Abstract
The lateral habenula (LHb) is part of the habenula complex of the dorsal thalamus. Recent studies of the LHb have focused on its projections to the ventral tegmental area (VTA) and rostromedial tegmental nucleus (RMTg), which contain GABAergic neurons that mediate reward prediction error via inhibition of dopaminergic activity. However, older studies in the rat have also identified LHb outputs to the lateral and posterior hypothalamus, median raphe, dorsal raphe, and dorsal tegmentum. Although these studies have shown that the medial and lateral divisions of the LHb have somewhat distinct projections, the topographic specificity of LHb efferents is not completely understood, and the relative extent of these projections to brainstem targets is unknown. Here we have used anterograde tracing with adeno-associated virus mediated expression of green fluorescent protein, combined with serial two-photon tomography, to map the efferents of the LHb on a standard coordinate system for the entire mouse brain, and reconstruct the efferent pathways of the LHb in three dimensions. Using automated quantitation of fiber density, we show that in addition to the RMTg, the median raphe, caudal dorsal raphe, and pontine central gray are major recipients of LHb efferents. Using retrograde tract tracing with cholera toxin subunit B, we show that LHb neurons projecting to the hypothalamus, VTA, median raphe, and caudal dorsal raphe, and pontine central gray reside in characteristic, but sometimes overlapping regions of the LHb. Together these results provide the anatomical basis for systematic studies of LHb function in neural circuits and behavior in mice.
Introduction
The habenula is a paired nucleus residing in the dorsal thalamus. It consists of medial and lateral subnuclei (MHb, LHb), both of which project to the ventral midbrain via a prominent tract, the fasciculus retroflexus (FR). Beyond this common output tract, the MHb primarily innervates the interpeduncular nucleus (IP), while the LHb has more diverse outputs to the tegmentum, midbrain and pontine raphe, and hypothalamus (Herkenham and Nauta, 1979). Classic lesion studies of the habenula have led to the assignment of diverse roles to this nucleus, including modulation of reproductive behavior, feeding, sleep-wake cycles, stress responses, attention, learning, and the behavioral effects of nicotine and other drugs of abuse (Klemm, 2004). Few of these studies have assigned these behavioral functions specifically to the MHb or LHb, yet there is little reason to believe that these nuclei have the same function.
Recent studies of habenula function have focused on the inhibition of dopamine-mediated reward signals by the LHb. Because LHb neurons projecting to the VTA are excitatory (Geisler et al., 2007), GABAergic neurons are required to convert the LHb signal to an inhibitory one. In the rat, these inhibitory neurons reside just caudal to the DA neurons of the VTA, in a region termed the rostromedial tegmental nucleus (RMTg), or “GABAergic tail” of the VTA (Perrotti et al., 2005; Jhou et al., 2009a; Jhou et al., 2009b). Studies in primates have elegantly demonstrated that DA neurons in the VTA fire in the presence of reward-predicting cues, while LHb neurons fire in the absence of an expected reward, or with punishment, apparently acting via RMTg interneurons to suppress the DA system (Matsumoto and Hikosaka, 2007; 2009; Hong et al., 2011). Consistent with this, stimulation of the LHb neurons projecting to the RMTg/VTA also induces behavioral avoidance in mice (Lammel et al., 2012; Stamatakis and Stuber, 2012). A role for the LHb-VTA pathway has also been described for learned helplessness in rats, a model of depression (Li et al., 2011; Winter et al., 2011), and in mediating the delayed aversive effects that follow an acute dose of cocaine (Jhou et al., 2013).
Despite the recent emphasis on the LHb > RMTg > VTA pathway, older studies in the rat have demonstrated a much more diverse set of LHb output pathways, including efferents to the lateral and posterior hypothalamus (LH, PH), the median raphe (MnR), dorsal raphe (DR), and pontine central gray (CGPn, Herkenham and Nauta, 1979; Sutherland, 1982; Araki et al., 1988). However, these studies did not place the LHb efferent pathways in any standardized neuroanatomical coordinate system, define LHb subdomains with respect to their projections, nor correlate LHb efferents with the neurotransmitter phenotype of their target nuclei. Therefore, in contrast to the LHb > RMTg pathway, very little is known about the function of the LHb projections to the hypothalamus and raphe.
Here we have used anterograde and retrograde tract tracing to define LHb efferent pathways in the mouse. First, we have mapped LHb projections globally, using tract-tracing with adeno-associated virus (AAV) mediated expression of green fluorescent protein (GFP), combined with serial two-photon tomography and automated quantitation of efferent fiber density. These methods have allowed the reconstruction of LHb projections in a standardized three-dimensional space for the entire brain, and this is the first study to quantitate habenula efferents to specific targets in any species. Second, we have used retrograde labeling of the LHb from each of its major targets, including the hypothalamus, VTA/RMTg, MnR, DR, and CGPn, to reveal a topographic map of LHb neurons onto their target nuclei. Use of transgenic GFP markers and neurotransmitter biosynthesis enzyme immunofluorescence for specific cell types in these LHb target regions has allowed refinement of the LHb projection map to functional brain regions. Together these results provide the anatomical basis for future studies of LHb function in neural circuits and behavior in mice.
Materials and Methods
Animals
Anterograde tracing was performed using male C57BL/6 mice (Jackson Labs) aged P56 +/− 2 days. Retrograde tract tracing was performed in C57BL/6 mice aged 2–6 months (Charles River). For the identification of GABAergic neurons in retrograde tracing experiments we employed mice expressing GFP under control of the Gad1/Gad67 gene locus, generously provided by Prof. Yuchio Yanagawa (Tamamaki et al., 2003). For Cre-mediated anterograde tract tracing Grm2Cre mice (STOCK Tg(Grm2-cre)MR90Gsat/Mmucd), originating in the GENSAT project, and Ntrk1Cre mice (B6;129S4-Ntrk1Tm1(cre)Lfr/Mmucd), originating in the laboratory of Louis Reichardt, were obtained from the Mutant Mouse Regional Resource Centers (https://www.mmrrc.org/).
Viral injections for anterograde tract-tracing
Anterograde tract tracing in wild-type mice in cases LHb/a1 and LHb/a2 was performed with the viral vector rAAV2/1.hSynapsin.EGFP.WPRE.bGH, encoding eGFP under the regulation of a human synapsin promoter. Tract tracing in the wild-type mouse in case LHb/a3 was performed with the viral vector AAV1.hSyn.ChR2(H134R)-eYFP.WPRE.hGH, encoding eYFP linked to the channelrhodopsin variant ChR2(H134R). Tract tracing in Cre-recombinase expressing mice was performed with the Cre-inducible viral vector rAAV2/1.pCAG.FLEX.EGFP.WPRE.bGH. Viral stocks were prepared at the University of Pennsylvania Gene Therapy Program Vector Core (http://www.med.upenn.edu/gtp/vectorcore/). The detailed methods used here for anterograde tract tracing with iontophoretic injection of AAV have recently been published in conjunction with the Allen Mouse Brain Connectivity Atlas (ACA, Harris et al., 2012; Oh et al., 2014). The stereotaxic coordinates targeted for case LHb/a1 (ACA case 147353537) were: AP -1.70 (mm caudal to bregma), ML 0.42 (mm lateral to midline), and DV 2.60 (mm ventral to lambda-bregma line, based on standard atlas coordinates, Paxinos and Franklin, 2001). The observed center of the injected area for case LHb/a1, a large injection in the dorsal LHb, was at: AP -1.9, ML 0.4, and DV 2.6. The targeted coordinates for case LHb/a2 (ACA case 120875111) were: AP -1.58, ML 1.00, using an injection angle of 20 degrees in the coronal plane, and DV 2.90. The observed center of the injected area for case LHb/a2, a relatively small injection in the ventromedial LHb, was at: AP -1.6, ML 0.2, DV 2.8. The targeted coordinates for case PV/a1(ACA case 175739791) were: AP -1.58, ML 0.35, and DV 2.73. The coordinates for case PV/a2 (ACA case 263106751) were: AP -1.06, ML 1.15, using an injection angle of 20 degrees in the coronal plane, and DV 3.35. The center coordinates of the injected area for case LHb/a3, which labeled the LHb extensively, were AP -1.85, ML 0.35, and DV -2.59. In cases PV/a1 (Grm2Cre) and PV/a2 (Ntrk1Cre) the expression of GFP from the injected virus was limited by the restricted expression of Cre-recombinase in the PV.
Anterograde tracing in cases LHb/a1 and LHb/a2 were analyzed by serial two-photon tomography (Ragan et al., 2012), using automated vibratome sectioning in the coronal plane, which allows the collection of 140 inherently pre-aligned images collected every 100μm through the entire rostral-to-caudal extent of the mouse brain. The resolution in the plane of section was 0.35μm. The Multi-photon image acquisition for the viral tract-tracing cases was accomplished using the TissueCyte 1000 system (TissueVision, Cambridge, MA) coupled with a Mai Tai HP DeepSee laser (Spectra Physics, Santa Clara, CA). Case LHb/a3 was analyzed by conventional epifluorescence microscopy and by confocal microscopy using a Zeiss 710 confocal microscope. Confocal images are Z-stacks encompassing the entire thickness of the 25μm section.
Quantitative analysis of fiber density in anterograde experiments
Each case in the ACA is processed through an automated data processing pipeline to quantify labeled fiber density for each structure in the Allen Reference Atlas (ARA) ontology. A detailed description of the design and implementation of the Connectivity Informatics Data Pipeline is available online (http://help.brain-map.org/display/mouseconnectivity/Documentation). In brief, a segmentation algorithm based on edge detection and morphological filtering was applied to each image to classify every pixel as either signal or background. The images are then registered to a 3D reference space integrated with the ARA annotation. For each structure, the number of segmented pixels is tallied for each hemisphere and multiplied by the resolution (pixel size) and z-sampling interval, to yield the “projection volume”. Note that the segmentation algorithm does not distinguish between passing fibers and terminals; however, signal falling within fiber tract regions annotated in the ARA is excluded from the structural computation.
Because the RMTg is not assigned in the ARA, the segmented pixels representing labeled fibers in this area was measured in a separate analysis. The boundaries of RMTg were first identified on images from case 147353537 (Figures 9–11) and then transformed to the 3D ARA reference space. In standard atlas coordinates, the RMTg extended from bregma -3.64mm to bregma -4.60mm. However, the caudal part of the RMTg, from bregma -4.16mm to bregma -4.60mm, also corresponds to the region designated as the anterior tegmental nucleus (ATg) in a standard atlas (Paxinos and Franklin, 2001). The equivalent region is not designated ATg in a standard rat atlas (Paxinos and Watson, 2005), or the ARA, and the assignment of this structure apparently extends too far in the rostral direction in the mouse atlas. The rostral RMTg thus defined spans ARA structures VTA, PRNr, CSm, and unassigned tegmental gray, while the caudal RMTg (“ATg”) spans structures PRNr and CSi. Using the 3D RMTg annotation, segmented projection volumes were then tallied for both parts of this structure in both cases. In Figure 8 the projection volume of each structure is shown as percentage of total projection volume in combined midbrain and hindbrain domains. Projections to the diencephalon were not included in the quantitative analysis.
