroke, and vascular death.6
A multidisciplinary approach should be used for diabetic patients with PAD. The aim is to maintain capillary blood glucose levels (Hb A1c) below 7% while avoiding hypoglycemic episodes.6 Recent trials have shown that there is no significant benefit with intensive glycemic control, especially in long-standing type 2 diabetics (in whom it could increase the mortality risk) and suggest a more tailored approach.48 49 50 As with blood pressure control, the choice of intervention (diet control, sulphonylureas or insulin) is less relevant than achieving target glycemic control.
A further consideration with metformin relates to the use of iodinated contrast; guideline recommendations vary significantly.51 The latest guidance from the Royal College of Radiologists suggests that there is no need to stop metformin after contrast exposure in patients with normal serum creatinine and or eGFR > 60. However, if the serum creatinine is elevated or the eGFR is < 60, then the referring clinician should be involved in the decision of withholding metformin for 48 hours.52
Antiplatelet/Anticoagulation
Atherosclerosis is a slowly progressive disease with acute exacerbations due to changes in plaque morphology, ulceration, rupture, and thrombosis. These acute exacerbations lead to vascular events such as MI, stroke, or acute limb ischemia. Antiplatelet and anticoagulant agents reduce the risk of thrombus formation, leading to a reduction in serious vascular events in PAD patients.5 53 Aspirin therapy leads to a 25% relative risk reduction in ischemic stroke, MI, and vascular death. They also reduce the risk of limb deterioration requiring revascularization in patients with intermittent claudication.54
The pharmacology of the inhibition of platelet activation is complex and drugs act on a variety of sites, hence, there is the possibility of synergistic effects when agents are combined. The commonly used medications are cyclooxygenase inhibitors (aspirin), and inhibitors of various platelet surface receptors including P2Y12 (clopidogrel, prasugrel, and ticagrelor), GPIIb/IIIa receptor antagonists (abciximab, tirofiban, and eptifibatide), dipyridamole, phosphodiesterase 3 (PDE3) inhibitor (cilostazol), warfarin, direct thrombin inhibitors (dabigatran and bivalirudin), factor Xa inhibitors (rivaroxaban and apixaban), and heparin (unfractionated, low-molecular-weight heparin).55
The optimal choice and dosage of antiplatelet agents has been long debated. The CAPRIE trial showed that clopidogrel is more effective than a medium dose (325 mg daily) aspirin in reducing serious vascular events and had a similar safety profile.56 Preoperative use of clopidogrel does not increase the incidence of per-operative bleeding.57 NICE considers clopidogrel to be cost effective in patients with PAD.58 In the authors practice, because of historical cost consideration, we use aspirin (75 mg) as first-line antiplatelet for all patients with PAD, with clopidogrel second line in aspirin-sensitive cases. However, current guidance would support a generic clopidogrel-first strategy in all patients with PAD.58
Aspirin and clopidogrel resistance are a growing concern with an increased risk of cardiovascular events, including in stent stenosis in patients with antiplatelet resistance.59 60 Both metabolic and genetic factors are implicated.60 61 The newer antiplatelet agents such as prasugrel and ticagrelor are more efficient with less incidence of resistance.62 Although individualized therapy with platelet function testing seems to be the way forward, there are several hurdles to be overcome.63 64
A Cochrane systematic review has confirmed that antiplatelet or anticoagulation therapy improves the patency after peripheral endovascular interventions.65 Aspirin is commonly used for this purpose and is continued life long.6 Although lacking evidence and guidance,3 6 66 a second antiplatelet agent such as clopidogrel (dual antiplatelet therapy) is prescribed by some interventionists with variable duration after endovascular procedures.67 In the authors practice, all patients will be on aspirin even before therapy and continue lifelong. Following SFA stenting or infrapopliteal endovascular interventions, the authors advocate dual therapy for 3 months and for high-risk patients may extend this to 12 months. There is no benefit in continuing dual therapy beyond 12 months.68 69 Dual therapy is considered better than single therapy, but increases the risk of adverse events related to bleeding.65 Further randomized trials and consensus guidelines are required to guide this practice.70
There is no role for routine usage of warfarin and aspirin in patients with PAD.66 Similarly, anticoagulants are not routinely recommended after endovascular intervention.66 In patients who are high risk for bypass occlusion and limb loss, warfarin can be considered after infrainguinal vein bypass.3 66
Drug Therapy for Symptomatic Relief
During the initial phases of the disease, patients experience pain only on walking (claudication pain) but with disease progression they also experience pain at rest. There are only five drugs licensed in the United Kingdom for relieving these symptoms,5 and in the authors' practice naftidrofuryl oxalate is a first choice drug followed by cilostazol.
