Abstract
Peripheral artery disease (PAD), secondary to atherosclerotic disease, is currently the leading cause of morbidity and mortality in the western world. While PAD is common, it is estimated that the majority of patients with PAD are undiagnosed and undertreated. The challenge to the treatment of PAD is to accurately diagnose the symptoms and determine treatment for each patient. The varied presentations of peripheral vascular disease have led to numerous classification schemes throughout the literature. Consistent grading of patients leads to both objective criteria for treating patients and a baseline for clinical follow-up. Reproducible classification systems are also important in clinical trials and when comparing medical, surgical, and endovascular treatment paradigms. This article reviews the various classification systems for PAD and advantages to each system.
Keywords: peripheral artery disease, chronic limb ischemia, acute limb ischemia, diabetic foot ulcer, TASC II, Rutherford, interventional radiology
Peripheral arterial disease (PAD) is extremely common, particularly in the elderly patients.1 2 The prevalence of PAD is increasing as “baby boomers” enter high-risk age groups. Despite its common occurrence, it is estimated that the majority of patients with PAD are undiagnosed and undertreated.3 Risk factors for PAD include age, race, smoking, hypertension, and hyperlipidemia.4 5 6 7
The presentation of PAD varies considerably and includes asymptomatic, acute, or chronic presentations. Numerous schemes have been developed to objectively classify patients for clinical, prognostic, or research purposes. Classification schemes can broadly be broken into stratification based on patient presentation or symptomatology, anatomic distribution of disease, or a combination of clinical factors such as the presence of wounds and infection. Consistent grading of patients provides physicians with objective criteria for patient evaluation, treatment, and clinical follow-up. Reproducible classification systems are also important in research when comparing medical, surgical, and endovascular treatment paradigms. This article reviews the various classification systems for PAD and advantages of each system.
Clinical Presentation
The basic clinical presentation of PAD is often most helpful in categorizing the disease and defining treatment algorithms. The American College of Cardiology/American Heart Association Practice Guidelines defines the presentation of PAD by four categories: asymptomatic, claudication, critical limb ischemia, and acute limb ischemia (ALI).8 9 Patients who are asymptomatic do not have typical claudication symptoms. Identification of asymptomatic PAD in these patients establishes that atherosclerosis is present and warrants risk reduction strategies to decrease cardiovascular risk factors. Claudication is defined as fatigue, discomfort, or pain in the lower extremities, typically the calves, which is reproducibly brought on by exercise and relieved by rest. Critical limb ischemia is defined by chronic ischemic rest pain, nocturnal recumbent pain, or ischemic skin lesions that may include ulcers or frank gangrene.3 Symptoms typically are present for at least 2 weeks. ALI refers to patients with a sudden decrease in limb perfusion causing an immediate threat to limb viability.3 Presentation can occur up to 2 weeks from the onset of symptoms. ALI may present with the “6 Ps” of pain, paralysis, paresthesia, pulselessness, poikilothermia, and pallor.
Fontaine Classification
The first classification system emerged from the European Society of Cardiovascular Surgery in 1952 and was published in 1954 by Fontaine et al.10 This classification system grades the clinical presentation of patients to four stages. The system is solely based on clinical symptoms, without other diagnostic tests, and is typically used for clinical research and not routinely used in patient care (Table 1).
Table 1. Fontaine classification10 .
| Grade | Symptoms |
|---|---|
| Stage I | Asymptomatic, incomplete blood vessel obstruction |
| Stage II | Mild claudication pain in limb |
| Stage IIA | Claudication at a distance > 200 m |
| Stage IIB | Claudication at a distance < 200 m |
| Stage III | Rest pain, mostly in the feet |
| Stage IV | Necrosis and/or gangrene of the limb |
Rutherford Classification
The symptomatic classification was adapted by Rutherford in 1986,11 with revision in 1997.12 Rutherford classified PAD into acute and chronic limb ischemia, emphasizing that each presentation requires different treatment algorithms. Rutherford also associated patient clinical symptoms with objective findings, including Doppler, arterial brachial indices (ABI), and pulse volume recordings. Acute versus chronic presentation implies timing of symptom onset; however, Rutherford did not include stringent temporal criteria in the definitions. Both classifications have been used widely in clinical settings to direct patient management as well as for research purposes.
Rutherford's chronic limb ischemia classification most resembles Fontaine's classification, with the addition of objective noninvasive data.12 The evaluation for any patient with chronic limb pain should include evaluation of the symptoms described in Rutherford's classification. The character of the patients' pain and onset should be evaluated. Claudication onset should be determined, and can be reliably verified by walking/treadmill tests in the noninvasive vascular diagnostic laboratory. Treadmill exercise testing with and without preexercise and postexercise ABIs helps differentiate claudication from pseudoclaudication in patients with exertional leg symptoms. Treadmill exercise testing may be useful to diagnose PAD with a normal resting ABI but a reduced postexercise ABI. Treadmill exercise testing may objectively document the magnitude of symptom limitation in patients with claudication. Treadmill protocols are well described in other publications.9 13 Patients who cannot perform treadmill testing can undergo similar stress testing using plantar flexion or thigh blood pressure cuff compression to cause reactive hyperemia (Table 2).