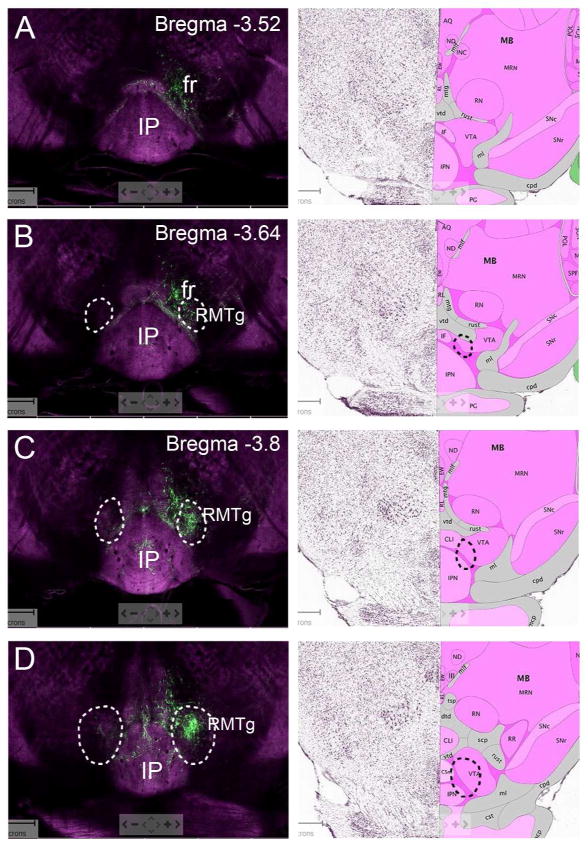
Figure 9. LHb projections to the rostral RMTg mapped onto the Allen Reference Atlas.
Case LHb/a1, with a strong ipsilateral projection to the RMTg, was used to assign the location of this nucleus in Figures 9–11. The circled areas on the Allen Reference Atlas show the area of integration for the quantitative analysis of the LHb projections to the RMTg in cases LHb/a1 and LHb/a2 (Figure 8). The designated levels relative to bregma correspond to the nearest levels annotated in a standard mouse brain atlas (Paxinos and Franklin, 2001). The correspondence is not exact because the data are acquired at 100μm intervals corresponding to the Allen Reference Atlas, and the standard atlas intervals are irregular. The most rostral RMTg fiber terminals are identified at bregma -3.64. Beginning at bregma -4.16, the strong ipsilateral projections from the LHb coincide with a region designated anterior tegmental nucleus (“ATg”) in a standard atlas (Paxinos and Franklin, 2001). However, in a standard atlas of the rat brain (Paxinos and Watson, 2005), the ATg is not annotated until a more caudal position relative to other structures (rat bregma -7.64mm, approximately equivalent to mouse bregma -4.40), where the strong ipsilateral projection in case LHb/a1 ends. Thus we infer that regions between bregma -4.16 and bregma -4.48 innervated by the strong ipsilateral projection from the LHb corresponds to the caudal part of the RMTg (cRMTg), not the ATg. See text for discussion. Note also that at the level corresponding to bregma -4.72, the location of the ATg (AT) is Allen Reference Atlas is designated VTg in the standard atlas. A full list of abbreviations used in the Allen Reference Atlas appear in the Atlas documentation at: http://help.brain-map.org/display/mousebrain/Documentation
Figure 11. LHb projections to the caudal RMTg mapped onto the Allen Reference Atlas.
See Figure 9 for legend.
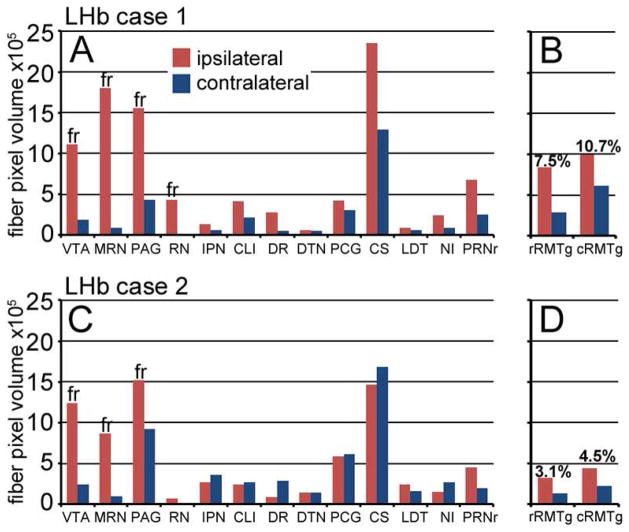
Figure 8. Quantitative analysis of LHb projections to the midbrain and pons.
Total fiber volume in each of the defined anatomical areas was determined from the segmentation views of case LHb/a1 and LHb/a2 (Methods). Percentage values were calculated based on the total fiber volume in the midbrain + pons. Only areas receiving greater than 2% of the LHb fibers are shown. Fibers detected in the diencephalon were not included in the analysis. (A,B) Distribution of fiber density in case LHb/a1, injected in the dorsolateral LHb. (C,D) Distribution of fiber density in case LHb/a2, injected in the ventromedial LHb. The RMTg was analyzed as a rostral (rRMTg) and a caudal (cRMTg) component. The caudal component has been assigned as ATg in a standard atlas, but this appears to be a misassignment of this structure (see text).
As discussed in Methods, because the FR is not precisely annotated in the Allen Reference Atlas, some fibers of passage within the FR were assigned to adjacent structures in this analysis. Areas which may have a significant contribution from these fibers of passage, always on the side ipsilateral to the injection, are annotated with “fr” on the corresponding bar in the graph. Because the quantitative analysis of fiber density was performed in the 3D Allen Reference Atlas space (Dong, 2008), the anatomical areas used here do not always correspond exactly to other published atlases, or may be differently named. Correspondence between the Allen Reference Atlas terms used here and Paxinos (2001) atlas nomenclature is as follows: Ventral tegmental area, VTA (same); Midbrain reticular nucleus, MRN (deep mesencephalic nucleus, DpMe); periaqueductal gray, PAG (same); red nucleus, RN (R); interpeduncular nucleus, IPN (IP), central linear n raphe, CLI (same); dorsal raphe, DR (same); dorsal tegmental nucleus, DTN (DTg); pontine central gray, PCG (CGPn); superior central nucleus raphe, CS (median raphe, MnR; paramedian raphe, PMnR); laterodorsal tegmental nucleus, LDT (LDTg); nucleus incertus, NI (includes multiple regions including the dorsal raphe, interfascicular, DRI); pontine reticular nucleus, PRNr (Pontine reticular n, caudal part, PnC).
Induction of cFos expression by amphetamine
Adult mice were injected intraperitoneally with 5mg/kg amphetamine or with saline vehicle (N=4 for each group, Colussi-Mas et al., 2007). Two hours after injection mice were sacrificed, perfused transcardially with 4% PFA. Coronal sections were cut on a cryostat at 50μm intervals from the level of the interpeduncular nucleus/RMTg to the caudal end of the dorsal tegmental nuclei. Floating sections were immunostained for cFos, mounted on slides, and imaged using fluorescence microscopy. Sections were co-immunostained for the dopamine transporter to reveal the location of dopaminergic cells and tracts. cFos-immunoreactive cells were counted in the area assigned as the RMTg based on the presence of LHb input fibers at three levels, corresponding to the rostral, central, and caudal parts of the RMTg, at the standard atlas levels bregma -3.8, -4.04 and -4.34, respectively.
Retrograde tract tracing and immunofluorescence
Retrograde tract tracing was performed using a 0.2–1% solution of cholera toxin B subunit (CTB; List Biological Labs), injected using standard stereotaxic coordinates (Paxinos and Franklin, 2001). Large areal injections of CTB were performed by pressure injection using a 33 gauge stainless steel needle and an automated syringe driver. For such injections 0.1μl CTB was infused over 1 min, followed by a 5 min rest period before the needle was retracted. Small focal injections were performed by iontopheresis using a pulled glass pipette, using published methods (Harris et al., 2012). In brief, the pipette was positioned using a stereotaxic frame, and current was delivered with a Midgard Precision current source. The current was set to 3μA, and was alternated for 7 seconds on and 7 seconds off for 5–12 minutes depending on the size of the structure labeled, followed by a 5 min rest period before the needle was retracted.
Antibody characterization
Primary antibodies for immunofluorescence are described in Table 1. Rabbit and guinea pig antibodies to Pou4f1/Brn3a, developed in the Turner laboratory, have been extensively characterized (Fedtsova and Turner, 1995; Quina et al., 2005). These antibodies reproduce the pattern of brain-specific staining shown by in situ hybridization, and show no tissue-specific signal in mice that are null mutants for the coding sequence of the Pou4f1 gene. Antibodies to enzymes in neurotransmitter synthesis pathways showed patterns of cellular staining consistent with in situ hybridization patterns for the corresponding genes. Antibodies to CTB showed no signal in the absence of injected CTB.
Table 1.
Table of primary antibodies used.
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Choline acetyltransferase | Human placental ChAT | EMD Millipore (Billerica, MA), goat polyclonal, AB144P RRID: AB_2079751 |
1:200 |
| Tyrosine hydroxylase | Denatured rat TH | EMD Millipore, rabbit polyclonal, AB152 RRID: AB_390204 |
1:2000 |
| Dopamine transporter DAT | N-terminus of human dopamine transporter | EMD Millipore, IgG2a rat monoclonal, MAB369 RRID: AB_2190413 |
1:1000 |
| Tryptophan hydroxylase 2 | KLH-conjugated peptide derived from human TPH2 (proprietary) | EMD Millipore, rabbit polyclonal, ABN60 RRID: AB_10806898 |
1:500 |
| cFos | Human cFos, N-terminal | Santa Cruz Biotechnology (Santa Cruz, CA) rabbit polyclonal #Sc52 RRID: AB_2106783 |
1:1000 |
| Cholera toxin subunit B | Purified toxin subunit (Choleragenoid) | List Biological Labs (Campbell, CA), goat polyclonal, #703 RRID: AB_10013220 |
1:1000 |
| Cholera toxin subunit B | Purified Choleragenoid | Abcam (Cambridge, MA), rabbit polyclonal, ab34992 RRID: AB_726859 |
1:2000 |
| Pou4f1/Brn3a | N-terminal GST fusion. See (Fedtsova and Turner, 1995) | Rabbit polyclonal, antigen affinity purified RRID: AB_2314040 |
1:500 |
| Pou4f1/Brn3a | As above, see (Quina et al., 2005) | Guinea pig polyclonal, antigen affinity purified | 1:500 |
Results
Anterograde tracing of LHb efferents
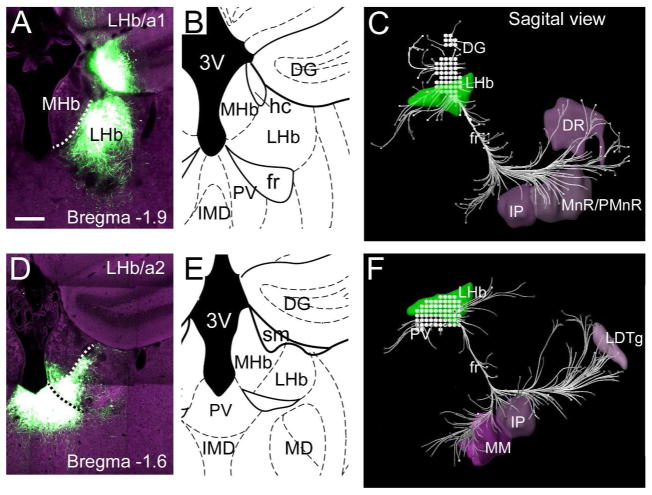
In order to obtain an overview of the output pathways of the LHb in the mouse, we first employed anterograde tracing with a recombinant adeno-associated virus (rAAV2/1) encoding eGFP, which has the advantages of robust native fluorescence and persistent labeling (Harris et al., 2012). These experiments were performed as a part of the Mouse Brain Connectivity Atlas (“Connectivity Atlas”) project at the Allen Institute for Brain Science (Oh et al., 2014). In case LHb/a1, a large injection was centered in the dorsal LHb at bregma -1.9, in the caudal third of the LHb (Figure 1A, B), which in the mouse extends from approximately bregma -1.0 to bregma -2.2. In case LHb/a2 a relatively small injection was centered in the ventromedial corner of the LHb at bregma -1.6, centrally located along the rostrocaudal extent of the nucleus (Figure 1D,E). Case LHb/a1 also significantly labeled the dentate gyrus of the hippocampus, and case LHb/a2 significantly labeled the paraventricular thalamic nucleus. Because neither of these adventitiously labeled areas project via the LHb output tract, the fasciculus retroflexus (FR), the projections of these areas were not further examined in this study.