Naftidrofuryl Oxalate
Naftidrofuryl oxalate is a selective serotonin (5-hydrroxytryptamine 2 [5-HT2]) receptor antagonist acting on endothelial cells and platelets. It inhibits the serotonin-induced contraction in human vessels, increases the efficiency of aerobic metabolism, reduces erythrocyte rigidity, and improves the transcutaneous oxygen pressure in areas of ischemia.71 It is the authors first choice drug in those patients who have failed to respond to supervised exercise and who are either unsuitable for, or have expressed a desire to avoid intervention. It is prescribed orally at a dose of 100 to 200 mg three times a day for a minimum of 3 months. The adverse effects include skin rash, diarrhea, nausea, and vomiting. Calcium oxalate kidney stones have been reported rarely.
A meta-analysis of randomized controlled trials (RCTs) showed that naftidrofuryl significantly improved the pain-free walking distance compared with placebo. The ratio of relative improvement was 1.37 with an absolute difference of 22.3%.72 It is more cost effective than other drugs with an estimated cost of £6,070 gained per quality adjusted life year; for these reasons, and hence it is recommended by NICE.73 74 75 76 Intravenous Naftidrofuryl oxalate is no longer used in the treatment of critical limb ischemia.77
Cilostazol
Cilostazol is a PDE3 inhibitor with antiplatelet, vasodilator, and antiproliferative activity.6 78 It is prescribed at 100 mg twice daily, and a meta-analysis of RCTs showed that it improves claudication distance and quality of life.79 There is emerging interest in its use for critical limb ischemia, and it is shown to have a beneficial effect on preventing and healing arterial leg ulcers.80 It reduces restenosis and improves long-term patency after infrainguinal endovascular interventions.81 82 83 84 85 As it is expensive and less cost-effective compared with naftidrofuryl, its routine use in patients with PVD is not recommended in the United Kingdom.76 It is contraindicated in patients with congestive heart failure.
Other Drugs
Pentoxifylline affects the rheology of blood cells and lowers blood viscosity. It is associated with some improvement in walking distance, but this has limited clinical benefit and cost effectiveness.73 74 75 86 Hence, it is not recommended for routine use.5 6
Inositol nicotinate has vasodilatory, fibrinolytic, and hypolipidemic effects. Its benefit over placebo is not clear in patients with PAD, and it is not recommended for routine use.5 Similarly, Cinnarizine, although licensed, has no proven clinical benefit and hence is not recommended for routine use.5
Analgesics
In patients with rest pain analgesics play a major role in relieving symptoms before revascularization. In the authors' experience, simple analgesics such as paracetamol and ibuprofen are rarely sufficient and hence opioids (transdermal or oral) remain the drug of choice. Buprenorphine transdermal patches are effective and well tolerated in the outpatient setting. Peridural analgesia is an emerging treatment option, which requires further evidence.87 88 Neuromodulation using spinal cord simulators improves pain relief and hence limb salvage, it should be considered in refractory cases. Spinal cord stimulators are expensive and associated with device specific complications such as infection of leads, which occurs in up to 17% of cases.89
Phantom limb pain can be very disabling and several analgesics including IV ketamine, oral or IV morphine, bupivacaine, and gabapentin have been shown to be effective in short- and long-term management.90 91 However, there is no evidence to support preemptive analgesia in the prevention of phantom pain.92
Prostanoids
Prostaglandin and prostacyclin (e.g., iloprost or beraprost), are used in the treatment of critical limb ischemia due to their potent vasodilator, antiplatelet, and antiproliferative properties. They improve the rest pain, rate of ulcer healing and quality of life, and decrease the amputation rate,93 94 but they are generally reserved for patients not suitable for surgical or endovascular revascularization.95 96
Novel Therapies—Therapeutic Angiogenesis
There are currently three therapeutic strategies being investigated for stimulation of collateral vessel formation in patients with PAD.97
Growth Factors
Several growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor, and placental growth factor have been shown to induce angiogenesis in PAD experimental models.97 Only intra-arterial injection of recombinant FGF (bFGF-2) was tested clinically and shown to improve walking time.98 Because of significant nephrotoxicity and marginal clinical benefit, no further clinical trials were performed.