Table 2. Rutherford classification for chronic limb ischemia11 12 .
| Grade | Category | Clinical description | Objective criteria |
|---|---|---|---|
| 0 | 0 | Asymptomatic—no hemodynamically significant occlusive disease | Normal treadmill or reactive hyperemia test |
| 1 | Mild claudication | Completes treadmill exercise; AP after exercise > 50 mm Hg but at least 20 mm Hg lower than resting value | |
| I | 2 | Moderate claudication | Between categories 1 and 3 |
| 3 | Severe claudication | Cannot complete standard treadmill exercise, and AP after exercise < 50 mm Hg | |
| II | 4 | Ischemic rest pain | Resting AP < 40 mm Hg, flat or barely pulsatile ankle or metatarsal PVR; TP < 30 mm Hg |
| III | 5 | Minor tissue loss—nonhealing ulcer, focal gangrene with diffuse pedal ischemia | Resting AP < 60 mm Hg, ankle or metatarsal PVR flat or barely pulsatile; TP < 40 mm Hg |
| 6 | Major tissue loss—extending above TM level, functional foot no longer salvageable | Same as category 5 |
Abbreviations: AP, ankle pressure; PVR, pulse volume recording; TM, transmetatarsal; TP, toe pressure.
Rutherford's ALI classification divides an extremity into viable, threatened, or irreversibly damaged categories (Table 3). All patients with ALI are initially managed with intravenous heparin unless there is a contraindication. Patients with category I and IIa ischemia with onset within 14 days and low risk of myonecrosis or ischemic nerve damage are often treated with endovascular methods including catheter-directed thrombolysis. Category IIb patients require more immediate revascularization due to higher risks of permanent nerve/tissue injury and muscle necrosis; this is often accomplished with operative thrombectomy and fasciotomy when clinically indicated. Patients with category III ischemia are nonviable and are treated with amputation.
Table 3. Rutherford classification for acute limb ischemia11 12 .
| Category | Description/Prognosis | Findings | Doppler signal | ||
|---|---|---|---|---|---|
| Sensory loss | Muscle weakness | Arterial | Venous | ||
| I. Viable | Not immediately threatened | None | None | Audible | Audible |
| II. Threatened | |||||
| a. Marginally | Salvageable if promptly treated | Minimal (toes) or none | None | Inaudible | Audible |
| b. Immediately | Salvageable with immediate revascularization | More than toes, associated rest pain | Mild, moderate | Inaudible | Audible |
| III. Irreversible | Major tissue loss or permanent nerve damage inevitable | Profound, anesthetic | Profound, paralysis | Inaudible | Inaudible |
Bollinger Angiographic Classification
Fontaine and Rutherford's classifications are based on clinical symptomatology. In contrast, other systems have been developed based on location and severity of atherosclerotic lesions. Anatomic classification systems have usually been based on catheter-directed angiography. The first angiographic-based system was proposed by Vogelberg in 1975.14 This system divides the peripheral circulation into pelvic, thigh, and calf vessels. Each segment is given a score of 1 to 9 depending on atherosclerotic disease burden. Each leg can be given a score of 1 to 27, with a bilateral total score up to 54.
Bollinger et al proposed a similar angiographic methodology for classification, but differentiates the lower extremity arteries into smaller defined segments (Fig. 1).15 Each segment is given an additive score of four categories of severity: occlusion, luminal stenosis greater than 50% of the lumen, stenosis 25 to 49% of the lumen, and plaques <25% of the lumen. The angiogram is also graded by the number of lesions: single lesion, multiple lesions encompassing less than half of the diseased segment, and multiple lesions encompassing more than half of the diseased segment. Severity scores are given in Table 4. In the presence of an occlusion, the stenosis and plaques are not considered. If there are stenoses and plaques, an additive score is given. For example, an occlusion less than half of the segment receives a score of 13. If a segment has multiple stenoses over the length of the vessel causing 25 to 49% stenosis and if there is an additional single 75% stenosis, the total score would be 8 (4 + 4). On repeat angiograms, changes in occlusion are considered. For occlusion length increases over 2 cm, one point is added to the score. Conversely, for decreases in length over 2 cm, one point is subtracted from the score. Bollinger et al also described a vector method of scoring angiograms, listing each subcategory of PAD in each column of subcategory.15
Figure 1.