Figure 1. Anterograde tract tracing injection sites and LHb pathway reconstruction.
An rAAV expressing an eGFP tract-tracing protein (Methods) was injected into the LHb, and the LHb output pathways were reconstructed using serial two-photon fluorescence tomography. (A–C) rAAV tracing of LHb/a1 (Allen case 147353537). The rAAV injection was positioned in the dorsolateral quadrant of the caudal LHb at bregma -1.9mm. Tracer expression was also evident in the DG, which did not contribute to brainstem projections. (A) Fluorescence signal at injection site. Dashed white line designates the border between MHb and LHb. (B) Anatomical map of the injected area, adapted from a standard atlas (Paxinos and Franklin, 2001). (C) Sagittal reconstruction of fiber tracts. Stippling indicates the area of viral spread. (D–F) rAAV tracing of LHb/a2 (Allen case 120875111). The rAAV injection was positioned in the ventromedial quadrant of the central LHb at bregma -1.6. Tracer expression was also evident in the PV, which did not contribute to brainstem projections. (D) Fluorescence signal at injection site. Dashed white line designates the border between the MHb and LHb; dashed black line indicates border between the LHb and the PV. (E) Anatomical map of the injected area. (F) Sagittal reconstruction of fiber tracts. An extensive projection from PV to the cerebral ganglia and rostral hippocampus is not shown. Nomenclature is derived from Paxinos (2001), see Table.
Initial imaging of the eGFP label was performed using automated serial two-photon tomography (Ragan et al., 2012) at 100μm intervals, allowing the entire brain to be imaged coronally in about 140 sections at a resolution of approximately 0.35μm/pixel. A segmentation algorithm based on edge detection and morphological filtering was then applied to each image to classify a pixel as either signal or background, thus generating “segmentation views” in which the fiber signal is digitally rescaled and thresholded. The images were then registered to a 3D reference space integrated with the structures defined in the Allen Mouse Brain Reference Atlas (“Allen Reference Atlas”, http://help.brain-map.org/display/mousebrain/Documentation). The Brain Explorer (Lau et al., 2008) software was then used to visualize 3D reconstructions of the LHb afferents (Figure 1C,F), juxtaposed with anatomical regions as defined in the Allen Reference Atlas.
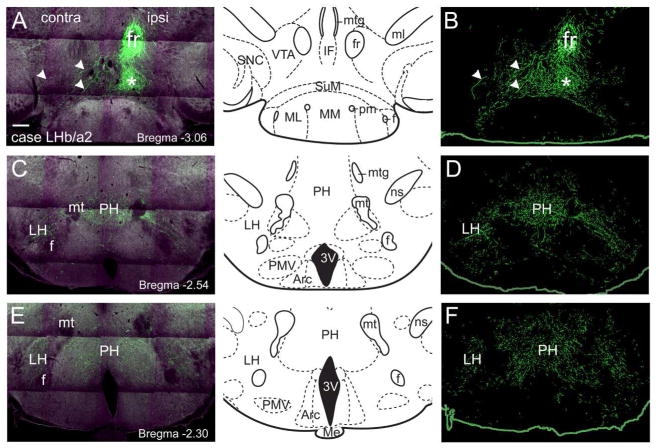
We next examined the detailed projections of LHb efferents to each of the main target areas in the hypothalamus, midbrain tegmentum, and pons. Projections to the hypothalamic area were much greater in LHb/a2 than in LHb/a1 (Figure 2). In LHb/a2, a bundle of fibers exited the FR prior to reaching the interpeduncular nucleus (IP), and turned rostrally toward the diencephalon (Figure 2A,B). Some of these fibers could be seen to cross the midline near bregma -3.0 (Figure 2B), and consistent with this, labeling of the lateral hypothalamus (LH) both ipsilateral and contralateral to the injection was observed. LHb efferent fibers were observed throughout the caudal part of the LH and the posterior hypothalamus (PH, Figure 2C–F). Because these areas were sparsely innervated relative to brainstem areas, the fibers were more easily visualized in the segmentation views (Figure 2B,D,F; Methods).
Figure 2. LHb projections to the hypothalamus.
The LHb was injected with a tract-tracing AAV as described in Figure 1. Only case LHb/a2, labeling the ventromedial LHb, showed significant output to the hypothalamus, and is shown here. Views shown are from 2-photon fluorescence imaging (left) and fiber segmentation analysis (right, Methods), and are shown in caudal to rostral order, progressively farther from their source. (A,B) Labeled fibers in the rostral tegmentum, just caudal to the LH, compared to a standard atlas view at bregma -3.06. Ascending fibers that have separated from the FR and turned rostrally are marked with an asterisk. Decussating fibers are marked with arrowheads. Only rare labeled fibers are observed in the VTA at this level. (C,D) Labeled fibers in the PH, compared to a standard atlas view at bregma -2.54. Labeled fibers are also observed in the LH bilaterally. (E,F) Labeled fibers at the level of the median eminence, compared to a standard atlas view at bregma -2.30. Labeled fibers are observed mainly in PH. Scale: 200μm.
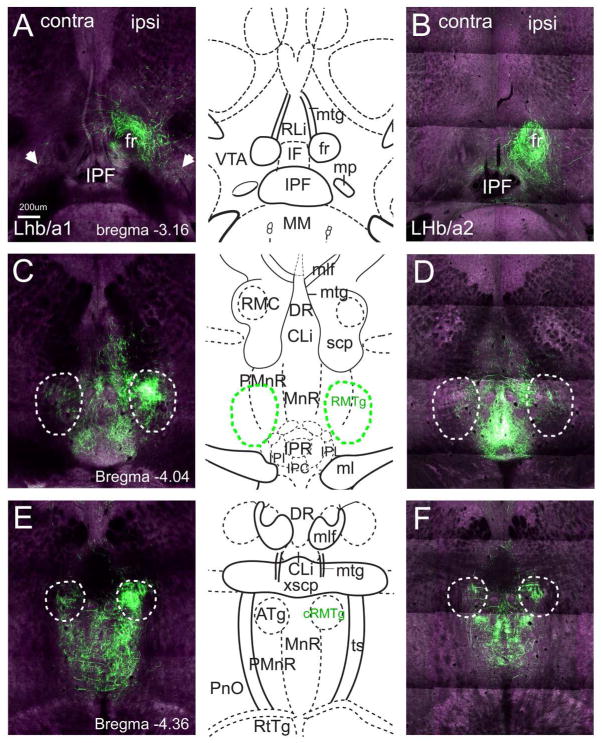
Examination of LHb projections to the ventral midbrain and pons in LHb/a1 and LHb/a2 revealed common and distinct areas of labeling. As expected, both cases sent their caudal projections through the FR, and only sparse labeling was observed in the VTA (Figure 3A,B). Although the RMTg is not identified in standard atlases of the mouse brain (Paxinos and Franklin, 2001; Dong, 2008), a cluster of fibers was observed just dorsolateral to the IP, the location of the LHb-recipient neurons of the rostral part of the RMTg in the rat (Jhou et al., 2009b). The fibers in the area of the RMTg were much more prominent in case LHb/a1 than in LHb/a2, and in LHb/a1 they were predominantly ipsilateral (Figure 3C,D). In case LHb/a2, significant labeling of the central part of the IP was also observed (IPR, IPC, Figure 3D); this was probably due to the viral infection of a small part of the ventral MHb, which is known to project to this area (Hsu et al., 2013). At more caudal tegmental levels, strong fiber labeling was observed in the median raphe (MnR) and paramedian raphe (PMnR) in both cases (Figure 3E,F). Case LHb/a1 also showed strong ipsilateral labeling of a region coinciding with the anterior tegmental nucleus (ATg), as identified in a standard atlas (Paxinos and Franklin, 2001). However, this area appears to be continuous with the RMTg, and like the RMTg, it receives mainly ipsilateral LHb projections (Figure 3E). We also note that in a standard rat atlas, the ATg is not identified at the equivalent rostrocaudal level to the mouse, near the oculomotor (3) nucleus, but instead begins more caudally, posterior to the trochlear (4) nucleus. Thus we hypothesized that the region identified as ATg in the standard atlas is misclassified, and instead shares most of its anatomic features with the RMTg. To address this issue, in the studies below we refer to this region dorsolateral to the MnR that receives strong ipsilateral LHb input as the “caudal RMTg” and the region dorsolateral to the IP nucleus as the “rostral RMTg”. However, this designation should not be taken to imply any functional distinction between these subregions.
Figure 3. LHb projections to the rostral brainstem.
The LHb was injected with a tract-tracing AAV as described in Figure 1. Labeled fibers are shown for case LHb/a1, injected in the dorsolateral LHb, and LHb/a2, injected in the ventromedial LHb. Images here and in Figure 6 are shown in rostral to caudal order, progressively farther from their source. (A,B) Labeled fibers in the descending FR, compared to a standard atlas view at bregma -3.16. Fibers of passage dominate at this level, but sparse terminal fibers can be seen in the VTA bilaterally (arrows, A). (C,D) Labeled fibers in the RMTg, compared to a standard atlas view at bregma -4.04. The anatomical localization of the RMTg is based on prior studies in the rat (Jhou et al., 2009b; Kaufling et al., 2009). The intensity of the specific ipsilateral projection to the RMTg is much stronger in case LHb/a1, injected in the dorsolateral LHb. Although fibers of passage cannot be definitively distinguished from terminal fibers using anterograde tracing with AAV, the dense labeling of the IP in LHb/a2 is likely to represent fibers of passage projecting to more caudal regions (Figures 6,7). (E, F) Labeled fibers in the MnR and PMnR compared to a standard atlas view at bregma -4.36. In case LHb/a1 there is a strong ipsilateral projection to a region annotated as ATg. However, this appears to be a caudal extension of the RMTg (cRMTg). See text for discussion. Scale: 200μm.
The RMTg is defined as a group of tegmental GABAergic neurons that project to the VTA, but reside caudal to most of the DA neurons in this structure. In order to determine whether the caudal RMTg, as defined in Figure 3E,F, contains neurons that meet these criteria, we performed CTB injections into the VTA (Figure 4A), and examined the rostral and caudal RMTg areas for retrograde labeling. In order to easily identify GABAergic neurons, injections were performed in mice expressing a GFP marker targeted to the Gad1 gene locus (Gad1GFP, Tamamaki et al., 2003).