Gene Therapy
In gene therapy, one or more therapeutic genes are delivered into the somatic cells in the hypoxic tissue (leg) using vectors.99 VEGF gene with the help of adenoviral vector has been extensively investigated in limb ischemia models. Other genes investigated include bFGF, hypoxia-inducible factor 1α (HIF-1α), and angiopoietin-1 (Ang-1).97 Initial clinical trials used VEGF 165 gene plasmid, transferred intra-arterially by coating an angioplasty balloon.100 Later, direct injection into the ischemic muscle was shown to be feasible.101 These trials, along with placebo-controlled randomized trials, confirmed the efficiency of gene transfer on improving vascularity in ischemic tissues.102 103 104 105 However, significant side effects and a failure to translate into improved ulcer healing or reduced amputation rate means that this remains an experimental treatment.99 106
Stem Cell Therapy
The isolation of endothelial progenitor cells in 1997 opened a new chapter in therapeutic angiogenesis.107 These cells migrate to ischemic tissues and are capable of neovascularization. Several animal model experiments have supported this view.97 In the therapeutic angiogenesis using cell transplantation study (TACT) in 2002, bone marrow-derived mononuclear cells (BM-MNC) were injected into the gastrocnemius muscle of diabetic patients with PAD and improvement in an ankle brachial index, pain-free walking time, and rest pain was noted.108 These findings were confirmed in several other small trials.109 110 In the PROVASA study, there was improved ulcer healing and reduced rest pain within 3 months of intra-arterial administration of BM-MNC.111 This technology is well tolerated and has good safety profile, but it needs a larger RCTs to practice evidence-based medicine.112 A summary of the authors current practice is shown in Fig. 1.
Figure 1.
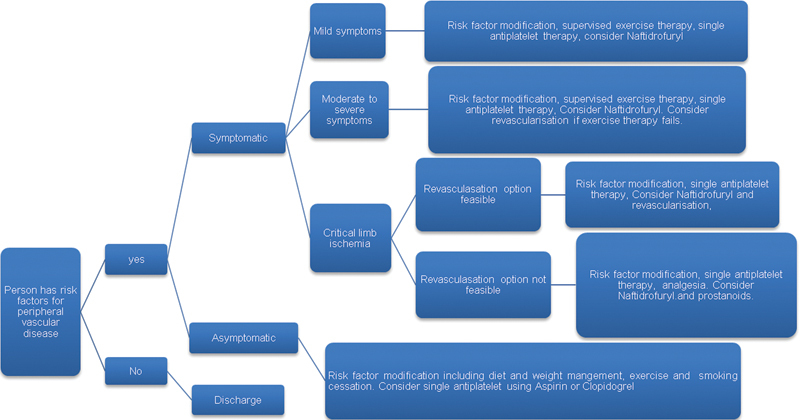
Flow chart showing the management of persons with peripheral arterial disease.
Conclusion
There is good evidence to support therapy to reduce vascular risk factors in patients with peripheral vascular disease. The mainstay of medical therapy for all patients should include lipid lowering, blood pressure control, and an antiplatelet agent. In patients with rest pain, analgesic drugs are essential but revascularization is usually required. Drugs providing symptomatic relief for claudication are usually reserved for patients who fail supervised exercise training. Novel approaches such as angiogenesis may in the future provide an alternative to amputation in patients with critical limb ischemia who lack an option for revascularization.