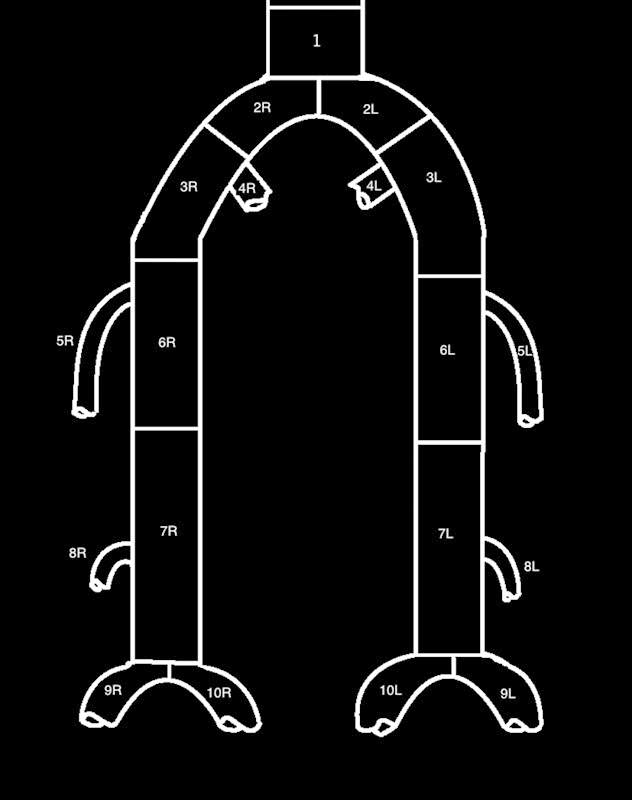
Bollinger classification. 1—abdominal aorta; 2—common iliac; 3—external iliac; 4—internal iliac; 5—profunda; 6—superficial femoral; 7—popliteal; 8—anterior tibial; 9—peroneal; 10—posterior tibial; R—right; L—left.
Table 4. Bollinger scoring system15 .
| Bollinger classification card | |||
|---|---|---|---|
| Location | Occlusive pattern | ||
| Plaque < 25% | Stenosis ≤ 50% | Stenosis > 50% | |
| Single | 1 | 2 | 4 |
| Multiple ≤ 50% segment | 2 | 3 | 5 |
| Multiple > 50% segment | 3 | 4 | 6 |
| Occlusions | < 50% = 13 | ||
| ≥ 50% = 15 | |||
| Follow-up: 2+ cm decrease = − 1; 2+ cm increase = +1 | |||
Notes: Evaluate each Bollinger segment (Fig. 1) and score based on occlusions and stenoses. In the presence of occlusions, plaques and stenoses are not considered. On follow-up examinations, if occlusion segment length increases over 2 cm, it adds a point (e.g., occlusion initially receiving score of 13 would be graded as 14). Occlusion segment decrease of 2 cm would subtract one point.
Bollinger methods of classification of PAD were used in the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial to characterize and follow patients. The classification system is used for chronic limb ischemia, and has not been validated for ALI. The classification system might be useful in computed tomographic angiography or magnetic resonance angiography; however, no validation for these imaging modalities has been published. This system is not used clinically.
Graziani's Morphologic Categorization
PAD in patients with diabetes has a different presentation than critical limb ischemia related purely to atherosclerotic disease. Critical limb ischemia was first defined solely in patients without diabetes as it, “…was generally agreed that diabetic patients who have a varied clinical picture of neuropathy, ischemia and sepsis make definition even more difficult and it is desirable that these patient be excluded … or should be clearly defined as a separate category.”16 Jude et al demonstrated that in diabetic patients, foot ulcers and gangrene were more prevalent than rest pain in patients with PAD without diabetes.17 In addition, the distribution of occlusive disease in diabetics was different from that in nondiabetics. Given the differences in PAD patients with and without diabetes, separate classification systems have been proposed that address these differences. The categorization by Graziani is based on an anatomic distribution.18 This classification system places greater emphasis on the below the knee vessels than previous anatomic classifications. Classification is also graded on the basis of catheter-directed angiography (Table 5). The initial cohort of patient for Graziani's classification included 417 patients, all with ulcers or gangrene. In the population studied by Graziani, the majority of patients with diabetes had two or three of the tibial/peroneal arteries occluded with femoral and popliteal stenosis and/or occlusions.
Table 5. Graziani's morphologic categorization of disease severity18 .
| Class | Angiographic finding |
|---|---|
| 1 | Isolated, one vessel tibial or peroneal artery obstruction |
| 2a | Isolated femoral/popliteal artery or two below knee arteries obstructed but with patency of one of the two tibial arteries |
| 2b | Isolated femoral/popliteal artery or two below knee tibial arteries obstructed but with patency of the peroneal artery |
| 3 | Isolated, one artery occluded and multiple stenosis of tibial/peroneal and/or femoral/popliteal arteries |
| 4 | Two arteries occluded and multiple stenoses of tibial/peroneal and/or femoral/popliteal vessels |
| 5 | Occlusion of all tibial and peroneal arteries (below knee cross-sectional occlusion) |
| 6 | Three arteries occluded and multiple stenosis of tibial/peroneal and/or femoral/popliteal arteries |
| 7 | Multiple femoropopliteal obstructions with no visible below the knee arterial segments |
Notes: Anatomic classification of patients with diabetes with foot ulcers or gangrene. Increasing class is associated with increasing disease severity.