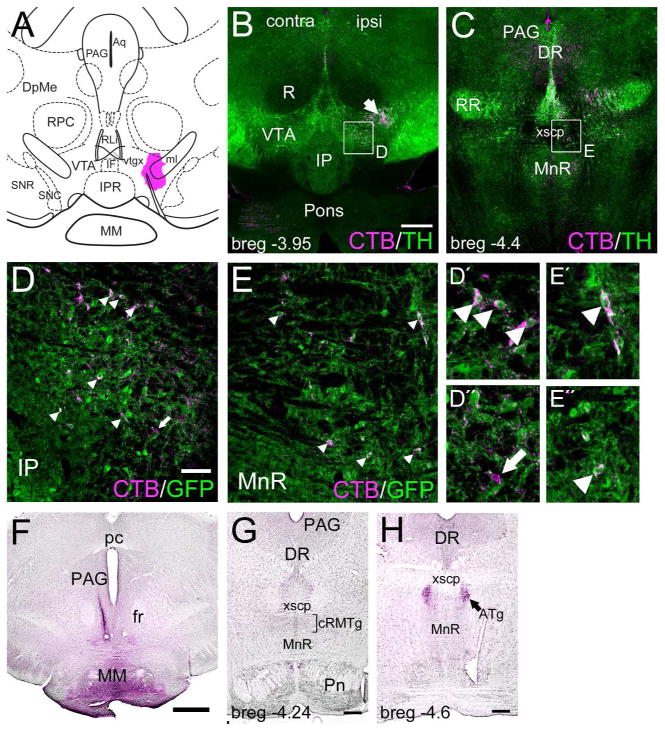
Figure 4. Identification of RMTg neurons in the pontine tegmentum.
Retrograde labeling using CTB was used to identify VTA-projecting neurons in the rostral and caudal parts of the RMTg. The injection was performed in Gad1GFP transgenic mice to facilitate detection of GABAergic neurons. (A) Location of a focal injection of CTB in the VTA. (B) Low-power image of the region containing the rostral RMTg at bregma -3.95. Arrow indicates an area of labeling contiguous with the injection site. Boxed area is enlarged in (D). (C) Low-power image of the region containing the caudal RMTg at bregma -4.4. Boxed area is enlarged in (E). (D) Confocal image of the area boxed in (B), including the rostral RMTg, showing immunofluorescent staining for CTB and GFP. The image is a Z-stack of four adjacent 1μm optical sections and is thus unlikely to include superimposed cells from different levels in the Z-dimension. Gad1GFP-label is expressed in GABAergic cell somata and diffusely in the neuropil. Gad1-negative cells appear as dark “holes”. Examples of CTB-labeled Gad1GFP-expressing cells are marked with arrowheads (inset view D′). A single CTB-labeled Gad1GFP-negative cell is marked with an arrow (inset view D″). (E) Confocal image of the area boxed in (C), including the CTB-labeled cells in the caudal RMTg, an area identified as ATg in standard atlases. Examples of CTB-labeled Gad1GFP-expressing cells are marked with arrowheads (inset views E′, E″). The image is a Z-stack of six 1μm optical sections. (F) Location of CTB injection in the mammillary body near bregma -2.70. (G) Immunostaining of case shown in (F) sectioned at bregma -4.24 (standard atlas coordinate) shows little retrograde transport of CTB in the area of the caudal RMTg (bracket). (H) Case shown in (F) sectioned at bregma -4.60 (standard atlas coordinate) shows retrograde transport of CTB in the ATg (arrow). Scale: B,C,F, 400μm; D,E, 50μm; G,H, 200μm.
As expected, retrograde transport of CTB labeled a population of neurons in the rostral RMTg, just lateral to the IP, and most of the labeled cells expressed Gad1GFP (Figure 4B,D). Retrograde labeling with CTB was also observed in the area we have designated the caudal RMTg, at the dorsal border of the MnR/PMnR at bregma -4.4 (Figure 4C), in the area designated ATg in a standard atlas (Figure 3E). CTB-labeled neurons in this area also expressed expressed Gad1GFP (Figure 4E). It is clear from case LHb/a1 that this area also receives strong LHb fiber input, and thus shares efferent connectivity, afferent connectivity, and neurotransmitter phenotype with the rostral RMTg. The most caudal extent of GABAergic neurons in this area that were retrogradely labeled from the VTA was approximately bregma -4.5. To further distinguish the caudal RMTg from ATg, we injected CTB into a rostral midline portion of the mammillary bodies (Figure 4F), a target of ATg efferents (T.J. unpublished observations). Retrogradely labeled neurons were observed in a bilateral cluster just caudal to the level of the trochlear nucleus, but not at more rostral levels (Figure 4G,H), consistent with the ATg placement in the rat, and significantly caudal to its identification in the standard mouse atlas. Thus we conclude that the mouse RMTg extends along the dorsal margin of the MnR/PMnR to approximately bregma -4.5, and more caudally, this position is occupied by the ATg.
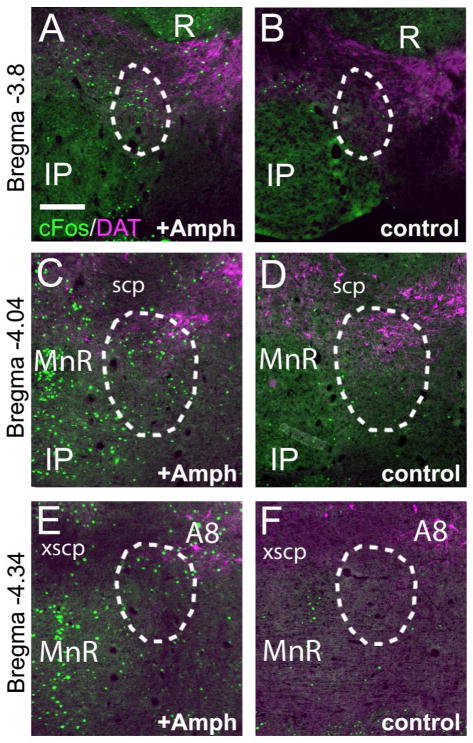
In addition to the receipt of a strong ipsilateral projection from the LHb and efferent projections to the VTA, the RMTg has been defined in the rat by the induction of cFos expression following the acute administration of amphetamine (Amph, Colussi-Mas et al., 2007; Jhou et al., 2009b). In order to determine whether the neurons defined here as the mouse RMTg also share this property, we compared the expression of cFos protein in Amph-treated and saline-injected control mice. The expression of cFos was examined throughout the tegmentum and pons, and as expected was found to be induced by Amph in several nuclei. The area defined as the rostral end of the RMTg (Figure 5A,B), the central part of the nucleus (Figure 5C,D) and the part identified here as the caudal RMTg, but annotated ATg in a standard atlas (Figure 5E,F), all showed significant induction of cFos following Amph treatment relative to saline controls. The results for the counts of cFos immunoreactive cells/section in one hemisphere of the area defined as RMTg based on input LHb projections were as follows: Rostral RMTg (bregma -3.8), Amph 17.3 ± 5.9, control 4.5 ± 3.7, p=0.01; central RMTg (bregma -4.04), Amph 40.5 ± 6.2, control 17.5 ± 14.5, p = 0.03; caudal RMTg (bregma -4.34, assigned as ATg in standard atlas), Amph 23 ± 4.2, control 7.5 ± 2.6, p = 0.0008 (N=4 Amph cases and 4 controls, mean ± SD, unpaired t-test).
Figure 5. Induction of cFos expression in the mouse RMTg by acute administration of amphetamine.
Mice were injected with Amph or saline (N=4 per group), sacrificed after 2 hours, and cFos immunoreactivity was examined throughout the tegmentum and pons. Co-immunostaining for the dopamine transporter (DAT) was performed to localize dopaminergic neurons in the areas examined. (A,B) Expression of cFos at the rostral end of the RMTg. (C,D) Expression of cFos in the central part of the RMTg. (E,F) Expression of cFos in the caudal part of the RMTg, identified as ATg in standard atlases. Scale: A, 200μm.
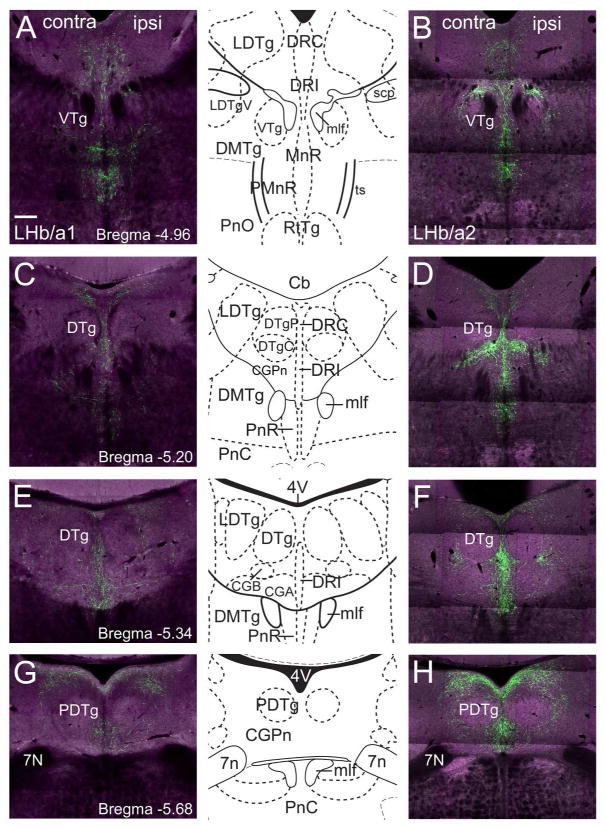
Both anterograde LHb cases also labeled fibers in the dorsal raphe (DR) and dorsal tegmental areas. Fiber labeling in the rostral part of the dorsal raphe was sparse (data not shown), and most projections to the DR terminated in the caudal (DRC) or interfascicular (DRI) parts of the DR (Figure 6A,B). Fibers were densely labeled in midline structures, and more sparsely labeled in the laterodorsal tegmental nuclei (LDTg), but the core of the dorsal tegmental nuclei did not contain labeled fibers (VTg, DTgP, DTgC, Figure 6C–F). The most caudal labeled fibers were observed in the pontine central gray (CGPn), surrounding the posterodorsal tegmental nucleus (PDTg, Figure 6G,H), and no labeled fibers were observed caudal to the 7th nerve. Projections to the DRI and dorsal pontine gray were much greater in LHb/a2, injected in the ventromedial LHb, than in LHb/a1, injected in the dorsal LHb, suggesting partial topographic specificity of the LHb projections to these areas.
Figure 6. LHb projections to the dorsal raphe and dorsal tegmental area.
The LHb was injected with a tract-tracing AAV as described in Figure 1. Labeled fibers are shown for case LHb/a1, injected in the dorsolateral LHb, and LHb/a2, injected in the ventromedial LHb. (A,B) Labeled fibers in the DRC, DRI and MnR, compared to a standard atlas view at bregma -4.96. Caudal to this level, the extent of labeling in LHb/a2 is much greater than in LHb/a1. (C,D) Labeled fibers in the DRC, DRI, and adjacent dorsal pontine central gray, compared to a standard atlas view at bregma -5.20. (E,F) Labeled fibers in the DRI and pontine central grey, compared to a standard atlas view at bregma -5.34. Note that fibers are observed in the pontine central gray medial and lateral to the DTg nuclei, but not in their core. (G,H) Labeled fibers in the pontine central grey at the most caudal extent of the LHb projection, compared to a standard atlas view at bregma -5.68. Scale: 200μm.