References
- 1.Diehm C, Allenberg J R, Pittrow D. et al. German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence. Lower limb peripheral arterial disease: diagnosis and management (CG147) London: National Institute for Health and Care Excellence; 2012Available at: https://www.nice.org.uk/guidance/cg147. Accessed June 30, 2014 [Google Scholar]
- 3.Tendera M, Aboyans V, Bartelink M L. et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(22):2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 4.2011 WRITING GROUP MEMBERS; 2005 WRITING COMMITTEE MEMBERS; ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines Circulation 2011124182020–2045. [DOI] [PubMed] [Google Scholar]
- 5.Edinburgh: Scottish Intercollegiate Guidelines Network; 2006. Scottish Intercollegiate Guidelines Network. Diagnosis and Management of Peripheral Arterial Disease: A National Clinical Guideline Number 89. [Google Scholar]
- 6.Norgren L Hiatt W R Dormandy J A Nehler M R Harris K A Fowkes F G; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg 200745(Suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Mackay D F, Pell J P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014;100(5):414–423. doi: 10.1136/heartjnl-2013-304082. [DOI] [PubMed] [Google Scholar]
- 8.Katsiki N, Papadopoulou S K, Fachantidou A I, Mikhailidis D P. Smoking and vascular risk: are all forms of smoking harmful to all types of vascular disease? Public Health. 2013;127(5):435–441. doi: 10.1016/j.puhe.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Mackay D F, Pell J P. Association between level of exposure to secondhand smoke and peripheral arterial disease: cross-sectional study of 5,686 never smokers. Atherosclerosis. 2013;229(2):273–276. doi: 10.1016/j.atherosclerosis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Mackay D F, Pell J P. Secondhand smoke exposure and intermittent claudication: a Scotland-wide study of 4231 non-smokers. Heart. 2013;99(18):1342–1345. doi: 10.1136/heartjnl-2013-304226. [DOI] [PubMed] [Google Scholar]
- 11.Willigendael E M, Teijink J A, Bartelink M L, Peters R J, Büller H R, Prins M H. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg. 2005;42(1):67–74. doi: 10.1016/j.jvs.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med. 2011;171(11):983–989. doi: 10.1001/archinternmed.2011.97. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Smoking cessation services (PH10) London: National Institute for Health and Care Excellence; 2013. Available at: http://www.nice.org.uk/guidance/ph102013. Accessed June 30, 2014 [Google Scholar]
- 14.Carson K V, Brinn M P, Robertson T A. et al. Current and emerging pharmacotherapeutic options for smoking cessation. Subst Abus. 2013;7:85–105. doi: 10.4137/SART.S8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills E J, Thorlund K, Eapen S, Wu P, Prochaska J J. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28–41. doi: 10.1161/CIRCULATIONAHA.113.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murabito J M, D'Agostino R B, Silbershatz H, Wilson W F. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96(1):44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Feringa H H, Karagiannis S E, van Waning V H. et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45(5):936–943. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Stalenhoef A F. The benefit of statins in non-cardiac vascular surgery patients. J Vasc Surg. 2009;49(1):260–265. doi: 10.1016/j.jvs.2008.11.070. [DOI] [PubMed] [Google Scholar]
- 19.Momsen A H, Jensen M B, Norager C B, Madsen M R, Vestersgaard-Andersen T, Lindholt J S. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg. 2009;38(4):463–474. doi: 10.1016/j.ejvs.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Westin G G, Armstrong E J, Bang H. et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63(7):682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schanzer A, Hevelone N, Owens C D, Beckman J A, Belkin M, Conte M S. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 22.O'Neil-Callahan K, Katsimaglis G, Tepper M R. et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45(3):336–342. doi: 10.1016/j.jacc.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Poldermans D, Bax J J, Kertai M D. et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation. 2003;107(14):1848–1851. doi: 10.1161/01.CIR.0000066286.15621.98. [DOI] [PubMed] [Google Scholar]