Limitation of the classification system by Graziani is that the system was not validated in a separate population of diabetics for predicting symptom severity. In addition, diabetics without tissue loss were not studied. Most importantly, anatomic distribution of occlusions and stenosis may be present in asymptomatic diabetics, the significance of which is not addressed by Graziani's morphologic categorization of disease severity.
Trans-Atlantic Inter-Society Consensus Document II
Fourteen societies representing disciplines in medicine, vascular surgery, interventional radiology, and cardiology from Europe and North America came together in 2000 to form a consensus in the classification and treatment of patients with PAD. The focus was to provide recommendations in the epidemiology of PAD, clinical evaluation, diagnosis, treatment, and follow-up of patients with intermittent claudication, ALI, and chronic limb ischemia. The resulting document was referred to as the Trans-Atlantic Inter-Society Consensus Document (TASC).19 In 2007, the consensus was updated and involved additional representatives from Australia, South Africa, and Japan and is referred to as TASC II.3 TASC II is comprehensive in reviewing the literature relating to PAD up to 2007.
While TASC II addresses all aspects of PAD, the anatomic classification detailed in TASC II has received the significant focus of the review as well as considerable criticism of the recommendations. Specific categories are assigned treatment algorithms (surgical vs. endovascular) based on lesion classification. TASC II divides anatomic distribution of lesions into aorto-iliac and femoral popliteal (Figs. 2 and 3). Lesion patterns are grouped into A–D lesions. Based on this group recommendation, TASC A lesions are those that should have excellent results from endovascular management alone. TASC B lesions are those that should have good results from endovascular management, and endoluminal interventions should be the first treatment approach. TASC C lesions are those for which surgical management provides superior long-term results and endovascular techniques should be reserved for patients who are surgically high risk. TASC D lesions should be treated by open surgery. While TASC II provides a framework to compare therapeutic techniques, advancement of endovascular techniques have led to many trials suggesting that endovascular management of TASC II C and D lesions is a potential alternative treatment to open strategies.20 21 22 23 24 25 26 27 28 29
Figure 2.
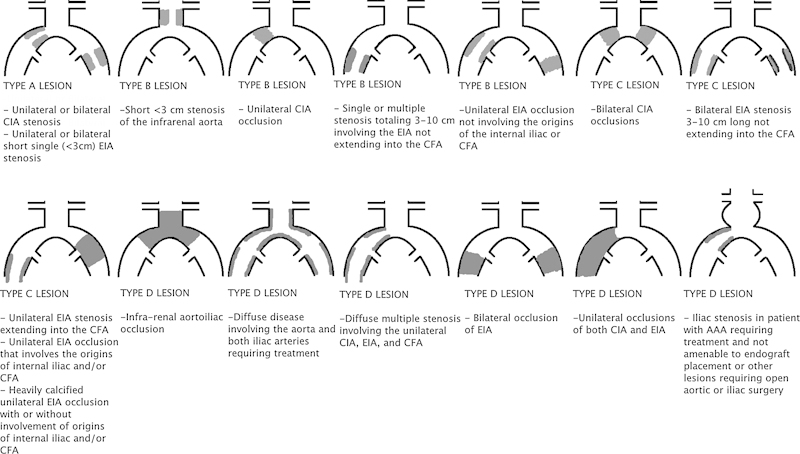
Trans-Atlantic Inter-Society Consensus Document classification of aortoiliac lesions. CIA, common iliac artery; EIA, external iliac artery; CFA, common femoral artery; AAA, abdominal aortic aneurysm.
Figure 3.
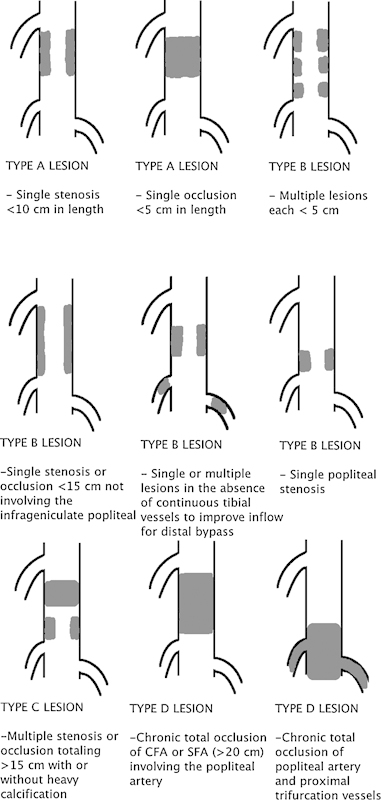
Trans-Atlantic Inter-Society Consensus Document classification of femoral popliteal lesions. CFA, common femoral artery; SFA, superficial femoral artery.