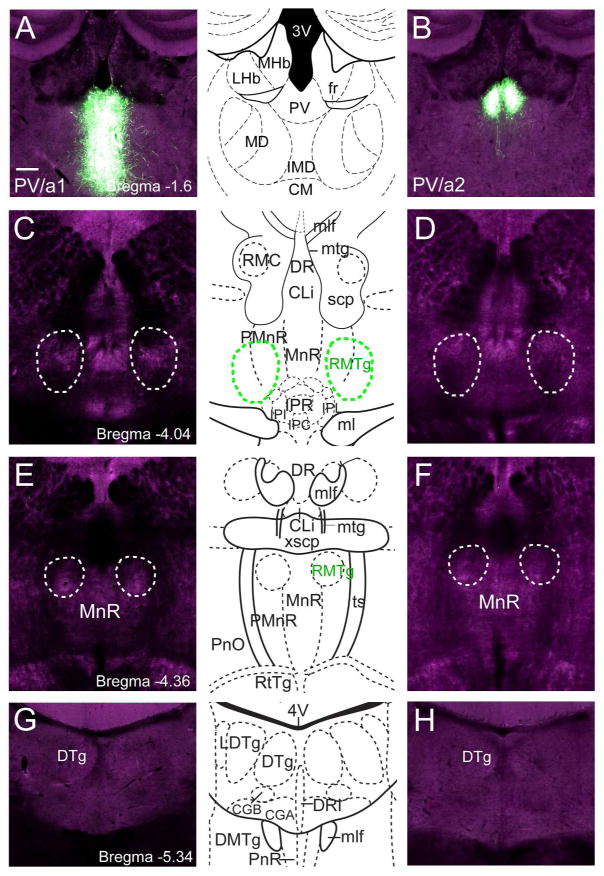
Case LHb/a2 was selected for analysis because it strongly and specifically labeled the ventromedial part of the LHb, but spread of the virus in this case also resulted in labeling of the adjacent PV. For this reason, we considered the possibility that some of the efferent fibers detected in the brainstem in case LHb/a2 might actually originate in the PV. In order to rule this out, we examined two cases in which viral expression was restricted to the PV, sparing the LHb (Figure 7A,B). The expression of the viral marker was restricted to the PV in these cases because the injected animals expressed Cre-recombinase expressed under the regulation of two genes, Ntrk1 and Grm2, which are expressed in the PV but not in the LHb. These mice were injected with a “Flex” virus in which GFP expression is induced by Cre-recombinase (Methods). No labeled fibers were noted in these cases in any of the brainstem targets of LHb innervation, including the FR, RMTg, MnR, or DRI (Figure 7C–H).
Figure 7. Anterograde labeling of paraventricular thalamic nucleus.
Specific labeling of the PV was achieved using two mouse strains Grm2Cre (case PV/a1) and Ntrk1cre (case PV/a2) that express Cre-recombinase in the PV but not in the LHb. No anterograde labeling of fibers was seen in any of the hindbrain areas receiving LHb efferents. (A,B) Injection sites in the PV in case PV/a1 and PV/a2. Grm2Cre activates fairly widespread expression in the medial thalamus. Ntrk1Cre activates restricted expression in the PV. (C,D) Sections at the level of the rostral part of the RMTg, at a level matched to Figure 3C,D. (E,F) Sections at the level of the caudal part of the RMTg, at a level matched to Figure 3E,F. (G,H) Sections at the level of the DTg/DRI, at a level matched to Figure 6E,F.
In the rat, some neurons in the central and lateral LHb project to the corresponding regions of the LHb on the contralateral side, via the habenula commissure (Kim, 2009). No such projections between LHb hemispheres were observed in cases LHb/a1 and LHb/a2 (data not shown, available online at http://connectivity.brain-map.org/) although injections into other regions of mouse LHb do give rise to commissural projections (T.J. unpublished results).
Quantitation of LHb efferents to the hindbrain
Although dense innervation of the RMTg was observed in case LHb/a1, global tracing of LHb projections in cases LHb/a1 and LHb/a2 suggests that the RMTg is only one of several significant targets. In order to quantify the relative magnitude of the LHb projections to brainstem targets, the segmented image files from cases LHb/a1 and LHb/a2 were registered to a 3D reference space integrated with the Allen Reference Atlas (ARA, Methods). For each annotated structure receiving LHb inputs, a pixel count from the segmentation views of the fiber projections were tallied for each hemisphere, and multiplied by the resolution and z-sampling interval to yield a “projection volume” in voxels for the LHb fibers in that structure. This quantitative analysis was only performed for midbrain and pontine structures because no projections were observed in the medulla or cerebellum, and in the diencephalon and forebrain, the incidental viral labeling of neurons outside the LHb (e.g. hippocampus, PVT), prevented an unambiguous assignment of the source of the fibers in those structures. The total segmented volume of the fibers projecting to the midbrain and pons was in each case used as a denominator to calculate the fraction (percentage) of the total brainstem LHb fiber output that projected to each of the ARA annotated areas. The anatomical areas defined in the ARA differ somewhat from standard atlases (Paxinos and Franklin, 2001) and the correspondence is discussed here and in Figure 8.
Four rostral regions examined in the quantitative analysis, including the VTA, the midbrain reticular nucleus (MRN; standard nomenclature, deep mesencephalic nucleus, DpMe), the periaqueductal gray (PAG), and the red nucleus (RN), were adjacent to some part of the FR. Because of this, LHb efferent fibers of passage in the FR were clearly assigned in error to each of these areas by the automated registration algorithm, always on the side ipsilateral to the injection (Figure 8A,C). Quantitative assignment to other regions appeared to be consistent with a visual section-by-section examination of the segmentation views. Automated analysis of fiber density identified the superior central nucleus raphe (CS; standard nomenclature, MnR/PMnR), the pontine central gray (containing in part the DRI, which is not specifically annotated in the ARA), the nucleus incertus (NI, also containing part of the DRI), and the pontine reticular nucleus, rostral part (PRNr, containing in part the ATg), as the predominant areas receiving LHb fiber inputs.
We then independently performed automated analysis of LHb efferent fiber density in the RMTg, which is not identified in the ARA or in any published standard atlas. Because the ARA annotations could not be used directly for the quantitation of fiber density in these areas, we first defined the regions of interest in the three-dimensional ARA space (Figure 9–11). The location of the rostral RMTg was suggested by prior work in the rat, where there are dense, mainly ipsilateral LHb fiber terminals just dorsal and lateral to the IP (Jhou et al., 2009b). The strong labeling of this area in LHb/a1 provided a template for the assignment of the mouse RMTg (Figures 3C, 9), and was confirmed by the induction of cFos expression by amphetamine (Figure 5). The area we designated as the caudal RMTg (Figure 3E), which lies dorsolateral to the MnR, is annotated as the ATg in a standard mouse atlas (Paxinos and Franklin, 2001), but as discussed above this appears to be an incorrect assignment of this structure. Automated quantitation of fiber density was performed for the rostral and caudal RMTg, thus defined, for LHb/a1 and LHb/a2 (Figure 8B,D). Quantitative comparison of cases LHb/a1 and LHb/a2 confirms that the RMTg projections originating in the medial division of the LHb are relatively sparse (i.e. RMTg projections in case LHb/a1, Figure 8B, are greater than in LHb/a2, Figure 8D), and also that the LHb input to the RMTg is predominantly ipsilateral. Normalization of the fiber volume in the RMTg to the total fiber volume in the midbrain+pons showed that 7.5% of total fibers mapped to the rostral RMTg and 10.7% to the caudal RMTg in case LHb/a1 (total fraction of LHb hindbrain efferents: 18.2%), and 3.1% of total fibers mapped to the rostral RMTg and 4.5% to the caudal RMTg in case LHb/a2 (total fraction of LHb hindbrain efferents, 7.6%). Thus we conclude that while the functional significance of the LHb-RMTg-VTA pathway is well established, the RMTg is but one of several significant targets, with no single region receiving more than about 25% of the LHb efferents.
Although the tools used in the automated analysis are not capable of distinguishing terminal fibers from fibers of passage quantitatively, close examination of each of the major areas receiving LHb efferents using conventional methods showed varicosities consistent with terminal fibers. Further indirect evidence for terminal fibers in each of the areas receiving LHb inputs is provided by the efficient retrograde transport of iontophoretically injected CTB from each of these areas, as described below. Tests of CTB injected iontophoretically to minimize fiber damage have shown minimal labeling of fibers of passage (Luppi et al., 1990), implying that fiber terminals in each of these areas account for the CTB uptake.
The major regions innervated by the LHb contain axon terminals
The two anterograde tracing cases examined for quantitative analysis by two-photon microscopy (LHb/a1 and LHb/a2) contained focal injections with partial coverage of the LHb, and could in principle fail to identify some targets of LHb efferents. In addition, the two-photon imaging platform used for quantitative analysis was not ideal for distinguishing axon fiber terminals from fibers of passage in the areas innervated by the LHb. For these reasons we also examined LHb projections in a case in which viral expression of the tracer spread throughout the LHb at a central position along its rostrocaudal axis (case LHb/a3, Figure 12,13), and examined the target fields by conventional confocal fluorescence microscopy. More extensive labeling of the LHb did not reveal any additional areas of LHb innervation beyond those identified in cases LHb/a1 and LHb/a2, and labeling in this case appeared to be a composite of the two focally labeled cases. LHb axon terminals in the VTA are well characterized in the rat (Omelchenko et al., 2009), and were not examined here. To determine whether the other areas receiving LHb inputs contain terminal fibers we performed confocal imaging of each of the major LHb target areas, including the LH (Figure 12C,D), the central RMTg (Figure 12E,F), the caudal RMTg (Figure 12G,H), the MnR/PMnR (Figure 13A,B), the DRC (Figure 13C,D), the pontine raphe (Figure 13E,F) and the caudal dorsal tegmentum (Figure 13G,H). Axon fibers in each of these areas exhibited a beaded appearance consistent with terminal fibers, also observed in terminal fibers in the LHb projection to the VTA in the rat (Omelchenko et al., 2009).
Figure 12. LHb efferent terminal fibers in the hypothalamus and rostral tegmentum.
The LHb was injected with an AAV virus encoding a YFP tracer (Methods, case LHb/a3), and YFP-labeled fibers were examined in the tegmentum and pons. (A) Extent of YFP expression in the central LHb, encompassing both the medial and lateral divisions. (B) Extent of YFP expression in the caudal LHb. (C,D) LHb projections to the hypothalamus. Fibers showing a beaded morphology, indicative of terminal fibers, can be seen in the enlargement of the boxed area shown in D (40x, confocal image). (E,F) LHb projections to the central part of the RMTg. Short fibers with punctate areas of intense fluorescence suggestive of fiber terminals characterize this area (F). (G,H) LHb projections to the caudal part of the RMTg. Fibers in this area frequently show a beaded morphology indicative of terminals. Scale: A, C 100μm; D, 25μm.
Figure 13. LHb efferent terminal fibers in the MnR and DTg.
Continuation of case LHb/a3 shown in Figure 12. (A,B) LHb projections to the MnR/PMnR. Fibers showing a beaded morphology, indicative of terminal fibers, can be seen in the enlargement of the boxed area shown in B (40x, confocal image). (C,D) LHb projections to the DRC. (E,F) LHb projections to the pontine raphe. (G,H) LHb projections to the area surrounding the PDTg. Scale: A, 100μm; B, 25μm.