- 24.Heart Protection Study Collaborative Group . Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45(4):645–654. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 25.Ardati A K, Kaufman S R, Aronow H D. et al. The quality and impact of risk factor control in patients with stable claudication presenting for peripheral vascular interventions. Circ Cardiovasc Interv. 2012;5(6):850–855. doi: 10.1161/CIRCINTERVENTIONS.112.975862. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Thapa R, Jeevanantham V. et al. Comparison of lipid management in patients with coronary versus peripheral arterial disease. Am J Cardiol. 2014;113(8):1320–1325. doi: 10.1016/j.amjcard.2014.01.405. [DOI] [PubMed] [Google Scholar]
- 27.Blum A S. Management of dyslipidemia with statins in the patient with peripheral arterial disease. Tech Vasc Interv Radiol. 2006;9(2):50–55. doi: 10.1053/j.tvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.London: National Institute for Health and Care Excellence; 2010. National Institute for Health and Care Excellence. Lipid Modification Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease (CG 67) [PubMed] [Google Scholar]
- 29.National Institute for Health and Clinical Excellence. Statins for the prevention of cardiovascular events (TA94) London: National Institute for Health and Care Excellence; 2008. Available at: http://www.nice.org.uk/guidance/ta94. Accessed June 30, 2014 [Google Scholar]
- 30.Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 31.London: National Institute for Health and Care Excellence; 2007. National Institute for Health and Care Excellence. Ezetimibe for the treatment of primary (heterozygous-familial and non-familial hypercholesterolaemia (TA132) [Google Scholar]
- 32.Joosten M M, Pai J K, Bertoia M L. et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308(16):1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapsomaniki E, Timmis A, George J. et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler A I, Stratton I M, Neil H A. et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams B, Poulter N R, Brown M J. et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18(3):139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Clinical Excellence. Hypertension: clinical management of primary hypertension in adults (CG 127) London: National Institute for Health and Care Excellence; 2011. Available at: http://www.nice.org.uk/guidance/cg127. Accessed June 30, 2014 [Google Scholar]
- 37.Singer D R, Kite A. Management of hypertension in peripheral arterial disease: does the choice of drugs matter? Eur J Vasc Endovasc Surg. 2008;35(6):701–708. doi: 10.1016/j.ejvs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Lane D A, Lip G Y. Treatment of hypertension in peripheral arterial disease. Cochrane Database Syst Rev. 2013;12:CD003075. doi: 10.1002/14651858.CD003075.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Ostergren J, Sleight P, Dagenais G. et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25(1):17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Shahin Y, Barnes R, Barakat H, Chetter I C. Meta-analysis of angiotensin converting enzyme inhibitors effect on walking ability and ankle brachial pressure index in patients with intermittent claudication. Atherosclerosis. 2013;231(2):283–290. doi: 10.1016/j.atherosclerosis.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 41.Høgh A, Lindholt J S, Nielsen H, Jensen L P, Johnsen S P. Use of angiotensin-converting enzyme inhibitors and cardiovascular outcomes following primary vascular surgery: a nationwide propensity score matched follow-up study. Vasc Endovascular Surg. 2012;46(7):515–523. doi: 10.1177/1538574412455229. [DOI] [PubMed] [Google Scholar]
- 42.Paravastu S C, Mendonca D A, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev. 2013;9:CD005508. doi: 10.1002/14651858.CD005508.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 44.Axelrod D A, Upchurch G R Jr, DeMonner S. et al. Perioperative cardiovascular risk stratification of patients with diabetes who undergo elective major vascular surgery. J Vasc Surg. 2002;35(5):894–901. doi: 10.1067/mva.2002.123681. [DOI] [PubMed] [Google Scholar]
- 45.Goodney P P, Nolan B W, Schanzer A. et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51(1):71–78. doi: 10.1016/j.jvs.2009.07.123. [DOI] [PubMed] [Google Scholar]