Angiosomes
One classification system that has been described in the plastic surgery literature and has gained acceptance in PAD is the concept of arterial perfusion via angiosomes. The concept was first described by Taylor and Palmer.30 Taylor evaluated cadaveric specimens looking at cross-sectional cadaveric slices and die-injected radiographs. After extensive analysis, the group suggested 40 vascular territories or “angiosomes.” The territories correlate strongly with neurologic dermatomes in the torso and head, but deviate from dermatomes in the extremities. Each angiosome comprises the muscle and overlying subcutaneous tissue and dermis; six angiosomes define the lower extremities.30 The posterior tibial artery feeds three angiosomes: the medial calcaneal artery angiosome, the medial plantar artery angiosome, and the lateral plantar artery angiosome. The anterior tibial artery has one angiosome: the anterior tibial artery–dorsalis pedis artery angiosome. The peroneal artery feeds two angiosomes: the lateral calcaneal artery angiosome and anterior perforator artery angiosome. The adjacent angiosomes can be feed by collateral vessels in the presence of necrosis, termed by Taylor as “choke vessels.”30
The conventional endovascular plan to heal foot ulcers and gangrene is to improve whichever vessel is easiest to recanalize and allow collateral flow to heal an ulcer. Several groups have looked at whether recanalizing the direct vessel to the affected angiosome has improved efficacy over “indirect” or nonselective revascularization. No randomized control study has been performed to evaluate this hypothesis; however, a meta-analysis by Biancariand Juvonen included nine studies that met their inclusion criteria (comparing direct vs. indirect revascularization).31 The direct revascularization showed significantly improved wound healing (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.52–0.80), lower risk of amputation (HR, 0.72; 95% CI, 0.50–1.04), and higher limb salvage rates (HR, 0.43; 95% CI, 0.24–0.77).31 While this summary of studies is promising, randomized control studies are needed to validate the angiosome classification for revascularization paradigms.
Wound, Ischemia, and Foot Infection
In response to the increasing number of diabetics comprising patients with critical limb ischemia, the Society for Vascular Surgery proposed a new classification scheme that combines the classification schemes based on PAD perfusion patterns with foot ulcer schemes. Several grading systems exist to characterize foot ulcers including PEDIS (perfusion, extent/size, depth/tissue low, infection, sensation),32 UT (University of Texas),33 Wagner,34 SAD (sepsis, arteriopathy, denervation),35 and Saint Elian.36 Diabetic foot ulcer schemas are based on size and depth of ulcers as well as foot gangrene.
The new classification system takes into account foot wounds and infection as well as limb perfusion and is titled WIfI (wound, ischemia, and foot infection).37 The Society of Vascular Surgery document addresses the importance of all three components of ulcer, concomitant infection, and limb vascularity in the treatment and outcomes of critical limb ischemia. The WIfI system categorizes patients in a system similar to a TNM (tumor, nodes, metastasis) system common in malignancies. A separate grade is given to the wound (the presence and depth of ulcer), ischemia (based on ABI, toe pressure, or transcutaneous oximetry (TcPO2), and infection (local to systemic) (Table 6). The three grades are combined to give a risk of amputation and estimated benefit of revascularization (Table 7).
Table 6. Society for Vascular Surgery WIfI (wound, ischemia, foot infection) classification38 .