Topographic organization of LHb projections to the brainstem
Anterograde tracing from the dorsolateral LHb (LHb/a1) and the ventromedial LHb (LHb/a2) suggest that these regions of the LHb project preferentially to different brainstem areas. However, these AAV anterograde experiments do not have the spatial resolution to test this definitively. For this reason we also performed retrograde tracing from all of the major regions innervated by the LHb. In each case, markers for specific neurotransmitter systems, including dopaminergic (DA, marked by expression of tyrosine hydroxylase, TH), serotonergic (5HT, marked by tryptophan hydroxylase 2, Tph2) and cholinergic (marked by choline acetyltransferase, ChAT), were used to functionally define the injected areas. Large areas of interest were labeled using pressure injection of cholera toxin, subunit b (CTB) and small areas of interest were labeled using iontophoretic injection of this marker (Methods). In each case the habenula was sectioned in the coronal plane, and the first appearance of the medial habenula (bregma -0.9) and the habenula commissure (bregma -2.3) were used as rostral and caudal landmarks, respectively, for the alignment of the sections to a standard atlas (Paxinos and Franklin, 2001). CTB labeling of LHb neurons at rostral (bregma -1.4), central (bregma -1.6), and caudal (bregma -1.9) levels was examined in detail. The mouse brain coronal level bregma -1.6 is approximately equivalent to the rat axial level bregma -3.2, which has been used as a reference level for the definition of rat lateral habenula subnuclei (Geisler et al., 2003). Habenula sections were co-stained for CTB and Pou4f1 (Brn3a), a transcription factor that is expressed in numerous LHb neurons that are distributed throughout the caudal two-thirds of the ganglion, and thus allowing the borders of the LHb to be visualized at these levels.
To assess LHb efferents projecting to the hypothalamus, focal injections of CTB were placed in the PH (Figure 14A) and LH (Figure 14B). Both CTB injections labeled numerous LHb neurons, confirming that the fiber projections to the hypothalamus observed in case LHb/a2 originate in the the LHb, and not in adjacent thalamic nuclei also infected with AAV (Figure 1D). Both PH and LH injections labeled numerous neurons in the rostral LHb (Figure 14C,F). At a central and caudal levels of the LHb, neurons retrogradely labeled from the PH were confined largely to the medial division of the nucleus (Figure 14D,E). LHb neurons labeled from the LH were more widely distributed, in both the medial and lateral divisions (Figure 14G,J). However, we note that the caudal border of the LH injection is adjacent to the VTA, and we cannot rule out that some of the transported CTB originates in the VTA, not the LH (see Figure 15 for VTA injection). In contrast, the PH injection is not adjacent to the VTA, and represents labeling of LHb neurons projecting exclusively to the hypothalamus. We conclude that the projections of the LHb to the hypothalamus originate predominantly in the rostral and medial parts of the nucleus, adjacent to the MHb. In order to address whether the expression of Pou4f1 identifies a subset of LHb neurons with a specific projection pattern, we also examined the co-localization of the CTB retrogradely transported from the LH with the expression of Pou4f1 in the LHb using confocal microscopy (Figure 14H,I). Very few of the LH-projecting LHb neurons express Pou4f1.
Figure 14. Retrograde tracing of the LHb-hypothalamic pathway.
LHb projections to the tegmentum were traced by the focal injection of CTB. Sections are counterstained for Pou4f1(Brn3a) which is expressed in the nuclei of neurons distributed through the caudal 2/3 of the LHb. In the rostral LHb, where Pou4f1-expressing neurons are sparse, the lateral border of the LHb is traced with a dashed line. (A,B) Locations of small focal injections of CTB in the PH (standard atlas bregma -2.54) and LH (standard atlas bregma -2-3), respectively. (C–E) Location of neurons labeled from the PH in the rostral, central and caudal LHb, respectively. In the central LHb, labeled neurons were largely confined to the medial division of the nucleus. (F,G) Location of neurons labeled from the LH in the rostral and central LHb, respectively. The area labeled ATg in a standard atlas corresponds to the caudal RMTg (cRMTg, see text). (H,I) Confocal imaging of CTB-labeled neurons in (G). Rare examples of neurons that show co-localization of CTB and Pou4f1 are indicated by arrowheads. (J) Location of neurons labeled from the LH in the caudal LHb. Scale: C, F 100μm; H, 50μm.
Figure 15. Retrograde tracing of LHb projections to the VTA and RMTg.
LHb projections to the tegmentum were traced by the focal injection of CTB and counterstained for Pou4f1 as described in Figure 14. (A,B) Location of a small focal injection of CTB in the VTA, compared to a standard atlas view at bregma -3.64. DA neurons in the injected area are labeled for tyrosine hydroxylase (TH) immunoreactivity. (C,D) Location of neurons labeled from the VTA in the central and caudal LHb; few labeled cells were seen in rostral part of the LHb (not shown). (E) Confocal image of CTB and Pou4f1 expression in the area boxed in (D). In the central LHb bilaterally, 15/32 nuclei of CTB-labeled neurons were Pou4f1+. (F,G) Location of a large injection of CTB in the RMTg, overlapping the adjacent MnR/PMnR, compared to a standard atlas view at bregma -4.04. DA neurons are not present in the area of injection. (H–J) Location of neurons labeled from the RMTg in the rostral, central, and caudal LHb. Scale: B, G 400μm; C, H 100μm; E, 50μm.
Focal injections of CTB were also used to examine LHb projections to the VTA and RMTg (Figure 15). The VTA contains DA neurons of the mesolimbic dopamine system, and the RMTg contains GABAergic neurons that project to the VTA. However, the border between the VTA and RMTg is not well defined. Indeed, the VTA contains intermingled GABAergic neurons (Omelchenko et al., 2009) and the RMTg has also been called the “GABAergic tail of the VTA” (Kaufling et al., 2009). The locations of the CTB injection sites in the tegmentum were examined by counterstaining for TH immunoreactivity to identify DA neurons. The VTA injection shown here was surrounded by DA neurons (Figure 15A,B), while the center of the RMTg injection was in a more caudal area devoid of DA neurons (Figure 15F,G) but may have slightly overlapped the DA region. Injections of the RMTg also unavoidably overlapped areas designated as the PMnR/MnR in standard atlases, since the RMTg itself is not defined in these references. Thus these cases are not absolutely specific for the VTA and RMTg, and should be viewed as tegmental injections labeling LHb neurons that skew toward projecting to either DA or non-DA areas of the VTA/RMTg system.
Retrograde labeling from the VTA labeled a relatively small number of LHb neurons, and these were located predominantly in the lateral division of the caudal half of the LHb (Figure 15C,D). The small number of labeled cells may be due in part to the small size of the injected area, but is also consistent with the observation that projections to the VTA are quite sparse in the anterograde tracing cases (data not shown; available online at http://connectivity.brain-map.org/). Nearly all LHb neurons retrogradely labeled from the VTA also expressed Pou4f1 (Figure 15E). CTB injections in the RMTg and adjacent structures (Figure 15F,G) labeled a larger fraction of LHb neurons than injections in the VTA (albeit from a larger injected area). Unlike the VTA case, injection of the RMTg produced significant labeling of the rostral LHb (Figure 15H). At central and caudal levels, labeling predominated in the lateral LHb and was sparse in the ventromedial area (Figure 15I,J), similar to the VTA case. Consistent with the lateralization of the LHb projections to the VTA and RMTg in the anterograde labeling case LHb/a1 (Figure 3), many more LHb neurons were labeled ipsilateral to the RMTg CTB injection than on the contralateral side.
In contrast to the cases injected in the VTA/RMTg, injection of CTB into the MnR resulted in strong labeling of the medial LHb (Figure 16). Labeling in the rostral LHb was seen mainly in the ventromedial area (Figure 16C). This is not surprising, because the dorsal LHb at this level is comprised mostly of incoming tracts of the striae medularis, and contains few cell bodies to label. In the central and caudal LHb, frequent labeling was observed in the most dorsal part of the nucleus, adjacent to the habenula commissure, as well as the ventromedial LHb (Figure 16D,E). An injection overlapping the MnR, centered predominantly in the area identified in a standard atlas as the ATg (Figure 16F,G) but identified here as the caudal RMTg, gave a composite pattern of labeling. Labeling of the medial LHb in this case resembled the MnR injection (rostral, Figure 16H, and bilateral at more caudal levels, Figure 16I,J), while labeling of the lateral LHb resembled the RMTg injection (predominantly ipsilateral, Figure 16I,J). It appears likely that the injection in this area has labeled a mixture of LHb neurons with MnR-like and RMTg-like projection patterns. In a case with a small injection in the PMnR (Figure 17A), which did not overlap the area of serotonergic MnR neurons (Figure 17B), scattered labeling was observed with a bias toward the medial LHb (Figure 17C).
Figure 16. Retrograde tracing of LHb projections to the median raphe and anterior tegmental nucleus.
LHb projections to the tegmentum were traced by the focal injection of CTB and counterstained for Pou4f1 as described in Figure 14. (A,B) Location of a large injection of CTB in the MnR, compared to a standard atlas view at bregma -4.36. (C–E) Location of neurons labeled from the MnR in the rostral, central, and caudal LHb. (F,G) Location of a small focal injection of CTB in the MnR plus the area identified as ATg in a standard atlas, here designated the “caudal RMTg” (Figure 3E, 10, see text), compared to a standard atlas view at bregma -4.16. (H–J) Location of neurons labeled from the caudal RMTg/ATg in the rostral, central and caudal LHb. Arrowheads in (I,J) indicate labeled neurons in a dorsomedial position that are not evident in VTA/RMTg-injected cases and may be attributable to spread of the label into the dorsal MnR. Scale: B, 400μm; C,H 100μm; G, 200μm.
Figure 17. Retrograde tracing of LHb projections to the paramedian raphe and rostral dorsal tegmentum.
LHb projections to the tegmentum were traced by the focal injection of CTB and counterstained for Pou4f1 as described in Figure 14. (A) Location of a small CTB injection in the PMnR, corresponding to bregma -4.96 in a standard atlas. (B) Relationship of the injected area to 5HT neurons of the MnR, marked by immunofluorescence for Tph2. (C) Retrograde labeling of LHb neurons in the central LHb. Labeled cells are seen mainly in the medial division of the LHb. Few labeled neurons were seen at rostral levels in this case (not shown). (D) Retrograde labeling of LHb neurons in the caudal LHb. (E) Location of a large CTB injection in the rostral part of the dorsal tegmentum (primarily LDTg), corresponding to bregma -5.02 in a standard atlas. (F–H) Retrograde labeling of neurons in the rostral, central, and caudal LHb, respectively. Scale: B,C,F 100μm.
To assess LHb projections to the dorsal tegmentum, we first placed a large injection in the rostral part of the dorsal tegmental nucleus (primarily LDTg, Figure 17D). Retrogradely labeled neurons were concentrated mainly in the rostral LHb (Figure 17E) and the medial division of the LHb at central and caudal levels (Figure 17F,G). LHb input fibers are rather sparse in this region, and correspondingly, the number of labeled LHb cell bodies were relatively few considering the extent of the injected area.
The most caudal LHb projections observed in the anterograde labeling cases densely innervate the dorsal pons, including the LDTg, the caudal and interfascicular (inferior) parts of the dorsal raphe (DRC, DRI), and the pontine central gray (CGPn) surrounding the dorsal tegmental nucleus (DTg). Anterograde tracing shows that LHb fibers innervate mainly the midline, and areas surrounding the DTg nuclei, rather than the nuclei themselves (Figure 6). Focal injection of CTB at the midline of the dorsal pons (Figure 18A), in an area populated by 5HT neurons of the DRI, labeled LHb neurons largely confined to the medial division of the nucleus. Although expression of Pou4f1 is relatively weak in the ventromedial LHb, the majority of DTg-projecting neurons were Pou4f1+ (Figure 18E). A larger CTB injection in the LDTg (Figure 18F), in an area containing cholinergic neurons (Figure 18G), produced more extensive labeling of the LHb. LDTg-projecting neurons included a significant number in the rostral LHb (Figure 18H), and the labeled cells were found primarily in the ventromedial part of the nucleus at central and caudal levels (Figure 18I,J).