- 46.Abularrage C J Conrad M F Hackney L A et al. Long-term outcomes of diabetic patients undergoing endovascular infrainguinal interventions J Vasc Surg 2010522314–322., e1–e4 [DOI] [PubMed] [Google Scholar]
- 47.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 48.Pozzilli P, Strollo R, Bonora E. One size does not fit all glycemic targets for type 2 diabetes. J Diabetes Investig. 2014;5(2):134–141. doi: 10.1111/jdi.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2:CD009122. doi: 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemmingsen B, Lund S S, Gluud C. et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;11:CD008143. doi: 10.1002/14651858.CD008143.pub3. [DOI] [PubMed] [Google Scholar]
- 51.Goergen S K, Rumbold G, Compton G, Harris C. Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology. 2010;254(1):261–269. doi: 10.1148/radiol.09090690. [DOI] [PubMed] [Google Scholar]
- 52.London: The Royal College of Radiologists; 2010. The Royal College of Radiologists. Standards for Intravascular Contrast Agent Administration to Adult Patients, Second edition. [Google Scholar]
- 53.Robless P, Mikhailidis D P, Stansby G. Systematic review of antiplatelet therapy for the prevention of myocardial infarction, stroke or vascular death in patients with peripheral vascular disease. Br J Surg. 2001;88(6):787–800. doi: 10.1046/j.0007-1323.2001.01774.x. [DOI] [PubMed] [Google Scholar]
- 54.Wong P F, Chong L Y, Mikhailidis D P, Robless P, Stansby G. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272. doi: 10.1002/14651858.CD001272.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Whayne T F. A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol. 2012;21(4):187–194. doi: 10.1055/s-0032-1330232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Committee C S; CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet 199634890381329–1339. [DOI] [PubMed] [Google Scholar]
- 57.Saadeh C, Sfeir J. Discontinuation of preoperative clopidogrel is unnecessary in peripheral arterial surgery. J Vasc Surg. 2013;58(6):1586–1592. doi: 10.1016/j.jvs.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 58.Greenhalgh J, Bagust A, Boland A. et al. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal No. 90): a systematic review and economic analysis. Health Technol Assess. 2011;15(31):1–178. doi: 10.3310/hta15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krasopoulos G, Brister S J, Beattie W S, Buchanan M R. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336(7637):195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laine M, Arméro S, Peyrol M. et al. Clinical impact of genetically determined platelet reactivity. J Cardiovasc Transl Res. 2013;6(3):398–403. doi: 10.1007/s12265-012-9421-4. [DOI] [PubMed] [Google Scholar]
- 61.Mijajlovic M D, Shulga O, Bloch S, Covickovic-Sternic N, Aleksic V, Bornstein N M. Clinical consequences of aspirin and clopidogrel resistance: an overview. Acta Neurol Scand. 2013;128(4):213–219. doi: 10.1111/ane.12111. [DOI] [PubMed] [Google Scholar]
- 62.Kastrati A. New anti-platelet agents: the end of resistance? Thromb Res. 2012;130 01:S53–S55. doi: 10.1016/j.thromres.2012.08.275. [DOI] [PubMed] [Google Scholar]
- 63.Cattaneo M. The role of laboratory monitoring in antiplatelet therapy. Handbook Exp Pharmacol. 2012;(210):471–494. doi: 10.1007/978-3-642-29423-5_19. [DOI] [PubMed] [Google Scholar]
- 64.Janssen P W, ten Berg J M. Platelet function testing and tailored antiplatelet therapy. J Cardiovasc Transl Res. 2013;6(3):316–328. doi: 10.1007/s12265-013-9458-z. [DOI] [PubMed] [Google Scholar]
- 65.Robertson L, Ghouri M A, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev. 2012;8:CD002071. doi: 10.1002/14651858.CD002071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alonso-Coello P, Bellmunt S, McGorrian C. et al. Antithrombotic therapy in peripheral artery disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(Suppl):e669S–690S. doi: 10.1378/chest.11-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allemang M T, Rajani R R, Nelson P R, Hingorani A, Kashyap V S. Prescribing patterns of antiplatelet agents are highly variable after lower extremity endovascular procedures. Ann Vasc Surg. 2013;27(1):62–67. doi: 10.1016/j.avsg.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Lee C W, Ahn J M, Park D W. et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129(3):304–312. doi: 10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 69.Park S J, Park D W, Kim Y H. et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 70.Matas M, Domínguez González J M, Montull E. Antiplatelet therapy in endovascular surgery: the RENDOVASC study. Ann Vasc Surg. 2013;27(2):168–177. doi: 10.1016/j.avsg.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 71.Barradell L B, Brogden R N. Oral naftidrofuryl. A review of its pharmacology and therapeutic use in the management of peripheral occlusive arterial disease. Drugs Aging. 1996;8(4):299–322. doi: 10.2165/00002512-199608040-00005. [DOI] [PubMed] [Google Scholar]