| Wound | |||
|---|---|---|---|
| Grade | Ulcer | Gangrene | |
| 0 | No ulcer | No gangrene | |
| 1 | Small, shallow ulcer on distal leg or foot; no exposed bone, unless limited to distal phalanx | No gangrene | |
| 2 | Deeper ulcer with exposed bone, joint, or tendon; generally not involving the heel; shallow heel ulcer, without calcaneal involvement | Gangrenous changes limited to digits | |
| 3 | Extensive, deep ulcer involving forefoot and/or midfoot; deep, full-thickness heel ulcer ± calcaneal involvement | Extensive gangrene involving the forefoot/midfoot; full-thickness heel necrosis ± calcaneal involvement | |
| Ischemia | |||
| Grade | ABI | Ankle systolic pressure | TP, TcPO2 |
| 0 | ≥ 0.80 | > 100 mm Hg | ≥ 60 mm Hg |
| 1 | 0.6–0.79 | 70–100 mm Hg | 40–59 mm Hg |
| 2 | 0.4–0.59 | 50–70 mm Hg | 30–39 mm Hg |
| 3 | ≤ 0.39 | < 50 mm Hg | < 30 mm Hg |
| Infection | |||
| Grade | Clinical manifestation of infection | ||
| 0 | No symptoms or signs of infection Infection present, as defined by the presence of at least two of the following items: • Local swelling or induration • Erythema 0.5–2 cm around the ulcer • Local tenderness or pain • Local warmth • Purulent discharge (thick, opaque to white, or sanguineous secretion) |
||
| 1 | Local infection involving only the skin and the subcutaneous tissue Exclude other causes of an inflammatory response of the skin (trauma, gout, acute Charcot, fracture, thrombosis, venous stasis) |
||
| 2 | Local infection with erythema >2 cm, or involving structures deeper than skin and subcutaneous tissues, and no systemic inflammatory response signs | ||
| 3 | No systemic inflammatory response signs Local infection with the signs of SIRS, as manifested by two or more of the following: • Temperature > 38 or < 36°C • Heart rate > 90 beats/min • Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg • White blood cell count > 12,000 or < 4,000 cu/mm or 10% immature bands |
||
Abbreviations: ABI, ankle brachial index; PaCO2, partial pressure of carbon dioxide; SIRS, systemic inflammatory response syndrome; TcPO2, transcutaneous oximetry; TP, toe pressure.
Notes: Patient's symptoms are graded by three categories: foot wound severity, tissue perfusion by ABI or transcutaneous oximetry, and the presence of infection.
Table 7. Society for Vascular Surgery WIfI estimations.
| Estimate risk of amputation at 1 y | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemia 0 | Ischemia 1 | Ischemia 2 | Ischemia 3 | |||||||||||||
| W-O | VL | VL | L | M | VL | L | M | H | L | L | M | H | L | M | M | H |
| W-1 | VL | VL | L | M | LV | L | M | H | L | M | H | H | M | M | H | H |
| W-2 | L | L | M | H | M | M | H | H | M | H | H | H | H | H | H | H |
| W-3 | M | M | H | H | H | H | H | H | H | H | H | H | H | H | H | H |
| fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | |
| Estimate likelihood of benefit of/requirement for revascularization (assuming infection can be controlled first) | ||||||||||||||||
| Ischemia 0 | Ischemia 1 | Ischemia 2 | Ischemia 3 | |||||||||||||
| W-O | VL | VL | VL | VL | VL | L | L | M | L | L | M | M | M | H | H | H |
| W-1 | VL | VL | VL | VL | L | M | M | M | M | H | H | H | H | H | H | H |
| W-2 | VL | VL | VL | VL | M | M | H | H | H | H | H | H | H | H | H | H |
| W-3 | VL | VL | VL | VL | M | M | M | H | H | H | H | H | H | H | H | H |
| fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | fL0 | fL1 | fL2 | fL3 | |
Abbreviations: fL, foot infection; H, high = clinical stage 4; L, low = clinical stage 2; M, moderate = clinical stage 3; VL, very low = clinical stage 1; W, wound.
Notes: Clinical stage 5 signifies unsalvageable foot. Grades from the WIfI evaluation are summarized and can be used to estimate the risk of amputation at 1 year for the patient. The same data can be used to estimate the utility of revascularization for the individual patient.
AMA Criteria for Lower Extremity Impairment
One final categorization that can be of interest to the interventional radiologist is the classification of PAD by the American Medical Association (AMA).38 The purpose of the AMA classification is to determine an individual's health impairment due to the disease. The AMA classification combines disease due to PAD and venous insufficiency. Questionnaires are available to narrow the patients' symptoms and classify their improvement. The classification is presented in Table 8. Other systems that have similar categories include workers' compensation, United States Social Security Administration classification, and private insurers.
Table 8. American Medical Association Whole Person Impairment Classification38 .
| Class | WPI | Signs and symptoms |
|---|---|---|
| 0 | 0% | Patient does not have claudication or pain at rest Patient experiences transient edema and one of the following is present: • Loss of pulses • Minimal loss of subcutaneous tissue • Calcification of arteries detected on radiographic examination • Asymptomatic dilation of arteries or veins not requiring surgery and not resulting in curtailment of activities |
| 1 | 2–10% | Patient has at least one of the following: • Intermittent claudication walking at least 100 yards at average pace • Moderate edema persists and is incompletely controlled by elastic supports • Evidence of tissue damage such as healed amputation (single digit) or healed ulceration |
| 2 | 11–24% | Patient has at least one of the following: • Intermittent claudication on walking 25–100 yards at average pace • Marked edema present that is only partially controlled by elastic supports • Evidence of tissue damage such as healed amputations (2+ digits single extremity) or healed ulceration |
| 3 | 25–44% | Patient has at least one of the following: • Intermittent claudication walking <25 yards • Intermittent pain at rest • Marked edema that cannot be controlled by elastic supports • Amputation at or above an ankle of one extremity, or amputation of 2+ digits of two extremities with persistent vascular disease with persistent widespread or deep ulceration involving one extremity |
| 4 | 45–65% | Patient has at least one of the following: • Severe and constant pain at rest • Tissue damage such as amputation at or above the ankles of both extremities, or amputation of all digits of two or more extremities and evidence of widespread or deep ulceration involving two or more extremities |
Abbreviation: WPI, Whole Person Impairment.