Figure 18. Retrograde tracing of LHb projections to the dorsal tegmentum and caudal dorsal raphe.
The fiber inputs to this area can be seen in Figure 6E–H. (A) Location of a CTB injection in the dorsal pontine gray/DRI at bregma -5.3. (B) Relationship of the injection in (A) to 5HT neurons in the DRI. (C,D) Location of neurons labeled from the DRI in the central and caudal LHb. Nearly all labeled neurons are in the medial division of the LHb. Few labeled neurons were seen at rostral levels in this case (not shown). (E) Confocal image of inset area shown in (D). Some fibers of passage labeled in this section (examples indicated by arrowheads) are not counted as CTB-labeled neurons. (F) Location of a CTB injection in the dorsal pontine gray, near the most caudal extent of LHb projections, at bregma -5.6. (G) Relationship of the CTB injection in (F) to cholinergic neurons in the LDTg at bregma -5.3, slightly rostral to the center of the injection shown in (F). (H-J) Location of neurons labeled from the LDTg in the rostral, central and caudal LHb. Scale: B,C,E,G 100μm.
Taken together, these retrograde tracing results suggest a topographic organization of the LHb and its projections to the tegmentum/pons. In order to better compare the LHb neurons projecting to specific nuclei, we mapped neurons labeled by the injection of CTB in each LHb target region on a standard atlas map of the central LHb (Figure 19). Only neurons labeled completely enough to show a full or partial profile of the cell nucleus were mapped. Retrograde labeling from the LH marked a band of neurons across the middle of the LHb, in both the medial and lateral division (Figure 19A). Retrograde labeling patterns from the VTA and RMTg were similar, predominantly in the lateral part of the LHb (Figure 19B,C). However, the projection to the RMTg originates mainly ipsilaterally, while the projection to the VTA is less lateralized. In contrast, retrograde labeling from the MnR labeled neurons predominantly in the medial LHb (Figure 19D). Retrograde labeling from the caudal RMTg (i.e. the reassigned “ATg”) labeled the LHb widely (Figure 19E), in a pattern resembling a composite of the RMTg- and MnR-labeled cases. However, it is notable that this injection site spills over slightly into the adjacent caudal PMnR which also receives LHb projections. Note that if the dorsomedial LHb is excluded, retrograde labeling from the caudal RMTg has an ipsilateral bias similar to that observed for the rostral RMTg (34 contralateral, 72 ipsilateral). Taken together with the strong ipsilateral projections to the rostral and caudal RMTg observed in the anterograde tract-tracing experiments (Figure 3), we conclude that the large caudal RMTg injection has probably labeled two components: a mainly ipsilaterally projecting group of cells in the lateral LHb, resembling the RMTg projection, and a bilaterally projecting group of neurons in the medial LHb, resembling the MnR/PMnR projection. Consistent with this idea, a small focal injection in the caudal PMnR labeled cells distributed throughout the medial division (Figure 19F), without a lateral bias.
Figure 19. Topography of LHb projections to brainstem targets.
The location of LHb neurons retrogradely labeled from each of the stated structures is shown. Only cell bodies showing a partial or complete nuclear outline were recorded. All maps are taken from the corresponding views of the central LHb (bregma -1.6 to -1.7) in Figures 15–18. Maps for midline injections show only a composite of both hemispheres. (A) Location of LHb neurons projecting to the LH. (B) LHb neurons projecting to VTA (derived from Figure 15C). (C) LHb neurons projecting to RMTg (Figure 15I). (D) LHb neurons projecting to the MnR (Figure 16D). (E) LHb neurons projecting to the caudal RMTg (Figure 16I). (F) LHb neurons projecting to the caudal PMnR (Figure 17C). (G) LHb neurons projecting to the CGPn/DRI (Figure 18C). (H) LHb neurons projecting to the LDTg (Figure 18I). (I) Subdomains of the central LHb defined by projections to specific targets. (J–M) Overlay of LHb neurons projecting to RMTg with those projecting to other areas.
In contrast, retrograde labeling from the CGPn/DRI predominantly labeled neurons in the medial LHb (Figure 19G), and labeling from the LDTg identified cells confined to the ventromedial LHb (Figure 19H), which were not strongly lateralized. These specific subnuclear labeling patterns suggest that the central LHb can be functionally divided into at least three domains, dorsomedial, ventromedial, and lateral, based on projection patterns (Figure 19I). These domains can be better visualized using overlays of labeled neurons from each of the major hindbrain regions receiving LHb projections (Figure 19J–M). Neurons projecting to the CGPn/DRI and LDTg define medial and ventromedial domains which have little overlap with neurons projecting to the VTA or RMTg from the lateral LHb. Neurons labeled from the MnR and ATg are more widely distributed, and overlap the distribution of those projecting to the RMTg. However, MnR/ATg injections label the dorsomedial LHb, while RMTg injections do not. Finally, both anterograde and retrograde tracing support a strong ipsilateral preference for the RMTg and caudal RMTg projections, while the projections to all other areas appear to be less lateralized.
Discussion
Although the majority of anatomical and functional studies of the habenula to date have been performed in the rat, mice offer experimental advantages for several types of functional studies. These include detailed, global gene expression data in the mouse, the availability of genetic models that affect habenula development or function (Quina et al., 2009), the availability of Cre-recombinase driver lines that allow cell-specific gene expression in many neural types, including the habenula (Hsu et al., 2013), and the availability of direct and inducible optogenetic systems for studies of neural function (Ren et al., 2011; Madisen et al., 2012). The efferent pathway of the MHb is relatively well-defined in both rat and mouse, with known outputs primarily from the dorsal MHb to the lateral part of the IP and from the ventral MHb to the central part of this nucleus (Kawaja et al., 1991; Kim, 2009; Quina et al., 2009). Here we have focused on analysis of the efferent projections of the LHb, which are more complex, and less well-defined. Although the MHb and LHb efferents share a common output tract, the fasciculus retroflexus, there appears to be little overlap in the areas they innervate and there is no reason to suppose they have the same function.
Early anatomical studies in the rat, using traditional tract-tracing methods, first identified the descending pathways from the LHb to the hypothalamus, midbrain tegmentum, midbrain/pontine raphe, and dorsal pontine central gray (Aghajanian and Wang, 1977; Herkenham and Nauta, 1979; Sutherland, 1982; Araki et al., 1988). The principal LHb outputs described here in the mouse are globally concordant with those defined in prior rat studies. However, here we have used brain-wide connectomics to place the LHb efferents on a standard coordinate system, which facilitates the use of this map as a template for functional studies. Global, quantitative analysis of the entire LHb output system on an automated analytical platform has also allowed us to place relative weights on LHb outputs to various brain regions. These methods confirm that the best understood LHb output system, the LHb-RMTg pathway, exhibits a high density of LHb efferent fibers. It also shows that the RMTg, defined by multiple criteria, extends more caudally that would be supposed from standard anatomical atlases. However, this pathway accounts for less than 20% of total LHb output to the hindbrain.
Topographic organization of LHb efferents
More recent anterograde and retrograde tracing studies of the rat LHb system support a topographic organization of the rat LHb that is substantially similar to that presented here for the mouse, with medial LHb neurons projecting primarily to the MnR and dorsal tegmentum/pons, and a predominance of lateral LHb neurons projecting to the VTA and RMTg. In one recent study of LHb projections in the rat (Kim, 2009), anterograde tracing from the lateral LHb showed a strong ipsilateral projection to the pontine tegmentum (an area subsequently identified as containing the RMTg), in a pattern strongly resembling Figures 3C,E shown here. Injections into central and medial LHb of the rat showed strong bilateral projections to the MnR, caudal DR (DRC/DRI), and pontine central gray surrounding the DTg, in a pattern very similar to Figure 6B,D,F,H.
Similar results were obtained in another study of the rat LHb (Goncalves et al., 2012), which first specifically mapped the position of the RMTg using retrograde tracing from the VTA, then analyzed for LHb projections to this and other target areas. Using anterograde tracing, these investigators showed that injections in the lateral LHb produced a strong ipsilateral projection to the RMTg, while injections in the medial LHb produced strong projections to the MnR and DRC, confirming a pattern similar to that shown here for the mouse (Figures 3 and 6). Retrograde tracing of LHb projections to the rat RMTg showed that these projections originate preferentially in the lateral LHb, again in agreement with our results (Figures 15I, 19B). While these investigators note that in the rat, retrograde labeling from the VTA primarily labeled neurons in the medial and caudal LHb, that study only examined projections to relatively restricted parts of VTA. In the present study, we also observed some neurons in the medial part of the caudal LHb that are retrogradely labeled from the tegmentum (Figure 15D,E,J). Yet when compared at equivalent rostrocaudal levels, LHb neurons labeled from the VTA and RMTg did not show a markedly different pattern (Figure 19B,C). We conclude that in the mouse, LHb neurons projecting to the VTA and RMTg do not have a strongly distinctive distribution. We also note that in the mouse, our anterograde and retrograde tracing experiments show that the LHb projections to the region of the VTA containing DA cell bodies, while present, are much sparser than the projections to the RMTg or MnR.
Another retrograde labeling study of the rat LHb used tracers with contrasting fluorescent labels to simultaneously label LHb neurons projecting to the VTA, and either the MnR or DR (Bernard and Veh, 2012). Whether the VTA injections in this study overlapped the RMTg was not specifically discussed. As in the present study, LHb neurons projecting to the VTA and MnR were widely distributed in the LHb. In sections centrally located along the rostrocaudal axis (rat coordinate bregma -3.2, approximately equivalent to mouse bregma -1.6), VTA-projecting neurons were somewhat preferentially found in the lateral division of the LHb, and MnR-projecting neurons in the medial division, generally consistent with results reported here. LHb neurons were rarely double labeled with the tracers injected into different areas, indicating that although intermingled, distinct populations of LHb neurons project to the RMTg and the MnR or DR.
The caudal extent of the RMTg in the mouse
Here, using AAV-mediated viral tract-tracing, we have identified a strong ipsilateral projection from the LHb that clearly innervates the RMTg/tVTA, as identified in the rat by tract tracing and psychostimulant induction of immediate early gene expression (Jhou et al., 2009b; Kaufling et al., 2009). This ipsilateral projection extends caudally to approximately -4.5mm relative to bregma (Figure 11), extending into an area just dorsal to the MnR/PMnR that is designated ATg in a standard mouse atlas (Paxinos and Franklin, 2001), but which we here referred to here as the caudal part of the RMTg. Caudal to this level, the LHb projections are distributed bilaterally, resembling the pattern seen throughout the MnR. Several lines of evidence suggest that this area is a functional continuation of the RMTg. First, we note that standard atlases annotate the mouse ATg at a significantly more rostral position relative to other structures in the mouse compared to the rat (Paxinos and Franklin, 2001; Paxinos and Watson, 2005). This may be an error in the assignment of this structure in the standard mouse atlas. Second, in the rat, neurons showing amphetamine- and cocaine-induced fos labeling, combined with retrograde labeling from the VTA (characteristics of the RMTg), are seen in the dorsal pons at this level identified as “ATg” in the mouse but not the rat (Colussi-Mas et al., 2007; Geisler et al., 2008). One study, using retrograde and anterograde tracing in the rat, has explicitly identified this area (near rat bregma -7.56, approximately equivalent to mouse bregma -4.5) as part of the rat RMTg (Goncalves et al., 2012). Finally, here we show that GABAergic neurons in this area projecting to the VTA extend caudally to about bregma -4.5, while neurons caudal to this level project to the mammillary bodies, consistent with an ATg identity. Thus we conclude that the mouse RMTg consists of a continuous column of neurons running rostrocaudally from the DA cell region of the VTA to approximately bregma -4.5.