- 72.de Backer T L, Vander Stichele R, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database Syst Rev. 2012;12:CD001368. doi: 10.1002/14651858.CD001368.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Squires H, Simpson E, Meng Y. et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess. 2011;15(40):1–210. doi: 10.3310/hta15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng Y, Squires H, Stevens J W. et al. Cost-effectiveness of cilostazol, naftidrofuryl oxalate, and pentoxifylline for the treatment of intermittent claudication in people with peripheral arterial disease. Angiology. 2014;65(3):190–197. doi: 10.1177/0003319712474335. [DOI] [PubMed] [Google Scholar]
- 75.Stevens J W, Simpson E, Harnan S. et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg. 2012;99(12):1630–1638. doi: 10.1002/bjs.8895. [DOI] [PubMed] [Google Scholar]
- 76.National institute for Health and Care Excellence. Cilostazol, Naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease (TA223) Available at: http://www.nice.org.uk/guidance/ta223. Accessed June 30, 2014
- 77.Smith F B, Bradbury A, Fowkes G. Intravenous naftidrofuryl for critical limb ischaemia. Cochrane Database Syst Rev. 2012;7:CD002070. doi: 10.1002/14651858.CD002070.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearce L, Ghosh J, Counsell A, Serracino-Inglott F. Cilostazol and peripheral arterial disease. Expert Opin Pharmacother. 2008;9(15):2683–2690. doi: 10.1517/14656566.9.15.2683. [DOI] [PubMed] [Google Scholar]
- 79.Robless P, Mikhailidis D P, Stansby G P. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2008;(1):CD003748. doi: 10.1002/14651858.CD003748.pub3. [DOI] [PubMed] [Google Scholar]
- 80.de Franciscis S Gallelli L Battaglia L et al. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease Int Wound J 2013. (e-pub ahead of print). doi:10.1111/iwj.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iida O, Yokoi H, Soga Y. et al. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol study. Circulation. 2013;127(23):2307–2315. doi: 10.1161/CIRCULATIONAHA.112.000711. [DOI] [PubMed] [Google Scholar]
- 82.Iida O, Nanto S, Uematsu M, Morozumi T, Kitakaze M, Nagata S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. J Vasc Surg. 2008;48(1):144–149. doi: 10.1016/j.jvs.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 83.Lee S W, Park S W, Kim Y H. et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients) J Am Coll Cardiol. 2008;51(12):1181–1187. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 84.Soga Y, Yokoi H, Kawasaki T. et al. Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol. 2009;53(1):48–53. doi: 10.1016/j.jacc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 85.Soga Y, Iida O, Kawasaki D, Hirano K, Yamaoka T, Suzuki K. Impact of cilostazol on angiographic restenosis after balloon angioplasty for infrapopliteal artery disease in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2012;44(6):577–581. doi: 10.1016/j.ejvs.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Salhiyyah K, Senanayake E, Abdel-Hadi M, Booth A, Michaels J A. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2012;1:CD005262. doi: 10.1002/14651858.CD005262.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Di Minno M N, Milone M, Russolillo A. et al. Ropivacaine infusion in diabetics subject with peripheral arterial disease. A prospective study. Exp Clin Endocrinol Diabetes. 2013;121(2):91–93. doi: 10.1055/s-0032-1327757. [DOI] [PubMed] [Google Scholar]
- 88.Aurilio C, Pace M C, Passavanti M B. et al. Treatment of ischemic pain in patients suffering from peripheral vasculopathy with transdermal buprenorphine plus epidural morphine with ropivacaine vs. epidural morphine with ropivacaine. Pain Pract. 2009;9(2):105–114. doi: 10.1111/j.1533-2500.2008.00237.x. [DOI] [PubMed] [Google Scholar]