Conclusion
Several classification systems have been described to stratify PAD. The heterogeneity of patient presentation, acute versus chronic limb ischemia, and the presence of diabetes contribute to the various classification systems. All physicians treating patients with PAD should be familiar with these classification systems and have a clear understanding of commonly used systems in the literature and clinical evaluation, such as the Rutherford classification and the TASC II classification. The recently proposed WIfI system may gain popularity in the future in the clinical evaluation of PAD, given the increasing percentage of patients with diabetes receiving treatment for chronic limb ischemia. Refer to Table 9 for comparison among the classification systems.
Table 9. Comparison of classification systems.
| Classification | Symptom based | Anatomic | Direct treatment | Apply to acute limb ischemia | Specifically for diabetes | Pros | Cons |
|---|---|---|---|---|---|---|---|
| Fontaine | Yes | No | Yes | Not classically | No | Historically proven; easy to apply to patient | No objective criteria |
| Rutherford | Yes | No | Yes | Yes | No | Historically proven, quickly apply to patient, objective | Classically should not be applied in diabetes, no consideration for wounds |
| Bollinger | No | Yes | No | No | No | Categorical variable can be used in research, allows for documentation of change in follow-up | No basis on symptoms, applied poorly to diabetes |
| Graziani | No | Yes | No | No | Yes | Application in diabetes | Does not address aortoileal disease, does not direct therapy |
| WIfI | Yes | No | Yes | No | Yes | Robust to account for several factors in PAD | New and not validated in many research studies |
| TASC II | No | Yes | Yes | Yes | No | Defined disease process, used in several research studies | Treatment recommendations not widely accepted and may need updating |
| Angiosome | No | Yes | Yes | Not described | No | May help optimize revascularization strategy | Needs further validation |
| AMA | Yes | No | No | Yes | No | Good for use in disability, reflects state of the patients global health | Not intended to direct treatment, mixed arterial and venous categories |
Abbreviations: AMA, American Medical Association; PAD, peripheral artery disease; TASC II, Trans-Atlantic Inter-Society Consensus Document; WIfI, wound, ischemia, foot infection.
References
- 1.Criqui M H, Fronek A, Barrett-Connor E, Klauber M R, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger T P. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt W R, Dormandy J A. et al. TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26(2):81–157. [PubMed] [Google Scholar]
- 4.Fowkes F GR, Housley E, Riemersma R A. et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol. 1992;135(4):331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- 5.Newman A B, Sutton-Tyrrell K, Kuller L H. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb. 1993;13(4):555–562. doi: 10.1161/01.atv.13.4.555. [DOI] [PubMed] [Google Scholar]
- 6.Olin J W. Masterclass series in peripheral arterial disease: Hypertension and peripheral arterial disease. Vasc Med. 2005;10(3):241–246. doi: 10.1191/1358863x05vm591xx. [DOI] [PubMed] [Google Scholar]
- 7.Price J F, Mowbray P I, Lee A J, Rumley A, Lowe G D, Fowkes F G. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20(5):344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 8.Rooke T W, Hirsch A T, Misra S. et al. American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(14):1555–1570. doi: 10.1016/j.jacc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch A T Haskal Z J Hertzer N R et al. American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations J Vasc Interv Radiol 20061791383–1397., quiz 1398 [DOI] [PubMed] [Google Scholar]
- 10.Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [in German] Helv Chir Acta. 1954;21(5–6):499–533. [PubMed] [Google Scholar]
- 11.Rutherford R B, Flanigan D P, Gupta S K. et al. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg. 1986;4(1):80–94. [PubMed] [Google Scholar]
- 12.Rutherford R B, Baker J D, Ernst C. et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 13.Høyer C, Sandermann J, Petersen L J. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg. 2013;58(1):231–238. doi: 10.1016/j.jvs.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Vogelberg K H, Berchtold P, Berger H. et al. Primary hyperlipoproteinemias as risk factors in peripheral artery disease documented by arteriography. Atherosclerosis. 1975;22(2):271–285. doi: 10.1016/0021-9150(75)90008-8. [DOI] [PubMed] [Google Scholar]