Subnuclear structure of the mouse LHb
Medial and lateral divisions of the LHb were recognized in early studies of the rat (Herkenham and Nauta, 1979). Subsequently, ultrastructural (Andres et al., 1999) and immunohistological (Geisler et al., 2003) studies in the rat have defined as many as four medial and five lateral LHb subnuclei evident at a central position along the rostrocaudal axis (rat bregma -3.2, mouse bregma -1.6), and a correlated subnuclear structure has been described in the mouse (Wagner et al., 2014). The tract-tracing studies reported here clearly support the existence of medial and lateral divisions of the central part of the mouse LHb, based on their topographically organized projections to the tegmentum, raphe, and pontine gray. Our retrograde labeling data also support division of the medial LHb into dorsal and ventral subdivisions, in that retrograde labeling from the LDTg labels only neurons in the ventromedial LHb (Figure 19H). However, retrograde tracing from specific hindbrain targets does not support the division of the central LHb into smaller subnuclei. In studies of the rat, the populations of neurons identified by retrograde labeling studies from specific LHb targets also frequently cross the subnuclear boundaries defined by cytochemistry (Bernard and Veh, 2012; Goncalves et al., 2012), and it has also been shown that LHb neurons in these anatomical subnuclei do not have distinctive electrophysiological properties (Weiss and Veh, 2011). In cases that produced significant labeling in the rostral 1/3 of the habenula (e.g. Figures 14C, 16H), the distribution of labeled cells does not suggest any clear subdivisions of the LHb, consistent with the reported lack of subnuclear structure at this level in the rat (Geisler et al., 2003). In practical terms, future experiments that address LHb function in the mouse will need to consider the major subnuclei of the LHb identified here in their design. For instance, viral injections to express effectors such as neurotransmitters, receptors, or optogenetic tools in the LHb will need to take into account the topographic map of the LHb onto its targets, in order to label specific LHb pathways.
Although recent progress has been made toward understanding the influence of the LHb on DA reward systems, the novel quantitative analysis of LHb efferents presented here, in which the RMTg is only one of several major recipients of LHb efferents, suggests that much remains to be learned about the functional pathways downstream of the LHb. Specifically, little is known about LHb signaling to the hypothalamus, MnR/PMnR, DRC/DRI and pontine central grey, which together receive the majority of the LHb efferent fibers. One recent study in the rat has implicated a circuit from the diagonal band of Broca, via the medial subnucleus of the LHb, to the raphe, in the regulation of theta oscillation in sleep (Aizawa et al., 2013). The LHb projections to the raphe may also be particularly relevant to behavioral responses to noxious stimuli. Stimulation of the LHb impairs the learning of active avoidance of electrical shock (Shumake et al., 2010), possibly by opposing the negative reinforcement received from successful escape. Increased excitatory synaptic inputs to VTA-projecting LHb neurons are associated with learned helplessness in response to inescapable shock, and behavioral aspects of this state are reversed by in vivo stimulation of the LHb, which may deplete these synaptic inputs (Li et al., 2011). Although both of these responses are thought to engage the LHb-VTA circuitry, the raphe nuclei, including the caudal regions of the DR strongly innervated by the LHb, have also been implicated in models of stress and depression (Hale et al., 2012). Lesions of the habenula (lateral + medial) prevent the rise in DR 5HT levels observed after inescapable shock, as well as reduce learned helplessness (Amat et al., 2001). Further studies should reveal whether the LHb serves to integrate DA and 5HT responses to aversive and rewarding behaviors, or instead show that these systems are modulated by distinct populations of LHb neurons in parallel pathways.
Figure 10. LHb projections to the central RMTg mapped onto the Allen Reference Atlas.
See Figure 9 for legend.
Acknowledgments
We would like to thank Dr. Yun-Wei “Toni” Hsu for helpful comments on the manuscript.
Support: This work was supported by NIMH awards R21 MH090478 and R01 MH093667 to E.T
Abbreviations
- 2Cb
2nd Cerebellar lobule
- 3V
3rd ventricle
- 4V
4th ventricle
- 6N
Abducens nucleus
- 7n
Facial nerve or its root
- A8
A8 dopamine cells
- Aq
Aqueduct (Sylvius)
- Arc
Arcuate hypothalamic nucleus
- ATg
Anterior tegmental nucleus
- Cb
Cerebellum
- CGA
Central gray, alpha part
- CGB
Central gray, beta part
- CGPn
Central gray of the pons
- CIC
Central nucleus of the inferior colliculus
- CLi
Caudal linear nucleus of the raphe
- cp
Cerebral peduncle, basal part
- DG
Dentate gyrus (of hippocampus)
- DMTg
Dorsomedial tegmental area
- DpMe
Deep mesencephalic nucleus
- DR
Dorsal raphe nucleus
- DRC
Dorsal raphe nucleus, caudal part
- DRI
Dorsal raphe nucleus, interfascicular part
- DTg
Dorsal tegmental nucleus
- DTgC
Dorsal tegmental nucleus, central part
- DTgP
Dorsal tegmental nucleus, pericentral part
- f
Fornix
- fr
Fasciculus retroflexus
- Gi
Gigantocellular reticular nucleus
- hc
Habenular commissure
- IF
Interfascicular nucleus
- IMD
Intermediodorsal thalamic nucleus
- IP
Interpeduncular nucleus
- IPC
Interpeduncular nucleus, caudal subnucleus
- IPF
Interpeduncular fossa
- IPI
Interpeduncular nucleus, intermediate subnucleus
- IPL
Interpeduncular nucleus, lateral subnucleus
- IPR
Interpeduncular nucleus, rostral subnucleus
- LC
Locus coeruleus
- LDTg
Laterodorsal tegmental nucleus
- LDTgV
Laterodorsal tegmental nucleus, ventral part
- LH
Lateral hypothalamic area
- LHb
Lateral habenula
- MD
Mediodorsal thalamic nucleus
- Me
Median eminence
- MHb
Medial habenula
- ml
Medial lemniscus
- ML
Medial mammillary nucleus, lateral part
- mlf
Medial longitudinal fasciculus
- MM
Medial mammillary nucleus, medial part
- MnR
Median raphe nucleus
- mp
Mammillary peduncle
- mt
Mammillothalamic tract
- mtg
Mammillotegmental tract
- ns
Nigrostriatal bundle
- PAG
Periaqueductal gray
- PDTg
Posterodorsal tegmental nucleus
- PF
Parafascicular thalamic nucleus
- PH
Posterior hypothalamic area
- pm
Principal mammillary tract
- PMnR
Paramedian raphe nucleus
- PMV
Premammillary nucleus, ventral part
- PN
Paranigral nucleus
- Pn
Pontine nuclei
- PnC
Pontine reticular nucleus, caudal part
- PnO
Pontine reticular nucleus, oral part
- PnR
Pontine raphe nucleus
- PPTg
Pedunculopontine tegmental nucleus
- PR
Prerubral field
- PV
Paraventricular thalamic nucleus
- py
Pyramidal tract
- R
Red nucleus
- RLi
Rostral linear nucleus of the raphe
- RMC
Red nucleus, magnocellular part
- RMg
Raphe magnus nucleus
- RMTg
Rostromedial tegmental nucleus (cRMTg, the caudal part of this nucleus)
- rs
Rubrospinal tract
- RtTg
Reticulotegmental nucleus of the pons
- scp
Superior cerebellar peduncle
- sm
Stria medullaris of thalamus
- SNC
Substantia nigra, compact part
- SNR
Substantia nigra, reticular part
- SuM
Supramammillary nucleus
- ts
Tectospinal tract
- VLPAG
Ventrolateral PAG
- VTA
Ventral tegmental area
- VTg
Ventral tegmental nucleus (of Gudden)
- vtgx
Ventral tegmental decussation
- xscp
Decussation of the superior cerebellar peduncle
Footnotes
Conflict of Interest Statetment: The authors declare that they have no conflicts of interest.
Role of Authors: Study concept and design: LQ, ET. Acquisition of data: LQ, LT, JH, SF. Analysis and interpretation of data: LQ, LN, JH, TJ, ET. Drafting of the manuscript: LQ, TJ, ET. Critical revision of the manuscript for important intellectual content: TJ, SF. Statistical analysis: LN, ET. Obtained funding: ET.
References
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122(2):229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Yanagihara S, Kobayashi M, Niisato K, Takekawa T, Harukuni R, McHugh TJ, Fukai T, Isomura Y, Okamoto H. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J Neurosci. 2013;33(20):8909–8921. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917(1):118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407(1):130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441(1–2):319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Bernard R, Veh RW. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J Comp Neurol. 2012;520(11):2545–2558. doi: 10.1002/cne.23080. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26(4):1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW. Allen reference atlas : a digital color brain atlas of the C57Black/6J male mouse. Hoboken, N.J: Wiley; 2008. p. ix.p. 366. [Google Scholar]
- Fedtsova N, Turner E. Brn-3.0 Expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mechanisms of Development. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Geisler S, Andres KH, Veh RW. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J Comp Neurol. 2003;458(1):78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33(11):2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. J Comp Neurol. 2012;520(6):1278–1300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32(5):695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Wook Oh S, Zeng H. Adeno-associated viral vectors for anterograde axonal tracing with fluorescent proteins in nontransgenic and cre driver mice. Curr Protoc Neurosci. 2012;Chapter 1(Unit 1):20, 21–18. doi: 10.1002/0471142301.ns0120s59. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187(1):19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31(32):11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YW, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE. Medial Habenula Output Circuit Mediated by alpha5 Nicotinic Receptor-Expressing GABAergic Neurons in the Interpeduncular Nucleus. J Neurosci. 2013;33(46):18022–18035. doi: 10.1523/JNEUROSCI.2927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513(6):566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33(17):7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513(6):597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hunt SP, Hrycyshyn AW. Substance P immunoreactivity in the rat interpeduncular nucleus: synaptic interactions between substance P-positive profiles and choline acetyltransferase- or glutamate decarboxylase-immunoreactive structures. Neuroscience. 1991;42(3):739–755. doi: 10.1016/0306-4522(91)90042-m. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513(2):173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10(11):RA261–273. [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Ng L, Thompson C, Pathak S, Kuan L, Jones A, Hawrylycz M. Exploration and visualization of gene expression with neuroanatomy in the adult mouse brain. BMC Bioinformatics. 2008;9:153. doi: 10.1186/1471-2105-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470(7335):535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534(1–2):209–224. doi: 10.1016/0006-8993(90)90131-t. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12(1):77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30(7):1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, Calif. London: Academic; 2001. p. xxv.p. 264. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam; Boston: Elsevier Academic Press; 2005. p. xliii.p. 166. [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21(10):2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25(50):11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quina LA, Wang S, Ng L, Turner EE. Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development. J Neurosci. 2009;29(45):14309–14322. doi: 10.1523/JNEUROSCI.2430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, Osten P. Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat Methods. 2012;9(3):255–258. doi: 10.1038/nmeth.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69(3):445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci. 2010;30(17):5876–5883. doi: 10.1523/JNEUROSCI.3604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15(8):1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6(1):1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467(1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Wagner F, Stroh T, Veh RW. Correlating habenular subnuclei in rat and mouse by using topographic, morphological, and cytochemical criteria. J Comp Neurol. 2014;522(11):2650–2662. doi: 10.1002/cne.23554. [DOI] [PubMed] [Google Scholar]
- Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2011;216(1):463–465. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]