- 89.Ubbink D T, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2013;2:CD004001. doi: 10.1002/14651858.CD004001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCormick Z, Chang-Chien G, Marshall B, Huang M, Harden R N. Phantom limb pain: a systematic neuroanatomical-based review of pharmacologic treatment. Pain Med. 2014;15(2):292–305. doi: 10.1111/pme.12283. [DOI] [PubMed] [Google Scholar]
- 91.Alviar M J, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2011;(12):CD006380. doi: 10.1002/14651858.CD006380.pub2. [DOI] [PubMed] [Google Scholar]
- 92.Ypsilantis E, Tang T Y. Pre-emptive analgesia for chronic limb pain after amputation for peripheral vascular disease: a systematic review. Ann Vasc Surg. 2010;24(8):1139–1146. doi: 10.1016/j.avsg.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 93.Ruffolo A J, Romano M, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev. 2010;(1):CD006544. doi: 10.1002/14651858.CD006544.pub2. [DOI] [PubMed] [Google Scholar]
- 94.Matsuo H, Shigematsu H. Patient-based outcomes using the Walking Impairment Questionnaire for patients with peripheral arterial occlusive disease treated with Lipo-PGE1. Circ J. 2010;74(2):365–370. doi: 10.1253/circj.cj-09-0376. [DOI] [PubMed] [Google Scholar]
- 95.Robertson L, Andras A. Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2013;4:CD000986. doi: 10.1002/14651858.CD000986.pub3. [DOI] [PubMed] [Google Scholar]
- 96.Hirsch A T, Haskal Z J, Hertzer N R. et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 97.Grochot-Przeczek A, Dulak J, Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene. 2013;525(2):220–228. doi: 10.1016/j.gene.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 98.Lederman R J, Mendelsohn F O, Anderson R D. et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359(9323):2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 99.Shimamura M, Nakagami H, Taniyama Y, Morishita R. Gene therapy for peripheral arterial disease. Expert Opin Biol Ther. 2014;14(8):1175–1184. doi: 10.1517/14712598.2014.912272. [DOI] [PubMed] [Google Scholar]
- 100.Isner J M, Pieczek A, Schainfeld R. et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348(9024):370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 101.Baumgartner I, Pieczek A, Manor O. et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97(12):1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 102.Kusumanto Y H, van Weel V, Mulder N H. et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17(6):683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 103.Grossman P M, Mendelsohn F, Henry T D. et al. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153(5):874–880. doi: 10.1016/j.ahj.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 104.Morishita R, Makino H, Aoki M. et al. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31(3):713–720. doi: 10.1161/ATVBAHA.110.219550. [DOI] [PubMed] [Google Scholar]
- 105.Mughal N A, Russell D A, Ponnambalam S, Homer-Vanniasinkam S. Gene therapy in the treatment of peripheral arterial disease. Br J Surg. 2012;99(1):6–15. doi: 10.1002/bjs.7743. [DOI] [PubMed] [Google Scholar]
- 106.Hammer A, Steiner S. Gene therapy for therapeutic angiogenesis in peripheral arterial disease - a systematic review and meta-analysis of randomized, controlled trials. Vasa. 2013;42(5):331–339. doi: 10.1024/0301-1526/a000298. [DOI] [PubMed] [Google Scholar]
- 107.Asahara T, Murohara T, Sullivan A. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 108.Tateishi-Yuyama E, Matsubara H, Murohara T. et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 109.Iafrati M D, Hallett J W, Geils G. et al. Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg. 2011;54(6):1650–1658. doi: 10.1016/j.jvs.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 110.Botham C M, Bennett W L, Cooke J P. Clinical trials of adult stem cell therapy for peripheral artery disease. Methodist Debakey Cardiovasc J. 2013;9(4):201–205. doi: 10.14797/mdcj-9-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walter D H, Krankenberg H, Balzer J O. et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4(1):26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 112.Moazzami K, Majdzadeh R, Nedjat S. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database Syst Rev. 2011;(12):CD008347. doi: 10.1002/14651858.CD008347.pub2. [DOI] [PubMed] [Google Scholar]