- 15.Bollinger A, Breddin K, Hess H. et al. Semiquantitative assessment of lower limb atherosclerosis from routine angiographic images. Atherosclerosis. 1981;38(3–4):339–346. doi: 10.1016/0021-9150(81)90050-2. [DOI] [PubMed] [Google Scholar]
- 16.Bell P, Charlesworth D, DePalma R. et al. The definition of critical ischaemia of a limb. Working party of the International Vascular Symposium. Br J Surg. 1982;69(S6):S2–S3. [Google Scholar]
- 17.Jude E B, Oyibo S O, Chalmers N, Boulton A J. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 18.Graziani L, Silvestro A, Bertone V. et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33(4):453–460. doi: 10.1016/j.ejvs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Dormandy J A, Rutherford R B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1, Pt 2):S1–S296. [PubMed] [Google Scholar]
- 20.Hans S S DeSantis D Siddiqui R Khoury M Results of endovascular therapy and aortobifemoral grafting for Transatlantic Inter-Society type C and D aortoiliac occlusive disease Surgery 20081444583–589., discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 21.Pulli R, Dorigo W, Fargion A. et al. Early and long-term comparison of endovascular treatment of iliac artery occlusions and stenosis. J Vasc Surg. 2011;53(1):92–98. doi: 10.1016/j.jvs.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Balzer J O, Gastinger V, Ritter R. et al. Percutaneous interventional reconstruction of the iliac arteries: primary and long-term success rate in selected TASC C and D lesions. Eur Radiol. 2006;16(1):124–131. doi: 10.1007/s00330-005-2736-7. [DOI] [PubMed] [Google Scholar]
- 23.Davaine J-M, Quérat J, Guyomarch B. et al. Primary stenting of TASC C and D femoropopliteal lesions: Results of the STELLA register at 30 months. Ann Vasc Surg. 2014 doi: 10.1016/j.avsg.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto N, Kawasaki R, Fukuda T, Yamaguchi M, Sugimura K, Sugimoto K. Endovascular treatment for unilateral chronic total occlusions of the iliac artery categorized as TASC II type D lesions. Surg Today. 2014:1–6. doi: 10.1007/s00595-014-0883-7. [DOI] [PubMed] [Google Scholar]
- 25.Aihara H, Soga Y, Mii S. et al. Comparison of long-term outcome after endovascular therapy versus bypass surgery in claudication patients with Trans-Atlantic Inter-Society Consensus-II C and D femoropopliteal disease. Circ J. 2014;78(2):457–464. doi: 10.1253/circj.cj-13-1147. [DOI] [PubMed] [Google Scholar]
- 26.Baril D T, Chaer R A, Rhee R Y, Makaroun M S, Marone L K. Endovascular interventions for TASC II D femoropopliteal lesions. J Vasc Surg. 2010;51(6):1406–1412. doi: 10.1016/j.jvs.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 27.Sixt S, Krankenberg H, Möhrle C. et al. Endovascular treatment for extensive aortoiliac artery reconstruction: a single-center experience based on 1712 interventions. J Endovasc Ther. 2013;20(1):64–73. doi: 10.1583/12-4014.1. [DOI] [PubMed] [Google Scholar]
- 28.Taurino M, Persiani F, Fantozzi C, Ficarelli R, Rizzo L, Stella N. Trans-Atlantic Inter-Society Consensus II C and D iliac lesions can be treated by endovascular and hybrid approach: a single-center experience. Vasc Endovascular Surg. 2014;48(2):123–128. doi: 10.1177/1538574413512381. [DOI] [PubMed] [Google Scholar]
- 29.Tewksbury R, Pearch B, Redmond K, Harper J, Klein K, Quinn J. Outcomes of infrapopliteal endoluminal intervention for transatlantic intersociety consensus C and D lesions in patients with critical limb ischaemia. ANZ J Surg. 2013 doi: 10.1111/ans.12460. [DOI] [PubMed] [Google Scholar]
- 30.Taylor G I, Palmer J H. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(2):113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 31.Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2014;47(5):517–522. doi: 10.1016/j.ejvs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Schaper N C. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20 01:S90–S95. doi: 10.1002/dmrr.464. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong D G, Lavery L A, Harkless L B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 34.Wagner F W Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 35.Macfarlane R, Jeffcoate W. Classification of diabetic foot ulcers: the S(AD) SAD system. The Diabetic Foot Journal. 1999;2:123–131. [Google Scholar]
- 36.Martínez-De Jesús F R. A checklist system to score healing progress of diabetic foot ulcers. Int J Low Extrem Wounds. 2010;9(2):74–83. doi: 10.1177/1534734610371594. [DOI] [PubMed] [Google Scholar]
- 37.Mills J L Sr Conte M S Armstrong D G et al. Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg 2014591220–234., e1–e2 [DOI] [PubMed] [Google Scholar]
- 38.Rondinelli R D Genovese E Brigham C R Association A M. Guides to the Evaluation of Permanent Impairment: American Medical Association; 2008 [Google Scholar]


